Integrated Transcriptomics and Metabolomics Analysis Promotes the Understanding of Adventitious Root Formation in Eucommia ulmoides Oliver
Abstract
1. Introduction
2. Results
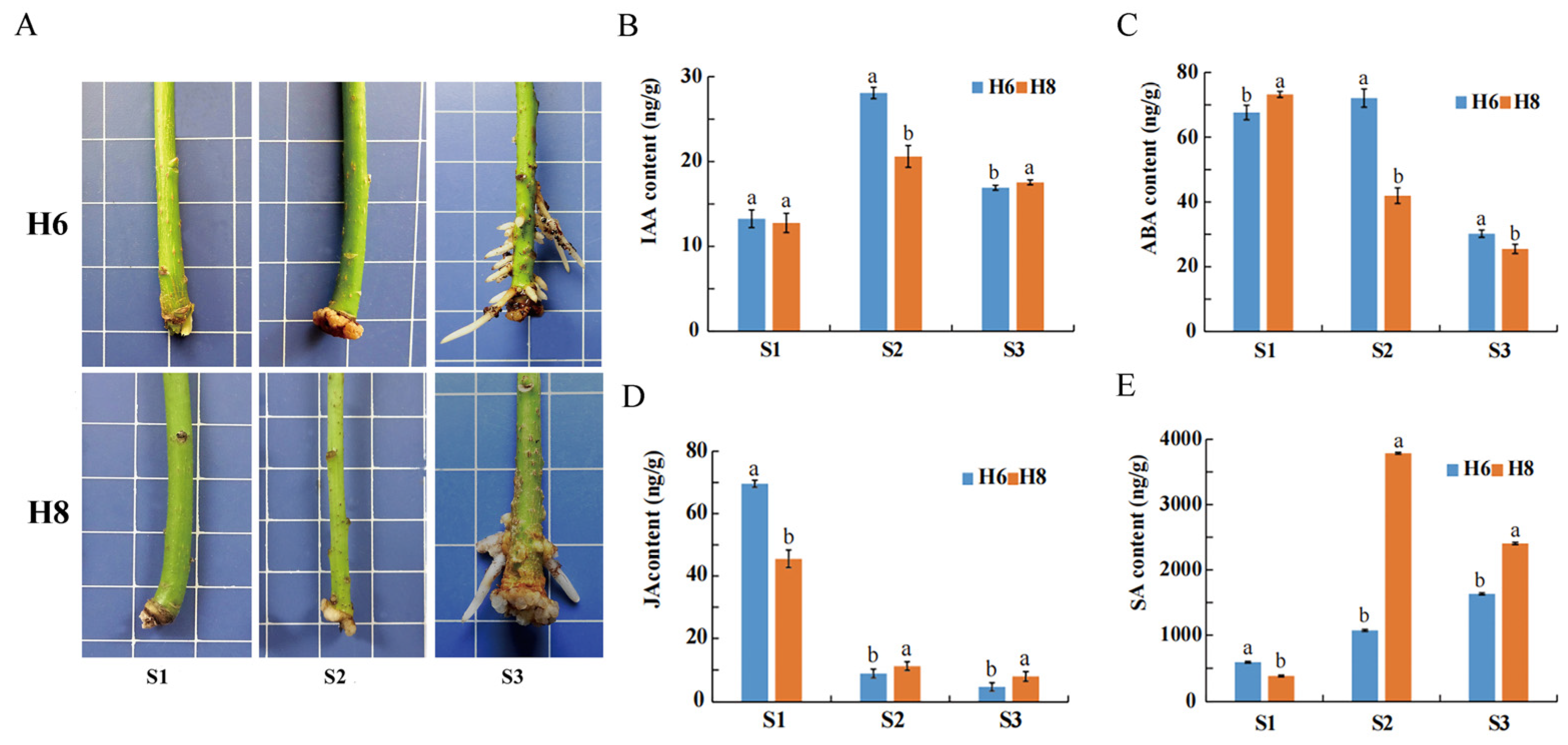
2.1. Morphological Characteristics and Hormone Content during AR Development
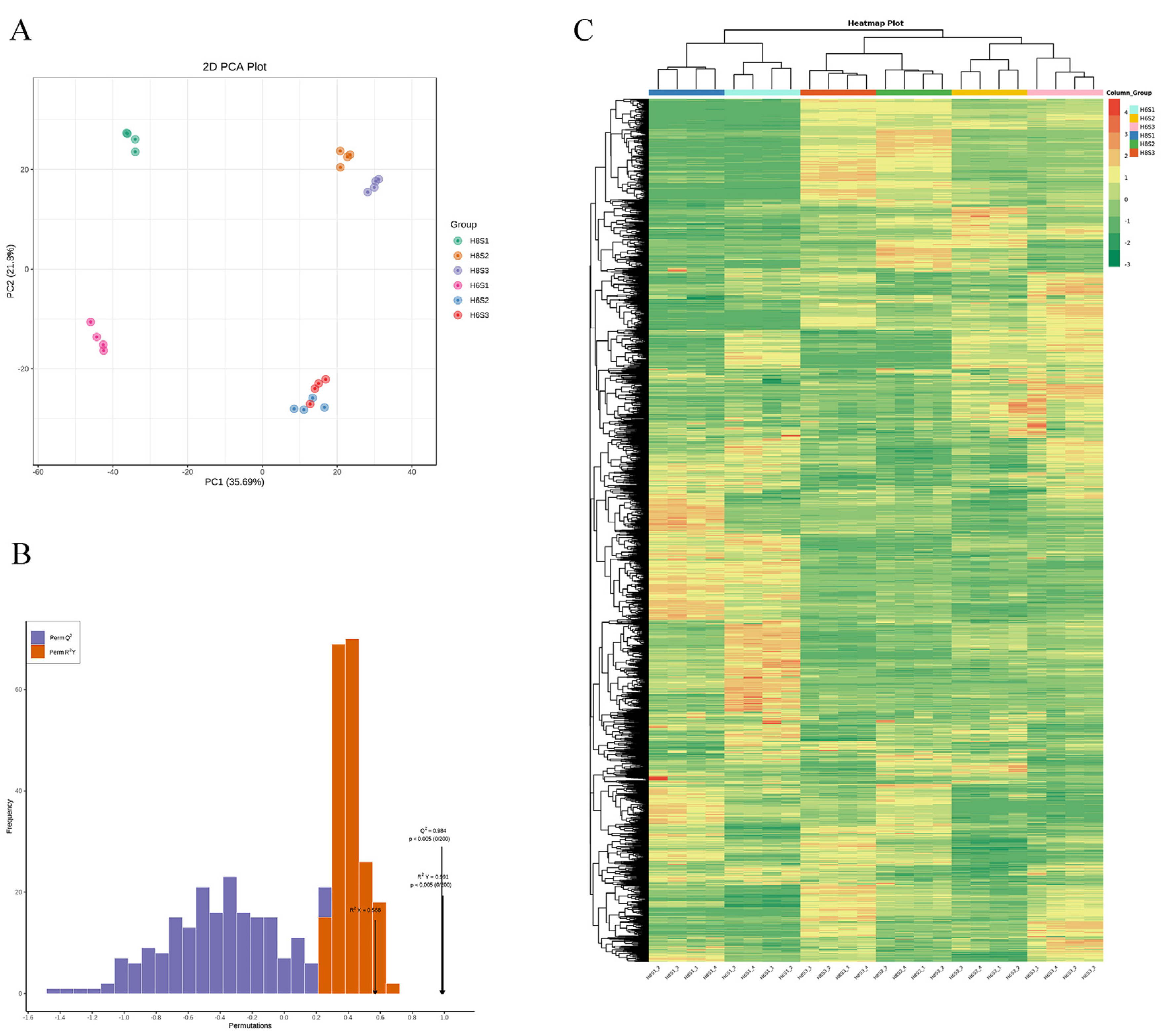
2.2. The DEGs and KEGG Pathways Enrichment during AR Development
2.3. Construction and Analysis of the WGCNA
2.4. Metabolome Profile of H8 and H6 during AR Development
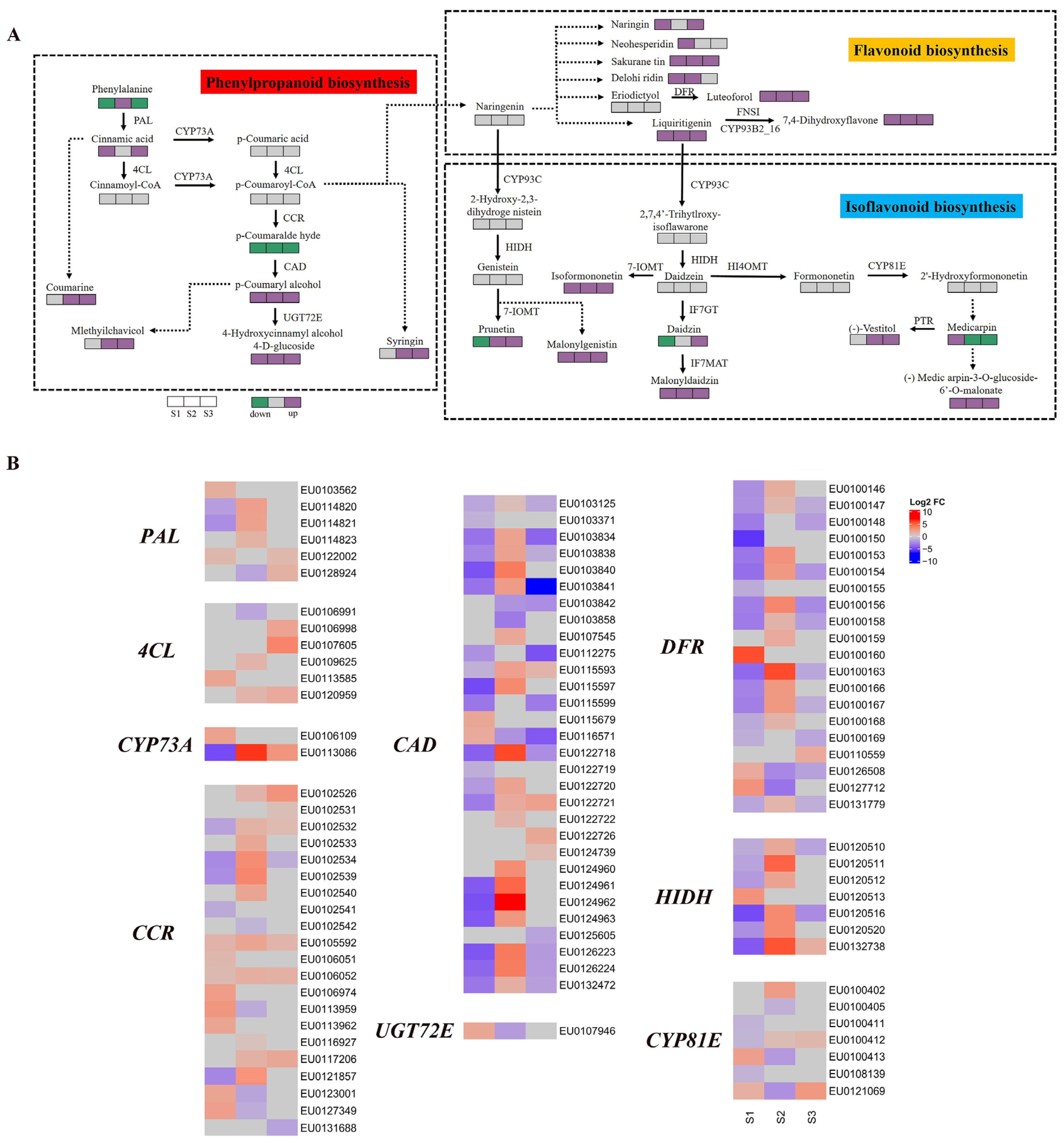
2.5. Transcriptome and Metabolome of Key Pathways duing AR Development
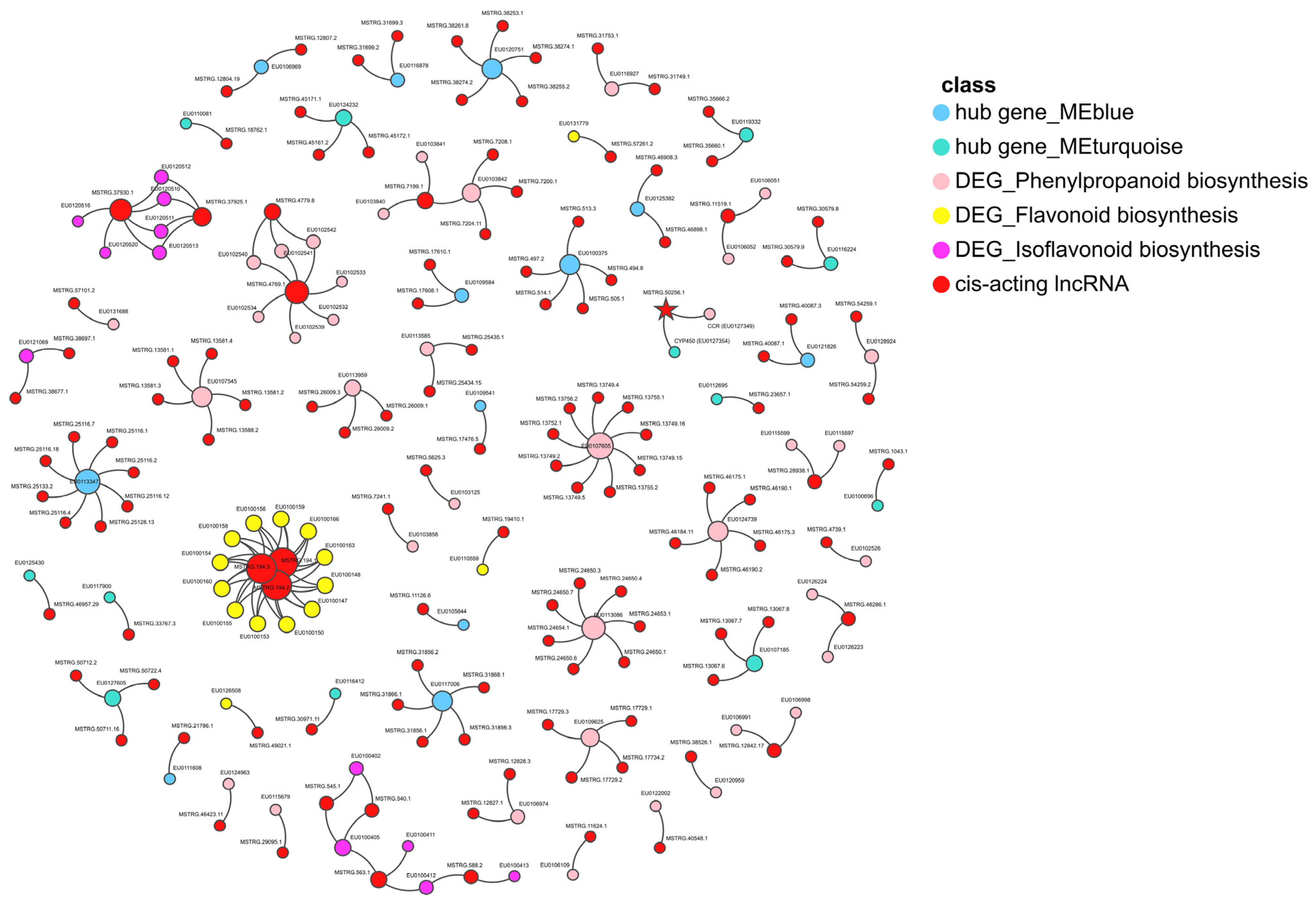
2.6. The Cis-Regulation of Hub Genes by lncRNAs
3. Discussion
3.1. Differences in Plant Hormone Content between Two E. ulmoides Cultivars
3.2. Several Hub Genes Regulate AR Development
3.3. Metabolic Pathway Enrichment of DEGs and DEMs during AR Development
3.4. LncRNAs Regulate Plant Hormone Signal Transduction during AR Development
4. Materials and Methods
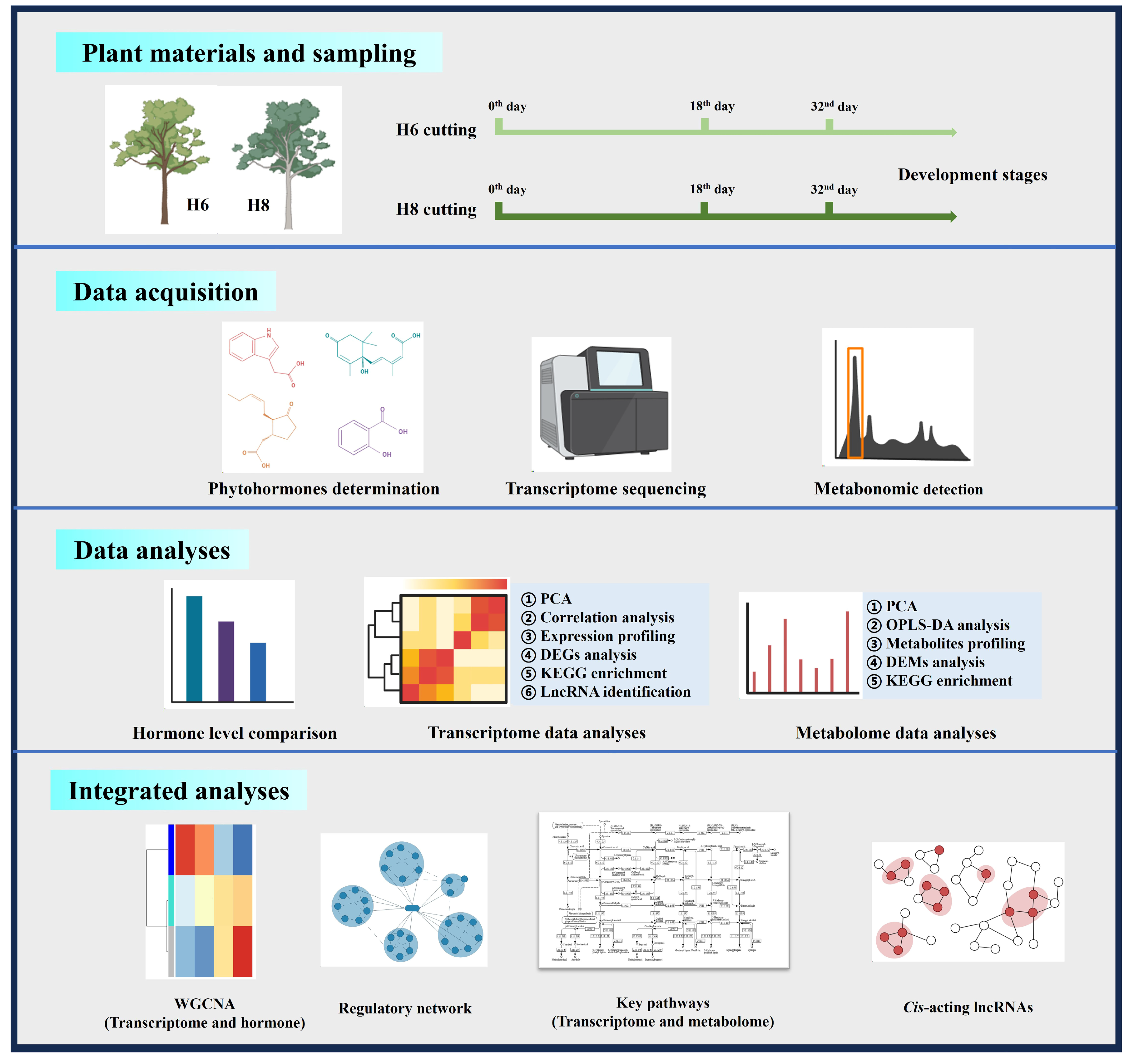
4.1. Plant Materials and Sampling
4.2. Determination of the Content of Plant Endogenous Hormones
4.3. RNA Extraction, Library Construction and Sequencing
4.4. Transcriptome Assembly, lncRNA Identification, and Differential Expression Analysis
4.5. WGCNA of Hormone Traits and All Expressed Genes
4.6. Metabolite Profiling and Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, C.B.; Zhang, Z.Y.; Shao, Q.Y.; Yao, T.; Hu, H.Y.; Huang, J.Z.; Liang, Z.Q.; Han, Y.F. Deciphering the effects of genetic characteristics and environmental factors on pharmacological active ingredients of Eucommia ulmoides. Ind. Crops Prod. 2022, 175, 114293. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.Z.; Kang, K.; Sun, G.T.; Zhu, M.Q. Review on extraction, characteristic, and engineering of the Eucommia ulmodies rubber for industrial application. Ind. Crops Prod. 2022, 180, 15. [Google Scholar] [CrossRef]
- Wang, C.C.; Gong, H.M.; Feng, M.; Tian, C.L. Phenotypic Variation in Leaf, Fruit and Seed Traits in Natural Populations of Eucommia ulmoides, a Relict Chinese Endemic Tree. Forests 2023, 14, 462. [Google Scholar] [CrossRef]
- He, X.R.; Wang, J.H.; Li, M.X.; Hao, D.J.; Yang, Y.; Zhang, C.L.; He, R.; Tao, R. Eucommia ulmoides Oliv.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 151, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Ruan, L.; Lu, L.; Zhao, X.Y.; Xiong, W.W.; Xu, H.Y.; Wu, S.Z. Effects of natural antioxidants on the oxidative stability of Eucommia ulmoides seed oil: Experimental and molecular simulation investigations. Food Chem. 2022, 383, 132640. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.N.; Peng, P.; Peng, F.; Dong, J.E. Natural Polymer Eucommia ulmoides Rubber: A Novel Material. J. Agric. Food Chem. 2021, 69, 3797–3821. [Google Scholar] [CrossRef] [PubMed]
- Wendling, I.; Trueman, S.J.; Xavier, A. Maturation and related aspects in clonal forestry—Part II: Reinvigoration, rejuvenation and juvenility maintenance. New Forest. 2014, 45, 473–486. [Google Scholar] [CrossRef]
