Hydrotime Model Parameters Estimate Seed Vigor and Predict Seedling Emergence Performance of Astragalus sinicus under Various Environmental Conditions
Abstract
1. Introduction
2. Results
2.1. Seed Germination in Response to Water Potential
2.2. Hydrotime Model Analysis for Seed Germination in Response to Water Potential
2.3. Effects of Different Environmental Conditions on Seedling Emergence Performance in Pot Experiments
2.4. Seedling Emergence Performance under Field Conditions
2.5. Correlation between Hydrotime Model Parameters and Seed Germination and Seedling Emergence Performance under Various Environmental Conditions
3. Discussion
4. Materials and Methods
4.1. Seed Materials
4.2. Germination Test
4.3. Pot Experiments
4.4. Field Experiments
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naito, Y.; Fujie, M.; Usami, S.; Murooka, Y.; Yamada, T. The involvement of a cysteine proteinase in the nodule development in Chinese milk vetch infected with Mesorhizobium huakuii subsp. rengei. Plant Physiol. 2000, 124, 1087–1095. [Google Scholar] [CrossRef]
- Chang, D.N.; Gao, S.J.; Zhou, G.P.; Deng, S.H.; Jia, J.Z.; Wang, E.T.; Cao, W.D. The chromosome-level genome assembly of Astragalus sinicus and comparative genomic analyses provide new resources and insights for understanding legume-rhizobial interactions. Plant Commun. 2022, 3, 100263. [Google Scholar] [CrossRef]
- Liu, J.X.; Ye, J.A.; Ye, H.W. The effects of supplementary Chinese milk vetch silage on the growth rate of cattle and their intake of ammoniated rice straw. Anim. Feed Sci. Technol. 1997, 65, 79–86. [Google Scholar] [CrossRef]
- Cho, H.; Brotherton, J.E.; Song, H.; Widholm, J.M. Increasing tryptophan synthesis in a forage legume Astragalus sinicus by expressing the tobacco feedback-insensitive anthranilate synthase (ASA2) gene. Plant Physiol. 2000, 123, 1069–1076. [Google Scholar] [CrossRef]
- Cho, H.; Widholm, J.M.; Tanaka, N.; Nakanishi, Y. Agrobacterium rhizogenes-mediated transformation and regeneration of the legume Astragalus sinicus (Chinese milk vetch). Plant Sci. 1998, 138, 53–65. [Google Scholar] [CrossRef]
- Qiao, J.; Zhao, D.; Zhou, W.; Yan, T.M.; Yang, L.Z. Sustained rice yields and decreased N runoff in a rice-wheat cropping system by replacing wheat with Chinese milk vetch and sharply reducing fertilizer use. Environ. Pollut. 2021, 288, 117722. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Zhou, L.; Li, Y.G.; Chen, D.S.; Tan, X.J.; Lei, L.; Zhou, J.C. A nodule-specific plant cysteine proteinase, AsNODF32, is involved in nodule senescence and nitrogen fixation activity of the green manure legume Astragalus sinicus. New Phytol. 2008, 180, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liao, Y.L.; Lu, Y.H.; Rees, R.M.; Cao, W.D.; Nie, J.; Li, M. Management of rice straw with relay cropping of Chinese milk vetch improved double-rice cropping system production in southern China. J. Integr. Agric. 2020, 19, 2103–2115. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, Y.; Xu, X.T.; Yang, B.J.; Aamer, M.; Zhang, P.; Huang, G.Q. Paddy-upland rotation with Chinese milk vetch incorporation reduced the global warming potential and greenhouse gas emissions intensity of double rice cropping system. Environ. Pollut. 2021, 276, 116696. [Google Scholar] [CrossRef]
