This study of edible species from the Peruvian coast was carried out in the context of taxonomic changes. It included not only yuyos (Chondracanthus) and cochayuyos (Porphyra/Pyropia) but also other seaweeds that had the potential to be introduced into people’s diets and that, due to their morphological complexity, could be subjected to taxonomic scrutiny, as is the case for species of the genera Ulva and Codium. Thus, before the chemical analysis, phylogenetic and taxonomic information was considered. Additionally, the localities of origin of the samples were taken into account when addressing the challenge of delimiting the taxa. This study confirmed the relationships between algal groups on the basis of the chemical analysis.
In this study, 37 algal specimens (red, green, and brown) collected from different sampling localities on the Peruvian coast were biochemically analyzed to determine their potential as food. The following species stood out as particularly nutritional and showed potential for use in human diets: C. chamissoi and Porphyra spp./Pyropia spp. (known edible); the brown algae Eisenia cokeri, Eisenia gracilis, and Lessonia berteroana; the red algae M. canaliculata, C. variegata, and R. corallina; and the green algae Ulva sp. and Codium sp. The numbers of species belonging to the red and green algal genera currently under taxonomic revision are not specified.
2.1. Proximal Composition
Table 1 shows the percentages of protein, carbohydrate, lipid, ash, and moisture in the seaweed specimens collected in Peru. Among the red algae, Bangiales algae presented the highest average percentage of protein (24.10%), followed by the green algae from Ulvales with 18.73% (
p = 0.000). There were no significant differences in the rest of the orders, for which values were in the range of 8.65–18.67%. In the case of the Laminariales brown algae of the genus
Eisenia, the frond had a higher percentage of proteins (14.11%) than the stipe (9.46%) (
p = 0.022). In general, these results agree with those of Thiviya et al. [
33], in which red and green algae had a higher percentage of protein (8–47%) compared to brown algae (4–24%). However, in our study, the percentages in Bryopsidales green algae and Gigartinales red algae were no greater than those in the Laminariales brown algae. In particular, for red algae, the average protein percentage in Bangiales algae from Peru, from the genera
Porphyra and
Pyropia, were in the range of 21.09–28.91% and had similar reference values (21.2–32.71%) to those reported for
Porphyra sp. from Japan, Korea, and China [
34];
Porphyra sp. from New Zealand [
35];
P. columbina from Argentina [
36];
Pyropia columbina from Chile [
37]; and
Porphyra sp. and
Porphyra umbilicalis from Portugal [
38,
39]. In the Gigartinales algae from Peru,
C. chamissoi, and
M. canaliculata, average protein concentrations of 12.02% and 11.25% were obtained, which are similar to the values obtained for
C. chamissoi from Peru (12.16%) [
26] and for
M. canaliculata (=
Chondrus canaliculatus) from Peru (12.48%) [
22]. The protein percentage of
C. variegata was lower (16.50%) in this study than that reported for
C. variegata from southern Chile, which ranged from 20 to 28% [
37,
40]. In the case of
R. corallina (Rhodymeniales), our results were half (14.58%) of those obtained by Rojas et al. [
23] for
R. corallina from Peru (28.56%, as
R. howeana).
In green algae, the average protein percentage of the
Ulva (Ulvales) algae from Peru in our study was in the range of 17.48–20.85%, which is within the range of reference values cited previously (8.65–27.2%) for
Ulva lactuca from northern Chile [
41], Iran [
42], Norway [
7], and Sweden [
8]; for
Ulva stenophylla from New Zealand [
35]; and for
Ulva compressa and
Ulva rigida from Portugal [
38,
39]. In
Codium sp. from Peru, our results showed a higher protein percentage (15.43%) than the average of 10.8% previously reported for
Codium fragile from northern Chile [
43]. In addition, for the brown algae Laminariales (
E. cokeri,
E. gracilis, and
L. berteroana) in this study, the range of protein percentages was 8.65–16.12%, which is within the range of reference values obtained for the other Laminariales species, including
E. arborea from Mexico, which had values between 5.5 and 11.7% [
44], and
L. berteroana from northern Chile, which had a value of 13.5% [
45]. For
E. cokeri and
E. gracilis from Peru, significant differences were found in functional morphological structure when comparing the percentage of proteins in the frond with that in the stipe (14.11% vs. 9.45%). Similar observations were previously reported for
L. berteroana from northern Chile [
45].