- Lv, G.X.; Qing, J.; Du, H.Y.; Du, Q.X.; Meng, Y.D.; He, F.; Liu, P.F.; Du, L.Y.; Wang, L. Comparing Rooting Ability and Physiological Changes of Two Eucommia ulmoides Improved Varieties. Forests 2021, 12, 1267. [Google Scholar] [CrossRef]
- Brunoni, F.; Vielba, J.M.; Sánchez, C. Plant Growth Regulators in Tree Rooting. Plants 2022, 11, 805. [Google Scholar] [CrossRef]
- Druege, U.; Hilo, A.; Pérez-Pérez, J.M.; Klopotek, Y.; Acosta, M.; Shahinnia, F.; Zerche, S.; Franken, P.; Hajirezaei, M.R. Molecular and physiological control of adventitious rooting in cuttings: Phytohormone action meets resource allocation. Ann. Bot. 2019, 123, 929–949. [Google Scholar] [CrossRef]
- Li, S.W. Molecular Bases for the Regulation of Adventitious Root Generation in Plants. Front. Plant Sci. 2021, 12, 614072. [Google Scholar] [CrossRef] [PubMed]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.T.; Xu, P.; Zhao, P.X.; Liu, R.; Yu, L.H.; Xiang, C.B. Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat. Commun. 2014, 5, 5833. [Google Scholar] [CrossRef] [PubMed]
- Lischweski, S.; Muchow, A.; Guthörl, D.; Hause, B. Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biol. 2015, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Bernabé-Antonio, A.; Castro-Rubio, C.; Rodríguez-Anda, R.; Silva-Guzmán, J.A.; Manríquez-González, R.; Hurtado-Díaz, I.; Sánchez-Ramos, M.; Hinojosa-Ventura, G.; Romero-Estrada, A. Jasmonic and Salicylic Acids Enhance Biomass, Total Phenolic Content, and Antioxidant Activity of Adventitious Roots of Acmella radicans (Jacq.) R.K. Jansen Cultured in Shake Flasks. Biomolecules 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Falasca, G.; Kevers, C.; Mainero Rocca, L.; Zadra, C.; Altamura, M.M. Adventitious rooting is enhanced by methyl jasmonate in tobacco thin cell layers. Planta 2009, 231, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Veloccia, A.; Della Rovere, F.; D’Angeli, S.; Falasca, G.; Altamura, M.M. Indole-3-butyric acid promotes adventitious rooting in Arabidopsis thaliana thin cell layers by conversion into indole-3-acetic acid and stimulation of anthranilate synthase activity. BMC Plant Biol. 2017, 17, 121. [Google Scholar] [CrossRef]
- Fattorini, L.; Hause, B.; Gutierrez, L.; Veloccia, A.; Della Rovere, F.; Piacentini, D.; Falasca, G.; Altamura, M.M. Jasmonate promotes auxin-induced adventitious rooting in dark-grown Arabidopsis thaliana seedlings and stem thin cell layers by a cross-talk with ethylene signalling and a modulation of xylogenesis. BMC Plant Biol. 2018, 18, 182. [Google Scholar] [CrossRef]
- Chen, K.M.; Guo, B.; Yu, C.M.; Chen, P.; Chen, J.K.; Gao, G.; Wang, X.F.; Zhu, A.G. Comparative Transcriptome Analysis Provides New Insights into the Molecular Regulatory Mechanism of Adventitious Root Formation in Ramie (Boehmeria nivea L.). Plants 2021, 10, 160. [Google Scholar] [CrossRef]
