Plant Iron Research in African Countries: Current “Hot Spots”, Approaches, and Potentialities
Abstract
:1. Introduction
2. Plant Fe Research Prominently Conducted in Africa, from 2018 to 2023
3. Research Hotspot: Fe Deficiency and Crops Biofortification
3.1. Rice
3.2. Wheat
3.3. Maize
3.4. Barley
3.5. Millet
3.6. Legumes
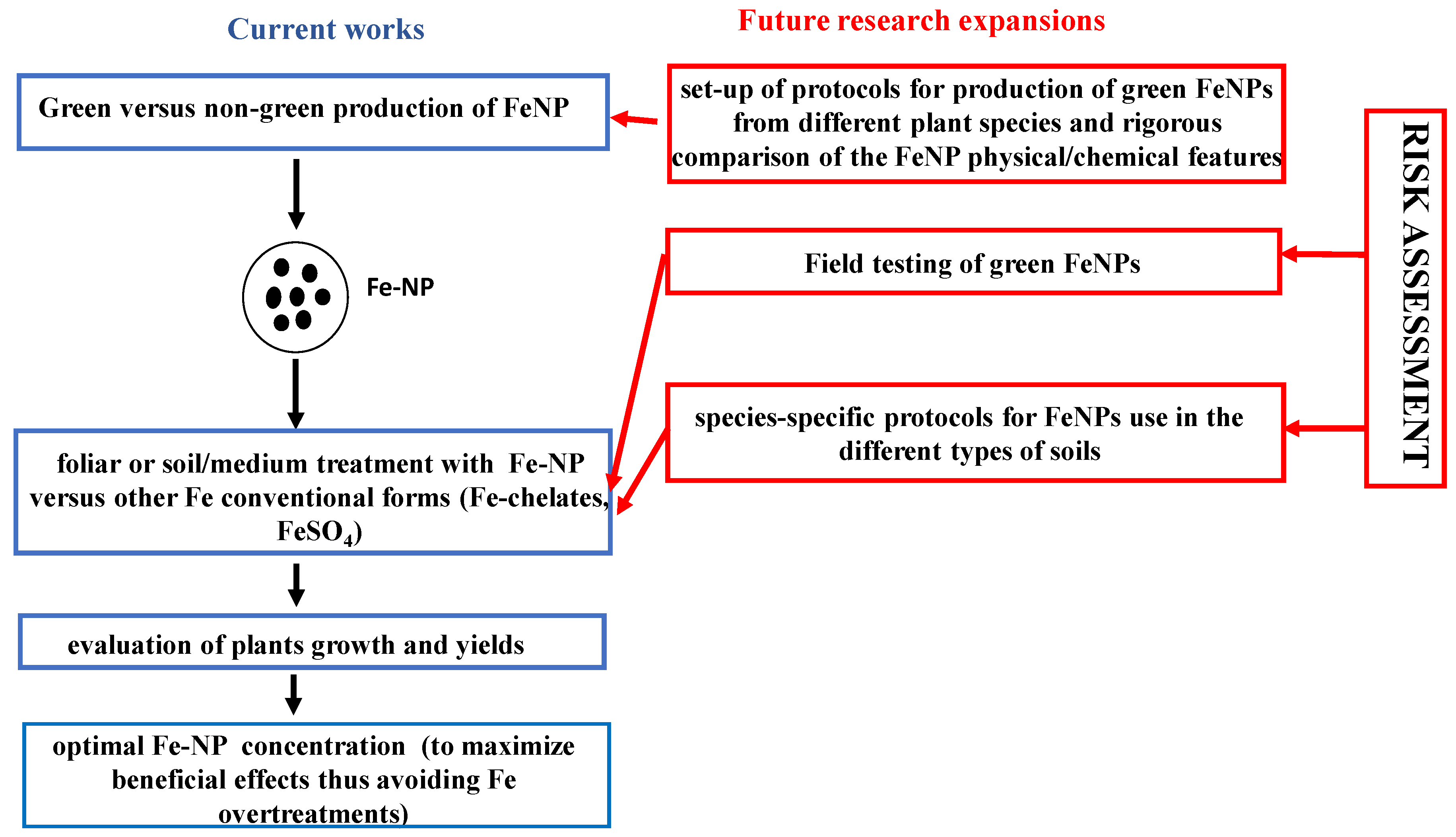
4. Research Hotspot: Sustainable Agriculture and Fe Nanoparticles
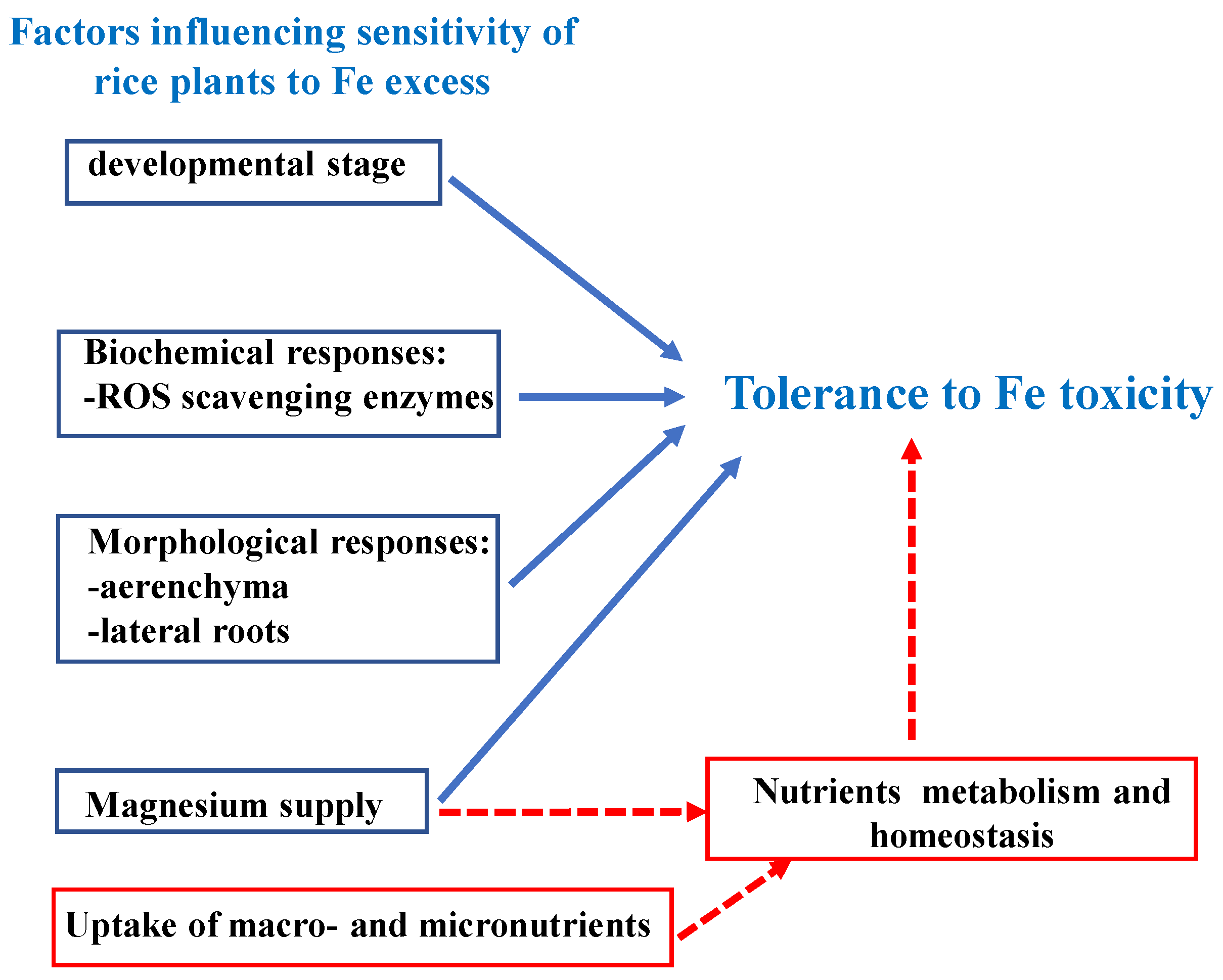
5. Research Hotspot: Fe Toxicity
6. Other Research Lines
7. Discussion
8. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajniak, J.; Giehl, R.F.; Chang, E.; Murgia, I.; von Wirén, N.; Sattely, E.S. Biosynthesis of redox-active metabolites in response to iron deficiency in plants. Nat. Chem. Biol. 2018, 14, 442–450. [Google Scholar] [CrossRef]
- Liang, G. Iron uptake, signaling, and sensing in plants. Plant Commun. 2022, 3, 100349. [Google Scholar] [CrossRef] [PubMed]
- Murgia, I.; Marzorati, F.; Vigani, G.; Morandini, P. Plant iron nutrition in the long road from soil to seeds. J. Exp. Bot. 2022, 73, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Murgia, I.; Midali, A.; Cimini, S.; De Gara, L.; Manasherova, E.; Cohen, H.; Paucelle, A.; Morandini, P. The Arabidopsis thaliana Gulono-1,4 γ-lactone oxidase 2 (GULLO2) facilitates iron transport from endosperm into developing embryos and affects seed coat suberization. Plant Physiol. Biochem. 2023, 196, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Morandini, P.; Murgia, I. Searching iron sensors in plants by exploring the link among 2′OG-dependent dioxygenases, the iron deficiency response and metabolic adjustments occurring under iron deficiency. Front. Plant Sci. 2013, 4, 169. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Murgia, I. Iron-requiring enzymes in the spotlight of oxygen. Trends Plant Sci. 2018, 23, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, L.; Simontacchi, M.; Murgia, I.; Zabaleta, E.; Lamattina, L. Nitric Oxide, Nitrosyl Iron complexes, ferritin and frataxin: A well equipped team to preserve plant iron homeostasis. Plant Sci. 2011, 181, 582–592. [Google Scholar] [CrossRef]
- Sági-Kazár, M.; Solymosi, K.; Solti, Á. Iron in leaves: Chemical forms, signalling, and in-cell distribution. J. Exp. Bot. 2022, 73, 1717–1734. [Google Scholar] [CrossRef]
- Harbort, C.J.; Hashimoto, M.; Inoue, H.; Niu, Y.; Guan, R.; Rombolà, A.D.; Kopriva, S.; Voges, M.J.E.E.E.; Sattely, E.S.; Garrido-Oter, R.; et al. Root-secreted coumarins and the microbiota interact to improve iron nutrition in Arabidopsis. Cell Host Microbe 2020, 28, 825–837. [Google Scholar] [CrossRef]
- Murgia, I.; Arosio, P.; Tarantino, D.; Soave, C. Biofortification for combating “hidden hunger” for iron. Trends Plant Sci. 2012, 17, 47–55. [Google Scholar] [CrossRef]
- Connorton, J.M.; Balk, J. Iron biofortification of staple crops: Lessons and challenges in plant genetics. Plant Cell Physiol. 2019, 60, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Stangoulis, J.C.R.; Knez, M. Biofortification of major crop plants with iron and zinc—Achievements and future directions. Plant Soil 2022, 474, 57–76. [Google Scholar] [CrossRef]
- Di Silvestre, D.; Vigani, G.; Mauri, P.; Hammadi, S.; Morandini, P.; Murgia, I. Network topological analysis for the identification of novel hubs in plant nutrition. Front. Plant Sci. 2021, 12, 629013. [Google Scholar] [CrossRef] [PubMed]
