Maintenance of Methyl-Esterified Pectin Level in Pollen Mother-Cell Stages Is Required for Microspore Development
Abstract
1. Introduction
2. Results
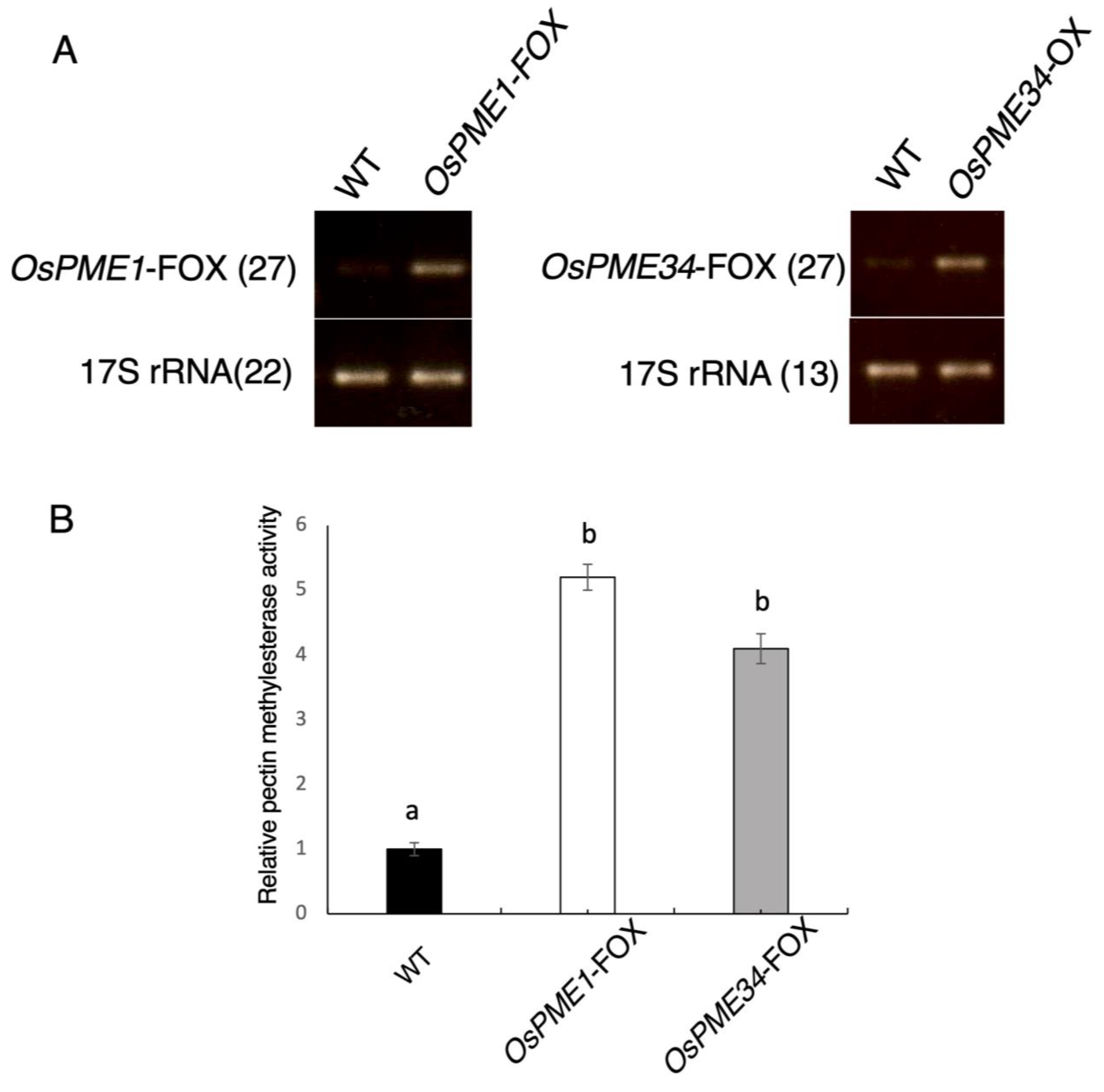
2.1. OsPME1-FOX and OsPME34-FOX Overexpressed PME
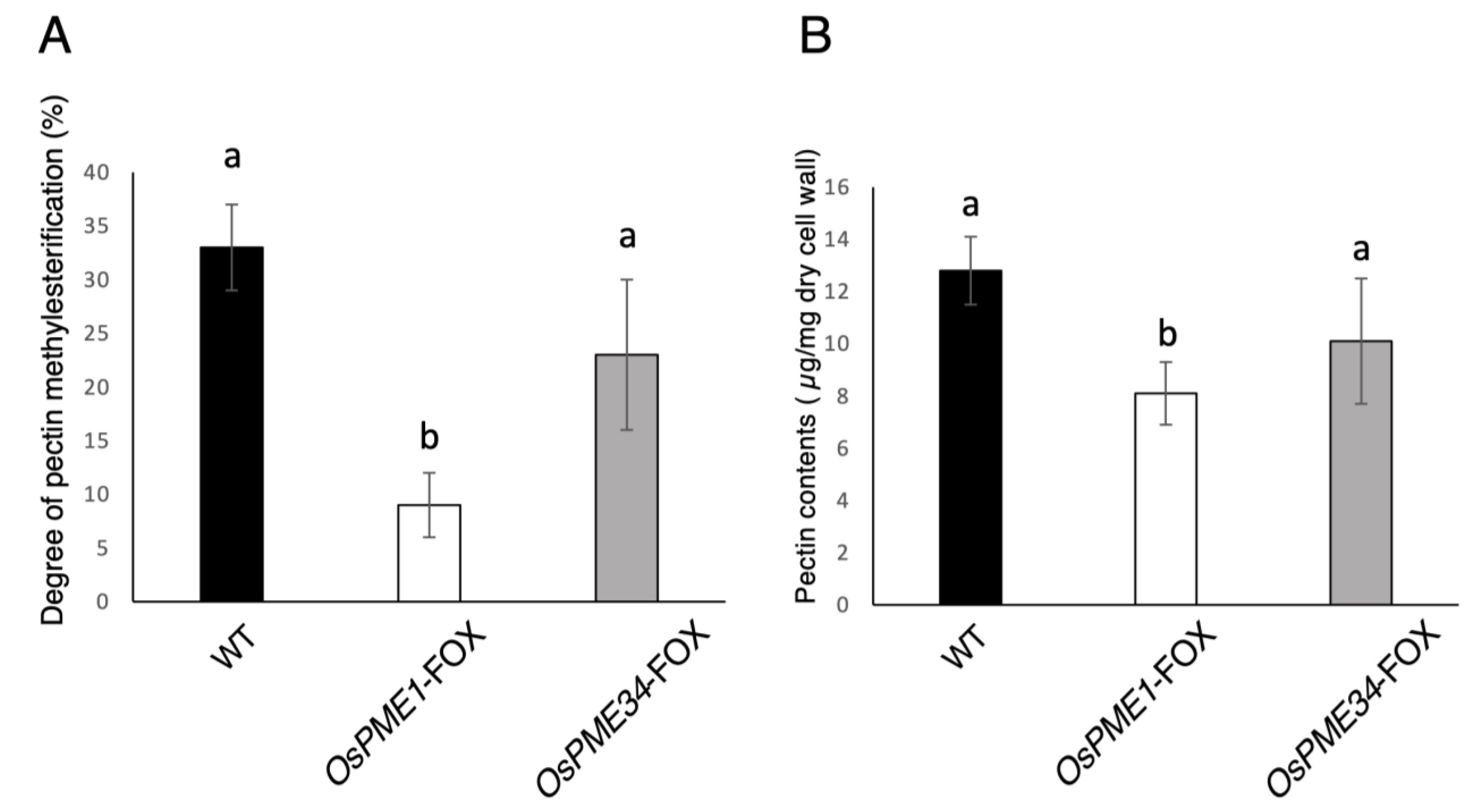
2.2. Effect of Overexpression of PME1 and PME34 on the Pectin of the Cell Wall
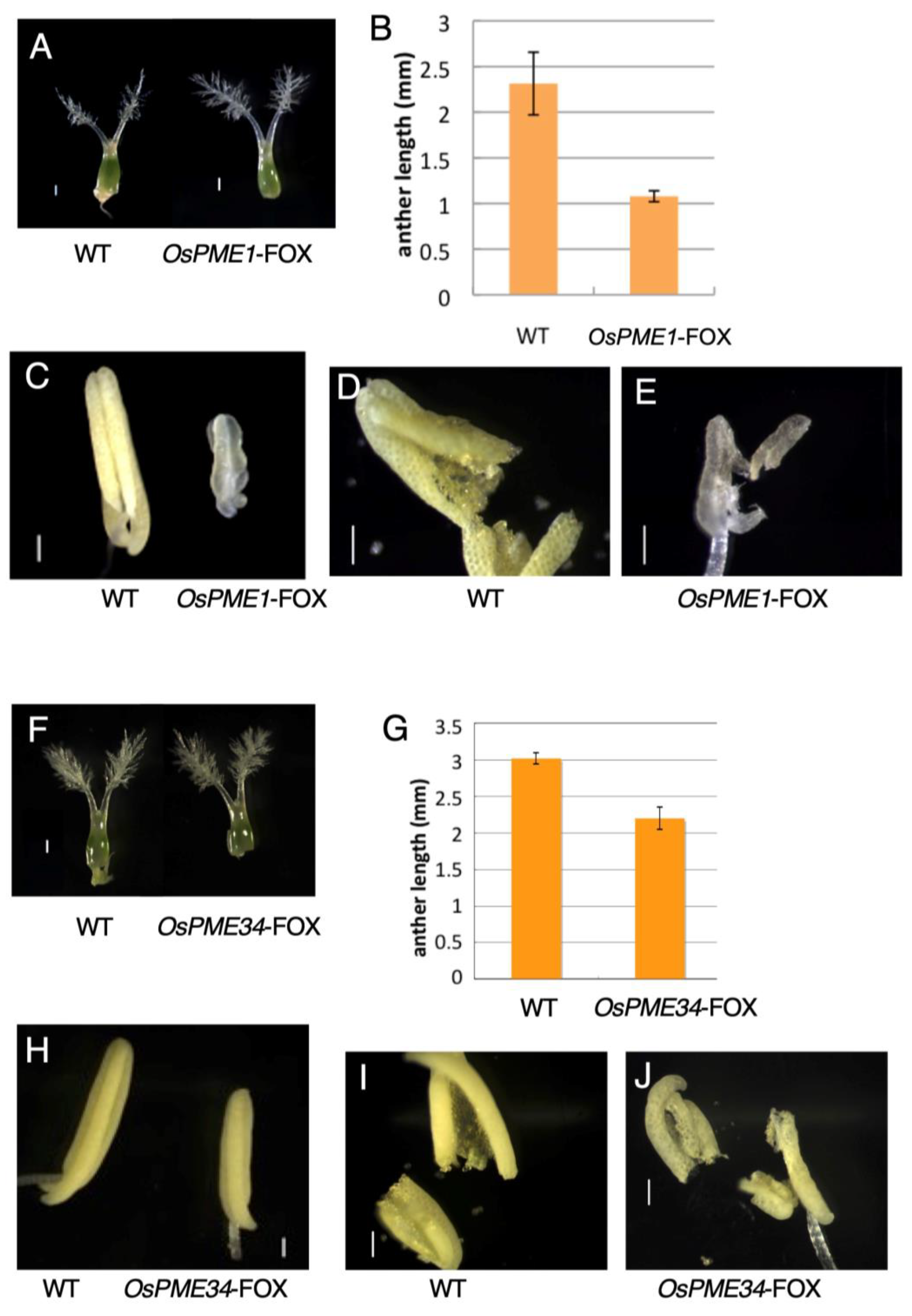
2.3. OsPME1-FOX and OsPME34-FOX Showed Abnormal Anther Development
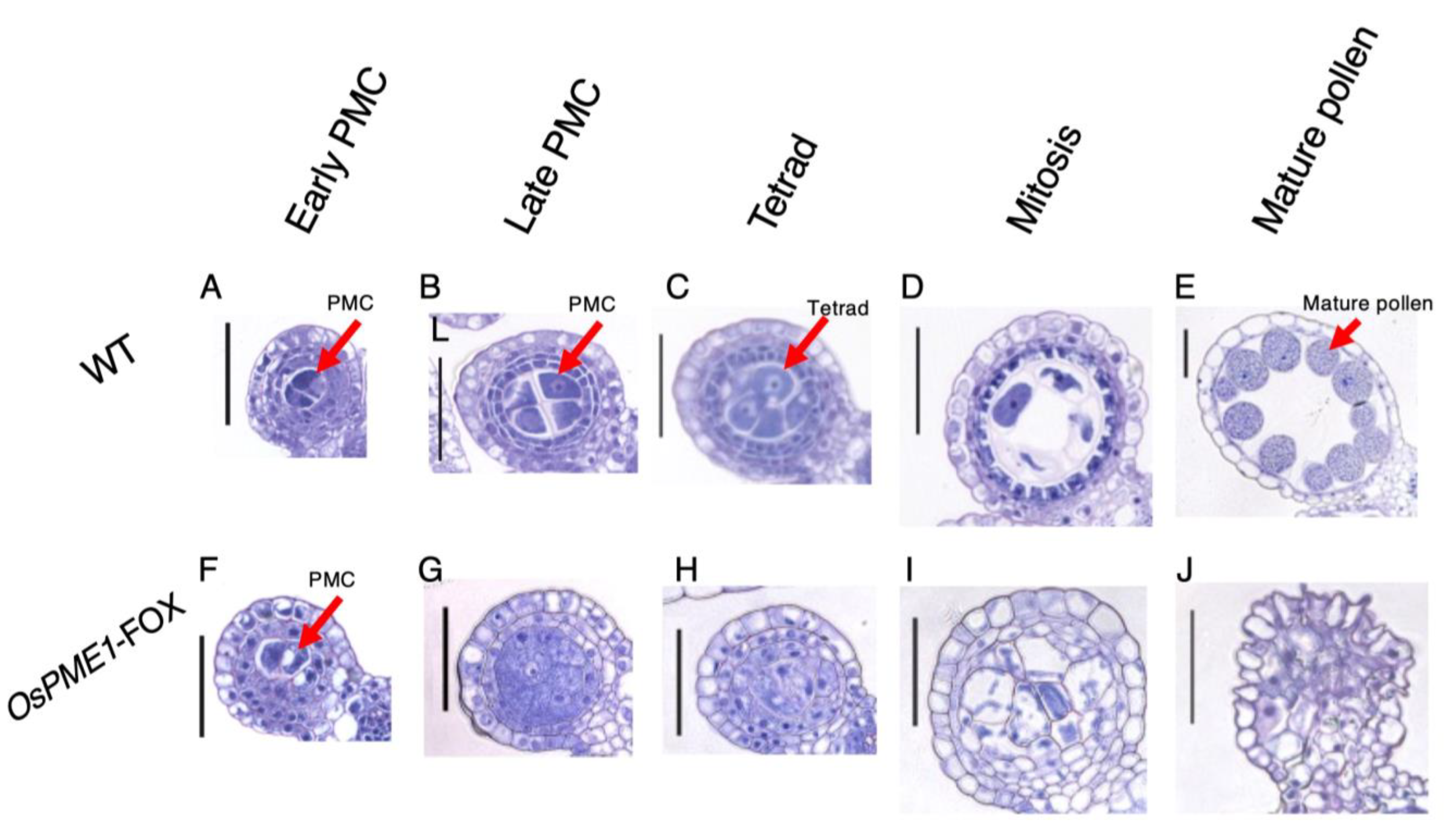
2.4. OsPME1-FOX Showed Abnormal Pollen Development
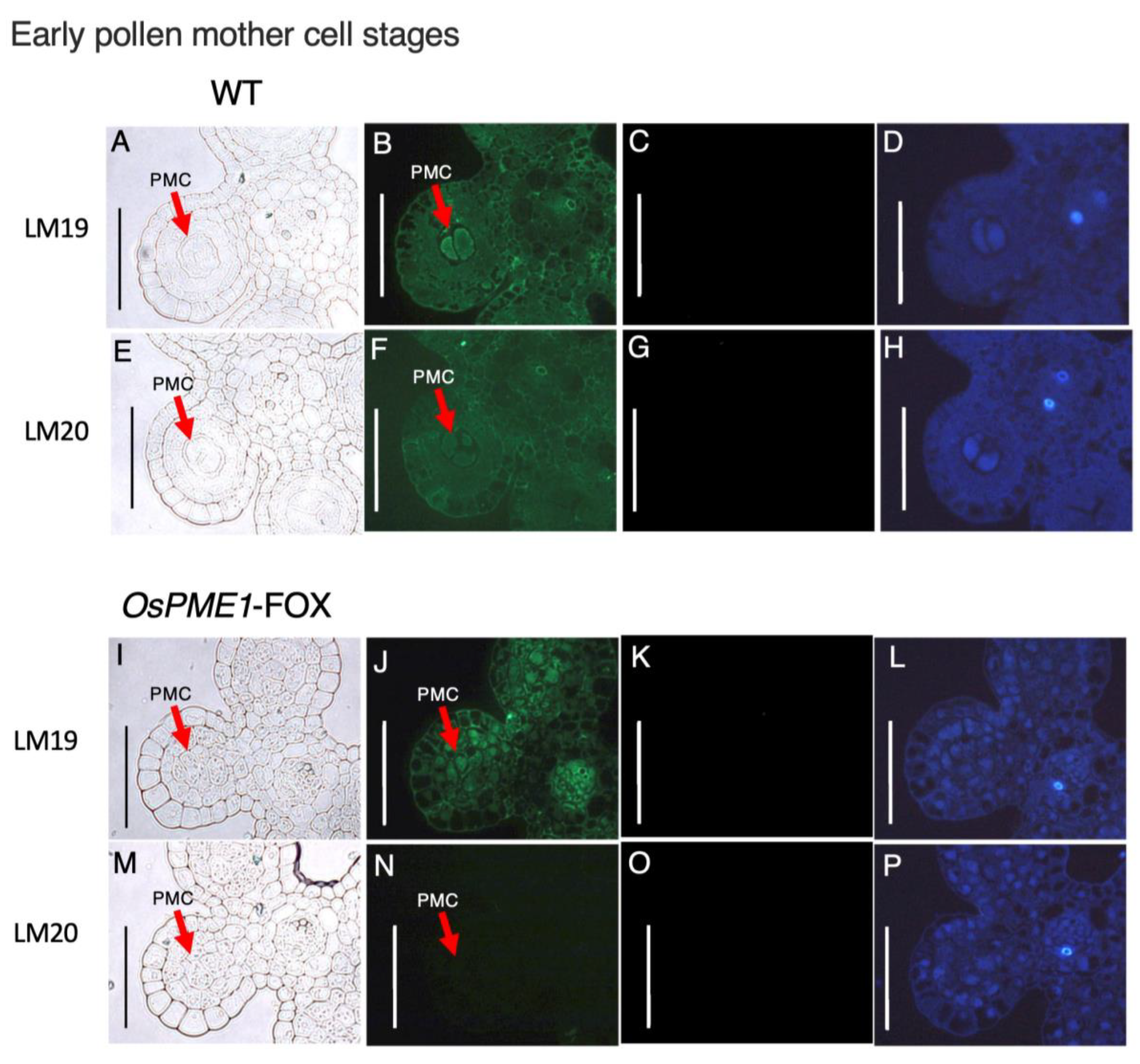
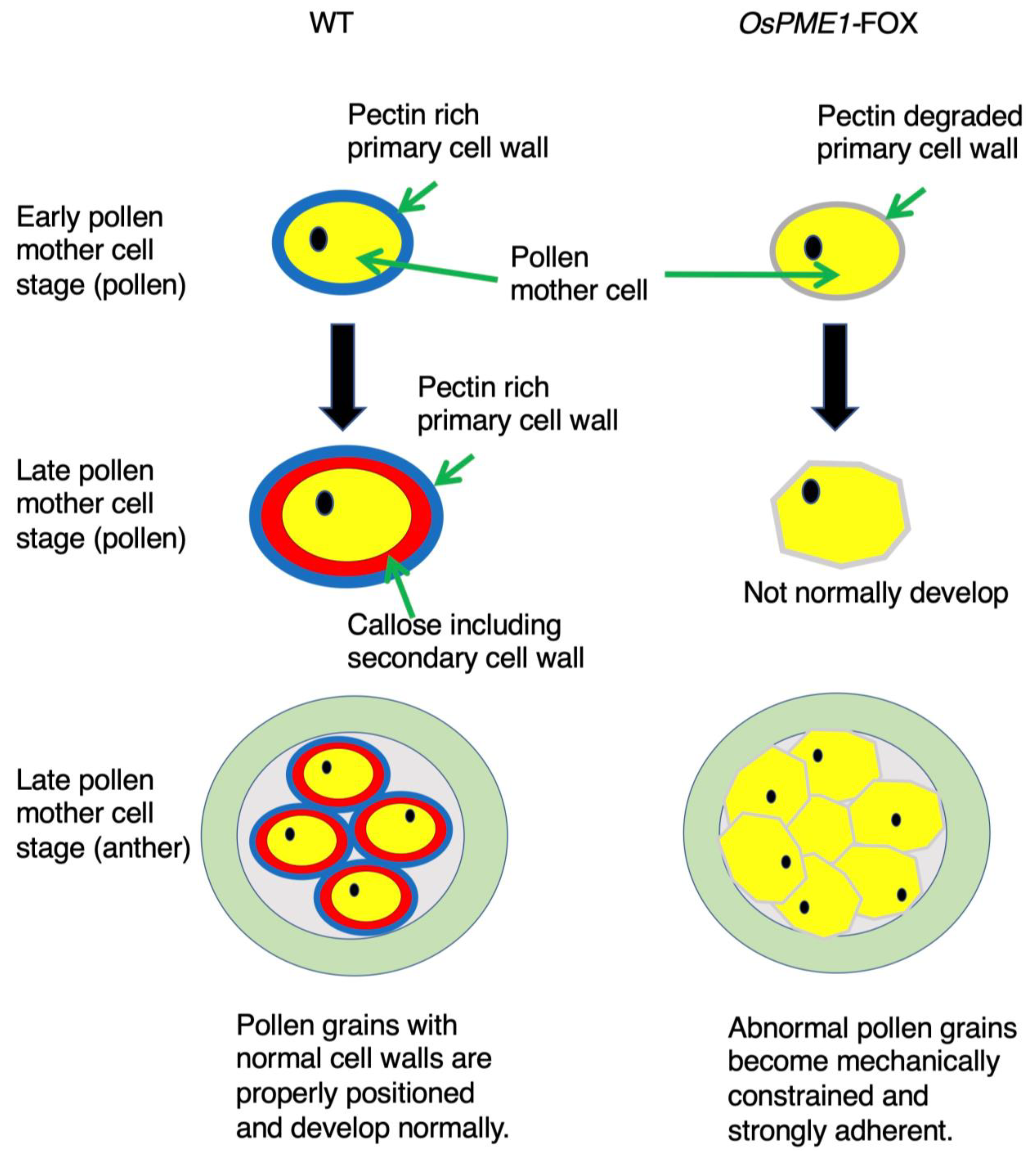
2.5. OsPME1-FOX Showed Abnormal Distribution of Methyl-Esterified and De-Methyl-Esterified Pectin in Pollen Mother-Cell Stage of Anther
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Monitoring of Anther Development
4.3. Extraction of RNA and Analysis of Gene Expression
4.4. Determination of Pectin Methylesterase Activity
4.5. Extraction and Analysis of Cell-Wall Polysaccharides
4.6. Determination of Uronic Acid
4.7. Determination of the Pectin Methyl Ester Content
4.8. Immunohistochemistry
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owen, H.A.; Makaroff, C.A. Ultrastructure of Microsporogenesis and Microgametogenesis In Arabidopsis thaliana (L.) Heynh. Ecotype Wassilewskija (Brassicaceae). Protoplasma 1995, 185, 7–21. [Google Scholar] [CrossRef]
- Akita, K.; Takagi, T.; Kobayashi, K.; Kuchitsu, K.; Kuroiwa, T.; Nagata, N. Ultrastructural Characterization of Microlipophagy Induced by the Interaction of Vacuoles and Lipid Bodies around Generative and Sperm Cells in Arabidopsis Pollen. Protoplasma 2021, 258, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Osborne, E.; Poindexter, P.D.; Somerville, C.R. Microspore Separation in the Quartet 3 Mutants of Arabidopsis Is Impaired by a Defect in a Developmentally Regulated Polygalacturonase Required for Pollen Mother Cell Wall Degradation. Plant Physiol. 2003, 133, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Takebe, N.; Nakamura, A.; Watanabe, T.; Miyashita, A.; Satoh, S.; Iwai, H. Cell Wall Glycine-Rich Protein2 Is Involved in Tapetal Differentiation and Pollen Maturation. J. Plant Res. 2020, 133, 883–895. [Google Scholar] [CrossRef]
- Luo, L.; Bai, J.; Yuan, S.; Guo, L.; Liu, Z.; Guo, H.; Zhang, T.; Duan, W.; Li, Y.; Zhao, C.; et al. Genome Wide Identification and Characterization of Wheat GH9 Genes Reveals Their Roles in Pollen Development and Anther Dehiscence. Int. J. Mol. Sci. 2022, 23, 6324. [Google Scholar] [CrossRef] [PubMed]
- Paxson-Sowders, D.M.; Dodrill, C.H.; Owen, H.A.; Makaroff, C.A. DEX1, a Novel Plant Protein, Is Required for Exine Pattern Formation during Pollen Development in Arabidopsis. Plant Physiol. 2001, 127, 1739–1749. [Google Scholar] [CrossRef]
- Fei, H.; Sawhney, V.K. Ultrastructural Characterization of Male Sterile33 (Ms33) Mutant in Arabidopsis Affected in Pollen Desiccation and Maturation. Can. J. Bot. 2001, 79, 118–129. [Google Scholar] [CrossRef]
- Brown, S.M.; Crouch, M.L. Characterization of a Gene Family Abundantly Expressed in Oenothera Organensis Pollen That Shows Sequence Similarity to Polygalacturonase. Plant Cell 1990, 2, 263–274. [Google Scholar] [CrossRef]
- Albani, D.; Altosaar, I.; Arnison, P.G.; Fabijanski, S.F. A Gene Showing Sequence Similarity to Pectin Esterase Is Specifically Expressed in Developing Pollen of Brassica Napus. Sequences in Its 5? Flanking Region Are Conserved in Other Pollen-Specific Promoters. Plant Mol. Biol. 1991, 16, 501–513. [Google Scholar] [CrossRef]