- Yang, W.; Yao, L.; Zhu, M.Z.; Li, C.W.; Li, S.Q.; Wang, B.; Dijkstra, P.; Liu, Z.Y.; Zhu, B. Replacing urea-N with Chinese milk vetch (Astragalus sinicus L.) mitigates CH4 and N2O emissions in rice paddy. Agric. Ecosyst. Environ. 2022, 336, 108033. [Google Scholar] [CrossRef]
- Wang, Y.R.; Yu, L.; Nan, Z.B.; Liu, Y.L. Vigor tests used to rank seed lot quality and predict field emergence in four forage species. Crop Sci. 2004, 44, 535–541. [Google Scholar] [CrossRef]
- Townsend, C.E.; McGinnies, W.J. Establishment of nine forage legumes in the central great plains. Agron. J. 1972, 64, 699–702. [Google Scholar] [CrossRef]
- Guo, Z.G.; Liu, H.X.; Wang, S.M.; Tian, F.P.; Cheng, G.D. Biomass, persistence and drought resistance of nine lucerne varieties in the dry environment of west China. Aust. J. Exp. Agric. 2005, 45, 59–64. [Google Scholar] [CrossRef]
- Shinohara, T.; Ducournau, S.; Matthews, S.; Wagner, M.H.; Powell, A.A. Early counts of radicle emergence, counted manually and by image analysis, can reveal differences in the production of normal seedlings and the vigour of seed lots of cauliflower. Seed Sci. Technol. 2021, 49, 219–235. [Google Scholar] [CrossRef]
- Ellis, R.H. Seed and seedling vigour in relation to crop growth and yield. Plant Growth Regul. 1992, 11, 249–255. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Wang, Y.R.; Powell, A.A. Frequent individual counts of radicle emergence and mean just germination time predict seed vigour of Avena sativa and Elymus nutans. Seed Sci. Technol. 2016, 44, 189–198. [Google Scholar]
- Akbarpour, M.; Khajeh-Hosseini, M.; Seifi, A. Potential of a single radicle emergence count in predicting field emergence of Desi chickpea seed lots as an alternative vigour test. Seed Sci. Technol. 2019, 47, 319–324. [Google Scholar] [CrossRef]
- Tao, Q.B.; Sun, J.P.; Zhang, Y.Q.; Sun, X.T.; Li, Z.Y.; Zhong, S.Z.; Sun, J. Single count of radicle emergence and mean germination time estimate seed vigour of Chinese milk vetch (Astragalus sinicus). Seed Sci. Technol. 2022, 50, 47–59. [Google Scholar] [CrossRef]
- Cheshmi, M.; Khajeh-Hosseini, M. Single count of radicle emergence, DNA replication during seed germination and vigour in alfalfa seed lots. Seed Sci. Technol. 2020, 48, 367–380. [Google Scholar] [CrossRef]
- dos Santos, L.A.; Carvalho, I.R.; Pinto, C.C.; Szareski, V.J.; Netto, J.F.; de Medeiros, L.R.; Martins, A.B.N.; Bilhalva, N.S.; Marchi, P.M.; Pimentel, J.R.; et al. Electrical conductivity test for measurement of white clover seeds vigor. J. Agric. Sci. 2019, 11, 40–49. [Google Scholar] [CrossRef]
- Artola, A.; Castañeda, G.C. The bulk conductivity test for birdsfoot trefoil seed. Seed Sci. Technol. 2005, 33, 231–236. [Google Scholar] [CrossRef]
- Cheshmi, M.; Khajeh-Hosseini, M. Effect of temperature on length of the lag period and its relationship with field performance of alfalfa (Medicago sativa) seeds. Seed Sci. Technol. 2018, 46, 317–326. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2015, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, A. Safflower (Carthamus tinctorius L.) seed vigor tests for the prediction of field emergence. Ind. Crops Prod. 2019, 131, 378–386. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Mo, Q.; Powell, A.A.; Wang, Y.R. DNA replication during seed germination, deterioration, and its relation to vigor in alfalfa and white clover. Crop Sci. 2018, 58, 1393–1401. [Google Scholar] [CrossRef]