- Li, S.W.; Leng, Y.; Feng, L.; Zeng, X.Y. Involvement of abscisic acid in regulating antioxidative defense systems and IAA-oxidase activity and improving adventitious rooting in mung bean Vigna radiata (L.) Wilczek seedlings under cadmium stress. Environ. Sci. Pollut. Res. 2014, 21, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.W.; Verstraeten, I.; Trinh, H.K.; Heugebaert, T.; Stevens, C.V.; Garcia-Maquilon, I.; Rodriguez, P.L.; Vanneste, S.; Geelen, D. Arabidopsis Hypocotyl Adventitious Root Formation Is Suppressed by ABA Signaling. Genes 2021, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic Acid Affects Root Meristem Patterning via Auxin Distribution in a Concentration-Dependent Manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.J.; Liu, X.Y.; Xie, L.L.; Wang, L.L.; Shang, Q.M. Salicylic acid regulates adventitious root formation via competitive inhibition of the auxin conjugation enzyme CsGH3.5 in cucumber hypocotyls. Planta 2020, 252, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, J.; Ge, Y.C.; Qin, P.; Xu, L. Pivotal role of LBD16 in root and root-like organ initiation. Cell Mol. Life Sci. 2018, 75, 3329–3338. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.B.; Shang, G.D.; Pan, Y.; Xu, Z.G.; Zhou, C.M.; Mao, Y.B.; Bao, N.; Sun, L.J.; Xu, T.D.; Wang, J.W. AP2/ERF Transcription Factors Integrate Age and Wound Signals for Root Regeneration. Plant Cell 2020, 32, 226–241. [Google Scholar] [CrossRef]
- Hu, X.M.; Xu, L. Transcription Factors WOX11/12 Directly Activate WOX5/7 to Promote Root Primordia Initiation and Organogenesis. Plant Physiol. 2016, 172, 2363–2373. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Pacurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Pacurar, M.; Demailly, H.; Geiss, G.; et al. Auxin Controls Arabidopsis Adventitious Root Initiation by Regulating Jasmonic Acid Homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef]
- Lee, H.W.; Cho, C.; Pandey, S.K.; Park, Y.; Kim, M.J.; Kim, J. LBD16 and LBD18 acting downstream of ARF7 and ARF19 are involved in adventitious root formation in Arabidopsis. BMC Plant Biol. 2019, 19, 46. [Google Scholar] [CrossRef]
- Trupiano, D.; Yordanov, Y.; Regan, S.; Meilan, R.; Tschaplinski, T.; Scippa, G.S.; Busov, V. Identification, characterization of an AP2/ERF transcription factor that promotes adventitious, lateral root formation in Populus. Planta 2013, 238, 271–282. [Google Scholar] [CrossRef]
- Yin, H.J.; Li, M.Z.; Lv, M.H.; Hepworth, S.R.; Li, D.D.; Ma, C.F.; Li, J.; Wang, S.M. SAUR15 Promotes Lateral and Adventitious Root Development via Activating H+-ATPases and Auxin Biosynthesis. Plant Physiol. 2020, 184, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Xie, Y.H.; Liu, W.S.; Tao, G.Y.; Sun, C.; Sun, X.M.; Zhang, S.G. Transcription factor LkWOX4 is involved in adventitious root development in Larix kaempferi. Gene 2020, 758, 144942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.Q.; Cao, P.; Xiao, Z.A.; Zhan, C.; Liu, M.F.; Nvsvrot, T.; Wang, N. The bZIP53-IAA4 module inhibits adventitious root development in Populus. J. Exp. Bot. 2020, 71, 3485–3498. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Cheng, J.F.; Chen, L.Q.; Zhang, G.F.; Huang, H.; Zhang, Y.J.; Xu, L. Auxin-Independent NAC Pathway Acts in Response to Explant-Specific Wounding and Promotes Root Tip Emergence during de Novo Root Organogenesis in Arabidopsis. Plant Physiol. 2016, 170, 2136–2145. [Google Scholar] [CrossRef]
- Du, X.L.; Cao, X.; Yin, C.R.; Tang, Z.; Du, W.; Ban, Y.Y.; Cheng, J.L. Comprehensive Analysis of R2R3-MYB Genes During Adventitious Root Formation in Cuttings of Morus alba. J. Plant Growth Regul. 2017, 36, 290–299. [Google Scholar] [CrossRef]
- Wang, P.; Ma, L.L.; Wang, S.A.; Li, L.F.; Wang, Q.; Yang, R.T.; Li, Y. Identification and Analysis of a Candidate WRKY Transcription Factor Gene Affecting Adventitious Root Formation Using Association Mapping in Catalpa Scop. DNA Cell Biol. 2019, 38, 297–306. [Google Scholar] [CrossRef]
- Ai, Y.; Qian, X.; Wang, X.Q.; Chen, Y.L.; Zhang, T.J.; Chao, Y.H.; Zhao, Y. Uncovering early transcriptional regulation during adventitious root formation in Medicago sativa. BMC Plant Biol. 2023, 23, 176. [Google Scholar] [CrossRef]
- Gaillochet, C.; Lohmann, J.U. The never-ending story: From pluripotency to plant developmental plasticity. Development 2015, 142, 2237–2249. [Google Scholar] [CrossRef]
- Vielba, J.M.; Rico, S.; Sevgin, N.; Castro-Camba, R.; Covelo, P.; Vidal, N.; Sánchez, C. Transcriptomics Analysis Reveals a Putative Role for Hormone Signaling and MADS-Box Genes in Mature Chestnut Shoots Rooting Recalcitrance. Plants 2022, 11, 3486. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B.S. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Sun, G.D.; Luo, Y.; Qian, W.J.; Fan, K.; Ding, Z.T.; Hu, J.H. Role of IAA and Primary Metabolites in Two Rounds of Adventitious Root Formation in Softwood Cuttings of Camellia sinensis (L.). Agronomy 2022, 12, 2486. [Google Scholar] [CrossRef]
- Chang, E.; Guo, W.; Dong, Y.; Jia, Z.R.; Zhao, X.L.; Jiang, Z.P.; Zhang, L.; Zhang, J.; Liu, J.F. Metabolic profiling reveals key metabolites regulating adventitious root formation in ancient Platycladus orientalis cuttings. Front. Plant Sci. 2023, 14, 1192371. [Google Scholar] [CrossRef] [PubMed]
- Petricka, J.J.; Winter, C.M.; Benfey, P.N. Control of Arabidopsis Root Development. Annu. Rev. Plant Biol. 2012, 63, 563–590. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Kjaer, J.E.; Ogorek, L.L.P.; Pellegrini, E.; Song, Z.W.; Pedersen, O.; Herzog, M. Responses of key root traits in the genus Oryza to soil flooding mimicked by stagnant, deoxygenated nutrient solution. J. Exp. Bot. 2023, 74, 2112–2126. [Google Scholar] [CrossRef]
- Liu, S.A.; Sun, Z.M.; Xu, M. Identification and characterization of long non-coding RNAs involved in the formation and development of poplar adventitious roots. Ind. Crops Prod. 2018, 118, 334–346. [Google Scholar] [CrossRef]