The green algae showed the highest average lipid percentages. Bryopsidales showed the highest percentage (5.38%), followed by Ulvales (1.58%) (
p = 0.000). The remaining orders of red and brown algae showed no significant differences, and their values ranged from 0.01 to 1.43%. The green seaweed
Codium sp. (Bryopsidales) from Peru had a lipid percentage three times higher than that reported for
C. fragile from northern Chile, which had a value of only 1.5% [
41]. Additionally, for
Ulva sp. (Ulvales) from Peru, the average lipid percentage was within the reference values (0.3–3.6%) obtained for
U. lactuca from northern Chile [
41], Iran [
42], and Norway [
7] and for
U. stenophylla from New Zealand [
35]. For red algae, the average lipid percentage in
Porphyra sp. (Bangiales) from Peru was 0.56%, which is similar to the range of 0.25–1.00% reported for
Porphyra sp. from China [
34] and for
Porphyra sp. from Argentina [
36] but lower than the range of 2.0–2.8% reported for
Porphyra sp. from Japan and Korea [
34] and for
Porphyra sp. from New Zealand [
35]. In brown algae, the average lipid content of Laminariales from Peru was 0.60%, which is similar to the range given for the fronds of
Eisenia arborea from Mexico [
44] of 0.45–0.66 and for the fronds and stipes of
L. berteroana from Chile [
45] of 0.7–1.3%.
As for carbohydrates, according to the Kruskal–Wallis test, the red macroalgae Bangiales (59.85%) and Gigartinales (58.73%,
p = 0.029) presented the highest medians, followed by the rest of the orders, with percentages ranging from 50.23 to 53.87%. For the Bangiales, our results were similar to the range of 39.85–60.36% reported for
Porphyra vietnamensis from India [
46]. In the case of Gigartinales, similar results were previously reported for
C. chamissoi (62.65%) [
26] and for
M. canaliculata (65.06%) [
22] from Peru. Additionally, for green algae, our results in
Ulva sp. and
Codium sp. were lower than those reported for
Ulva lactuca from Iran (59.1%) [
42] and from Chile (61.5%) [
41] and for
Codium fragile (66.8%) from Chile [
43]. In the case of brown algae, the range of 43.3–54.3% reported for
Eisenia arborea from Mexico [
44] was similar to our results in
Eisenia from Peru.
Regarding the percentage of ash, according to the Kruskal–Wallis test, the Bangiales red algae from Peru presented the lowest median (7.95%,
p = 0.000) among other orders of red, green, and brown algae, which had values in the range of 9.50–36.45%. The red algae
Porphyra sp. and
Pyropia sp. (Bangiales) from Peru had a range similar to that of
P. columbina from Argentina (average 6.46%) [
36] and
Pyropia vietnamensis (3.85–7.40%) [
46]. However, previous results for
Porphyra spp. from New Zealand [
35] and for
Py. columbina from Chile [
37] were higher than our results, with average percentages between 15.1 and 19.8% for
Porphyra sp. and
Py. columbina. For green algae, our results for
Ulva sp. were 11.0–32.2%, which are within the range established for
U. lactuca from northern Chile [
41], Iran [
42], Norway [
7], and Sweden [
8] and for
U. stenophylla from New Zealand [
35]. For the brown algae
L. berteroana,
E. cokeri, and
E. gracilis (Laminariales), a range of 9.50–36.45% was obtained for ash (%). These results were within the ranges from the references of 25.5–30.9 for the fronds of
E. arborea from Mexico [
44] and of 19.3–29.3% for the stipes and fronds of
L. berteroana from Chile [
45].
The moisture contents of all species ranged from 4 to 16%, and no relevant differences between the orders were demonstrated.