- Artola, A.; Castañeda, G.C. Accelerated aging time estimation for birdsfoot trefoil seed. Seed Sci. Technol. 2005, 33, 493–497. [Google Scholar] [CrossRef]
- Modarresi, R.; Damme, P.V. Application of the controlled deterioration test to evaluate wheat seed vigour. Seed Sci. Technol. 2003, 31, 771–775. [Google Scholar] [CrossRef]
- Luo, Y.; Guan, Y.J.; Huang, Y.T.; Li, J.; Li, Z.; Hu, J. Single counts of radicle emergence provides an alternative method to test seed vigour in sweet corn. Seed Sci. Technol. 2015, 43, 519–525. [Google Scholar] [CrossRef]
- Javaid, M.M.; Mahmood, A.; Alshaya, D.S.; AlKahtani, M.D.F.; Waheed, H.; Wasaya, A.; Khan, S.A.; Naqve, M.; Haider, I.; Shahid, M.A.; et al. Influence of environmental factors on seed germination and seedling characteristics of perennial ryegrass (Lolium perenne L.). Sci. Rep. 2022, 12, 9522. [Google Scholar] [CrossRef]
- Idris, L.M.; Nulit, R.; Zaman, F.Q.; Arifin, F.K.M. Hydrotime analysis of Amaranthus spp. Seed germination under salinity condition. J. Appl. Res. Med. Aromat. Plants 2020, 17, 100249. [Google Scholar] [CrossRef]
- Hu, X.W.; Fan, Y.; Baskin, C.C.; Baskin, J.M.; Wang, Y.R. Comparison of the effects of temperature and water potential on seed germination of Fabaceae species from desert and subalpine grassland. Am. J. Bot. 2015, 102, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Luo, K.; Chen, D.L.; Baskin, J.M.; Baskin, C.C.; Wang, Y.R.; Hu, X.W. Comparison of thermal and hydrotime requirements for seed germination of seven Stipa species from cool and warm habitats. Front. Plant Sci. 2020, 11, 560714. [Google Scholar] [CrossRef] [PubMed]
- Gummerson, R.J. The effect of constant temperatures and osmotic potentials on the germination of sugar beet. J. Exp. Bot. 1986, 37, 729–741. [Google Scholar] [CrossRef]
- Bradford, K.J. A water relation analysis of seed germination rates. Plant Physiol. 1990, 94, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Farahinia, P.; Sadat-Noori, S.A.; Mortazavian, M.M.; Soltani, E.; Foghi, B. Hydrotime model analysis of Trachyspermum ammi (L.) Sprague seed germination. J. Appl. Res. Med. Aromat. Plants 2017, 5, 88–91. [Google Scholar] [CrossRef]
- Shaygan, M.; Baumgartl, T.; Arnold, S. Germination of Atriplex halimus seeds under salinity and water stress. Ecol. Eng. 2017, 102, 636–640. [Google Scholar] [CrossRef]
- Bradford, K.J.; Still, D.W. Applications of hydrotime analysis in seed testing. Seed Technol. 2004, 26, 74–85. [Google Scholar]
- López, A.S.; López, D.R.; Arana, M.V.; Batlla, D.; Marchelli, P. Germination response to water availability in populations of Festuca pallescens along a Patagonian rainfall gradient based on hydrotime model parameters. Sci. Rep. 2021, 11, 10653. [Google Scholar] [CrossRef]
- Chen, X.L.; Wei, Z.C.; Chen, D.L.; Hu, X.W. Base water potential but not hydrotime predicts seedling emergence of Medicago sativa under water stress conditions. PeerJ 2022, 10, e13206. [Google Scholar] [CrossRef]
- Soltani, E.; Farzaneh, S. Hydrotime analysis for determination of seed vigour in cotton. Seed Sci. Technol. 2014, 42, 260–273. [Google Scholar] [CrossRef]
- Soltani, E.; Adeli, R.; Akbari, G.A.; Ramshini, H. Application of hydrotime model to predict early vigour of rapeseed (Brassica napus L.) under abiotic stresses. Acta Physiol. Plant 2017, 39, 252. [Google Scholar] [CrossRef]