- Vigani, G.; Di Silvestre, D.; Agresta, A.M.; Donnini, S.; Mauri, P.; Gehl, C.; Bittner, F.; Murgia, I. Molybdenum and iron mutually impact their homeostasis in cucumber (Cucumis sativus) plants. New Phytol. 2017, 213, 1222–1241. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Cózatl, D.G.; Gokul, A.; Carelse, M.F.; Jobe, T.O.; Long, T.A.; Keyster, M. Keep talking: Crosstalk between iron and sulfur networks fine-tunes growth and development to promote survival under iron limitation. J. Exp. Bot. 2019, 70, 4197–4210. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, M.N.; Mzembe, G.; Moya, E.; Verhoef, H. Iron deficiency anaemia in sub-Saharan Africa: A review of current evidence and primary care recommendations for high-risk groups. Lancet Haematol. 2021, 8, e732–e743. [Google Scholar] [CrossRef]
- Onyango, D.A.; Entila, F.; Dida, M.M.; Ismail, A.M.; Drame, K.N. Mechanistic understanding of iron toxicity tolerance in contrasting rice varieties from Africa: 1. Morpho-physiological and biochemical responses. Funct. Plant Biol. 2018, 46, 93–105. [Google Scholar] [CrossRef]
- Onyango, D.A.; Entila, F.; Egdane, J.; Pacleb, M.; Katimbang, M.L.; Dida, M.M.; Ismail, A.M.; Drame, K.N. Mechanistic understanding of iron toxicity tolerance in contrasting rice varieties from Africa: 2. Root oxidation ability and oxidative stress control. Funct. Plant Biol. 2020, 47, 145–155. [Google Scholar] [CrossRef]
- Kirk, G.J.D.; Manwaring, H.R.; Ueda, Y.; Semwal, V.K.; Wissuwa, M. Below-ground plant–soil interactions affecting adaptations of rice to iron toxicity. Plant Cell Environ. 2022, 45, 705–718. [Google Scholar] [CrossRef]
- Ullah, H.; Ahmed, S.F.; Santiago-Arenas, R.; Himanshu, S.K.; Mansour, E.; Chaum, S.; Datta, A. Tolerance mechanism and management concepts of iron toxicity in rice: A critical review. Adv. Agron. 2023, 177, 215–257. [Google Scholar]
- Melandri, G.; Sikirou, M.; Arbelaez, J.D.; Shittu, A.; Semwal, V.K.; Konaté, K.A.; Maji, A.T.; Ngaujah, S.A.; Akintayo, I.; Govindaraj, V.; et al. Multiple small-effect alleles of indica origin enhance high iron-associated stress tolerance in rice under field conditions in West Africa. Front. Plant Sci. 2021, 11, 604938. [Google Scholar] [CrossRef]
- Sakariyawo, O.S.; Oyedeji, O.E.; Soretire, A.A. Effect of iron deficiency on the growth, development and grain yield of some selected upland rice genotypes in the rainforest. J. Plant Nutr. 2020, 43, 851–863. [Google Scholar] [CrossRef]
- Van Oort, P.A.J. Mapping abiotic stresses for rice in Africa: Drought, cold, iron toxicity, salinity and sodicity. Field Crops Res. 2018, 219, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Rajonandraina, T.; Rakotoson, T.; Wissuwa, M.; Ueda, Y.; Razafimbelo, T.; Andriamananjara, A.; Kirk, G.J.D. Mechanisms of genotypic differences in tolerance of iron toxicity in field-grown rice. Field Crops Res. 2023, 298, 108953. [Google Scholar] [CrossRef]
- Rajonandraina, T.; Ueda, Y.; Wissuwa, M.; Kirk, G.J.D.; Rakotoson, T.; Manwaring, H.; Andriamananjara, A.; Razafimbelo, T. Magnesium supply alleviates iron toxicity-induced leaf bronzing in rice through exclusion and tissue-tolerance mechanisms. Front. Plant Sci. 2023, 14, 1213456. [Google Scholar] [CrossRef] [PubMed]
- Mayaba, T.; Sawadogo, N.; Ouédraogo, M.H.; Sawadogo, B.; Aziadekey, M.; Sié, M.; Sawadogo, M. Genetic diversity of African’s rice (Oryza glaberrima Steud.) accessions cultivated under iron toxicity. Aust. J. Crop Sci. 2020, 14, 415–421. [Google Scholar] [CrossRef]
- Nasar, J.; Wang, G.Y.; Zhou, F.J.; Gitari, H.; Zhou, X.B.; Tabl, K.M.; Hasan, M.E.; Ali, H.; Waqas, M.M.; Ali, I.; et al. Nitrogen fertilization coupled with foliar application of iron and molybdenum improves shade tolerance of soybean under maize-soybean intercropping. Front. Plant Sci. 2022, 13, 1014640. [Google Scholar] [CrossRef] [PubMed]
- Nasar, J.; Wang, G.-Y.; Ahmad, S.; Muhammad, I.; Zeeshan, M.; Gitari, H.; Adnan, M.; Fahad, S.; Khalid, M.H.B.; Zhou, X.-B.; et al. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 2022, 13, 988055. [Google Scholar] [CrossRef]
- Akhtar, S.; Osthoff, G.; Mashingaidze, K.; Labuschagne, M. Iron and zinc in maize in the developing world: Deficiency, availability, and breeding. Crop Sci. 2018, 58, 2200–2213. [Google Scholar] [CrossRef]