- Mu, J.-H.; Stains, J.P.; Kao, T. Characterization of a Pollen-Expressed Gene Encoding a Putative Pectin Esterase OfPetunia Inflata. Plant Mol. Biol. 1994, 25, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Tebbutt, S.J.; Rogers, H.J.; Lonsdale, D.M. Characterization of a Tobacco Gene Encoding a Pollen-Specific Polygalacturonase. Plant Mol. Biol. 1994, 25, 283–297. [Google Scholar] [CrossRef]
- Ariizumi, T.; Amagai, M.; Shibata, D.; Hatakeyama, K.; Watanabe, M.; Toriyama, K. Comparative Study of Promoter Activity of Three Anther-Specific Genes Encoding Lipid Transfer Protein, Xyloglucan Endotransglucosylase/Hydrolase and Polygalacturonase in Transgenic Arabidopsis Thaliana. Plant Cell Rep. 2002, 21, 90–96. [Google Scholar] [CrossRef]
- Amagai, M.; Ariizumi, T.; Endo, M.; Hatakeyama, K.; Kuwata, C.; Shibata, D.; Toriyama, K.; Watanabe, M. Identification of Anther-Specific Genes in a Cruciferous Model Plant, Arabidopsis Thaliana, by Using a Combination of Arabidopsis Macroarray and MRNA Derived from Brassica Oleracea. Sex. Plant Reprod. 2003, 15, 213–220. [Google Scholar] [CrossRef]
- Fujita, M.; Horiuchi, Y.; Ueda, Y.; Mizuta, Y.; Kubo, T.; Yano, K.; Yamaki, S.; Tsuda, K.; Nagata, T.; Niihama, M.; et al. Rice Expression Atlas In Reproductive Development. Plant Cell Physiol. 2010, 51, 2060–2081. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Iwai, H. Virtual Issue: Cell Wall Functions in Plant Growth and Environmental Responses. J. Plant Res. 2021, 134, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Pelloux, J.; Rusterucci, C.; Mellerowicz, E. New Insights into Pectin Methylesterase Structure and Function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef]