- Farzane, S.; Soltani, E. Relationships between hydrotime parameters and seed vigor in sugar beet. Seed Sci. Biotechnol. 2011, 5, 7–10. [Google Scholar]
- Tatari, S.; Ghaderi-Far, F.; Yamchi, A.; Siahmarguee, A.; Shayanfar, A.; Baskin, C.C. Application of the hydrotime model to assess seed priming effects on the germination of rapeseed (Brassica napus L.) in response to water stress. Botany 2020, 98, 283–291. [Google Scholar] [CrossRef]
- Romano, A.; Bravi, R. Hydrotime model to evaluate the effects of a set of priming agents on seed germination of two leek cultivars under water stress. Seed Sci. Technol. 2021, 49, 159–174. [Google Scholar] [CrossRef]
- Windauer, L.; Altuna, A.; Benech-Arnold, R. Hydrotime analysis of Lesquerella fendleri seed germination responses to priming treatments. Ind. Crops Prod. 2007, 25, 70–74. [Google Scholar] [CrossRef]
- Li, R.; Min, D.D.; Chen, L.J.; Chen, C.Y.; Hu, X.W. Hydropriming accelerates seed germination of Medicago sativa under stressful conditions: A thermal and hydrotime model approach. Legume Res. 2017, 40, 741–747. [Google Scholar] [CrossRef]
- Maleki, K.; Maleki, K.; Soltani, E.; Oveisi, M.; Gonzalez-Andujar, J.L. A model for changes in germination synchrony and its implements to study weed population dynamics: A case study of Brassicaceae. Plants 2023, 12, 233. [Google Scholar] [CrossRef]
- Min, X.Y.; Wang, Q.X.; Wei, Z.W.; Liu, Z.P.; Liu, W.X. Full-length transcriptional analysis reveals the complex relationship of leaves and roots in responses to cold-drought combined stress in common vetch. Front. Plant Sci. 2022, 13, 976094. [Google Scholar] [CrossRef]
- Bhat, K.A.; Mahajan, R.; Pakhtoon, M.M.; Urwat, U.; Bashir, Z.; Shah, A.A.; Agrawal, A.; Bhat, B.; Sofi, P.A.; Masi, A.; et al. Low temperature stress tolerance: An insight into the omics approaches for legume crops. Front. Plant Sci. 2022, 13, 888710. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Cui, H.T.; Li, M.N.; Yang, G.F.; Wang, Z.Y.; Zhang, K. Carex rigescens caffeic acid O-methyltransferase gene CrCOMT confer melatonin-mediated drought tolerance in transgenic tobacco. Front. Plant Sci. 2022, 13, 971431. [Google Scholar] [CrossRef]
- Wu, H.W.; Asaduzzaman, M.; Shephard, A.; Hopwood, M.; Ma, X.Y. Germination and emergence characteristics of prickly lettuce (Lactuca serriola L.). Crop Prot. 2020, 136, 105222. [Google Scholar] [CrossRef]
- Mavi, K.; Light, M.E.; Demir, I.; van Staden, J.; Yasar, F. Positive effect of smoke-derived butanolide priming on melon seedling emergence and growth. N. Z. J. Crop Hortic. Sci. 2010, 38, 147–155. [Google Scholar] [CrossRef]
- Müller, F.; Masemola, L.; Britz, E.; Ngcobo, N.; Modiba, S.; Cyster, L.; Samuels, I.; Cupido, C.; Raitt, L. Seed germination and early seedling growth responses to drought stress in annual Medicago L. and Trifolium L. forages. Agronomy 2022, 12, 2960. [Google Scholar] [CrossRef]
- Miano, A.C.; Augusto, P.E.D. From the sigmoidal to downward concave shape behavior during the hydration of grains: Effect of the initial moisture content on Adzuki beans (Vigna angularis). Food Bioprod. Process. 2015, 96, 43–51. [Google Scholar] [CrossRef]