- Akhtar, S.; Labuschagne, M.; Osthoff, G.; Mashingaidze, K.; Hossain, A. Xenia and deficit nitrogen influence the iron and zinc concentration in the grains of hybrid maize. Agronomy 2021, 11, 1388. [Google Scholar] [CrossRef]
- Akhtar, S.; Mekonnen, T.W.; Osthoff, G.; Mashingaidz, K.; Labuschagne, M. Genotype by environment interaction in grain iron and zinc concentration and yield of maize hybrids under low nitrogen and optimal conditions. Plants 2023, 12, 1463. [Google Scholar] [CrossRef] [PubMed]
- Goredema-Matongera, N.; Ndhlela, T.; van Biljon, A.; Kamutando, C.N.; Cairns, J.E.; Baudron, F.; Labuschagne, M. Genetic variation of zinc and iron concentration in normal, provitamin a and quality protein maize under stress and non-stress conditions. Plants 2023, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Manzeke-Kangara, M.G.; Mtambanengwe, F.; Watts, M.J.; Broadley, M.R.; Lark, R.M.; Mapfumo, P. Can nitrogen fertilizer management improve grain iron concentration of agro-biofortified crops in Zimbabwe? Agronomy 2021, 11, 124. [Google Scholar] [CrossRef]
- Udo, E.; Abe, A.; Meseka, S.; Mengesha, W.; Menkir, A. Genetic Analysis of zinc, iron and provitamin A content in tropical maize (Zea mays L.). Agronomy 2023, 13, 266. [Google Scholar] [CrossRef]
- Saradadevi, R.; Mukankusi, C.; Li, L.; Amongi, W.; Mbiu, J.P.; Raatz, B.; Ariza, D.; Beebe, S.; Varshney, R.K.; Huttner, E.; et al. Multivariate genomic analysis and optimal contributions selection predicts high genetic gains in cooking time, iron, zinc, and grain yield in common beans in East Africa. Plant Genome 2021, 14, e20156. [Google Scholar] [CrossRef] [PubMed]
- Katuuramu, D.N.; Wiesinger, J.A.; Luyima, G.B.; Nkalubo, S.T.; Glahn, R.P.; Cichy, K.A. Investigation of genotype by environment interactions for seed zinc and iron concentration and iron bioavailability in common bean. Front. Plant Sci. 2021, 12, 670965. [Google Scholar] [CrossRef] [PubMed]
- Nsiri, K.; Krouma, A. The key physiological and biochemical traits underlying common bean (Phaseolus vulgaris L.) response to iron deficiency, and related interrelationships. Agronomy 2023, 13, 2148. [Google Scholar] [CrossRef]
- Philipo, M.; Ndakidemi, P.A.; Mbega, E.R. Environmental and genotypes influence on seed iron and zinc levels of landraces and improved varieties of common bean (Phaseolus vulgaris L.) in Tanzania. Ecol. Genet. Genom. 2020, 15, 100056. [Google Scholar] [CrossRef]
- Gelaw, Y.M.; Eleblu, J.S.Y.; Ofori, K.; Fenta, B.A.; Mukankusi, C.; Offei, S. Genome-wide association study of grain iron and zinc concentration in common bean (Phaseolus vulgaris). Plant Breed. 2023, 142, 357–371. [Google Scholar] [CrossRef]
- Salama, Z.A.; El Fouly, M.M. Can copper and zinc in different chemical forms can improve iron deficient in phaseolus plant. Iraqi J. Agric. Sci. 2020, 51, 278–286. [Google Scholar]
- Mira, M.M.; Asmundson, B.; Renault, S.; Hill, R.D.; Stasolla, C. Suppression of the soybean (Glycine max) phytoglobin GmPgb1 improves tolerance to iron stress. Acta Physiol. Plant. 2021, 43, 147. [Google Scholar] [CrossRef]
- Haque, A.F.M.M.; Rahman, M.A.; Das, U.; Rahman, M.M.; Elseehy, M.M.; El-Shehawi, A.M.; Parvez, M.S.; Kabir, A.H. Changes in physiological responses and MTP (metal tolerance protein) transcripts in soybean (Glycine max) exposed to differential iron availability. Plant Physiol. Biochem. 2022, 179, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Knijnenburg, J.T.N.; Hilty, F.M.; Oelofse, J.; Buitendag, R.; Zimmermann, M.B.; Cakmak, I.; Grobler, A.F. Nano- and Pheroid technologies for development of foliar iron fertilizers and iron biofortification of soybean grown in South Africa. Chem. Biol. Technol. Agric. 2018, 5, 26. [Google Scholar] [CrossRef]
