Effects of Passovia ovata Mistletoe on Pro-Inflammatory Markers In Vitro and In Vivo
Abstract
1. Introduction
2. Results
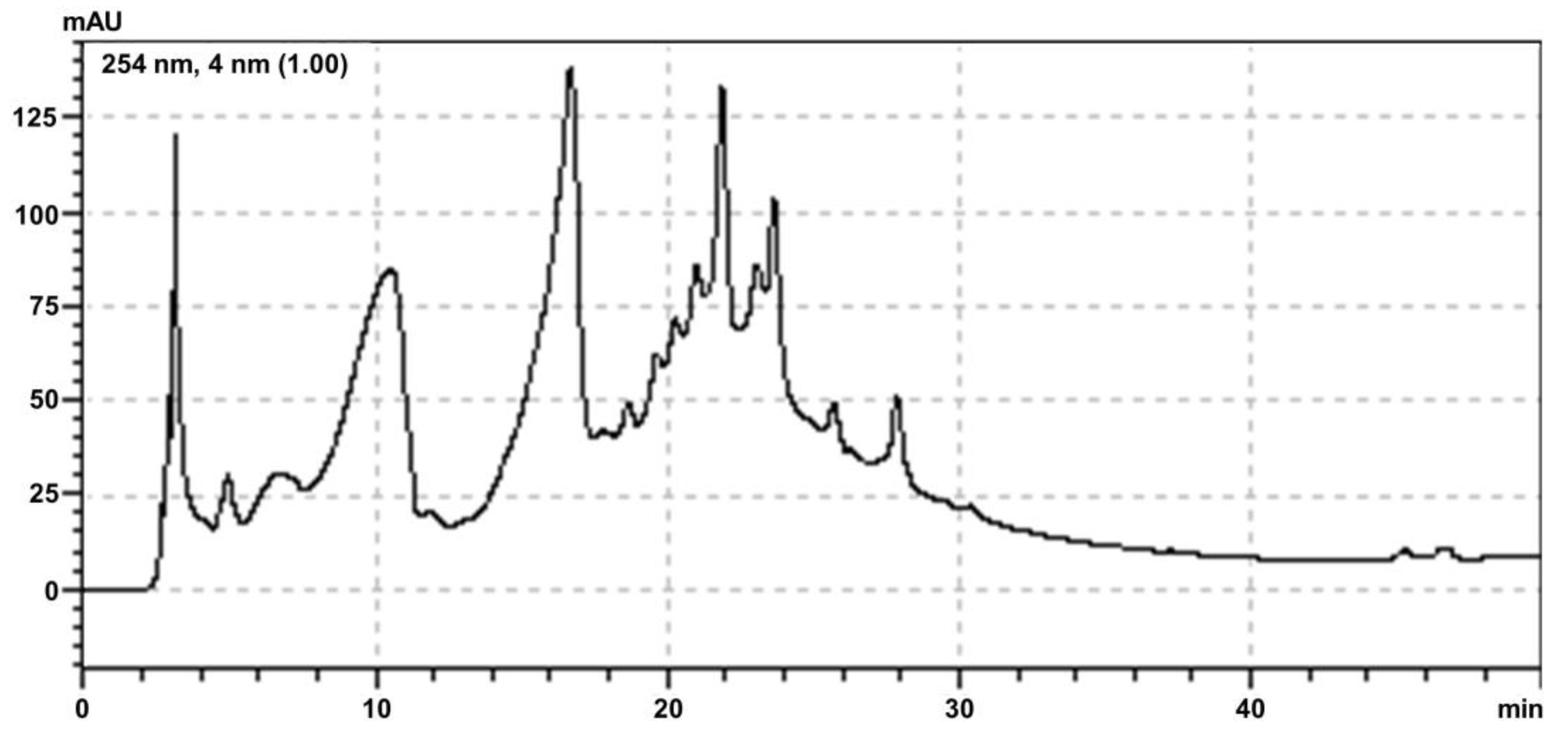
2.1. Yield and Physicochemical Properties
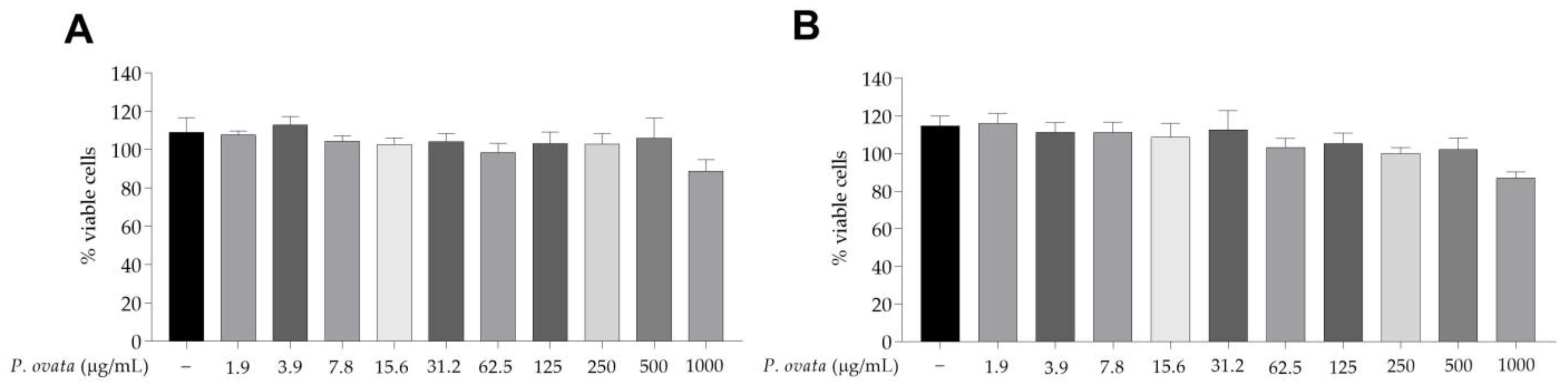
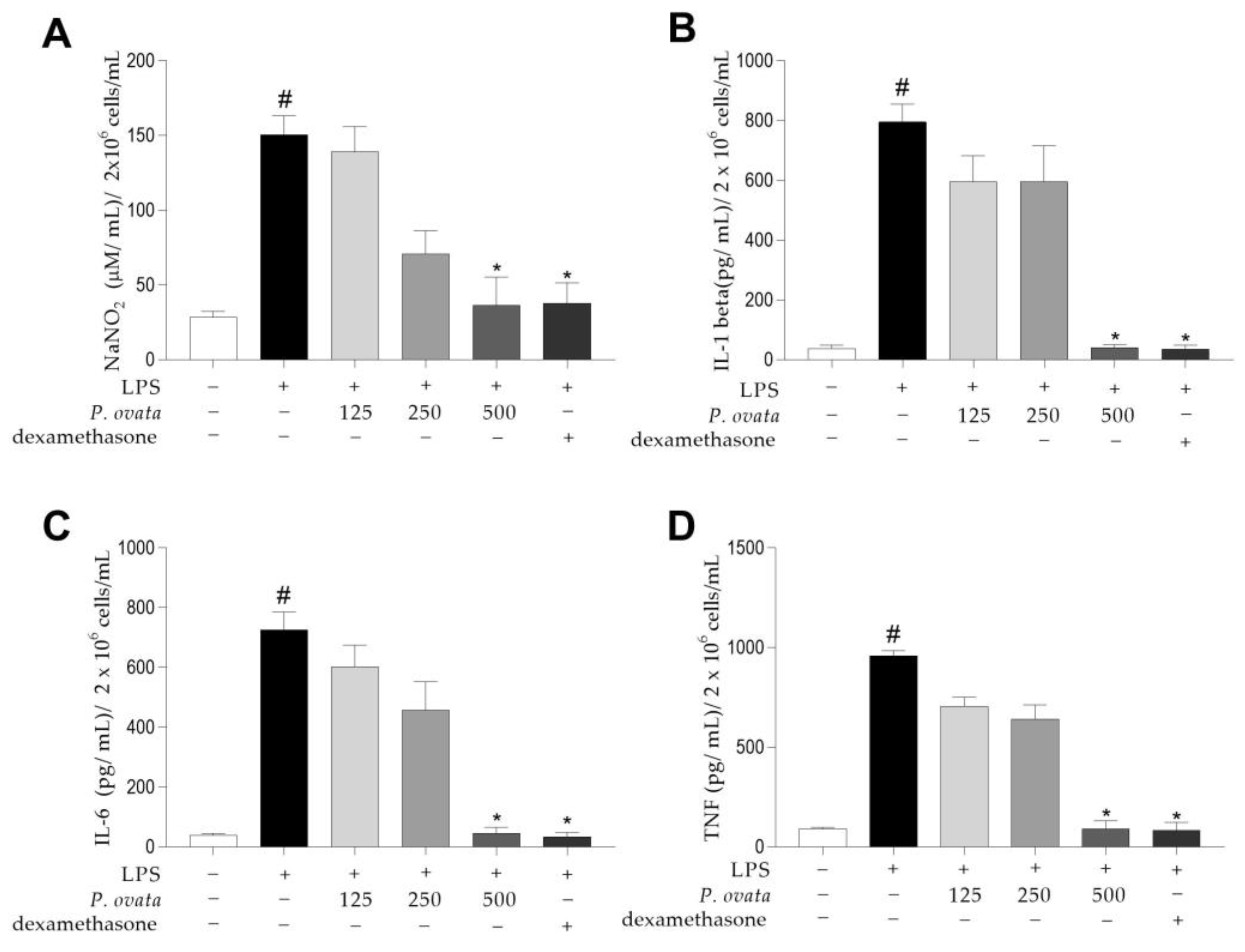
2.2. Effects of the Hydroethanolic Extract of P. ovata on Macrophages RAW 264.7
2.3. In Vivo Anti-Inflammatory Effect of POH
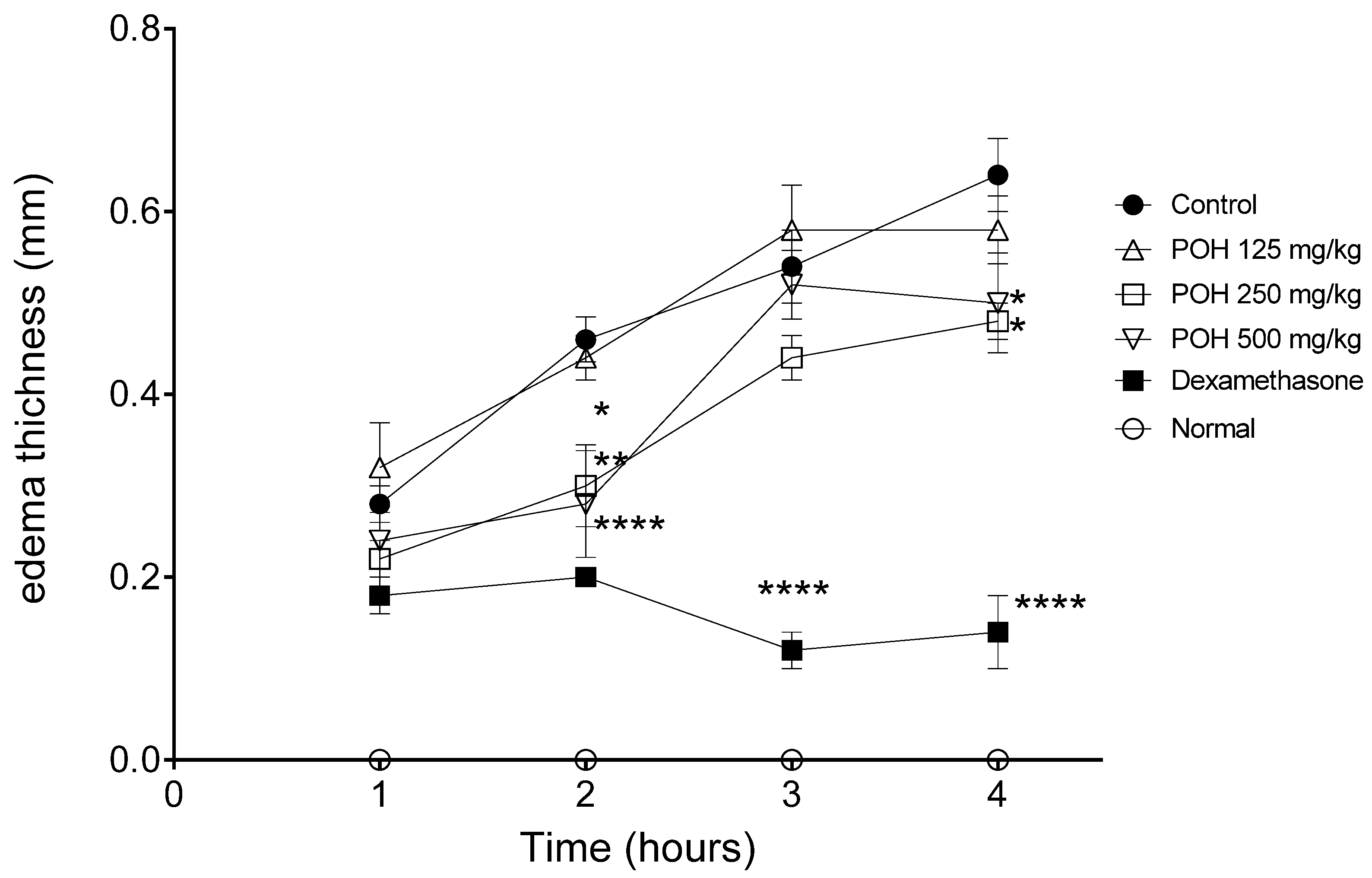
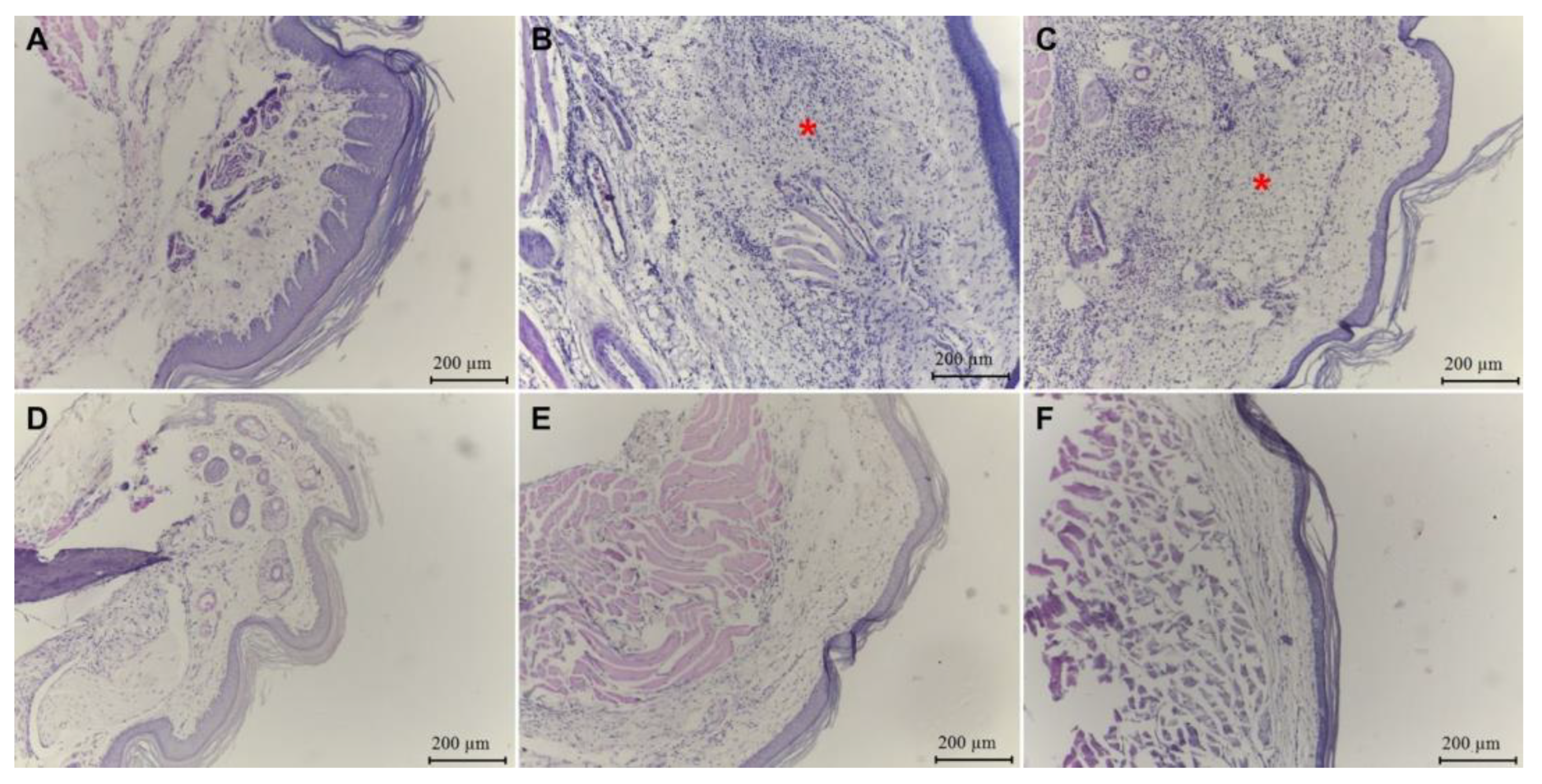
2.3.1. Paw Edema
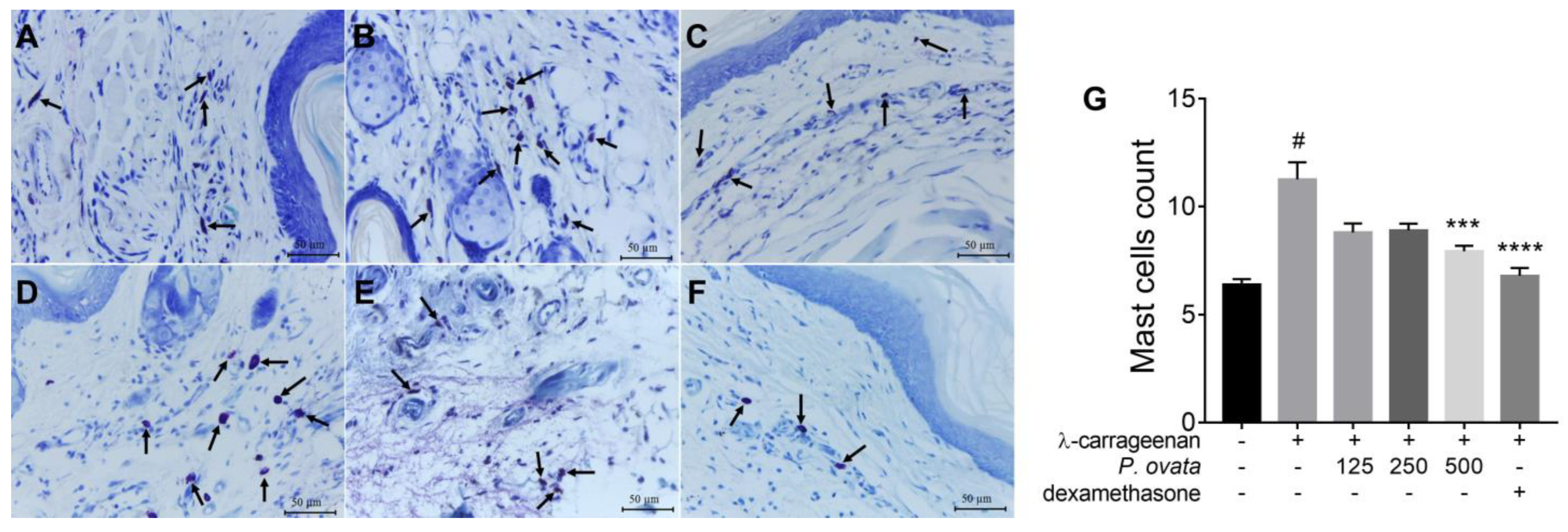
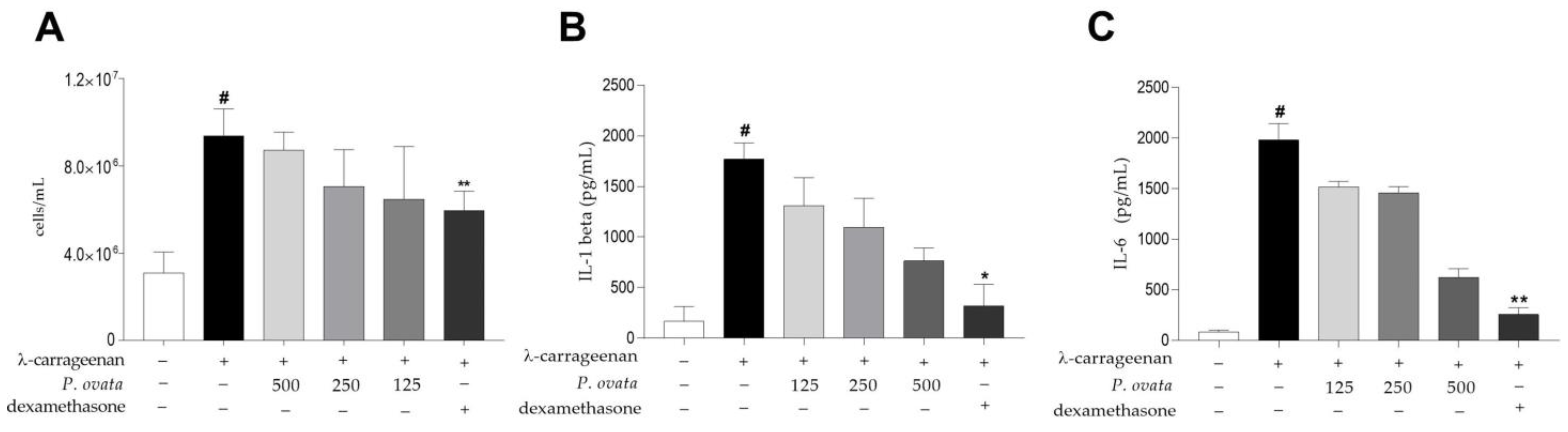
2.3.2. λ-Carrageenan-Induced Peritonitis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Hydroethanolic Extract Preparation
4.2. High Performance Liquid Chromatography (HPLC-UV)
4.3. Phytochemical Screening by Qualitative Analysis
4.3.1. Anthraquinones
4.3.2. Saponins
4.3.3. Tannins
4.3.4. Coumarins
4.3.5. Anthocyanins, Anthocyanidins, Flavone, Flavanols, Xanthones, Chalcones, Aurones, Leukoanthocyanidins, Catechins, Flavonols, and Flavanones
4.4. Flavonoid Quantification
4.5. RAW 264.7 Macrophages Cell Culture
4.6. Cytotoxicity of the Hydroethanolic Extract of P. ovata on RAW 264.7 Macrophages
4.7. Anti-Inflammatory Effect of the Hydroethanolic Extract of P. ovata in Lipopolysaccharide-Induced RAW 264.7 Macrophages
4.8. In Vivo Anti-Inflammatory Activity of Hydroethanolic Extract of P. ovata
4.8.1. Animals
4.8.2. Paw Edema Model
4.8.3. λ-Carrageenan-Induced Peritonitis
4.9. Nitrite and Cytokines Quantification