For species from Peru, it is necessary not only to introduce information regarding the chemical compositions of these edible seaweeds but also to discuss the species that could be included in the human diet because of their nutritional content. Leandro et al. [
47] stated that most seaweeds are edible and can provide the macro- and micronutrients necessary for good nutrition. According to the dietary reference intakes (DRI), the total caloric intake in a balanced adult diet should be 10–35% protein, 46%–65% carbohydrates, and 20–35% lipids [
48]. In this sense, seaweeds could represent an alternative to the animal products and legumes used as typical protein sources [
47]. Our results agree with the protein DRI requirements as the Bangiales red algae from Peru had a percentage of 21.09–28.91%, followed by the Ulvales green algae, which was in the range of 17.48–20.85%. On one hand, seaweeds have high amounts of carbohydrates (in some species, carbohydrates represent more than 50% of the dry weight) [
11]. According to our results, most of the analyzed samples had excellent carbohydrates content, which were similar to the values reported by DRI, especially the Bangiales (59.85%) and Gigartinales (58.73%) from Peru. On the other hand, seaweeds are known to be foods with low lipid contents [
7]. At the order level, we found that only the Bryopsidales green algae from Peru had a considerable amount of lipids 5.38%.
Finally, in terms of proximal composition, the seaweeds analyzed in this study are suitable for human consumption. Véliz et al. [
49] stated that the flours made from 11 species of seaweeds from Chile possessed chemical compositions suitable for use as ingredients in human and animal diets, including
Ulva sp. for green algae;
Durvillaea incurvata,
Lessonia spicata,
L. berteroana,
Lessonia trabeculata, and
M. pyrifera for brown algae; and
Gracilaria chilensis,
C. chamissoi,
Cryptonemia obovata,
Sarcodiotheca gaudichaudii, and
Acrosorium sp. for red algae.
2.2. Amino Acid Profile
The amino acid compositions of the lyophilized brown, red, and green algae are shown in
Table 2,
Table 3 and
