1. Introduction
Hevea brasiliensis belongs to the genus Hevea of the Euphorbiaceae family; it is a perennial cross-pollinated tree. The natural rubber it produces is a strategic industrial raw material, ideal for manufacturing aircraft and automobile tires, and has the advantages of high strength, good flexibility, and high wear resistance [
1]. Advanced tissue culture methods have enabled the large-scale propagation of Hevea seedlings, also leading to the creation of genetically modified clones with high yield and various disease- or pest-resistant cultivars [
2]. SE has received more attention for the mass production of Hevea tissue culture seedlings. Recently, transgenic research on Hevea has considerably explored the possibilities of somatic embryogenesis for the development of transgenic lines that are resistant to various biotic and abiotic challenges.
A number of in vitro approaches, such as anther culture [
3], inner-integument culture [
4], the regeneration of the fragile embryogenic callus [
5], and secondary embryo circulatory proliferation [
6] are being implemented in Hevea tissue culture for the regeneration of plantlets through somatic embryogenesis. Various researchers have already reported the future prospects of primary and secondary embryogenesis for regenerating Hevea seedlings on a commercial level [
7]. Transcriptional-level studies of SE in Hevea were conducted by [
8] for an actual understanding of molecular regulation during the rubber tree’s late SE.
Due to the seasonal nature of main donor explants, anthers, and inner-integument availability, the regeneration system is easily restricted by season and time, making it impossible to achieve year-round cultivation. Anther culture is limited by time, with only three flowering stages: spring, summer, and autumn flowers. The inner-integument sampling time is generally 45 to 75 days after natural pollination. In addition, for the secondary embryo cycle proliferation system, the primary embryo is derived from the anther or inner integument, which are also limited by the seasons. The above problems can be overcome by choosing the new explant ABs of
Hevea brasiliensis. In other crops such as cassava (
Manihot esculenta Crantz) [
9], banana (
Musa nana Lour.) [
10], poplar (
Populus L.) [
11], ginkgo (
Ginkgo biloba L.) [
12], and (
Primula × pubescens) [
13], an AB-based tissue culture system is a viable method for seedling breeding. Therefore, we must study the potential of
Hevea brasiliensis ABs for the seedling regeneration system through SE.
There are few studies on plant regeneration using RT ABs as explants. In the RT, adventitious buds were induced by stem tips and successfully regenerated seedlings with a rapid growth rate and no deformity [
14]. In another study, the leaves and ABs of
Hevea brasiliensis were used as explants to induce plant regeneration, and the callus induction rate of ABs was higher than that of leaves. ABs could directly induce small buds and developed into a good root system; however, they were still unable to induce somatic embryos successfully [
15]. Using the ABs of
Hevea brasiliensis as explants, regenerated plants were induced by the callus and somatic embryo; however, there was no detailed picture description [
16]. Thus, we designed the current study to give in-depth information about the possibility of AB swelling as a source plant for the complete callogenesis and somatic embryogenesis pathway for the large-scale regeneration of Hevea seedlings. We also analyzed the genetic stability of the regenerated plants to determine the genomic mechanism of the explant source to establish a plant regeneration system in Hevea. Thus, our study provides a technical reference for the production of in vitro seedlings as well as the transgenic plants of Hevea self-rooted juvenile clones in large-scale propagation.
3. Discussion
Due to Hevea’s heterozygous nature and long juvenile period, conventional breeding is highly complicated. The introduction of in vitro breeding techniques became a remedy to overcome these difficulties. However, most of the explants used in Hevea tissue culture techniques are seasonal, creating complexity in the continuation of production [
25]. Hence, it became important to establish a nonseasonal explant-based in vitro propagation system with high potential for the seedling regeneration. A successful micropropagation system relies considerably on five factors: explant preparation, the development of aseptic culture, multiplication of propagules, regeneration into a complete plantlet, and acclimatization to greenhouse conditions. Therefore, the most important objective in tissue culturing is to establish an effective protocol including all these stages [
26].