- Wuyun, T.N.; Wang, L.; Liu, H.M.; Wang, X.W.; Zhang, L.S.; Bennetzen, J.L.; Li, T.Z.; Yang, L.R.; Liu, P.F.; Du, L.Y.; et al. The Hardy Rubber Tree Genome Provides Insights into the Evolution of Polyisoprene Biosynthesis. Mol. Plant 2018, 11, 429–442. [Google Scholar] [CrossRef]
- Du, Q.X.; Wu, Z.X.; Liu, P.F.; Qing, J.; He, F.; Du, L.Y.; Sun, Z.Q.; Zhu, L.L.; Zheng, H.C.; Sun, Z.Y.; et al. The chromosome-level genome of Eucommia ulmoides provides insights into sex differentiation and a-linolenic acid biosynthesis. Front. Plant Sci. 2023, 14, 1118363. [Google Scholar] [CrossRef]
- Legué, V.; Rigal, A.; Bhalerao, R.P. Adventitious root formation in tree species: Involvement of transcription factors. Physiol. Plant. 2014, 151, 192–198. [Google Scholar] [CrossRef]
- Díaz-Sala, C. Direct reprogramming of adult somatic cells toward adventitious root formation in forest tree species: The effect of the juvenile-adult transition. Front. Plant Sci. 2014, 5, 310. [Google Scholar] [CrossRef]
- Vielba, J.M.; Vidal, N.; José, M.C.S.; Rico, S.; Sánchez, C. Recent Advances in Adventitious Root Formation in Chestnut. Plants 2020, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.T.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U.; et al. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 2009, 181, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Ahkami, A.; Scholz, U.; Steuernagel, B.; Strickert, M.; Haensch, K.T.; Druege, U.; Reinhardt, D.; Nouri, E.; von Wirén, N.; Franken, P.; et al. Comprehensive Transcriptome Analysis Unravels the Existence of Crucial Genes Regulating Primary Metabolism during Adventitious Root Formation in Petunia hybrida. PLoS ONE 2014, 9, e100997. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Hosseini, S.A.; Hajirezaei, M.R.; Druege, U.; Geelen, D. Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J. Exp. Bot. 2015, 66, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.J.; Zhou, Y.X.; Zhou, M.L.; Yan, J.; Khurshid, M.; Weng, W.F.; Cheng, J.P.; Zhang, K.X. Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.H. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef]
- Waadt, R.; Seller, C.A.; Hsu, P.K.; Takahashi, Y.; Munemasa, S.; Schroeder, J. Plant hormone regulation of abiotic stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 680–694. [Google Scholar] [CrossRef]
- Liu, H.; Timko, M.P. Jasmonic Acid Signaling and Molecular Crosstalk with Other Phytohormones. Int. J. Mol. Sci. 2021, 22, 2914. [Google Scholar] [CrossRef]
- Sendon, P.M.; Seo, H.S.; Song, J.T. Salicylic Acid Signaling: Biosynthesis, Metabolism, and Crosstalk with Jasmonic Acid. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 501–506. [Google Scholar] [CrossRef]
- Liu, L.J.; Sonbol, F.M.; Huot, B.; Gu, Y.N.; Withers, J.; Mwimba, M.; Yao, J.; He, S.Y.; Dong, X.N. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 13099. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Yoon, Y.; Choi, Y.D. Crosstalk with Jasmonic Acid Integrates Multiple Responses in Plant Development. Int. J. Mol. Sci. 2020, 21, 10305. [Google Scholar] [CrossRef] [PubMed]