- Micheli, F. Pectin Methylesterases: Cell Wall Enzymes with Important Roles in Plant Physiology. Trends Plant Sci. 2001, 6, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Hongo, S.; Sato, K.; Yokoyama, R.; Nishitani, K. Demethylesterification of the Primary Wall by PECTIN METHYLESTERASE35 Provides Mechanical Support to the Arabidopsis Stem. Plant Cell 2012, 24, 2624–2634. [Google Scholar] [CrossRef]
- Sénéchal, F.; Graff, L.; Surcouf, O.; Marcelo, P.; Rayon, C.; Bouton, S.; Mareck, A.; Mouille, G.; Stintzi, A.; Höfte, H.; et al. Arabidopsis PECTIN METHYLESTERASE17 Is Co-Expressed with and Processed by SBT3.5, a Subtilisin-like Serine Protease. Ann. Bot. 2014, 114, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- Levesque-Tremblay, G.; Pelloux, J.; Braybrook, S.A.; Müller, K. Tuning of Pectin Methylesterification: Consequences for Cell Wall Biomechanics and Development. Planta 2015, 242, 791–811. [Google Scholar] [CrossRef]
- Turbant, A.; Fournet, F.; Lequart, M.; Zabijak, L.; Pageau, K.; Bouton, S.; Van Wuytswinkel, O. PME58 Plays a Role in Pectin Distribution during Seed Coat Mucilage Extrusion through Homogalacturonan Modification. J. Exp. Bot. 2016, 67, 2177–2190. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Lin, S.; Yu, Y.; Huang, L.; Cao, J. The Putative Pectin Methylesterase Gene, BcMF23a, Is Required for Microspore Development and Pollen Tube Growth in Brassica Campestris. Plant Cell Rep. 2018, 37, 1003–1009. [Google Scholar] [CrossRef]
