Biochemical, Antioxidant Properties and Antimicrobial Activity of Epiphytic Leafy Liverwort Frullania dilatata (L.) Dumort
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Sample
4.2. Extraction Process
4.3. Microorganisms
4.4. Microorganism Inoculum Preparation
4.5. Antimicrobial Assay
4.6. Antioxidant Activity by DPPH Radical Scavenging Method
4.7. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- Asakawa, Y. Biologically active compounds from bryophytes. Pure Appl. Chem. 2001, 73, 507–515. [Google Scholar] [CrossRef]
- Glime, J.M. Chapter 10: Temperature: Effects. In Bryophyte Ecology; Michigan Technological University: Houghton, MI, USA; International Association of Bryologists: Seattle, WA, USA, 2017; Volume 1, Available online: http://digitalcommons.mtu.edu/bryophyte-ecology/ (accessed on 28 July 2022).
- Asakawa, Y.; Ludwiczuk, A. Chemical constituents of bryophytes: Structures and biological activity. J. Nat. Prod. 2017, 81, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y. Liverworts-Potential Source of Medicinal Compounds. Med. Aromat. Plants 2012, 1. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Novakovic, M.; Bukvicki, D.; Yongabi Anchang, K. Bis-bibenzyls, Bibenzyls, and Terpenoids in the Marchantiophyta (Liverworts): Structures, Synthesis, and Bioactivity. J. Nat. Prod. 2022, 85, 729–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dai, P.; Bao, L.; Guo, H.; Zhang, G.; Zhao, J. Neuroprotective Bibenzyls from the stems of Dendrobium ellipsophyllum. J. Nat. Prod. 2016, 79, 1969–1977. [Google Scholar]
- Forrest, L.L.; Villarreal, J.C. The role of bryophyte oil bodies in the early evolution of liverworts: Evidence from the Marchantiales. Flora. 2014, 209, 671–678. [Google Scholar]
- Tyagi, A.K.; Bukvicki, D.; Gottardi, D.; Veljic, M.; Guerzoni, M.E.; Malik, A.; Marin, P.D. Antimicrobial Potential and Chemical Characterization of Serbian Liverwort (Porella arboris-vitae): SEM and TEM Observations. Evid. Based Complement. Alternat. Med. 2013, 2013, 382927. [Google Scholar]
- Purkon, D.B.; Fadhlillah, F.M.; Maigoda, T.C.; Iwo, M.I.; Soemardji, A.A.; Nadhifah, A.; Sudaryat, Y. Phytochemical use in Ethnomedicine and Therapeutic Activities of Marchanita Genus. J. Vocat. Health Stud. 2022, 5, 174–185. [Google Scholar] [CrossRef]
- Stelmasiewicz, M.; Świątek, Ł.; Ludwiczuk, A. Phytochemical profile and anticancer potential of endophytic microorganisms from liverwort species, Marchantia polymorpha L. Molecules 2021, 27, 153. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Bhatia, R.; Narain, J.P. The growing challenge of antimicrobial resistance in the South-East Asia Region-Are we losing the battle? Indian J. Med. Res. 2010, 132, 482. [Google Scholar] [PubMed]
- Tunca-Pinarli, Y.; Benek, A.; Turu, D.; Bozyel, M.E.; Canli, K.; Altuner, E.M. Biological Activities and Biochemical Composition of Endemic Achillea fraasii. Microorganisms 2023, 11, 978. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The World Health Report 2002: Reducing Risks, Promoting Healthy Life; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Ludwiczuk, A.; Asakawa, Y. Chemical Diversity of Liverworts From Frullania Genus. Nat. Prod. Commun. 2021, 16, 1934578X21995381. [Google Scholar]
- Greiff, G. Studying bryophilous fungi on Frullania dilatata. Field Bryol. 2021, 126, 35–40. [Google Scholar]
- Nikolajeva, V.; Liepina, L.; Petrina, Z.; Krumina, G.; Grube, M.; Muiznieks, I. Antibacterial activity of extracts from some bryophytes. Adv. Microbiol. 2012, 2, 345. [Google Scholar] [CrossRef]