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hawiger, J.; Zienkiewicz, J. Decoding inflammation, its causes, genomic responses, and emerging countermeasures. Scand. J. Immunol. 2019, 90, e12812. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.L.; Mogildea, M.; Moreno, I.; Lopes, A. Acute Inflammation and Metabolism. Inflammation 2018, 41, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Margetic, S. Inflammation and haemostasis. Biochem. Med. 2012, 22, 49–62. [Google Scholar] [CrossRef]
- Machado, A.C.; Oliveira, R.C. Medicamentos fitoterápicos na Odontologia: Evidências e perspectivas sobre o uso da aroeira-do-sertão (Myracroduo murundeuva Allemão). Rev. Bras. Plantas Med. 2014, 16, 283–289. [Google Scholar] [CrossRef]
- Der, J.P.; Nickrent, D.L. A Molecular phylogeny of Santalaceae (Santalales). Syst. Bot. 2008, 33, 107–116. [Google Scholar] [CrossRef]
- Muche, M.; Muasya, A.M.; Tsegay, B.A. Biology and resource acquisition of mistletoes, and the defense responses of host plants. Ecol. Process. 2022, 11, 24. [Google Scholar] [CrossRef]
- Caires, C.S.; Gomes-Bezerra, K.M.; Machado, A.F.P.; Dettke, G.A. Nomenclatural novelties and synopsis of Passovia (Loranthaceae): New synonyms, new combinations and reinstated species. Rodriguésia 2021, 72, e01952019. [Google Scholar] [CrossRef]
- Venturelli, M. Estudos sobre Struthanthus vulgaris Mart.: Anatomia do fruto e semente e aspecto de germinação, crescimento e desenvolvimento. Rev. Bras. Bot. 1981, 4, 131–147. [Google Scholar]