- Ramadan, A.A.E.-M.; El-Bassiouny, H.M.S.; Bakry, B.A.; Abdallah, M.M.S.; El-Enany, M.A.M. Growth, yield and biochemical changes of soybean plant in response to iron and magnesium oxide nanoparticles. Pak. J. Biol. Sci. 2020, 23, 406–417. [Google Scholar] [CrossRef]
- Gangashetty, P.I.; Riyazaddin, M.; Sanogo, M.D.; Inousa, D.; Issoufou, K.A.; Asungre, P.A.; Sy, O.; Govindaraj, M.; Ignatius, A.I. Identification of high-yielding iron-biofortified open-pollinated varieties of pearl millet in West Africa. Front. Plant Sci. 2021, 12, 688937. [Google Scholar] [CrossRef] [PubMed]
- Asungre, P.A.; Akromah, R.; Kena, A.W.; Gangashetty, P. Genotype by environment interaction on grain yield stability and iron and zinc content in OPV of pearl millet in Ghana using the AMMI method. Int. J. Agron. 2021, 2021, 9656653. [Google Scholar] [CrossRef]
- Asungre, P.A.; Akromah, R.; Kena, A.W.; Gangashetty, P. Assessing the adaptability and stability of new pearl millet hybrids for grain yield, grain iron and zinc content in Ghana using AMMI analysis. J. Crop Sci. Biotechnol. 2022, 25, 501–514. [Google Scholar] [CrossRef]
- Gaoh, B.B.S.; Gangashetty, P.I.; Mohammed, R.; Dzidzienyo, D.K.; Tongoona, P. Generation mean analysis of pearl millet [Pennisetum glaucum (L.) R. Br.] grain iron and zinc contents and agronomic traits in West Africa. J. Cereal Sci. 2020, 96, 103066. [Google Scholar] [CrossRef]
- Teklu, D.; Gashu, D.; Joy, E.J.M.; Lark, R.M.; Bailey, E.H.; Wilson, L.; Amede, T.; Broadley, M.R. Genotypic response of finger millet to zinc and iron agronomic biofortification, location and slope position towards yield. Agronomy 2023, 13, 1452. [Google Scholar] [CrossRef]
- Wafula, W.N.; Korir, N.K.; Ojulong, H.F.; Siambi, M.; Gweyi-Onyango, J.P. Protein, calcium, zinc, and iron contents of finger millet grain response to varietal differences and phosphorus application in Kenya. Agronomy 2018, 8, 24. [Google Scholar] [CrossRef]
- Krouma, A. Differential response of pea (Pisum sativum L.) genotypes to iron deficiency in relation to the growth, rhizosphere acidification and ferric chelate reductase activities. Aust. J. Crop Sci. 2021, 15, 925–932. [Google Scholar] [CrossRef]
- Barhoumi, S.; Ellouzi, H.; Krouma, A. Functional analysis of the genotypic differences in response of pea (Pisum sativum L.) to calcareous-induced iron deficiency. Phyton-Int. J. Exp. Bot. 2023, 92, 521–536. [Google Scholar] [CrossRef]
- Coppa, E.; Vigani, G.; Aref, R.; Savatin, D.; Bigini, V.; Hell, R.; Astolfi, S. Differential modulation of Target of Rapamycin activity under single and combined iron and sulfur deficiency in tomato plants. Plant J. 2023, 115, 127–138. [Google Scholar] [CrossRef] [PubMed]
- El-Desouky, H.S.; Islam, K.R.; Bergefurd, B.; Gao, G.; Harker, T.; Abd-El-Dayem, H.; Ismail, F.; Mady, M.; Zewail, R.M.Y. Nano iron fertilization significantly increases tomato yield by increasing plants’ vegetable growth and photosynthetic efficiency. J. Plant Nutr. 2021, 44, 1649–1663. [Google Scholar] [CrossRef]
- Olowolaju, E.D.; Okunlola, G.O.; Ayeotan, O.J. Growth, yield and uptake of some nutrients by tomato as affected by iron concentration. Int. J. Veg. Sci. 2021, 27, 378–387. [Google Scholar] [CrossRef]
- Prity, S.A.; El-Shehawi, A.M.; Elseehy, M.M.; Tahura, S.; Kabir, A.H. Early-stage iron deficiency alters physiological processes and iron transporter expression, along with photosynthetic and oxidative damage to sorghum. Saudi J. Biol. Sci. 2021, 28, 4770–4777. [Google Scholar] [CrossRef] [PubMed]
- Maswada, H.F.; Djanaguiraman, M.; Prasad, P.V.V. Seed treatment with nano-iron (III) oxide enhances germination, seeding growth and salinity tolerance of sorghum. J. Agron. Crop Sci. 2018, 204, 577–587. [Google Scholar] [CrossRef]