Table 4, respectively. The amino acids exhibiting the highest average concentrations were glutamic acid (2.17 g/100 g) and aspartic acid (2.05 g/100 g), followed by alanine (1.54 g/100 g) and the essential amino acid leucine (1.35 g/100 g), while those with the lowest concentrations were tyrosine (0.52 g/100 g), methionine (0.30 g/100 g), and histidine (0.30 g/100 g,
p = 0.000). When analyzed by order, we found that green algae from Ulvales and red algae from Rhodymeniales and Bangiales presented the highest average concentrations of amino acids, with 1.34, 1.34, and 1.27 g/100 g, respectively, while the brown algae from Laminariales had the lowest average concentration (0.66 g/100 g,
p = 0.000).
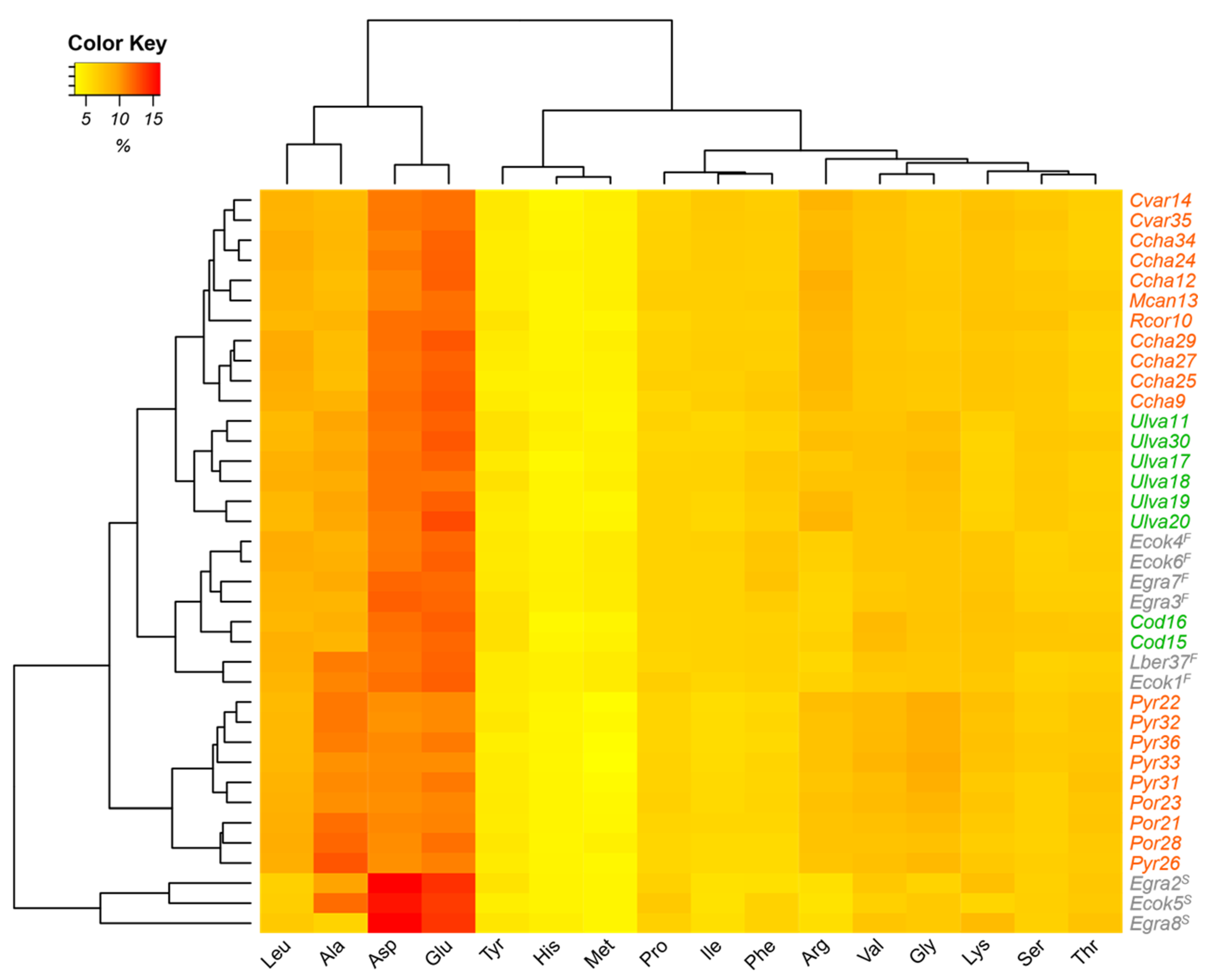
Furthermore, as can be seen from the heat map (
Figure 1), a distinction within the algal groups based on amino acid composition was observed. In the case of red algae, there were two groups. The first group, the upper group, had higher concentrations of glutamic acid and aspartic acid; a predominance of the genera
Callophyllis, Mazzaella, Chondracanthus, and
Rhodymenia; and an identical profile. The second group, the lower group, had a higher concentration of alanine; lower amounts of glutamic acid and aspartic acid; and, in it, the genera
Porphyra and
Pyropia were predominant. In green algae,
Ulva formed a separate group from
Codium specimens. The brown algae were grouped into two sets according to their morphological compartment, i.e., stipe or frond.
E. cokeri and
E. gracilis were analyzed as stipes and had high relative percentages of glutamic acid and aspartic acid, while the species corresponding to fronds,
Eisenia and
Lessonia, presented lower relative percentages of these amino acids. According to Hernández-Carmona et al. [
44], the nonessential amino acids most present in seaweeds are glutamic acid, aspartic acid, and alanine, and the most frequently present essential amino acid is leucine [
33]. Several studies have indicated higher concentrations of these amino acids in green algae, followed by red and, to a lesser extent, brown algae [
9,
10,
23,
39,
41,
50,
51,
52,
53]. In particular, the large amounts of amino acids such as glutamic acid are responsible for the special salty or umami taste of various algae [
54].