- De Souza, M.C.; Scalon, M.C.; Poschenrieder, C.; Tolrà, R.; Venâncio, T.; Teixeira, S.P.; Da Costa, F.B. Aluminium detoxification in facultative (Passovia ovata (Pohl ex DC.) Kuijt and Struthanthus polyanthus Mart.—Loranthaceae) and dependent (Psittacanthus robustus (Mart.) Marloth—Loranthaceae) Al-accumulating mistletoe species from the brazilian savanna. Phytochemistry 2018, 153, 58–63. [Google Scholar] [CrossRef]
- Jain, M.; Kapadia, R.; Albert, S.; Mishra, S.H. Standardization of Feronia limonia L. leaves by HPLC, HPTLC, physicochemical and histological parameters. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 525–535. [Google Scholar]
- Xiang, H.; Cao, F.; Ming, D.; Zheng, Y.; Dong, X.; Zhong, X.; Mu, D.; Li, B.; Zhong, L.; Cao, J.; et al. Aloe-emodin inhibits Staphylococcus aureus biofilms and extracellular protein production at the initial adhesion stage of biofilm development. App. Microbiol. Biotechnol. 2017, 101, 6671–6681. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lei, Y.; Kang, M.; Xiao, Y.L.; Chen, Z.X. Molecular characterisation of clinical Cryptococcus neoformans and Cryptococcus gattii isolates from Sichuan province China. Mycoses 2017, 58, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xin, X.; Zhang, K.; Cui, T.; Peng, Y.; Zheng, J. Cytochrome P450 mediated metabolic activation of chrysophanol. Chem. Biol. Interact. 2018, 289, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Luiz, R.L.; Vila, T.V.; de Mello, J.C.; Nakamura, C.V.; Rozental, S.; Ishida, K. Proanthocyanidins polymeric tannin from Stryphnodendron adstringens are active against Candida albicans biofilms. BMC Complement. Altern. Med. 2015, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Ding, Y.S.; Zhang, Y.; Chen, J.B.; Cui, B.S.; Bai, J.Y.; Lin, M.B.; Hou, Q.; Zhang, P.C.; Li, S. Anti-inflammatory hydrolyzable tannins from Myricaria bracteata. J. Nat. Prod. 2015, 78, 1015–1025. [Google Scholar] [CrossRef]