- Abou-Baker, N.H.; Hussein, M.M.; El-Ashry, S.M. Comparison between nano iron and iron EDTA as foliar fertilizers under salt stress conditions. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 17–32. [Google Scholar]
- Bhattacharya, S.; Das, A.; Banerjee, J.; Mandal, S.N.; Kumar, S.; Gupta, S. Elucidating genetic variability and genotype × environment interactions for grain iron and zinc content among diverse genotypes of lentils (Lens culinaris). Plant Breed. 2022, 141, 786–800. [Google Scholar] [CrossRef]
- Gupta, S.; Das, S.; Dikshit, H.K.; Mishra, G.P.; Aski, M.S.; Bansal, R.; Tripathi, K.; Bhowmik, A.; Kumar, S. Genotype by environment interaction effect on grain iron and zinc concentration of indian and mediterranean lentil genotypes. Agronomy 2021, 11, 1761. [Google Scholar] [CrossRef]
- Mannan, M.A.; Tithi, M.A.; Islam, M.R.; Al Mamun, M.A.; Mia, S.; Rahman, M.Z.; Awad, M.F.; ElSayed, A.I.; Mansour, E.; Hossain, M.S. Soil and foliar applications of zinc sulfate and iron sulfate alleviate the destructive impacts of drought stress in wheat. Cereal Res. Commun. 2022, 50, 1279–1289. [Google Scholar] [CrossRef]
- Gorafi, Y.S.A.; Ishii, T.; Kim, J.-S.; Elbashir, A.A.E.; Tsujimoto, H. Genetic variation and association mapping of grain iron and zinc contents in synthetic hexaploid wheat germplasm. Plant Genet. Resour. Characterisation Util. 2018, 16, 9–17. [Google Scholar] [CrossRef]
- Kallala, N.; M’sehli, W.; Hammi, K.M.; Abid, G.; Mhadhbi, H. Efficiency of antioxidant system in barrel medic (Medicago truncatula) genotypes and the orchestration of their key components under iron deficiency. Crop Pasture Sci. 2021, 73, 138–148. [Google Scholar] [CrossRef]
- Kallala, N.; M’sehli, W.; Jelali, K.; Batnini, M.; Badri, M.; Nouairi, I.; Mhadhbi, H. Biodiversity within Medicago truncatula genotypes toward response to iron deficiency: Investigation of main tolerance mechanisms. Plant Species Biol. 2019, 34, 95–109. [Google Scholar] [CrossRef]
- Ifie, J.E.; Ifie-Etumah, S.; Ikhajiagbe, B. Physiological and biochemical responses of selected cowpea (Vigna unguiculata (L.) Walp.) accessions to iron toxicity [Fiziološki in biokemični odziv akcesij kitajske vinje (Vigna unguiculata (L.) Walp.) na toksičnost železa]. Acta Agric. Slov. 2020, 115, 25–38. [Google Scholar] [CrossRef]
- Sileshi, F.; Nebiyu, A.; Van Geel, M.; Abeele, S.V.; Du Laing, G.; Boeckx, P. Spatial variability of iron, zinc and selenium content in faba bean (Vicia faba L.) seeds from central and southwestern highlands of Ethiopia. Plant Soil 2022, 473, 351–368. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Ayad, A.A.; Abdel-Aziz, H.S.M.; Williams, L.L.; El-Shazoly, R.M.; Abdel-Wahab, A.; Abdeldaym, E.A. Foliar application of different iron sources improves morpho-physiological traits and nutritional quality of broad bean grown in sandy soil. Plants 2022, 11, 2599. [Google Scholar] [CrossRef]
- Nyiraguhirwa, S.; Grana, Z.; Ouabbou, H.; Iraqi, D.; Ibriz, M.; Mamidi, S.; Udupa, S.M. A genome-wide association study identifying single-nucleotide polymorphisms for iron and zinc biofortification in a worldwide barley collection. Plants 2022, 11, 1349. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Maqbool, M.; Akram, R.; Shahid, A.; Shokralla, S.; Al-Ghobari, H.; Alataway, A.; Dewidar, A.Z.; El-Sabrout, A.M.; et al. Seed priming with iron oxide nanoparticles raises biomass production and agronomic profile of water-stressed flax plants. Agronomy 2022, 12, 982. [Google Scholar] [CrossRef]
- Shareef, H.J.; Hzaa, A.Y.L.; Elsheery, N.I. Foliar iron and zinc nano-fertilizers enhance growth, mineral uptake, and antioxidant defense in date palm (Phoenix dactylifera L.) seedlings. Folia Oecologica 2023, 50, 185–195. [Google Scholar] [CrossRef]
- Barhoumi, Z.; Atia, A.; Hussain, A.A.; Maatallah, M.; Alalmaie, A.; Alaskri, K.I.; Assiri, A.M. Effects of salinity and iron deficiency on growth and physiological attributes of Avicennia marina (Forssk.) Vierh. Arch. Agron. Soil Sci. 2023, 69, 2753–2766. [Google Scholar] [CrossRef]