- Lakehal, A.; Chaabouni, S.; Cavel, E.; Le Hir, R.; Ranjan, A.; Raneshan, Z.; Novák, O.; Pacurar, D.I.; Perrone, I.; Jobert, F.; et al. A Molecular Framework for the Control of Adventitious Rooting by TIR1/AFB2-Aux/IAA-Dependent Auxin Signaling in Arabidopsis. Mol. Plant 2019, 12, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wen, S.S.; Sun, T.T.; Wang, R.; Zuo, W.T.; Yang, T.; Wang, C.; Hu, J.J.; Lu, M.Z.; Wang, L.Q. PagWOX11/12a positively regulates the PagSAUR36 gene that enhances adventitious root development in poplar. J. Exp. Bot. 2022, 73, 7298–7311. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.R.; Bai, T.H.; Gao, H.Y.; Cui, Y.J.; Zhou, R.L.; Wang, Z.Y.; Song, S.W.; Jiao, J.; Wang, M.M.; Wan, R.; et al. Genome-wide identification of LBD transcription factors in apple and the function of MdLBD16a in adventitious rooting and callus development. Sci. Hortic. 2023, 317, 112048. [Google Scholar] [CrossRef]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 Regulate Lateral Root Formation via Direct Activation of LBD/ASL Genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef]
- Agrawal, R.; Singh, A.; Giri, J.; Magyar, Z.; Thakur, J.K. MEDIATOR SUBUNIT17 is required for transcriptional optimization of root system architecture in Arabidopsis. Plant Physiol. 2023, 192, 1548–1568. [Google Scholar] [CrossRef]
- Feng, K.; Hou, X.L.; Xing, G.M.; Liu, J.X.; Duan, A.Q.; Xu, Z.S.; Li, M.Y.; Zhuang, J.; Xiong, A.S. Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef]
- Neogy, A.; Garg, T.; Kumar, A.; Dwivedi, A.K.; Singh, H.; Singh, U.; Singh, Z.; Prasad, K.; Jain, M.; Yadav, S.R. Genome-Wide Transcript Profiling Reveals an Auxin-Responsive Transcription Factor, OsAP2/ERF-40, Promoting Rice Adventitious Root Development. Plant Cell Physiol. 2019, 60, 2343–2355. [Google Scholar] [CrossRef]
- Zhu, H.C.; Li, C.; Gao, C.X. Applications of CRISPR-Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Bio. 2020, 21, 661–677. [Google Scholar] [CrossRef]
- Wang, P.; Ma, L.L.; Li, Y.; Wang, S.A.; Li, L.F.; Yang, R.T.; Ma, Y.Z.; Wang, Q. Transcriptome profiling of indole-3-butyric acid-induced adventitious root formation in softwood cuttings of the Catalpa bungei variety ‘YU-1’ at different developmental stages. Genes Genom. 2016, 38, 145–162. [Google Scholar] [CrossRef]
- Munir, M.Z.; Din, S.U.; Imran, M.; Zhang, Z.J.; Pervaiz, T.; Han, C.; Nisa, Z.U.; Bakhsh, A.; Muneer, M.A.; Sun, Y.H.; et al. Transcriptomic and Anatomic Profiling Reveal Etiolation Promotes Adventitious Rooting by Exogenous Application of 1-Naphthalene Acetic Acid in Robinia pseudoacacia L. Forests 2021, 12, 789. [Google Scholar] [CrossRef]
- Mobin, M.; Wu, C.H.; Tewari, R.K.; Paek, K.Y. Studies on the glyphosate-induced amino acid starvation and addition of precursors on caffeic acid accumulation and profiles in adventitious roots of Echinacea purpurea (L.) Moench. Plant Cell Tiss. Org. 2015, 120, 291–301. [Google Scholar] [CrossRef]