Regarding the genera studied, the high and low amounts of amino acids reported were similar to those reported by other authors for the green algae
Ulva fasciata from Brazil [
55];
U. capensis,
U. rigida, and
U. lactuca from South Africa [
56];
U. lactuca from Norway [
7];
U. lactuca and
Ulva intestinalis from Norway [
10];
U. lactuca from China [
21];
U. rigida from Portugal [
39]; and
Codium decorticatum,
C. spongiosum, and
C. taylorii from Brazil [
55]. They were also similar to those reported for species of red algae including
Porphyra sp. from Japan [
57];
P. acanthophora from Brazil [
55];
Porphyra sp. from Japan, Korea, and China [
34];
P. columbina from Argentina [
36];
P. dioica,
P. purpurea, and
P. umbilicalis from Norway [
10];
Porphyra spp. from Portugal [
58];
P. umbilicalis from Portugal [
39]; and
C.
chamissoi from southern Peru [
26]. With respect to brown algae,
E. cokeri and E. gracilis from Peru presented the same ranges as were reported for
E. arborea from Mexico [
44] for glutamic acid (0.55–4.27 g/100 g), aspartic acid (0.40–3.67 g/100 g), alanine (0.22–4.30 g/100 g), and leucine (0.29–3.89 g/100 g).
Concerning the essential amino acids ratio (EAA%), the highest average was found in red algae from Gigartinales, with 48.65% (
p = 0.000). The other orders did not show significant differences among them, with averages being between 43.71 and 45.98%. These values are higher than those reported for soy protein (39%) and very close to those for egg protein (47%) [
59]. In addition, our results show that the red alga
C. chamissoi collected from different localities in Peru had a higher amount of EAA% (average 48.41%) than that reported for
C. chamissoi collected in Moquegua, in southern Peru (31.07%) [
26]. Similarly,
Porphyra sp./
Pyropia sp. in our study had an EAA% of 44.48–46.65%, while the published values are in the range of 31.07–44.44% for
Porphyra sp. from Japan, Korea, and China [
34]; for
Porphyra spp. from Portugal [
58]; and for
P. umbilicalis from Portugal [
39]. This was the same for the green alga
Ulva sp., which in our study had an EAA% of 44.54–46.22%, which is higher than the range determined (40.30–40.79%) for
U. lactuca from Norway [
7] and for
U. rigida from Portugal [
39].
Additionally, in terms of amino acid score (AAS), considering all of the taxa analyzed, the essential amino acids with the highest average scores were lysine, threonine, valine, and leucine (AAS > 0.96), followed by phenylalanine + tyrosine (0.91), and finally, isoleucine and histidine (0.82 > AAS > 0.80,
p = 0.000). Numerically, the lowest average AAS was that of histidine from the Bryopsidales green algae (0.65). The latter result is in agreement with data previously reported in studies suggesting that sulfur-containing amino acids such as histidine are the major limiting amino acids in the proteins of some seaweeds [
60].
Regarding the essential amino acid index (EAAI), the highest average percentages corresponded to Ulvales, Laminariales, Gigartinales, and Rhodymeniales (EAAI > 0.92), while the lowest percentages corresponded to Bryopsidales and Bangiales (0.90 > EAAI > 0.88,
p = 0.000). Furthermore, in our study, the EAAI of
Porphyra sp./
Pyropia sp. was in the range of 0.87–0.90, which is slightly lower than the results obtained for
Porphyra sp. from Japan, Korea, and China [
34], and for
P. umbilicalis and
P. dioica from Portugal [
39] was between 0.89 and 0.96, which is in agreement with those authors who have stated that the limiting essential amino acids are methionine, tryptophan, leucine, and isoleucine. Additionally, our EAAI results for the green alga
Ulva sp. were in the range of 0.91–0.95, which is similar to the value reported by other authors indicating an EAAI between 0.92 and 1.23, with methionine as the limiting essential amino acid, in
U. lactuca from Norway [
7] and
U. rigida from Portugal [
39]. According to our results, most of the analyzed samples presented high and moderate quality with an EAAI value of >0.80 [
61,
62,
63]. In addition, they had excellent profiles, with EAAI near that of egg protein and AASs higher than those of other plant proteins [
33].
2.3. Fatty Acid Composition
Table 5,
Table 6 and
Table 7 show the fatty acid compositions of brown, red, and green algae. The highest average content of fatty acids corresponded to polyunsaturated fatty acids (PUFAs, 44.06%), followed by saturated fatty acids (SFAs, 34.56%), and finally monounsaturated fatty acids (MUFAs, 21.17%) (
p = 0.000). The percentages of PUFAs in the references consulted for all seaweed groups were in the range of 30–60% [
7,
16,
34,
37,
43]. However, values below 22% were also found [
26,
41,
50,
64]. The PUFAs found in the highest proportion were linolenic acid (C18:3n − 3) in the green algae group, arachidonic acid (ARA, C20:4n − 6) in the brown and red algae groups, and EPA (C20:5n − 3) in the red algae group.