- Ndaba, B.; Roopnarain, A.; Vatsha, B.; Marx, S.; Maaza, M. Synthesis, characterization, and evaluation of Artemisia afra-mediated iron nanoparticles as a potential nano-priming agent for seed germination. ACS Agric. Sci. Technol. 2022, 2, 1218–1229. [Google Scholar] [CrossRef]
- Alharbi, K.; Alshallash, K.S.; Hamdy, A.E.; Khalifa, S.M.; Abdel-Aziz, H.F.; Sharaf, A.; Abobatta, W.F. Magnetic iron–improved growth, leaf chemical content, yield, and fruit quality of chinese mandarin trees grown under soil salinity stress. Plants 2022, 11, 2839. [Google Scholar] [CrossRef] [PubMed]
- Mnafgui, W.; Rizzo, V.; Muratore, G.; Hajlaoui, H.; Schinoff, B.D.O.; Mnafgui, K.; Elleuch, A. Trigonella foenum-graecum morphophysiological and phytochemical processes controlling iron uptake and translocation. Crop Pasture Sci. 2022, 73, 957–968. [Google Scholar] [CrossRef]
- El-Sonbaty, A.E.; Farouk, S.; Al-Yasi, H.M.; Ali, E.F.; Abdel-Kader, A.A.S.; El-Gamal, S.M.A. Enhancement of rose scented geranium plant growth, secondary metabolites, and essential oil components through foliar applications of iron (nano, sulfur and chelate) in alkaline soils. Agronomy 2022, 12, 2164. [Google Scholar] [CrossRef]
- Saudy, H.S.; El–Samad, G.A.A.; El–Temsah, M.E.; El–Gabry, Y.A.E.-G. Effect of iron, zinc, and manganese nano-form mixture on the micronutrient recovery efficiency and seed yield response index of sesame genotypes. J. Soil Sci. Plant Nutr. 2022, 22, 732–742. [Google Scholar] [CrossRef]
- Salhi, K.; Hajlaoui, H.; Krouma, A. Genotypic differences in response of durum wheat (Triticum durum Desf.) to lime-induced iron chlorosis. Plant Direct 2022, 6, e377. [Google Scholar] [CrossRef]
- El-Shehawi, A.M.; Rahman, M.A.; Elseehy, M.M.; Kabir, A.H. Mercury toxicity causes iron and sulfur deficiencies along with oxidative injuries in alfalfa (Medicago sativa). Plant Biosyst. 2022, 156, 284–291. [Google Scholar] [CrossRef]
- El-Gioushy, S.F.; Ding, Z.; Bahloul, A.M.E.; Gawish, M.S.; Abou El Ghit, H.M.; Abdelaziz, A.M.R.A.; El-Desouky, H.S.; Sami, R.; Khojah, E.; Hashim, T.A.; et al. Foliar application of nano, chelated, and conventional iron forms enhanced growth, nutritional status, fruiting aspects, and fruit quality of washington navel orange trees (Citrus sinensis L. Osbeck). Plants 2021, 10, 2577. [Google Scholar] [CrossRef]
- El-Monem, A.A.A.; Abdallah, M.M.S.; Bakry, B.A.; El-Bassiouny, H.M.S.; Sadak, M.S. Physiological role of iron chelators and/or arginine for improving yield and active constituents of roselle sepals. Asian J. Plant Sci. 2020, 19, 77–90. [Google Scholar]
- González, M.R.; Hailemichael, G.; Catalina, Á.; Martín, P. Combined effects of water status and iron deficiency chlorosis on grape composition. Sci. Agric. 2019, 76, 473–480. [Google Scholar] [CrossRef]
- Ben Abdallah, H.; Mai, H.J.; Slatni, T.; Fink-Straube, C.; Abdelly, C.; Bauer, P. Natural variation in physiological responses of tunisian Hedysarum carnosum under iron deficiency. Front. Plant Sci. 2018, 9, 1383. [Google Scholar] [CrossRef] [PubMed]
- Wissal, M.; Nadia, K.; Haythem, M. Legumes: Model plants for sustainable agriculture in phosphorus and iron deficient soils. Agric. Sci. Dig. 2020, 40, 445–447. [Google Scholar] [CrossRef]
- Garcia-Oliveira, A.L.; Chander, S.; Ortiz, R.; Menkir, A.; Gedil, M. Genetic basis and breeding perspectives of grain iron and zinc enrichment in cereals. Front. Plant Sci. 2018, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Mc Inturf, S.A.; Khan, M.A.; Gokul, A.; Castro-Guerrero, N.A.; Höhner, R.; Li, J.; Marjault, H.-B.; Fichman, Y.; Kunz, H.-H.; Goggin, F.L.; et al. Cadmium interference with iron sensing reveals transcriptional programs sensitive and insensitive to reactive oxygen species. J. Exp. Bot. 2022, 73, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nishizawa, N.K. Iron sensors and signals in response to iron deficiency. Plant Sci. 2014, 224, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Riaz, N.; Guerinot, M.L. All together now: Regulation of the iron deficiency response. J. Exp. Bot. 2021, 72, 2045–2055. [Google Scholar] [CrossRef]