- Ducatti, K.R.; Batista, T.B.; Hirai, W.Y.; Luccas, D.A.; Moreno, L.A.; Guimarães, C.C.; Bassel, G.W.; da Silva, E.A.A. Transcripts expressed during germination Sensu Stricto are associated with vigor in soybean seeds. Plants 2022, 11, 1310. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.; Khajeh-Hosseini, M. Length of the lag period of germination and metabolic repair explain vigour differences in seed lots of maize (Zea mays). Seed Sci. Technol. 2007, 35, 200–212. [Google Scholar] [CrossRef]
- Mavi, K.; Demir, I.; Matthews, S. Mean germination time estimates the relative emergence of seed lots of three cucurbit crops under stress conditions. Seed Sci. Technol. 2010, 38, 14–25. [Google Scholar] [CrossRef]
- Yan, H.F.; Yu, X.D.; Jia, W.; Mao, P.S. Length of the lag period of germination predicts the vigour differences and field emergence potential in Italian ryegrass (Lolium multiflorum) seed lots. Seed Sci. Technol. 2017, 45, 238–242. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, C.; Fu, Y.Y.; Huang, Y.T.; He, F.; Guan, Y.J.; Hu, J. Single counts of radicle emergence can be used as a fast method to test seed vigour of indica rice. Seed Sci. Technol. 2017, 45, 222–229. [Google Scholar] [CrossRef]
- Mao, P.S.; Zhang, X.Y.; Sun, Y.; Zhang, W.X.; Wang, Y.W. Relationship between the length of lag period of germination and the emergence performance of oat (Avena sativa) seeds. Seed Sci. Technol. 2013, 41, 281–291. [Google Scholar] [CrossRef]
- Venuste, M.; Chen, D.L.; Hu, X.W. Detection of seed vigour differences in Festuca sinensis seed lots. Seed Sci. Technol. 2022, 50, 61–75. [Google Scholar]
- Venuste, M.; Li, D.M.; Jia, P.; Hu, X.W. Various vigour test methods to rank seed lot quality and predict field emergence in two forage grasses. Seed Sci. Technol. 2022, 50, 345–356. [Google Scholar]
- Patanè, C.; Saita, A.; Tubeileh, A.; Cosentino, S.L.; Cavallaro, V. Modeling seed germination of unprimed and primed seeds of sweet sorghum under PEG-induced water stress through the hydrotime analysis. Acta Physiol. Plant. 2016, 38, 115. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Gholamhossieni, M. Quantification of soybean seed germination response to seed deterioration under PEG-induced water stress using hydrotime concept. Acta Physiol. Plant. 2018, 40, 126. [Google Scholar] [CrossRef]
- Krichen, K.; Mariem, H.B.; Chaieb, M. Ecophysiological requirements on seed germination of a Mediterranean perennial grass (Stipa tenacissima L.) under controlled temperatures and water stress. S. Afr. J. Bot. 2014, 94, 210–217. [Google Scholar] [CrossRef]
- Cavallaro, V.; Barbera, A.C.; Maucieri, C.; Gimma, G.; Scalisi, C.; Patanè, C. Evaluation of variability to drought and saline stress through the germination of different ecotypes of carob (Ceratonia siliqua L.) using a hydrotime model. Ecol. Eng. 2016, 95, 557–566. [Google Scholar] [CrossRef]
- Windauer, L.B.; Martinez, J.; Rapoport, D.; Wassner, D.; Benech-Arnold, R. Germination responses to temperature and water potential in Jatropha curcas seeds: A hydrotime model explains the difference between dormancy expression and dormancy induction at different incubation temperatures. Ann. Bot. 2012, 109, 265–273. [Google Scholar] [CrossRef]
- Schellenberg, M.P.; Biligetu, B.; Wei, Y. Predicting seed germination of slender wheatgrass [Elymus trachycaulus (Link) Gould subsp. trachycaulus] using thermal and hydro time models. Can. J. Plant Sci. 2013, 93, 793–798. [Google Scholar] [CrossRef]