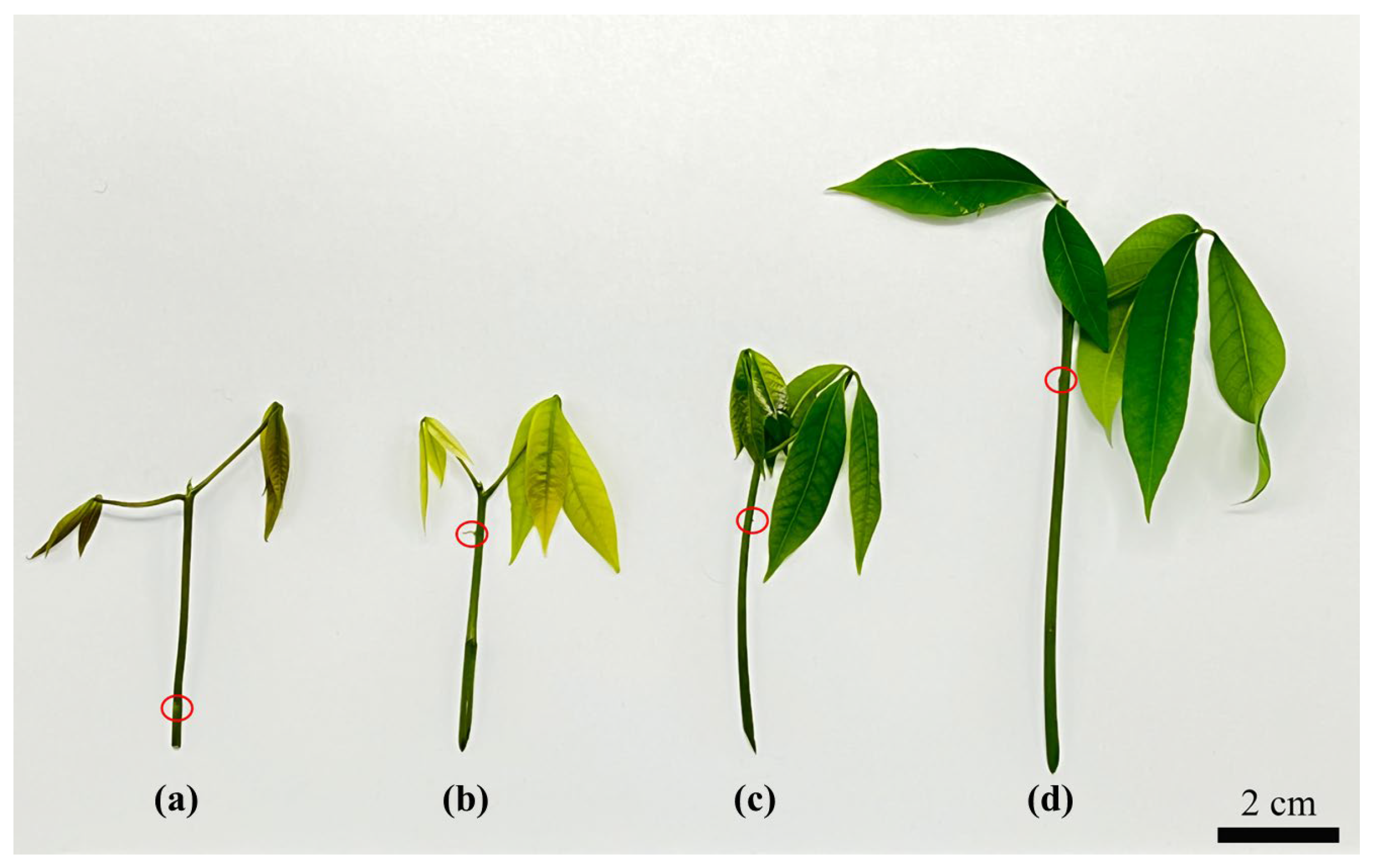
In the present study, an axillary-bud-based seedling regeneration system was established for the propagation of in vitro seedlings to overcome complications with seasonal explants. ABs (0~3 d) had a short swelling time from the stem segment of an in vitro seedling, which was inoculated for the AB swelling in the MS media, and this swift AB response exhibited vigorous meristematic ability for further development [
27]. It has been reported that, with this potential, ABs can develop into shoots or leaves, and the level of mitogens in endogenous cells will be increased during the development of ABs [
28]. Alterations in mitogens, such as cytokinin content, regulate the differentiation and overgrowth of ABs [
29,
30]. This mechanism is crucial in axillary-bud-based tissue culture techniques.