- Chen, J.; Duan, W.; Bai, R.; Yao, H.; Shang, J.; Xu, J. Design, synthesis and antioxidant activity evaluation of novel β-elemene derivatives. Bioorg. Med. Chem. Lett. 2014, 24, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Q.; Ding, X.; Jia, Y.C.; Huang, C.X.; Wang, Y.Z.; Xu, Y.H. Anti-tumor effect of β-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008, 264, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, G.; Zhao, J.; Ding, H.; Cunningham, C.; Chen, F.; Flynn, D.C.; Reed, E.; Li, Q.Q. Antiproliferative effect of β-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cell. Mol. Life Sci. 2005, 62, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.K.; Kang, K.Y.; Jang, H.Y.; Hwang, Y.H.; Hong, S.G.; Kim, S.J.; Kim, Y.M.; Yee, S.T. Atraric acid exhibits anti-inflammatory effect in lipopolysaccharide-stimulated RAW264.7 cells and mouse models. Int. J. Mol. Sci. 2020, 21, 7070. [Google Scholar] [CrossRef]
- Chimplee, S.; Graidist, P.; Srisawat, T.; Sukrong, S.; Bissanum, R.; Kanokwiroon, K. Anti breast cancer potential of frullanolide from Grangea maderaspatana plant by inducing apoptosis. Oncol. Lett. 2019, 17, 5283–5291. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar] [PubMed]
- Librán-Pérez, M.; Pereiro, P.; Figueras, A.; Novoa, B. Antiviral activity of palmitic acid via autophagic flux inhibition in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 95, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Felgentreff, F.; Schröder, H.; Meier, B.; Brattström, A. The anxiolytic effects of a Valerian extract is based on valerenic acid. BMC Complement. Altern. Med. 2014, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Ciric, A.; Glamoclija, J.; Nikolic, M.; Sokovic, M. In vitro anti-quorum sensing activity of phytol. Nat. Prod. Res. 2015, 29, 374–377. [Google Scholar] [CrossRef]
- Ghaneian, M.T.; Ehrampoush, M.H.; Jebali, A.; Hekmatimoghaddam, S.; Mahmoudi, M. Antimicrobial activity, toxicity and stability of phytol as a novel surface disinfectant. Environ. Health Eng. Manag. J. 2015, 2, 13–16. [Google Scholar]
- Fagali, N.; Catalá, A. Antioxidant activity of conjugated linoleic acid isomers, linoleic acid and its methyl ester determined by photoemission and DPPH techniques. Biophys. Chem. 2008, 137, 56–62. [Google Scholar] [CrossRef]
- Taylor, T.A.; Unakal, C.G. Staphylococcus Aureus; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Chen, M.; Zhang, J.; Qi, J.; Dong, R.; Liu, H.; Wu, D.; Shao, H.; Jiang, X. Boronic Acid-Decorated Multivariate Photosensitive Metal–Organic Frameworks for Combating Multi-Drug-Resistant Bacteria. ACS Nano 2022, 16, 7732–7744. [Google Scholar] [CrossRef]
- Stock, I.; Wiedemann, B. Natural antibiotic susceptibility of Providencia stuartii, P. rettgeri, P. alcalifaciens and P. rustigianii strains. J. Med. Microbiol. 1998, 47, 629–642. [Google Scholar] [CrossRef]
- Lopez-Valladares, G.; Danielsson-Tham, M.L.; Tham, W. Implicated Food Products for Listeriosis and Changes in Serovars of Listeria monocytogenes Affecting Humans in Recent Decades. Foodborne Pathog. Dis. 2018, 15, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Duze, S.T.; Marimani, M.; Patel, M. Tolerance of Listeria monocytogenes to Biocides Used in Food Processing Environments. Food Microbiol. 2021, 97, 103758. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- O’Toole, R.F.; Leong, K.W.; Cumming, V.; van Hal, S.J. Vancomycin-Resistant Enterococcus faecium and the Emergence of New Sequence Types Associated with Hospital Infection. Res. Microbiol. 2023, 174, 104046. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Phytochemical and Biological Studies of Bryophytes. Phytochemistry 2013, 91, 52–80. [Google Scholar] [CrossRef]