- Nazaré, C.A.N. Avaliação da Atividade Antioxidante de uma Espécie de Loranthaceae. I Simpósio Internacional em Investigações Quimico-Farmacêuticas; Univali: Itajaí, Brasil, 2017. [Google Scholar]
- Alharits, L.; Handayani, W.; Yasman, Y.; Hemelda, N.M. Phytochemical analysis and antioxidant activity of leaves and flowers extracts of mistletoe (Dendrophthoe pentandra (L.) Miq.), collected from UI Campus, Depok. AIP Conf. Proc. 2019, 2168, 020101. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric Oxide in macrophage immunometabolism: Hiding in sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Marques, F.M.; da Costa, M.R.; Vittorazzi, C.; Gramma, L.S.D.S.; Barth, T.; de Andrade, T.U.; Endringer, D.C.; Scherer, R.; Fronza, M. In vitro and in vivo anti-inflammatory effects of Struthanthus vulgaris. Planta Med. 2017, 83, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Ohikhena, F.U.; Wintola, O.A.; Afolayan, A.J. quantitative phytochemical constituents and antioxidant activities of the mistletoe, Phragmanthera capitata (Sprengel) Balle extracted with different solvents. Pharmacogn. Res. 2018, 10, 16–23. [Google Scholar]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491–503. [Google Scholar] [CrossRef]
- Cavaillon, J.M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Pengal, R.A.; Cao, X.; Ganesan, L.P.; Wewers, M.D.; Marsh, C.B.; Tridandapani, S. Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP. J. Immunol. 