- Bosch, M.; Hepler, P.K. Silencing of the Tobacco Pollen Pectin Methylesterase NtPPME1 Results in Retarded in Vivo Pollen Tube Growth. Planta 2006, 223, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhuang, X.; Cai, Y.; Cheung, A.Y.; Jiang, L. Apical F-Actin-Regulated Exocytic Targeting of NtPPME1 Is Essential for Construction and Rigidity of the Pollen Tube Cell Wall. Plant J. 2013, 76, 367–379. [Google Scholar] [CrossRef]
- Francis, K.E.; Lam, S.Y.; Copenhaver, G.P. Separation of Arabidopsis Pollen Tetrads Is Regulated by QUARTET1, a Pectin Methylesterase Gene. Plant Physiol. 2006, 142, 1004–1013. [Google Scholar] [CrossRef]
- Preuss, D.; Rhee, S.Y.; Davis, R.W. Tetrad Analysis Possible in Arabidopsis with Mutation of the QUARTET (QRT) Genes. Science 1994, 264, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Somerville, C.R. Tetrad Pollen Formation in Quartet Mutants of Arabidopsis Thaliana Is Associated with Persistence of Pectic Polysaccharides of the Pollen Mother Cell Wall. Plant J. 1998, 15, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Hakata, M.; Nakamura, H.; Iida-Okada, K.; Miyao, A.; Kajikawa, M.; Imai-Toki, N.; Pang, J.; Amano, K.; Horikawa, A.; Tsuchida-Mayama, T.; et al. Production and Characterization of a Large Population of CDNA-Overexpressing Transgenic Rice Plants Using Gateway-Based Full-Length CDNA Expression Libraries. Breed. Sci. 2010, 60, 575–585. [Google Scholar] [CrossRef]
- Itoh, J.-I.; Nonomura, K.-I.; Ikeda, K.; Yamaki, S.; Inukai, Y.; Yamagishi, H.; Kitano, H.; Nagato, Y. Rice Plant Development: From Zygote to Spikelet. Plant Cell Physiol. 2005, 46, 23–47. [Google Scholar] [CrossRef]