- Therby-Vale, R.; Lacombe, B.; Rhee, S.Y.; Nussaume, L.; Rouached, H. Mineral nutrient signaling controls photosynthesis: Focus on iron deficiency-induced chlorosis. Trends Plant Sci. 2022, 27, 502–509. [Google Scholar] [CrossRef]
- Jones, M.P.; Dingkuhn, M.; Aluko, G.K.; Semon, M. Interspecific Oryza sativa L. X O. glaberrima Steud. progenies in upland improvement. Euphytica 1997, 92, 237–246. [Google Scholar] [CrossRef]
- Heuer, S.; Miézan, K.M.; Sié, M.; Gaye, S. Increasing biodiversity of irrigated rice in Africa by interspecific crossing of Oryza glaberrima (Steud.) x O. sativa (L.). Euphytica 2003, 132, 31–40. [Google Scholar] [CrossRef]
- Orr, S.; Sumberg, J.; Erenstein, O.; Oswald, A. Funding international agricultural research and the need to be noticed. A case study of NERICA Rice. Outlook Agric. 2008, 37, 159–168. [Google Scholar] [CrossRef]
- Olivares Diaz, E.; Kawamura, S.; Koseki, S. Physical Properties of NERICA Compared to Indica and Japonica types of rice. Agric. Mech. Asia Afr. Lat. Am. 2018, 49, 68–73. [Google Scholar]
- Matsuoka, Y.; Takumi, S.; Kawahara, T. Natural variation for fertile triploid F1 hybrid formation in allohexaploid wheat speciation. Theor. Appl. Genet. 2007, 115, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Satyavathi, C.T.; Ambawat, S.; Khandelwal, V.; Srivastava, R.K. Pearl Millet: A climate-resilient nutricereal for mitigating hidden hunger and provide nutritional security. Front. Plant Sci. 2021, 12, 659938. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Babu, S.; Singh, R.; Yadav, D.; Singh Rathore, S.; Raj, R.; Avasthe, R.; Yadav, S.K.; Das, A.; Yadav, V.; Yadav, B.; et al. Nanofertilizers for agricultural and environmental sustainability. Chemosphere 2022, 292, 133451. [Google Scholar] [CrossRef]
- Zhu, M.T.; Wang, B.; Wang, Y.; Yuan, L.; Wang, H.J.; Wang, M.; Ouyang, H.; Chai, Z.F.; Feng, W.Y.; Zhao, Y.L. Endothelial dysfunction and inflammation induced by iron oxide nanoparticle exposure: Risk factors for early atherosclerosis. Toxicol. Lett. 2011, 203, 162–171. [Google Scholar] [CrossRef]
- Maher, B.A.; González-Maciel, A.; Reynoso-Robles, R.; Torres-Jardón, R.; Calderón-Garcidueñas, L. Iron-rich air pollution nanoparticles: An unrecognised environmental risk factor for myocardial mitochondrial dysfunction and cardiac oxidative stress. Environ. Res. 2020, 188, 109816. [Google Scholar] [CrossRef]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef]
- Aung, M.S.; Masuda, H. How does rice defend against excess iron? Physiological and molecular mechanisms. Front. Plant Sci. 2020, 11, 1102. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Chen, Y. Green synthesis of iron nanoparticles and their environmental application and implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Zhou, Q.; Zheng, T.; Mo, F.; Ouyang, S. Iron oxide nanoparticles in the soil environment: Adsorption, transformation, and environmental risk. J. Hazard. Mater. 2023, 459, 132107. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, T.G.; Stueckle, T.A.; Antonini, J.A.; Rojanasakul, Y.; Castranova, V.; Yang, Y.; Wang, L. Potential toxicity and underlying mechanisms associated with pulmonary exposure to iron oxide nanoparticles: Conflicting literature and unclear risk. Nanomaterials 2017, 7, 307. [Google Scholar] [CrossRef]