Usually, AB tissue culture is a two-step process: shoot proliferation and rooting, then obtaining a complete plant [
31]. Nevertheless, the current study has been targeted at primary somatic embryogenesis and whole-plant regeneration through callus proliferation from in vitro-induced ABs. To find the best donor plant for AB induction, phenologically different in vitro seedlings were selected. From the results obtained, we confirmed that the phenological impacts were very high on the induction of ABs. Explant age is a crucial factor in the success of plant micropropagation [
32,
33]. The most suitable phenological stage was the mature period (70.83 ± 7.22), which exhibited the best AB swelling rates compared with the bronze period, color-change period and pale-green period. The use of test tube seedling–stem segments resulted in contamination-free cultures during AB swelling. In vitro culture contamination is considered the major stumbling block in tissue culture experiments.
6-benzyladine is a popular and highly efficient synthetic cytokinin mainly used for crop production. Based on many previous reports, it has the potential to enhance non-meristem differentiation and lateral bud outgrowth [
34,
35]. Some researchers found that
Acacia auriculiformis bud growth was greatly influenced by 6-BA (1.5 mg/L) [
36]. In this study, the swelling rate of rubber tree ABs could be significantly increased by increasing the 6-BA concentration (up to 85.83% at 2 mg/L), which is consistent with the research findings of [
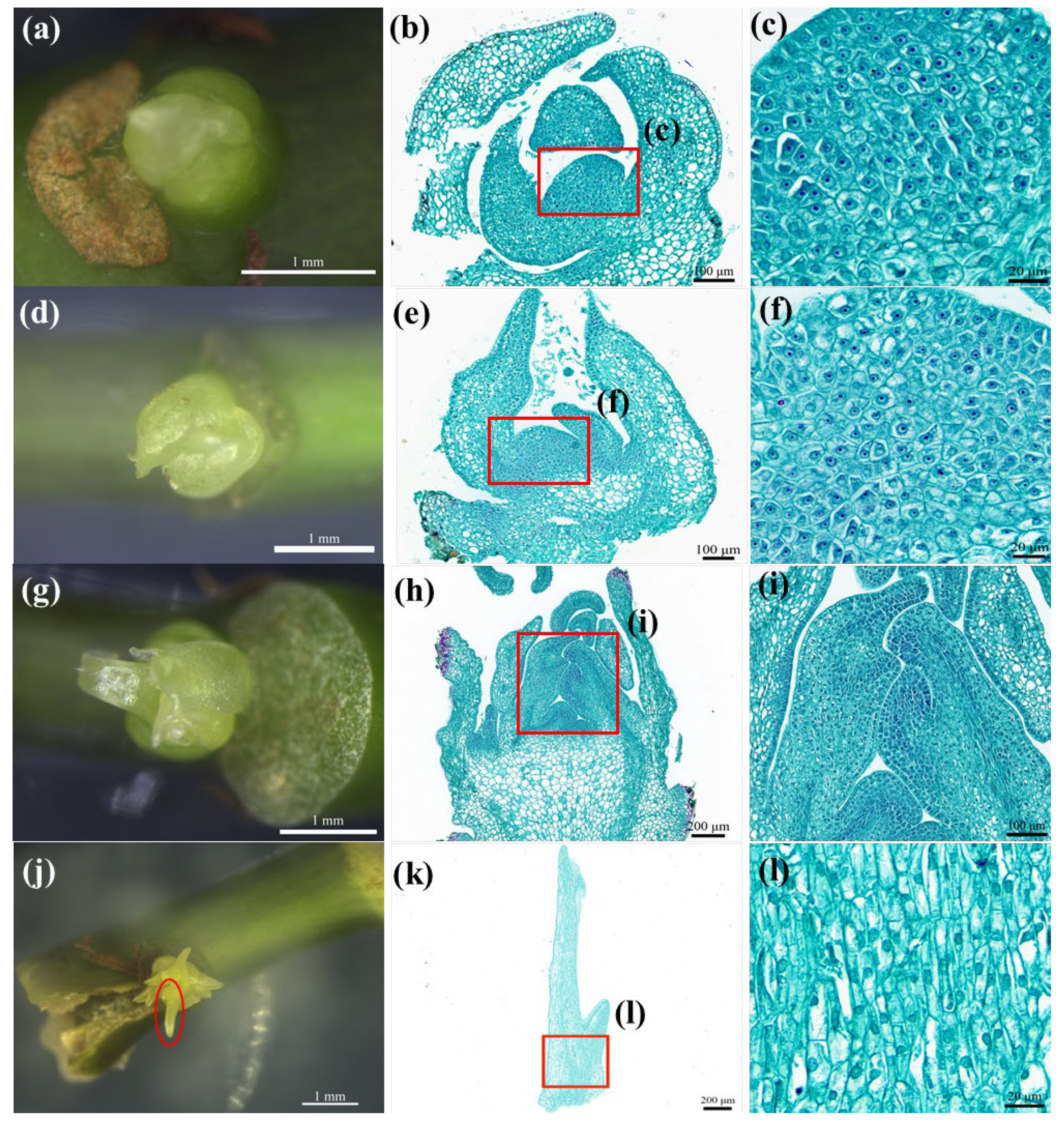
37]. The anatomical results of the ABs showed that the 3 d ABs had meristems with dense cytoplasm, and the callus occurred at the central growth point with meristems. The 10 d ABs had young leaves extending outward. The callus incidence rate of the 3 d ABs (48.72%) was higher than the incidence of the AB callus at 10 d (12.22%). The histological section showed that the swelling times with high callus incidence corresponded to the induction days with vigorous meristems, indicating that the callus incidence was related to the swelling times of ABs.