- Andrews, J.M. BSAC Standardized Disc Susceptibility Testing Method (Version 6). J. Antimicrob. Chemother. 2003, 60, 20–41. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.D.; Coube, C.S.; Leitão, S.G. Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Tunç, K.; Semerci, A.B.; Okur, İ. Antioxidant Activity of the Fruits of Pyracantha coccinea Using Ethanolic Extract Method. Food Health 2020, 6, 35–40. [Google Scholar]
- Bharat, C.R.; Krishna, G.D. GC-MS Analysis of Young Leaves of Allophylus cobbe (L.) Raeusch. and Allophylus serratus (Roxb.) Kurz. Indian J. Pharm. Educ. Res. 2017, 51, 472–479. [Google Scholar] [CrossRef]
| Microorganisms | 50 µL | 100 µL | 200 µL | p-Value * | GEN (10 µg) |
|---|---|---|---|---|---|
| Acinetobacter baumannii CECT 9111 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | 13 |
| Acinetobacter baumannii MDR | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | 10 |
| Candida albicans DSMZ 1386 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | 12 |
| Candida tropicalis CI | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | 0 |
| Escherichia coli ATCC 25922 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | 22 |
| Enterococcus faecalis ATCC 29212 | 7.00 ± 0.00 | 8.00 ± 0.00 | 9.00 ± 0.00 | <2.2 × 10−16 ** | 12 |
| Enterococcus faecium FI | 7.00 ± 0.00 | 8.00 ± 0.00 | 8.00 ± 0.58 | 0.125 | 28 |
| Klebsiella pneumoniae CI | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | 18 |
| Listeria monocytogenes ATCC 7644 | 8.00 ± 0.00 | 9.00 ± 0.00 | 9.00 ± 0.58 | 0.125 | 28 |
| Pseudomonas aeruginosa DSMZ 50071 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | 15 |
| Providencia rustigianii MDR | 7.00 ± 0.00 | 8.00 ± 0.00 | 9.00 ± 0.00 | <2.2 × 10−16 ** | 16 |
| Staphylococcus aureus ATCC 25923 | 9.00 ± 0.58 | 10.00 ± 0.00 | 11.00 ± 0.00 | 0.016 | 21 |
| Staphylococcus hominis ATCC 27844 | 7.00 ± 0.00 | 8.00 ± 0.00 | 0.00 ± 0.00 | <2.2 × 10−16 *** | 18 |
| Streptococcus pneumoniae MDR | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | 10 |
| Salmonella typhimurium SL 1344 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | - | 24 |
| Microorganisms | Ethanol Extract MIC (mg/mL) | Water Extract MIC (mg/mL) |
|---|---|---|
| Acinetobacter baumannii CECT 9111 | - | - |
| Acinetobacter baumannii MDR | - | - |
| Candida albicans DSMZ 1386 | 0.257 | - |
| Candida tropicalis CI | 0.514 | - |
| Escherichia coli ATCC 25922 | - | - |
| Enterococcus faecalis ATCC 29212 | 0.1285 | - |
| Enterococcus faecium FI | 1.028 | - |
| Klebsiella pneumoniae CI | - | - |
| Listeria monocytogenes ATCC 7644 | 0.257 | 21.44 |
| Pseudomonas aeruginosa DSMZ 50071 | - | - |
| Providencia rustigianii MDR | 1.028 | - |
| Staphylococcus aureus ATCC 25923 | 0.257 | 21.44 |
| Staphylococcus hominis ATCC 27844 | - | - |
| Streptococcus pneumoniae MDR | - | - |
| Salmonella typhimurium SL 1344 | - | - |
| Concentration (µg/mL) | FD (%) | AA (%) | t-Value | p-Value |
|---|---|---|---|---|
| 200.000 | 89.294 | 94.515 | −4.702 | 0.063 |
| 100.000 | 85.654 | 93.654 | −4.728 | 0.063 |
| 50.000 | 65.456 | 92.878 | −13.916 | 0.012 |