- Koramutla, M.K.; Tuan, P.A.; Ayele, B.T. Salicylic Acid Enhances Adventitious Root and Aerenchyma Formation in Wheat under Waterlogged Conditions. Int. J. Mol. Sci. 2022, 23, 1243. [Google Scholar] [CrossRef] [PubMed]
- Cheniany, M.; Ganjeali, A. Developmental role of phenylalanine-ammonia-lyase (pal) and cinnamate 4-hydroxylase (c4h) genes during adventitious rooting of juglans regia l. microshoots. Acta Biol. Hung. 2016, 67, 379–392. [Google Scholar] [CrossRef]
- Tian, Y.Y.; Bai, S.L.G.; Dang, Z.H.; Hao, J.F.; Zhang, J.; Hasi, A. Genome-wide identification and characterization of long non-coding RNAs involved in fruit ripening and the climacteric in Cucumis melo. BMC Plant Biol. 2019, 19, 369. [Google Scholar] [CrossRef]
- Engreitz, J.M.; Haines, J.E.; Perez, E.M.; Munson, G.; Chen, J.; Kane, M.; McDonel, P.E.; Guttman, M.; Lander, E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 2016, 539, 452–455. [Google Scholar] [CrossRef]
- Long, Y.C.; Wang, X.Y.; Youmans, D.T.; Cech, T.R. How do lncRNAs regulate transcription? Sci. Adv. 2017, 3, eaao2110. [Google Scholar] [CrossRef]
- Patturaj, M.; Munusamy, A.; Kannan, N.; Ramasamy, Y. Biologia Futura: Progress and future perspectives of long non-coding RNAs in forest trees. Biol. Futur. 2022, 73, 43–53. [Google Scholar] [CrossRef]
- Chekanova, J.A. Long non-coding RNAs and their functions in plants. Curr. Opin. Plant Biol. 2015, 27, 207–216. [Google Scholar] [CrossRef]
- Hou, J.N.; Lu, D.D.; Mason, A.S.; Li, B.Q.; Xiao, M.L.; An, S.F.; Fu, D.H. Non-coding RNAs and transposable elements in plant genomes: Emergence, regulatory mechanisms and roles in plant development and stress responses. Planta 2019, 250, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Ran, N.; Liu, S.; Qi, H.R.; Wang, J.L.; Shen, T.F.; Xu, W.L.; Xu, M. Long Non-Coding RNA lncWOX11a Suppresses Adventitious Root Formation of Poplar by Regulating the Expression of PeWOX11a. Int. J. Mol. Sci. 2023, 24, 5766. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.R.; Wu, L.; Shen, T.F.; Liu, S.; Cai, H.; Ran, N.; Wang, J.L.; Xu, M. Overexpression of the long non-coding RNA lncWOX5 negatively regulates the development of adventitious roots in Populus. Ind. Crops Prod. 2023, 192, 116054. [Google Scholar] [CrossRef]
- Um, Y.; Lee, Y.; Kim, S.C.; Jeong, Y.J.; Kim, G.S.; Choi, D.W.; Cha, S.W.; Kim, O.T. Expression analysis of ginsenoside biosynthesis-related genes in methyl jasmonate-treated adventitious roots of Panax ginseng via DNA microarray analysis. Hortic. Environ. Biotechnol. 2017, 58, 376–383. [Google Scholar] [CrossRef]
- Zhao, X.H.; Zhao, Y.J.; Gou, M.Y.; Liu, C.J. Tissue-preferential recruitment of electron transfer chains for cytochrome P450-catalyzed phenolic biosynthesis. Sci. Adv. 2023, 9, eade4389. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.C.; Nelson, D.R.; Moller, B.L.; Werck-Reichhart, D. Plant cytochrome P450 plasticity and evolution. Mol. Plant 2021, 14, 1244–1265. [Google Scholar] [CrossRef] [PubMed]