Callus induction is the major step in the process of culturing AB tissue, and the use of an appropriate medium will be helpful for successful callus formation. Among the nine different basic media checked in the current study, ABs showed remarkable results in obtaining a faster response rate, the highest survival rate, and almost no browning in the basic MH medium. Based on these data, basic MH medium was selected for callus induction, and different combinations and concentrations of 2,4-D, NAA, KT, and sucrose were checked for the optimum combination of callus induction. For callus induction, 2,4-D and NAA are required, and kinetin stimulates the callus proliferation [
38,
39]. The present study indicated that MH medium supplemented with KT (1.5 mg/L), 2,4-D (1.5 mg/L), NAA (1.5 mg/L), and sucrose 70 g/L was the most suitable combination for inducing a callus from AB swelling. One of the major requirements for plant growth development is a carbon source. The presence of sucrose in the culture medium is the main energy source for the plant cells. The concentration of sucrose in the culture medium may vary in different plant species [
40,
41]. Recent studies have reported that plants growing in media containing high concentrations of sucrose display higher gains in dry weight and leaf development [
42]. Various studies have supported the beneficial effects of these hormones in inducing a callus in Hevea and other plant species [
43,
44,
45,
46]. The optimal regeneration potential was obtained in MH media supplemented with 1 mg/L 6-BA, 3 mg/L KT, 0.06 mg/L 2, 4-D, and 0.5 mg/L GA3. A complete plantlet was obtained within 175 d. Shoot elongation and rooting occurred in MH, which contained 0.5 mg/L KT, 3 mg/L GA
3, and 0.2 mg/L IAA. The development rate was equal with the BSs and SSESs.