2004, 173, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.N.; Heo, S.J.; Yoon, W.J.; Kang, S.M.; Ahn, G.; Yi, T.H.; Jeon, Y.J. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-kappaB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol. 2010, 649, 369–375. [Google Scholar] [CrossRef]
- Song, M.J.; Kim, K.H.; Yoon, J.M.; Kim, J.B. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 2006, 346, 739–745. [Google Scholar] [CrossRef]
- Kim, J.B. Immunological activities of korean mistletoe Extract (Viscum album coloratum; KM—110). Korean J. Immunol. 1997, 10, 571–582. [Google Scholar]
- Sobiepanek, A.; Kuryk, Ł.; Garofalo, M.; Kumar, S.; Baran, J.; Musolf, P.; Siebenhaar, F.; Fluhr, J.W.; Kobiela, T.; Plasenzotti, R.; et al. The multifaceted roles of mast cells in immune homeostasis, infections and cancers. Int. J. Mol. Sci. 2022, 23, 2249. [Google Scholar] [CrossRef]
- Galli, S.J.; Grimbaldeston, M.; Tsai, M. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Hofmann, A.M.; Abraham, S.N. New roles for mast cells in modulating allergic reactions and immunity against pathogens. Curr. Opin. Immunol. 2009, 21, 678–686. [Google Scholar] [CrossRef]
- Ansar, W.; Ghosh, S. Inflammation and inflammatory diseases, markers, and mediators: Role of CRP in some inflammatory diseases. In Biology of C Reactive Protein in Health and Disease; Springer: New Delhi, India, 2016. [Google Scholar]
- Rodrigues, T.S.; Guimarães, S.F.; Rodrigues-Das-Dôres, R.G.; Gabriel, J.V. Métodos de secagem e rendimento dos extratos de folhas de Plectranthus barbatus (boldo-da-terra) e P. ornatus (boldo-miúdo). Rev. Bras. Plantas Med. 2011, 13, 587–590. [Google Scholar] [CrossRef]