- Stieglitz, H. Role of β-1,3-Glucanase in Postmeiotic Microspore Release. Dev. Biol. 1977, 57, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Neelam, A.; Sexton, R. Cellulase (Endo β-1,4 Glucanase) and Cell Wall Breakdown during Anther Development in the Sweet Pea (Lathyrus odoratus L.): Isolation and Characterization of Partial CDNA Clones. J. Plant Physiol. 1995, 146, 622–628. [Google Scholar] [CrossRef]
- Hasegawa, K.; Kamada, S.; Takehara, S.; Takeuchi, H.; Nakamura, A.; Satoh, S.; Iwai, H. Rice Putative Methyltransferase Gene OsPMT16 Is Required for Pistil Development Involving Pectin Modification. Front. Plant Sci. 2020, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Kamada, S.; Takehara, S.; Takeuchi, H.; Nakamura, A.; Satoh, S.; Iwai, H. Rice Putative Pectin Methyltransferase Gene OsPMT 10 Is Required for Maintaining the Cell Wall Properties of Pistil Transmitting Tissues via Pectin Modification. Plant Cell Physiol. 2021, 62, 1902–1911. [Google Scholar] [CrossRef] [PubMed]
- Hyodo, H.; Terao, A.; Furukawa, J.; Sakamoto, N.; Yurimoto, H.; Satoh, S.; Iwai, H. Tissue Specific Localization of Pectin–Ca2+ Cross-Linkages and Pectin Methyl-Esterification during Fruit Ripening in Tomato (Solanum lycopersicum). PLoS ONE 2013, 8, e78949. [Google Scholar] [CrossRef]
- Albrecht, C.; Russinova, E.; Hecht, V.; Baaijens, E.; de Vries, S. The Arabidopsis thaliana SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASES1 and 2 Control Male Sporogenesis. Plant Cell 2005, 17, 3337–3349. [Google Scholar] [CrossRef] [PubMed]
- Canales, C.; Bhatt, A.M.; Scott, R.; Dickinson, H. EXS, a Putative LRR Receptor Kinase, Regulates Male Germline Cell Number and Tapetal Identity and Promotes Seed Development in Arabidopsis. Curr. Biol. 2002, 12, 1718–1727. [Google Scholar] [CrossRef]