In addition, a significant difference was observed in the MUFAs when orders were compared, with the stipe of the brown algae
Eisenia (Laminariales) having the highest median (31.27%), while red algae from Bangiales had the lowest (11.40%) (
p = 0.006). Previous studies have reported that Bangiales had a range of 14–21% [
34,
37,
64], which is higher than our results. The MUFA present in the highest quantity was oleic acid (C18:1n − 9) in the brown algae from Laminariales and in the red algae from Gigartinales and Rhodymeniales.
As for SFAs, the fatty acid present in the highest quantity was palmitic acid (C16:0) for the three groups of algae, with the highest content being found in the red algae
C. chamissoi (Gigartinales), with a median of 46.98%, and the lowest in the frond of the brown algae
Eisenia (Laminariales), with 25.51%. These results agree with those reported by Sohrabipour et al. [
65], wherein palmitic acid was the fatty acid with the highest amount, regardless of the species of seaweed studied.
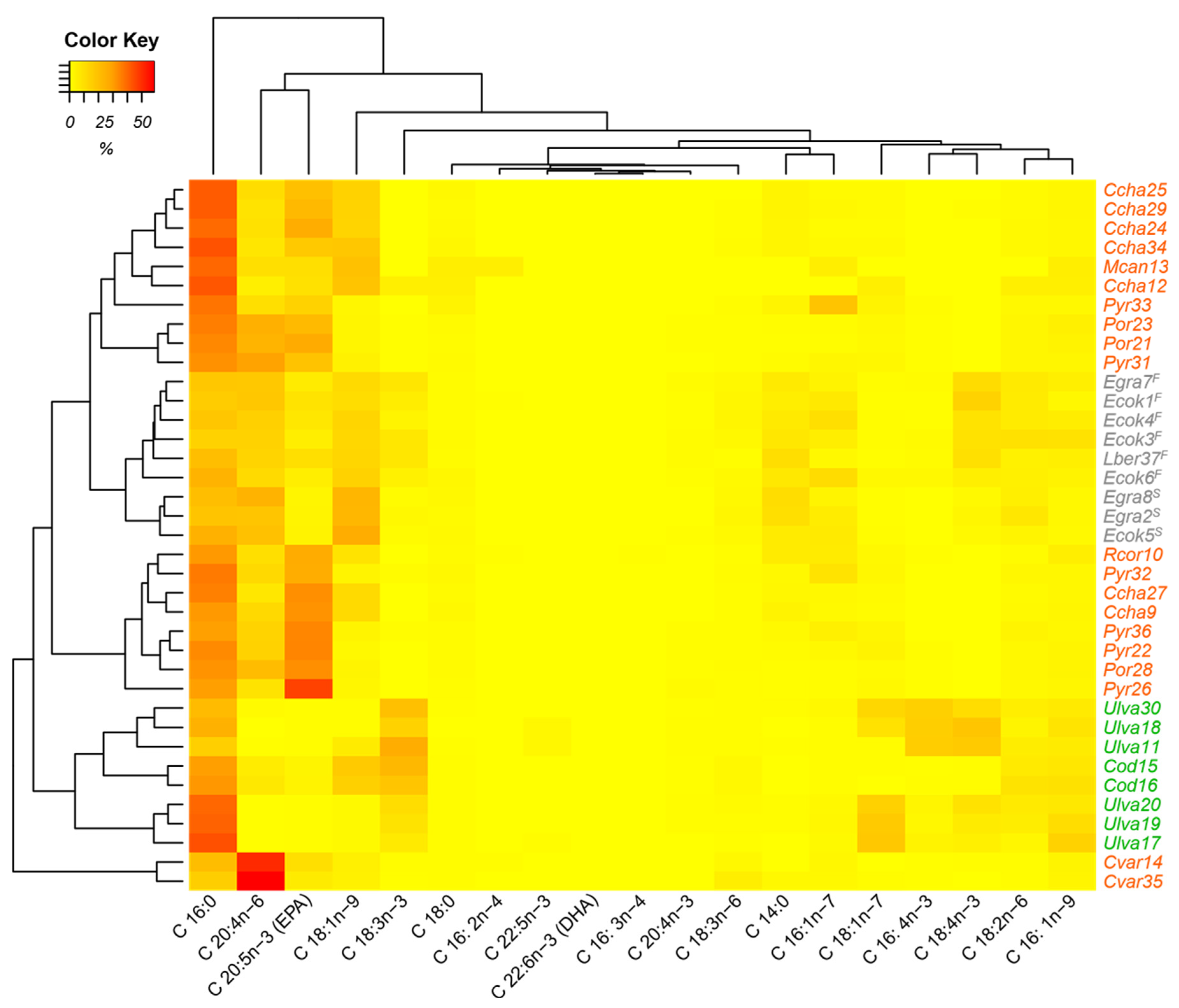
In the heat map (
Figure 2), the three groups of algae are organized according to the evaluated composition of the fatty acids. There are two clearly separate groups. One belongs to the green algae, which have a cluster related to C18:3n − 3 acid. The brown algae form another cluster, which is related to the fatty acids C20:4n − 6 and C18:1n − 9, whereby a higher percentage of the C18:1n − 9 fatty acid is presented in the stipe. This allows separation from the frond. Elsewhere, red algae form three clusters with three discriminating fatty acids, C16:0, EPA, and C20:4n − 6. The top cluster, formed by
C. chamissoi and
M. canaliculata (Gigartinales) and
Pyropia and
Porphyra (Bangiales), is distinguished by its higher percentage of C16:0; the central cluster, consisting of the red algae
C. chamissoi (Gigartinales),
Pyropia and
Porphyra (Bangiales), and
R. corallina (Rhodymeniales), presents higher percentages of EPA; and the lowest cluster, formed by the red alga