- Costa, A.F. Farmacognosia, 3rd ed.; Fundação Calouste Gulbenkian: Lisboa, Portugal, 1994. [Google Scholar]
- Matos, F.J.A. Introdução à Fitoquímica Experimental; Edições UFC: Fortaleza, Brasil, 1997; pp. 1–150. [Google Scholar]
- Acácio, R.; Franco, S.P.B.; Costa Filho, W.S.; Costa, J.G.; Santos, A.F.; Santana, H.G. Avaliação da atividade antioxidante do extrato etanólico de Melochia tomentosa LINAEUS (1735). Divers. J. 2018, 3, 412–428. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 56, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Le Bars, D.; Gozariu, M.; Cadden, S.W. Animal models of nociception. Pharmacol. Rev. 2001, 53, 597–652. [Google Scholar] [PubMed]
- Guerra, A.S.; Malta, D.J.; Laranjeira, L.P.; Maia, M.B.; Colaço, N.C.; de Lima, M.C.; Galdino, S.L.; Pitta Ida, R.; Gonçalves-Silva, T. Anti-inflammatory and antinociceptive activities of indole-imidazolidine derivatives. Int. Immunopharmacol. 2011, 11, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analyses of nitrate, nitrite and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
| Classes of Secondary Metabolites | Chemical Reagents | Result |
|---|---|---|
| Anthraquinones | NaOH | +++ |
| Saponins | Foam index | − |
| Tannins | Gelatin | +++ |
| Coumarins | KOH | − |
| Anthocyanins and anthocyanidins | NaOH | − |
| Flavone, flavanols and xanthones | NaOH | − |
| Chalcones and Aurones | NaOH | − |
| Leukoanthocyanidins | HCl | − |
| Catechins | HCl | +++ |
| Flavanols and flavanones | NaOH | +++ |
| Group | λ-Carrageenan | Dose (mg/kg) | Administration Time (% Inhibition of Edema) | |||