- Colcombet, J.; Boisson-Dernier, A.; Ros-Palau, R.; Vera, C.E.; Schroeder, J.I. Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASES1 and 2 Are Essential for Tapetum Development and Microspore Maturation. Plant Cell 2005, 17, 3350–3361. [Google Scholar] [CrossRef]
- Hord, C.L.H.; Chen, C.; DeYoung, B.J.; Clark, S.E.; Ma, H. The BAM1/BAM2 Receptor-Like Kinases Are Important Regulators of Arabidopsis Early Anther Development. Plant Cell 2006, 18, 1667–1680. [Google Scholar] [CrossRef]
- Schiefthaler, U.; Balasubramanian, S.; Sieber, P.; Chevalier, D.; Wisman, E.; Schneitz, K. Molecular Analysis of NOZZLE, a Gene Involved in Pattern Formation and Early Sporogenesis during Sex Organ Development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1999, 96, 11664–11669. [Google Scholar] [CrossRef]
- Zhao, D.-Z.; Wang, G.-F.; Speal, B.; Ma, H. The EXCESS MICROSPOROCYTES1 Gene Encodes a Putative Leucine-Rich Repeat Receptor Protein Kinase That Controls Somatic and Reproductive Cell Fates in the Arabidopsis Anther. Genes Dev. 2002, 16, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Catoire, L.; Pierron, M.; Morvan, C.; du Penhoat, C.H.; Goldberg, R. Investigation of the Action Patterns of Pectinmethylesterase Isoforms through Kinetic Analyses and NMR Spectroscopy. J. Biol. Chem. 1998, 273, 33150–33156. [Google Scholar] [CrossRef]
- Ohara, T.; Takeuchi, H.; Sato, J.; Nakamura, A.; Ichikawa, H.; Yokoyama, R.; Nishitani, K.; Minami, E.; Satoh, S.; Iwai, H. Structural Alteration of Rice Pectin Affects Cell Wall Mechanical Strength and Pathogenicity of the Rice Blast Fungus Under Weak Light Conditions. Plant Cell Physiol. 2021, 62, 641–649. [Google Scholar] [CrossRef]