C. variegata (Gigartinales), has a higher percentage of C20:4n − 6. The characteristic fatty acids found in the heat map for the algal clusters have also been reported in different publications: Green algae from Ulvales and Bryopsidales had a significant relative percentage of C18:3n − 3 between 7 and 27% for
U. lactuca from the USA [
66] and Norway [
7]; for
Ulva sp. from southern Chile [
67]; for
Codium fragile from northern Chile [
43]; and for
C. tomentosum from Portugal [
68]. Similarly, brown algae from Laminariales present significant relative percentages of C20:4n − 6 (9–34%) and C18:1n − 9 (9–17%) for
Lessonia flavicans from southern Chile [
67]. Finally, red algae from Gigartinales and Bangiales have significant relative percentages of C16:0 (30–64%), EPA (6–42%), and C20:4n − 6 (1–17%) for
P. tenera from Japan [
57]; for
Porphyra sp. from Japan, Korea, and China [
34]; for
C. variegata from Chile [
37]; for
P. columbina from Argentina [
36]; for
M. canaliculata from the central coast of Peru [
22]; and for
Py. columbina from southern Chile [
67].
With respect to omega-6 content (ω6), arachidonic acid (C20:4n − 6) was the compound with the highest amount, with 54.73% for the red alga
C. variegata (Gigartinales), followed by the stipe (20. 06%) and the frond (15.21%) of
Eisenia (Laminariales) and Bangiales (14.13%). The lowest median of C20:4n − 6 was found for the green algae from Bryopsidales (6.56%) and Ulvales (0.37%) (
p = 0.001). The relative percentage of ω6 for
C. variegata from Peru (58.81%) was three times higher than the value reported for
C. variegata from southern Chile, which was 19.15% [
37]. In the case of the Laminariales algae studied,
L. berteroana,
E. cokeri, and
E. gracilis, our results (18.03–31.04%) were similar to those reported for
L. flavicans from southern Chile, with a relative percentage between 15 and 43% [
67]. The green algae of the genus
Ulva had a relative percentage of ω6 between 3.61 and 8.19%, which is similar to the reference values (3 and 12%) for
U. lactuca from the USA [
66], northern Chile [
41], and Norway [
7];
U. armoricana from France [
16]; and
Ulva sp. from southern Chile [
67].