|---|---|---|---|---|---|---|
| 1 h | 2 h | 3 h | 4 h | |||
| Normal | PBS | PBS | 0.014 ± 0.03 | 0.014 ± 0.03 | 0.014 ± 0.03 | 0.014 ± 0.03 |
| Control | + | PBS | 0.28 ± 0.045 | 0.46 ± 0.054 | 0.54 ± 0.089 | 0.64 ± 0.089 |
| POH | + | 125 | 0.32 ± 0.109 | 0.44 ± 0.054 | 0.58 ± 0.109 | 0.58 ± 0.083 |
| + | 250 | 0.22 ± 0.044 | 0.3 ± 0.1 (34.78) * | 0.44 ± 0.054 | 0.48 ± 0.044 (25.0) * | |
| + | 500 | 0.24 ± 0.089 | 0.28 ± 0.13 (39.13) ** | 0.52 ± 0.083 | 0.5 ± 0.122 (21.87) * | |
| Dexa | + | 5 | 0.18 ± 0.044 | 0.02 ± 0.0 (56.52) **** | 0.12 ± 0.044 (77.77) **** | 0.14 ± 0.089 (78.12) **** |
| Group | λ-Carrageenan | Dose (mg/kg) | Classification of Inflammatory Infiltrate |
|---|---|---|---|
| Normal | PBS | PBS | Discreet |
| Control | + | PBS | Intense |
| POH | + | 125 | Intense |
| + | 250 | Intense | |
| + | 500 | Moderate | |
| Dexa | + | 5 | Discreet |
| Group | λ-Carrageenan | Dose (mg/kg) | RBC (Million/mm3) | HGB (g/dL) | HcT (%) | MCV (fm3) | MHC (pg) | MCHC (g/dL) | WBC (mil/mm3) | Platelet Count (mil/mm3) |
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | PBS | PBS | 9.27 ± 0.17 | 16.1 ± 0.31 | 37.46 ± 0.5 | 40.4 ± 1.15 | 17.26 ± 0.15 | 42.83 ± 1.15 | 9.91 ± 1.45 | 259 ± 14.1 |
| Control | + | PBS | 9.78 ± 0.13 | 17.6 ± 0.21 | 40.25 ± 1.02 | 41.17 ± 1.17 | 18.0 ± 0.33 | 43.77 ± 0.57 | 7.68 ± 0.97 | 122.25 ± 6.8 |
| POH | + | 125 | 9.49 ± 0.19 | 16.4 ± 0.52 | 37.86 ± 0.51 | 39.9 ± 0.3 | 17.26 ± 0.2 | 43.36 ± 0.77 | 10.76 ± 4.04 | 155.66 ± 7.6 |
| + | 250 | 9.69 ± 0.19 | 16.92 ± 0.71 | 38.66 ± 1.29 | 39.86 ± 0.55 | 17.44 ± 0.4 | 43.74 ± 0.43 | 10.06 ± 1.78 | 158.2 ± 6.1 | |