- Pruitt, R.E.; Vielle-Calzada, J.-P.; Ploense, S.E.; Grossniklaus, U.; Lolle, S.J. FIDDLEHEAD, a Gene Required to Suppress Epidermal Cell Interactions in Arabidopsis, Encodes a Putative Lipid Biosynthetic Enzyme. Proc. Natl. Acad. Sci. USA 2000, 97, 1311–1316. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, K.A.; Bennett, A.B. Polygalacturonases: Many Genes in Search of a Function1. Plant Physiol. 1998, 117, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, M.; Nakamura, A.; Nakamura, H.; Hakata, M.; Ichikawa, H.; Hirochika, H.; Ishii, T.; Satoh, S.; Iwai, H. Increase in Cellulose Accumulation and Improvement of Saccharification by Overexpression of Arabinofuranosidase in Rice. PLoS ONE 2013, 8, e78269. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasegawa, K.; Ichikawa, A.; Takeuchi, H.; Nakamura, A.; Iwai, H. Maintenance of Methyl-Esterified Pectin Level in Pollen Mother-Cell Stages Is Required for Microspore Development. Plants 2023, 12, 1717. https://doi.org/10.3390/plants12081717
Hasegawa K, Ichikawa A, Takeuchi H, Nakamura A, Iwai H. Maintenance of Methyl-Esterified Pectin Level in Pollen Mother-Cell Stages Is Required for Microspore Development. Plants. 2023; 12(8):1717. https://doi.org/10.3390/plants12081717
Chicago/Turabian StyleHasegawa, Kazuya, Ai Ichikawa, Haruki Takeuchi, Atsuko Nakamura, and Hiroaki Iwai. 2023. "Maintenance of Methyl-Esterified Pectin Level in Pollen Mother-Cell Stages Is Required for Microspore Development" Plants 12, no. 8: 1717. https://doi.org/10.3390/plants12081717
APA StyleHasegawa, K., Ichikawa, A., Takeuchi, H., Nakamura, A., & Iwai, H. (2023). Maintenance of Methyl-Esterified Pectin Level in Pollen Mother-Cell Stages Is Required for Microspore Development. Plants, 12(8), 1717. https://doi.org/10.3390/plants12081717