In terms of omega-3 (ω3) content, at the group level, using the Kruskal–Wallis test, the main fatty acids were found to be EPA (C20:5n − 3) in red algae, with a median of 24.19%, and C18:3n − 3 in green algae, with a median of 16.11%. In brown algae, EPA (C20:5n − 3) (5.28%) and stearidonic acid (C18:4n − 3) (8.73%) were important. Fatty acid ω3 content showed no significant differences when comparing orders (p = 0.180).
At the order level, using the Kruskal–Wallis test, the highest median ω6/ω3 ratio was found in the Gigartinales red alga
C. variegata (6.59), followed by stipes of
Eisenia (Laminariales) (3.78). For the rest of the algae, the ratio did not exceed the median value of 1 (
p = 0.001). The value of the ω6/ω3 ratio for the alga
C. variegata from the central coast of Peru was remarkable, with none of the references consulted approaching it in value. The ω6/ω3 ratios reported for the orders Ulvales, Bangiales, and Laminariales from southern Chile [
67] had the same tendencies, as shown in our results; the Laminariales had a ratio higher than 1, and the rest of the orders had a ratio lower than 1. Healthy diets have ω6/ω3 ratios with values lower than 10 [
69] or with a maximum of 4 [
70,
71] since higher ratios favor the risk of developing diseases [
72]. Based on these results, the red alga
C. variegata from two localities in Peru (Paracas and Marcona in Ica) could be used in food because of its high content of ω6, which increases the ratio ω6/ω3.
This study showed that Bangiales (cochayuyos; Porphyra sp. and Pyropia sp.) had the highest average percentage of protein and carbohydrates and the lowest values of ash. Additionally, Ulvales specimens (sea lettuce, Ulva sp.) were remarkable because of their protein content, and Bryopsidales (Codium sp.) were remarkable because of their lipid contents. The highest amounts of essential amino acids were found in Gigartinales (yuyo, C. chamissoi; alga flor, M. canaliculata; and carola, C. variegata), while the highest essential amino acid index score was found for Ulvales (Ulva sp.), Laminariales (“kelps” E. cokeri, E. gracilis, and L. berteroana), Gigartinales (C. chamissoi, M. canaliculata, and C. variegata), and Rhodymeniales (R. corallina). The major PUFAs were linolenic acid in the group of green algae, arachidonic acid in the group of brown and red algae, and EPA in the group of red algae. A major MUFA was oleic acid in Laminariales, Gigartinales, and Rhodymeniales. With respect to the SFAs, palmitic acid had the highest quantity in the Gigartinales specimens. With regard to the content of omega-6, ARA was predominantly found in in the red alga C. variegata (Gigartinales), while for omega-3, it was EPA in red and green algae.
Knowledge regarding edible algae is scarce in Peru, so it was considered necessary to provide detailed and complete information on the chemical compositions of seaweeds that are consumed and that have been consumed since pre-Inca times in Peru, such as yuyos and cochayuyos (red algae), as well as to include data on other potentially edible species. From here, it is possible to revalue these edible algae and emphasize the consumption of other groups (orders) of seaweeds that are nutritionally promising, such as green and brown algae, which are not commonly consumed but could be introduced into the diet not only as a protein source but also for their important contents of essential amino acids, polyunsaturated fatty acids, and carbohydrates. These findings provide, for the first time in Peru, relevant information on the chemical and nutritional composition of seaweeds that could potentially be used directly as food or as ingredients in human or animal diets. It is also a first step in connecting the differences and similarities in the chemical compounds of these taxa with their taxonomic positions since it is important to understand these entities at a specific level in order for them to be commercialized internally or externally. Despite the amount of information generated, the grouping of the different taxa did not reflect a well-defined taxonomic classification, although it allowed the chemical characterization of the edible species analyzed. With that, we reinforce the definition of integrative taxonomy [
73], in which species should be revised, analyzing different characteristics, using other methods, and applying other criteria to delimit species.