- Allen, P.S.; Thorne, E.T.; Gardner, J.S.; White, D.B. Is the barley endosperm a water reservoir for the embryo when germinating seeds are dried? Int. J. Plant Sci. 2000, 161, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Dahal, P.; Bradford, K.J. Effects of priming and endosperm integrity on germination rates of tomato genotypes. II. Germination at reduced water potential. J. Exp. Bot. 1990, 41, 1441–1453. [Google Scholar] [CrossRef]
- Bradford, K.J.; Somasco, O.A. Water relations of lettuce seed thermoinhibition. I. Priming and endosperm effects on base water potential. Seed Sci. Res. 1994, 4, 1–10. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Fallahi, H.; Aghhavani-Shajari, M.; Mohammadi, M.; Kadkhodaei-Barkook, R.; Zareei, E. Predicting of flixweed (Descurainia sophia (L.) Webb ex Prantl) germination response to temperature using regression models. J. Appl. Res. Med. Aromat. Plants 2017, 6, 131–134. [Google Scholar] [CrossRef]
- Tao, Q.B.; Lv, Y.Y.; Mo, Q.; Bai, M.J.; Han, Y.H.; Wang, Y.R. Impacts of priming on seed germination and seedling emergence of Cleistogenes songorica under drought stress. Seed Sci. Technol. 2018, 46, 239–258. [Google Scholar] [CrossRef]
- Yang, X.J.; Baskin, C.C.; Baskin, J.M.; Liu, G.Z.; Huang, Z.Y. Seed mucilage improves seedling emergence of a sand desert shrub. PLoS ONE 2012, 7, e34597. [Google Scholar] [CrossRef]
- Zhao, H.J.; Liu, Q.L.; Fu, H.W.; Hu, X.H.; Wu, D.X.; Shu, Q.Y. Effect of non-lethal low phytic acid mutations on grain yield and seed viability in rice. Field Crops Res. 2008, 108, 206–211. [Google Scholar] [CrossRef]
- Cheng, Z.; Bradford, K.J. Hydrothermal time analysis of tomato seed germination responses to priming treatments. J. Exp. Bot. 1999, 50, 89–99. [Google Scholar] [CrossRef]
- Huarte, R. Hydrotime analysis of the effect of fluctuating temperatures on seed germination in several non-cultivated species. Seed Sci. Technol. 2006, 34, 533–547. [Google Scholar] [CrossRef]
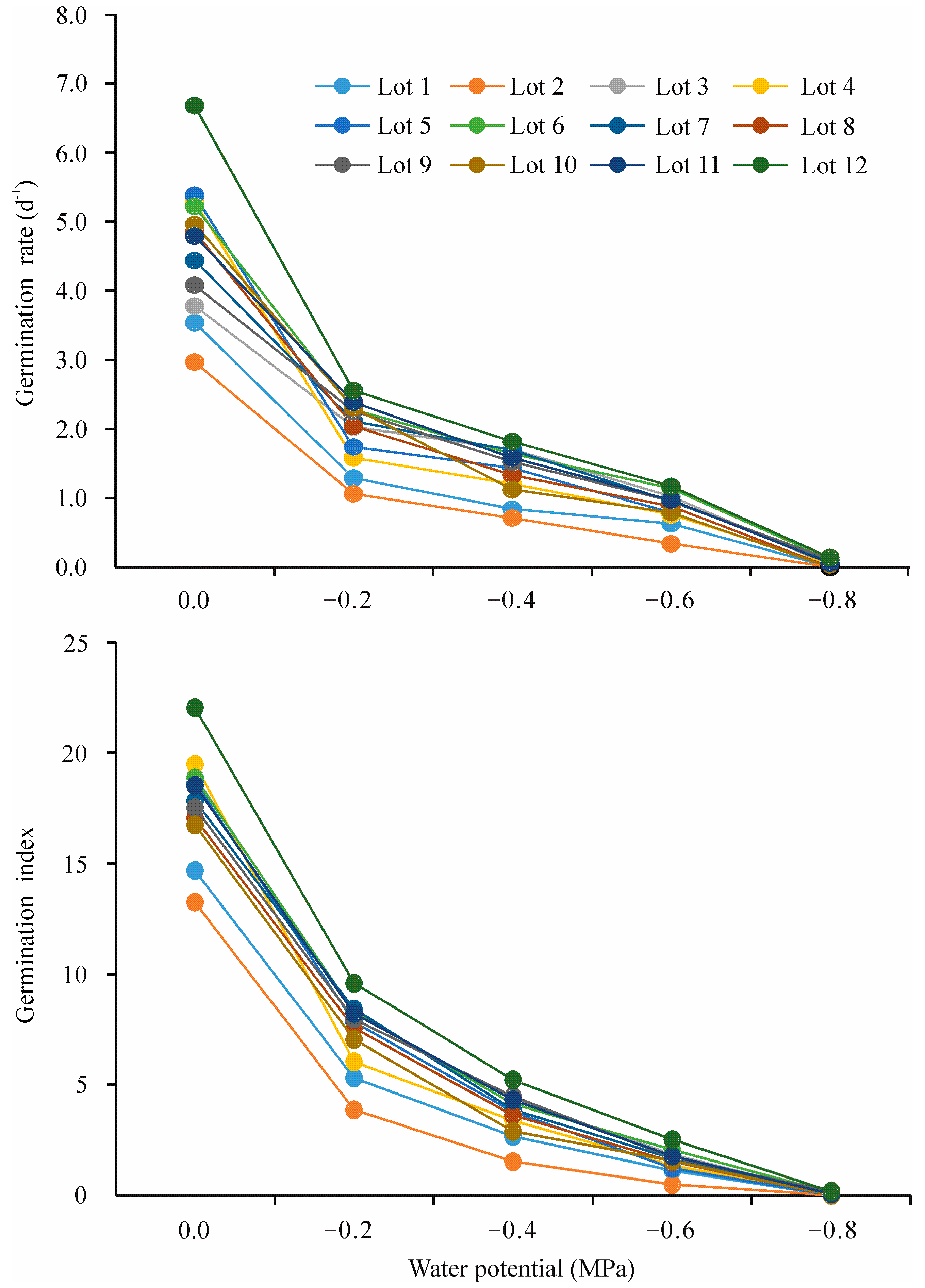
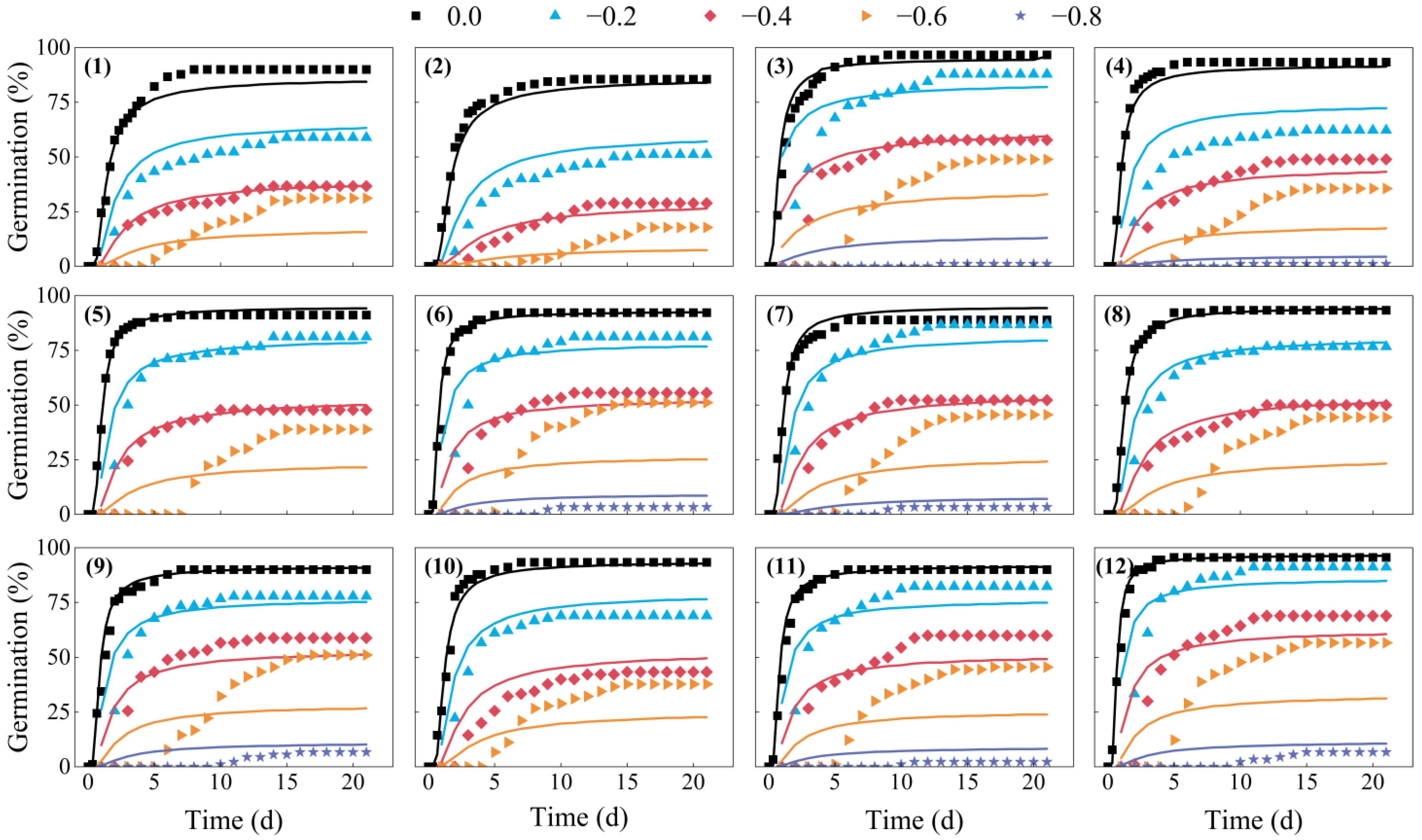


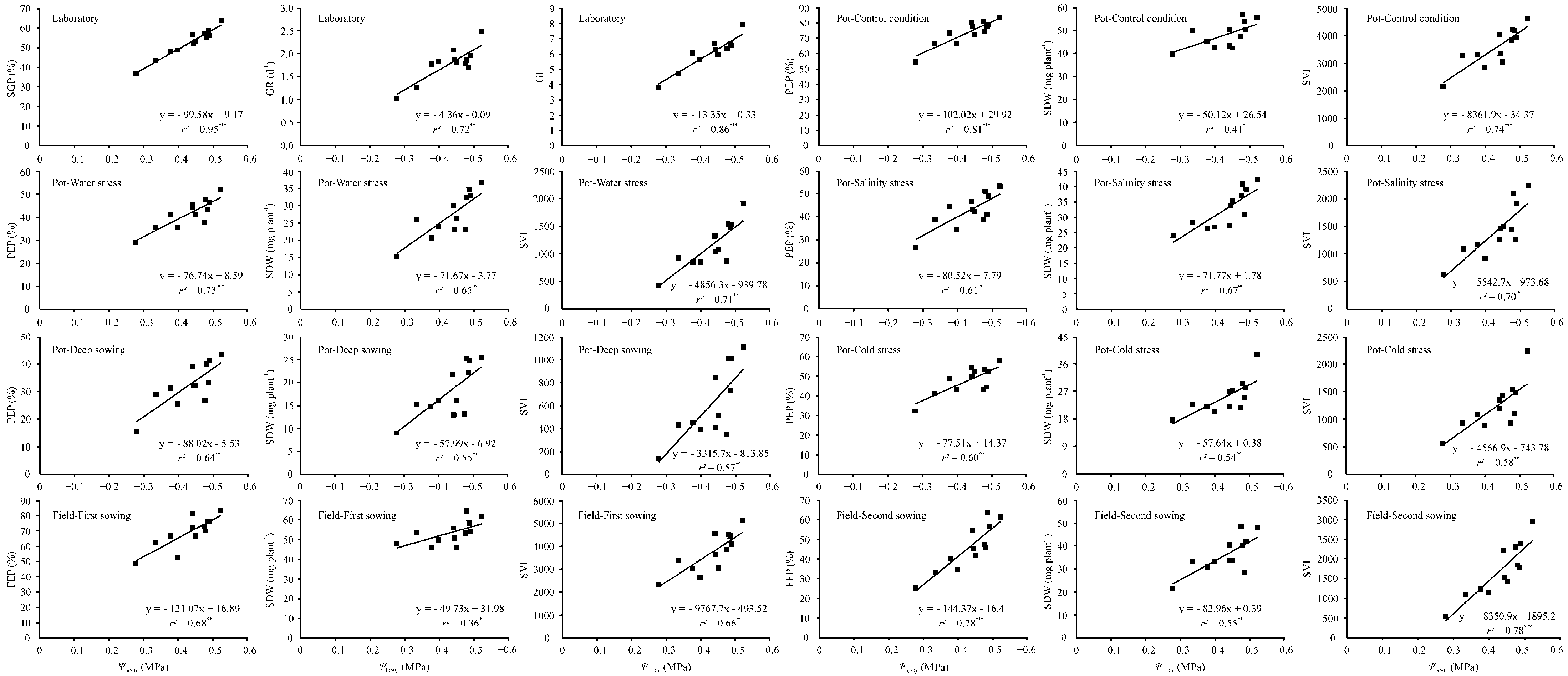
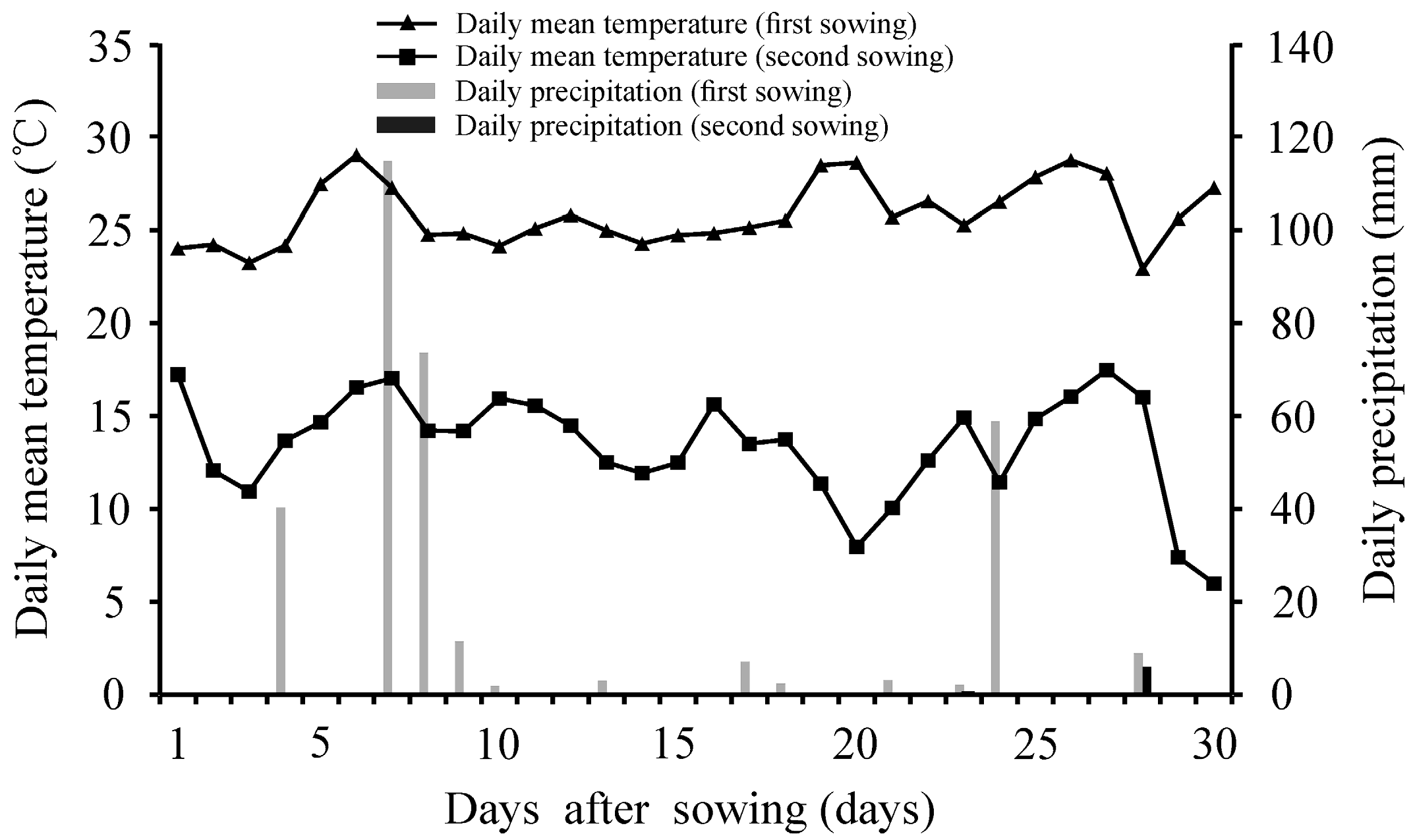
| Seed Lot | Water Potential (MPa) | ||||
|---|---|---|---|---|---|
| 0.0 | −0.2 | −0.4 | −0.6 | −0.8 | |
| 1 | 90.0 abc | 58.9 ef | 36.7 cd | 31.1 de | 0.0 c |
| 2 | 85.6 c | 51.1 f | 28.9 d | 17.8 e | 0.0 c |
| 3 | 96.7 a | 87.8 ab | 57.8 ab | 48.9 abc | 1.1 c |
| 4 | 93.3 abc | 62.2 ef | 48.9 bc | 35.6 cd | 1.1 c |
| 5 | 91.1 abc | 81.1 bc | 47.8 bcd | 38.9 bcd | 0.0 c |
| 6 | 92.2 abc | 81.1 bc | 55.6 abc | 51.1 abc | 3.3 abc |
| 7 | 88.9 bc | 86.7 abc | 52.2 abc | 45.6 abcd | 3.3 ab |
| 8 | 93.3 ab | 76.7 cd | 50.0 bc | 44.4 abcd | 0.0 c |
| 9 | 90.0 abc | 77.8 cd | 58.9 ab | 51.1 ab | 6.7 a |
| 10 | 93.3 abc | 68.9 de | 43.3 bcd | 37.8 bcd | 0.0 c |
| 11 | 90.0 abc | 82.2 bc | 60.0 ab | 45.6 abcd | 2.2 c |
| 12 | 95.6 ab | 91.1 a | 68.9 a | 56.7 a | 6.7 ab |
| Analysis of variance | |||||
| Source of variance | Degrees of freedom | Sum of squares | Mean square | F | p |
| Seed lot (SL) | 11 | 1.582 | 0.144 | 15.653 | <0.001 |
| Water potential (WP) | 4 | 28.077 | 7.019 | 764.101 | <0.001 |
| SL × WP | 44 | 0.892 | 0.020 | 2.206 | <0.001 |
| Seed Lot | θH (MPa·h) | Ψb(50) (MPa) | σφb | r2 |
|---|---|---|---|---|
| 1 | 14.171 | −0.335 | 0.309 | 0.885 |
| 2 | 14.770 | −0.278 | 0.276 | 0.906 |
| 3 | 13.606 | −0.485 | 0.283 | 0.876 |