The occurrence of genetic and epigenetic changes is the main limitation in the micropropagation of Hevea clones [
47]. Thus, it is important to confirm the genetic stability among the plants obtained through somatic embryogenesis [
48]. One of the most effective cytogenetic methods for the estimation of genome size and ploidy in plants is flow cytometry. This technique has been used to assess DNA content and ploidy variation among different species, including
Leucocoryneae [
49]. The technique was also used to analyze the ploidy levels of nine Chinese native species of Rosaceae (
Rosa sp.) [
50]. Moreover, flow cytometry is widely used to compare the relationships and origins of different materials within the same genus. In a previous study on wheat, the presence of an extra chromosome was identified based on karyotype analysis [
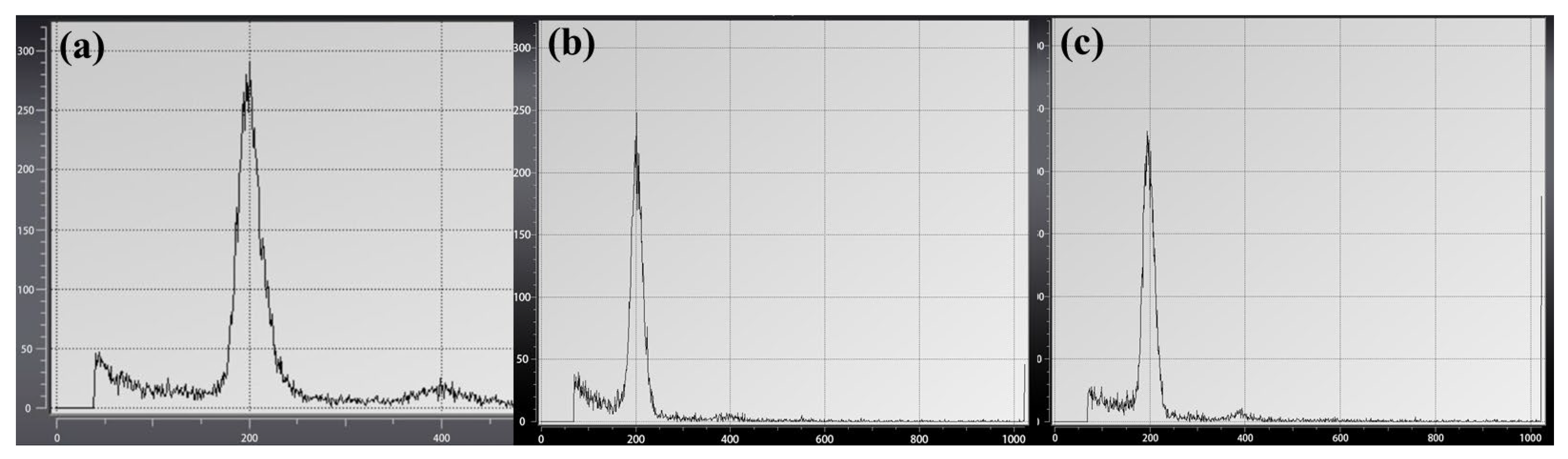
51]. In the current study, chromosome counting and assessment confirmed that ABRPs were diploid, similar to BSs and SSESs. Additionally, the relative genome sizes of ABRPs, BSs, and SSESs were similar, indicating the absence of any abnormality at the genome level for ABRPs.
Molecular tools and their applications are widely implemented on tissue culture seedlings, and their beneficial outputs have been well documented by various researchers worldwide [
52,
53]. According to [
54], it is necessary to estimate the genetic variations caused by the in vitro tissue culture techniques because there is a high possibility for soma clonal variation. In this study, analysis using ISSR markers revealed a low level of polymorphism between the test samples. Similar results were observed in many previous reports [
55,
56,
57]. In the genetic diversity assessments of Hevea ABRPs, BSs and SSESs using ISSR markers, 488 loci were amplified. When comparing ABRPs with BSs, only 10 (6.06%) were polymorphic, while with SSESs, the number rose to 13 (7.74%), indicating a low genetic diversity. Thus, a lower level of difference was observed among ABRPs, BSs, and SSESs. Therefore, we confirmed that AB swelling can be used for the mass regeneration of plantlets through the induction of the callus and somatic embryogenesis pathways without any seasonal barrier.