- Murgia, I.; Delledonne, M.; Soave, C. Nitric oxide mediates iron-induced ferritin accumulation in Arabidopsis. Plant J. 2002, 30, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, N.; Murgia, I.; Boucherez, J.; Briat, J.F.; Cellier, F.; Gaymard, F. An iron-induced nitric oxide burst precedes ubiquitin-dependent protein degradation for Arabidopsis AtFer1 ferritin gene expression. J. Biol. Chem. 2006, 281, 23579–23588. [Google Scholar] [CrossRef] [PubMed]
- Murgia, I.; Vazzola, V.; Tarantino, D.; Cellier, F.; Ravet, K.; Briat, J.F.; Soave, C. Knock-out of the ferritin AtFer1 causes earlier onset of age-dependent leaf senescence in Arabidopsis. Plant Physiol. Biochem. 2007, 45, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Briat, J.F.; Céline Duc, C.; Karl Ravet, K.; Gaymard, F. Ferritins and iron storage in plants. Biochim. Biophys. Acta (BBA) 2010, 1800, 806–814. [Google Scholar] [CrossRef]
- Briat, J.F.; Ravet, K.; Arnaud, N.; Duc, C.; Boucherez, J.; Touraine, B.; Cellier, F.; Gaymard, F. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann. Bot. 2010, 105, 811–822. [Google Scholar] [CrossRef]
- Vigani, G.; Tarantino, D.; Murgia, I. Mitochondrial ferritin is a functional iron-storage protein in cucumber (Cucumis sativus) roots. Front. Plant Sci. 2013, 4, 316. [Google Scholar] [CrossRef]
| Plant Species | Publications | Publications with African Prominent Authorship |
|---|---|---|
| rice (various Oryza species) | [17,18,19,20,21,22,23,24,25,26] | [17,18,21,22,23,24,25,26] |
| maize (Zea mays) | [27,28,29,30,31,32,33,34] | [29,30,31,32,33,34] |
| common bean (Phaseolus vulgaris) | [35,36,37,38,39,40] | [37,38,39,40] |
| soybean (Glycine max) | [27,41,42,43,44] | [44] |
| pearl millet (Pennisetum glaucum) | [45,46,47,48] | [45,46,47,48] |
| finger millet (Eleusine coracana) | [33,49,50] | [33,49,50] |
| pea (Pisum sativum) | [51,52] | [51,52] |
| tomato (Solanum lycopersicum) | [53,54,55] | [53,54,55] |
| sorghum (Sorghum bicolor) | [56,57,58] | [57,58] |
| lentil (Lens culinaris) | [59,60] | |
| wheat (various species) | [61,62] | [61,62] |
| barrel medic (Medicago truncatula) | [63,64] | [63,64] |
| cowpea (Vigna unguiculata) | [33,65] | [33,65] |
| broad bean (Vicia faba) | [66,67] | [66,67] |
| barley (Hordeum vulgare) | [68] | [68] |
| flax (Linum usitatissimum) | [69] | |
| date palm (Phoenix dactylifera) | [70] | |
| grey mangrove (Avicennia marina) | [71] | |
| African wormwood (Artemisia afra) | [72] | [72] |
| spinach (Spinacia oleracea) | [72] | [72] |
| carrot (Dacus carota) | [72] | [72] |
| Chinese mandarine (Citrus reticulata Blanco) | [73] | |
| fenugreek (Trigonella foenum-graecum) | [74] | [74] |
| rose-scented geranium (Pelargonium graveolens) | [75] | [75] |
| sesame (Sesamum indicum) | [76] | [76] |
| durum wheat (Triticum durum) | [77] | [77] |
| alfalfa (Medicago sativa) | [78] | |
| Washington navel orange (Citrus sinensis) | [79] | |
| roselle (Hibiscus sabdariffa) | [80] | [80] |
| grape (Vitis vinifera) | [81] | |
| Sulla carnosa (Hedysarum carnosum) | [82] | |
| legumes | [83] | [83] |
| cereals | [84] | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murgia, I.; Morandini, P. Plant Iron Research in African Countries: Current “Hot Spots”, Approaches, and Potentialities. Plants 2024, 13, 14. https://doi.org/10.3390/plants13010014
Murgia I, Morandini P. Plant Iron Research in African Countries: Current “Hot Spots”, Approaches, and Potentialities. Plants. 2024; 13(1):14. https://doi.org/10.3390/plants13010014
Chicago/Turabian StyleMurgia, Irene, and Piero Morandini. 2024. "Plant Iron Research in African Countries: Current “Hot Spots”, Approaches, and Potentialities" Plants 13, no. 1: 14. https://doi.org/10.3390/plants13010014
APA StyleMurgia, I., & Morandini, P. (2024). Plant Iron Research in African Countries: Current “Hot Spots”, Approaches, and Potentialities. Plants, 13(1), 14. https://doi.org/10.3390/plants13010014