| 4 | 10.086 | −0.377 | 0.270 | 0.844 |
| 5 | 11.819 | −0.443 | 0.286 | 0.917 |
| 6 | 8.897 | −0.441 | 0.305 | 0.783 |
| 7 | 13.618 | −0.479 | 0.319 | 0.859 |
| 8 | 14.114 | −0.450 | 0.291 | 0.847 |
| 9 | 13.763 | −0.475 | 0.322 | 0.838 |
| 10 | 12.814 | −0.398 | 0.267 | 0.866 |
| 11 | 13.636 | −0.489 | 0.333 | 0.879 |
| 12 | 8.799 | −0.522 | 0.299 | 0.833 |
| Seed Lot | Control Conditions | Water Stress | Salinity Stress | Deep Sowing | Cold Stress | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEP (%) | SDW (mg plant−1) | SVI | PEP (%) | SDW (mg plant−1) | SVI | PEP (%) | SDW (mg plant−1) | SVI | PEP (%) | SDW (mg plant−1) | SVI | PEP (%) | SDW (mg plant−1) | SVI | |
| 1 | 66.7 ab | 49.9 abc | 3280.1 de | 35.6 cd | 26.1 cd | 924.5 def | 38.9 bcd | 28.4 efg | 1088.3 cd | 28.9 cd | 15.3 b | 432.3 de | 41.1 cd | 22.7 cdef | 924.9 e |
| 2 | 54.4 b | 39.7 d | 2153.4 f | 28.9 d | 15.4 f | 433.5 g | 26.7 d | 24.1 g | 631.2 e | 15.6 e | 9.0 c | 135.2 f | 32.2 e | 17.7 f | 561.1 f |
| 3 | 77.8 a | 53.9 ab | 4175.9 ab | 43.3 abc | 34.6 ab | 1484.9 bc | 41.1 bc | 30.9 def | 1262.6 bcd | 33.3 abcd | 22.1 a | 729.4 c | 44.4 bc | 25.0 bcde | 1099.1 de |
| 4 | 73.3 a | 45.3 cd | 3306.8 de | 41.1 bc | 20.7 e | 849.4 f | 44.4 abc | 26.3 fg | 1173.8 bcd | 31.1 bcd | 14.7 b | 453.2 de | 48.9 abc | 22.0 def | 1079.3 de |
| 5 | 77.8 a | 43.4 cd | 3360.9 cde | 45.6 abc | 23.2 de | 1045.5 de | 43.3 abc | 33.7 cde | 1465.6 bc | 32.2 bcd | 12.9 b | 408.3 de | 50.0 abc | 27.0 bcd | 1350.1 bc |
| 6 | 80.0 a | 50.2 abc | 4029.1 abc | 44.4 abc | 29.9 bc | 1324.0 c | 46.7 abc | 27.3 fg | 1262.0 bcd | 38.9 abc | 21.9 a | 844.7 b | 54.4 ab | 22.0 def | 1194.5 cd |
| 7 | 74.4 a | 56.9 a | 4213.2 ab | 47.8 ab | 32.5 ab | 1540.0 b | 51.1 ab | 40.8 ab | 2093.2 a | 40.0 ab | 25.2 a | 1008.3 a | 53.3 ab | 29.5 b | 1538.2 b |
| 8 | 72.2 ab | 42.4 cd | 3048.9 e | 41.1 bc | 26.4 cd | 1081.2 d | 42.2 abc | 35.6 bcd | 1497.3 b | 32.2 bcd | 16.1 b | 508.7 d | 52.2 abc | 27.3 bcd | 1425.7 bc |
| 9 | 81.1 a | 47.3 bcd | 3846.3 bcd | 37.8 bcd | 23.1 de | 869.3 ef | 38.9 bcd | 37.2 abc | 1434.6 bc | 26.7 d | 13.1 b | 346.5 e | 43.3 bcd | 21.7 def | 927.8 e |
| 10 | 66.7 ab | 42.8 cd | 2848.3 e | 35.6 cd | 23.9 de | 848.0 f | 34.4 cd | 26.8 fg | 919.6 de | 25.6 d | 16.2 b | 396.8 de | 43.3 bcd | 20.4 ef | 886.1 e |
| 11 | 78.9 a | 50.2 abc | 3943.0 abcd | 46.7 ab | 32.9 ab | 1533.1 b | 48.9 ab | 39.2 abc | 1912.9 a | 41.1 ab | 24.7 a | 1011.0 a | 52.2 abc | 28.3 bc | 1471.9 b |
| 12 | 83.3 a | 55.6 a | 4629.4 a | 52.2 a | 36.8 a | 1906.0 a | 53.3 a | 42.2 a | 2241.6 a | 43.3 a | 25.6 a | 1108.4 a | 57.8 a | 39.0 a | 2235.9 a |
| ANOVA | |||||||||||||||
| SV | df | PEP | SDW | SVI | |||||||||||
| SL | 11 | *** | *** | *** | |||||||||||
| EC | 4 | *** | *** | *** | |||||||||||
| SL × EC | 44 | ns | *** | *** | |||||||||||
| Experiment Conditions | Variable | θH (MPa·h) | Ψb(50) (MPa) | σφb |
|---|---|---|---|---|
| Germination test | Germination percentage (%) | −0.463 | −0.972 (<0.001) | 0.424 |
| Germination rate (day−1) | −0.702 (0.011) | −0.847 (0.001) | 0.234 | |
| Germination index | −0.623 (0.030) | −0.929 (<0.001) | 0.312 | |
| Pot | Control conditions | |||
| Emergence percentage (%) | −0.531 | −0.900 (<0.001) | 0.476 | |
| Seedling dry weight (mg plant−1) | −0.268 | −0.642 (0.024) | 0.546 | |
| Simplified vigor index | −0.458 | −0.858 (<0.001) | 0.563 | |