| 25.000 | 43.099 | 90.501 | −11.787 | 0.016 |
| 12.500 | 35.813 | 68.095 | −5.075 | 0.058 |
| 6.250 | 16.224 | 47.973 | −2.321 | 0.144 |
| 3.125 | 10.436 | 28.418 | −3.293 | 0.097 |
| 1.075 | 10.002 | 20.788 | −1.607 | 0.232 |
| No | RT | Chemical Structure | Compound Name | Formula | Molecular Weight (g/mol) | Area (%) | Known Activity |
|---|---|---|---|---|---|---|---|
| 1 | 21.456 |  | Azulene,1,2,3,4,5,6,7,8-octahydro-1,4-dimethyl-7-(1-methylethenyl)-, (1S,4S,7R)- | C15H24 | 204.351 | 2.19 | - |
| 2 | 22.272 |  | Aciphyllene | C15H24 | 204.351 | 0.77 | - |
| 3 | 22.398 |  | .beta.-Elemene | C15H24 | 204.351 | 0.84 | Antioxidant activity [20], antitumor effect [21], antiproliferative effect [22] |
| 5 | 24.620 | 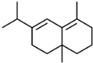 | delta-Selinene | C15H24 | 204.35 | 2.19 | - |
| 6 | 28.048 | Unknown | - | - | 0.5 | - | |
| 7 | 29.246 | 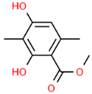 | Atraric acid | C10H12O4 | 196.200 | 0.92 | Anti-inflammatory effect [23] |
| 8 | 30.240 | Unknown | - | - | 1.73 | ||
| 9 | 31.547 |  | Neophytadiene | C20H38 | 278.516 | 1.18 | - |
| 10 | 32.321 | Unknown | - | - | 1.25 | ||
| 11 | 33.000 | Unknown | - | - | 3.39 | ||
| 12 | 33.157 | Unknown | - | - | 3.19 | ||
| 13 | 33.634 |  | Methyl linolenate | C19H32O2 | 292.456 | 0.59 | - |
| 14 | 33.793 |  | Frullanolide | C15H20O2 | 232.318 | 19.08 | Anti-breast cancer activity [24] |
| 15 | 33.921 | Unknown | - | - | 1.34 | ||
| 16 | 34.068 | 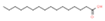 | Palmitic acid | C16H32O2 | 256.424 | 9.83 | Antitumor activity [25], antiviral activity [26] |
| 17 | 34.403 | 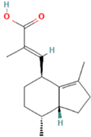 | Valerenic acid | C15H22O2 | 234.334 | 5.3 | Anxiolytic effect [27] |
| 18 | 34.862 |  | 2,3-Dimethylanisole | C9H12O | 136.191 | 15.21 | - |
| 19 | 36.885 |  | Phytol | C20H40O | 296.531 | 1.01 | Anti-quorum sensing activity [28], antimicrobial activity [29] |
| 20 | 37.530 |  | Linoleic acid | C18H32O2 | 280.445 | 11.11 | Antioxidant activity [30] |
| 21 | 41.087 | 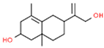 | Bicyclo [4.4.0]dec-5-ene,1,5-dimethyl-3-hydroxy-8-(1-methylene-2-hydroxyethyl-1)- | C15H24O2 | 236.350 | 3.89 | - |
| 22 | 45.183 | Unknown | - | - | 1.66 | - | |
| 23 | 45.866 | 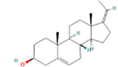 | (Z)-pregna-5,17(20)-dien-3beta-ol | C21H32O | 300.500 | 2.52 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simsek, O.; Canli, K.; Benek, A.; Turu, D.; Altuner, E.M. Biochemical, Antioxidant Properties and Antimicrobial Activity of Epiphytic Leafy Liverwort Frullania dilatata (L.) Dumort. Plants 2023, 12, 1877. https://doi.org/10.3390/plants12091877
Simsek O, Canli K, Benek A, Turu D, Altuner EM. Biochemical, Antioxidant Properties and Antimicrobial Activity of Epiphytic Leafy Liverwort Frullania dilatata (L.) Dumort. Plants. 2023; 12(9):1877. https://doi.org/10.3390/plants12091877
Chicago/Turabian StyleSimsek, Ozcan, Kerem Canli, Atakan Benek, Dilay Turu, and Ergin Murat Altuner. 2023. "Biochemical, Antioxidant Properties and Antimicrobial Activity of Epiphytic Leafy Liverwort Frullania dilatata (L.) Dumort" Plants 12, no. 9: 1877. https://doi.org/10.3390/plants12091877
APA StyleSimsek, O., Canli, K., Benek, A., Turu, D., & Altuner, E. M. (2023). Biochemical, Antioxidant Properties and Antimicrobial Activity of Epiphytic Leafy Liverwort Frullania dilatata (L.) Dumort. Plants, 12(9), 1877. https://doi.org/10.3390/plants12091877