- Gou, M.Y.; Ran, X.Z.; Martin, D.W.; Liu, C.J. The scaffold proteins of lignin biosynthetic cytochrome P450 enzymes. Nat. Plants 2018, 4, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Alber, A.V.; Renault, H.; Basilio-Lopes, A.; Bassard, J.E.; Liu, Z.; Ullmann, P.; Lesot, A.; Bihel, F.; Schmitt, M.; Werck-Reichhart, D.; et al. Evolution of coumaroyl conjugate 3-hydroxylases in land plants: Lignin biosynthesis and defense. Plant J. 2019, 99, 924–936. [Google Scholar] [CrossRef]
- Guttikonda, S.K.; Trupti, J.; Bisht, N.C.; Chen, H.; An, Y.Q.C.; Pandey, S.; Xu, D.; Yu, O. Whole genome co-expression analysis of soybean cytochrome P450 genes identifies nodulation-specific P450 monooxygenases. BMC Plant Biol. 2010, 10, 243. [Google Scholar] [CrossRef]
- Babu, P.R.; Rao, K.V.; Reddy, V.D. Structural organization and classification of cytochrome P450 genes in flax (Linum usitatissimum L.). Gene 2013, 513, 156–162. [Google Scholar] [CrossRef]
- Lin, C.; Ogorek, L.L.P.; Liu, D.; Pedersen, O.; Sauter, M. A quantitative trait locus conferring flood tolerance to deepwater rice regulates the formation of two distinct types of aquatic adventitious roots. New Phytol. 2023, 238, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, C.T.; de Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Yu, G.C.; Wang, L.G.; Han, Y.Y.; He, Q.Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Chen, T.; Liu, Y.X.; Huang, L. ImageGP: An easy-to-use data visualization web server for scientific researchers. iMeta 2022, 1, e5. [Google Scholar] [CrossRef]
- Lu, S.N.; Wang, J.Y.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Q.; Song, K.; Wang, L.; Du, L.; Du, H.; Li, B.; Li, H.; Yang, L.; Wang, Y.; Liu, P. Integrated Transcriptomics and Metabolomics Analysis Promotes the Understanding of Adventitious Root Formation in Eucommia ulmoides Oliver. Plants 2024, 13, 136. https://doi.org/10.3390/plants13010136
Du Q, Song K, Wang L, Du L, Du H, Li B, Li H, Yang L, Wang Y, Liu P. Integrated Transcriptomics and Metabolomics Analysis Promotes the Understanding of Adventitious Root Formation in Eucommia ulmoides Oliver. Plants. 2024; 13(1):136. https://doi.org/10.3390/plants13010136
Chicago/Turabian StyleDu, Qingxin, Kangkang Song, Lu Wang, Lanying Du, Hongyan Du, Bin Li, Haozhen Li, Long Yang, Yan Wang, and Panfeng Liu. 2024. "Integrated Transcriptomics and Metabolomics Analysis Promotes the Understanding of Adventitious Root Formation in Eucommia ulmoides Oliver" Plants 13, no. 1: 136. https://doi.org/10.3390/plants13010136
APA StyleDu, Q., Song, K., Wang, L., Du, L., Du, H., Li, B., Li, H., Yang, L., Wang, Y., & Liu, P. (2024). Integrated Transcriptomics and Metabolomics Analysis Promotes the Understanding of Adventitious Root Formation in Eucommia ulmoides Oliver. Plants, 13(1), 136. https://doi.org/10.3390/plants13010136