| Water stress | ||||
| Emergence percentage (%) | −0.557 | −0.853 (<0.001) | 0.391 | |
| Seedling dry weight (mg plant−1) | −0.290 | −0.807 (0.002) | 0.476 | |
| Simplified vigor index | −0.415 | −0.845 (0.001) | 0.462 | |
| Salinity stress | ||||
| Emergence percentage (%) | −0.552 | −0.780 (0.003) | 0.504 | |
| Seedling dry weight (mg plant−1) | −0.031 | −0.820 (0.001) | 0.666 (0.018) | |
| Simplified vigor index | −0.284 | −0.834 (0.001) | 0.616 (0.033) | |
| Deep sowing | ||||
| Emergence percentage (%) | −0.523 | −0.803 (0.002) | 0.518 | |
| Seedling dry weight (mg plant−1) | −0.347 | −0.742 (0.006) | 0.485 | |
| Simplified vigor index | −0.423 | −0.754 (0.005) | 0.529 | |
| Cold stress | ||||
| Emergence percentage (%) | −0.630 (0.028) | −0.776 (0.003) | 0.351 | |
| Seedling dry weight (mg plant−1) | −0.375 | −0.734 (0.007) | 0.341 | |
| Simplified vigor index | −0.508 | −0.763 (0.004) | 0.335 | |
| Field | First sowing | |||
| Emergence percentage (%) | −0.567 | −0.825 (0.001) | 0.519 | |
| Seedling dry weight (mg plant−1) | −0.191 | −0.600 (0.039) | 0.527 | |
| Simplified vigor index | −0.469 | −0.810 (0.001) | 0.566 | |
| Second sowing | ||||
| Emergence percentage (%) | −0.437 | −0.884 (<0.001) | 0.397 | |
| Seedling dry weight (mg plant−1) | −0.383 | −0.741 (0.006) | 0.700 (0.011) | |
| Simplified vigor index | −0.490 | −0.883 (<0.001) | 0.617 (0.033) |
| Seed Lot | Production Year | Storage Period (Years) | SMC (%) | TSW (g) | Proportion of HS (%) |
|---|---|---|---|---|---|
| 1 | 2018 | 4 | 8.76 | 3.359 | 2.2 |
| 2 | 2016 | 6 | 8.52 | 3.392 | 2.2 |
| 3 | 2017 | 5 | 8.69 | 3.383 | 0.0 |
| 4 | 2017 | 5 | 8.61 | 3.389 | 1.1 |
| 5 | 2019 | 3 | 8.69 | 3.501 | 2.2 |
| 6 | 2018 | 4 | 8.48 | 3.384 | 1.1 |
| 7 | 2018 | 4 | 8.57 | 3.462 | 4.4 |
| 8 | 2019 | 3 | 8.86 | 3.431 | 2.2 |
| 9 | 2019 | 3 | 8.88 | 3.480 | 2.2 |
| 10 | 2017 | 5 | 8.72 | 3.427 | 0.0 |
| 11 | 2021 | 1 | 9.10 | 3.390 | 3.3 |
| 12 | 2021 | 1 | 8.93 | 3.415 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Q.; Chen, D.; Bai, M.; Zhang, Y.; Zhang, R.; Chen, X.; Sun, X.; Niu, T.; Nie, Y.; Zhong, S.; et al. Hydrotime Model Parameters Estimate Seed Vigor and Predict Seedling Emergence Performance of Astragalus sinicus under Various Environmental Conditions. Plants 2023, 12, 1876. https://doi.org/10.3390/plants12091876
Tao Q, Chen D, Bai M, Zhang Y, Zhang R, Chen X, Sun X, Niu T, Nie Y, Zhong S, et al. Hydrotime Model Parameters Estimate Seed Vigor and Predict Seedling Emergence Performance of Astragalus sinicus under Various Environmental Conditions. Plants. 2023; 12(9):1876. https://doi.org/10.3390/plants12091876
Chicago/Turabian StyleTao, Qibo, Dali Chen, Mengjie Bai, Yaqi Zhang, Ruizhen Zhang, Xiaofei Chen, Xiaotong Sun, Tianxiu Niu, Yuting Nie, Shangzhi Zhong, and et al. 2023. "Hydrotime Model Parameters Estimate Seed Vigor and Predict Seedling Emergence Performance of Astragalus sinicus under Various Environmental Conditions" Plants 12, no. 9: 1876. https://doi.org/10.3390/plants12091876
APA StyleTao, Q., Chen, D., Bai, M., Zhang, Y., Zhang, R., Chen, X., Sun, X., Niu, T., Nie, Y., Zhong, S., & Sun, J. (2023). Hydrotime Model Parameters Estimate Seed Vigor and Predict Seedling Emergence Performance of Astragalus sinicus under Various Environmental Conditions. Plants, 12(9), 1876. https://doi.org/10.3390/plants12091876