| + | 500 | 9.42 ± 0.37 | 16.20 ± 0.86 | 37.74 ± 1.75 | 40.06 ± 0.57 | 17.18 ± 0.34 | 42.9 ± 0.72 | 10.39 ± 2.69 | 141 ± 25.8 | |
| Dexa | + | 5 | 9.64 ± 0.31 | 17.03 ± 0.89 | 39.03 ± 1.5 | 40.46 ± 0.25 | 17.63 ± 0.37 | 43.56 ± 0.77 | 11.32 ± 4.51 | 158.3 ± 36.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, I.d.F.B.; Figueirêdo, A.L.M.; da Silva, E.M.; de Miranda, A.A.B.; da Rocha, C.Q.; da Silva Calabrese, K.; Almeida-Souza, F.; Abreu-Silva, A.L. Effects of Passovia ovata Mistletoe on Pro-Inflammatory Markers In Vitro and In Vivo. Plants 2023, 12, 1814. https://doi.org/10.3390/plants12091814
Magalhães IdFB, Figueirêdo ALM, da Silva EM, de Miranda AAB, da Rocha CQ, da Silva Calabrese K, Almeida-Souza F, Abreu-Silva AL. Effects of Passovia ovata Mistletoe on Pro-Inflammatory Markers In Vitro and In Vivo. Plants. 2023; 12(9):1814. https://doi.org/10.3390/plants12091814
Chicago/Turabian StyleMagalhães, Isadora de Fátima Braga, Ana Letícia Marinho Figueirêdo, Elizeu Mendes da Silva, Adryan Adam Batalha de Miranda, Cláudia Quintino da Rocha, Katia da Silva Calabrese, Fernando Almeida-Souza, and Ana Lúcia Abreu-Silva. 2023. "Effects of Passovia ovata Mistletoe on Pro-Inflammatory Markers In Vitro and In Vivo" Plants 12, no. 9: 1814. https://doi.org/10.3390/plants12091814
APA StyleMagalhães, I. d. F. B., Figueirêdo, A. L. M., da Silva, E. M., de Miranda, A. A. B., da Rocha, C. Q., da Silva Calabrese, K., Almeida-Souza, F., & Abreu-Silva, A. L. (2023). Effects of Passovia ovata Mistletoe on Pro-Inflammatory Markers In Vitro and In Vivo. Plants, 12(9), 1814. https://doi.org/10.3390/plants12091814







