Bio-Fabrication of Silver Nanoparticles Using Citrus aurantifolia Fruit Peel Extract (CAFPE) and the Role of Plant Extract in the Synthesis
Abstract
1. Introduction
2. Results
2.1. Phytochemical Screening of Citrus aurantifolia Fruit Peel Extract
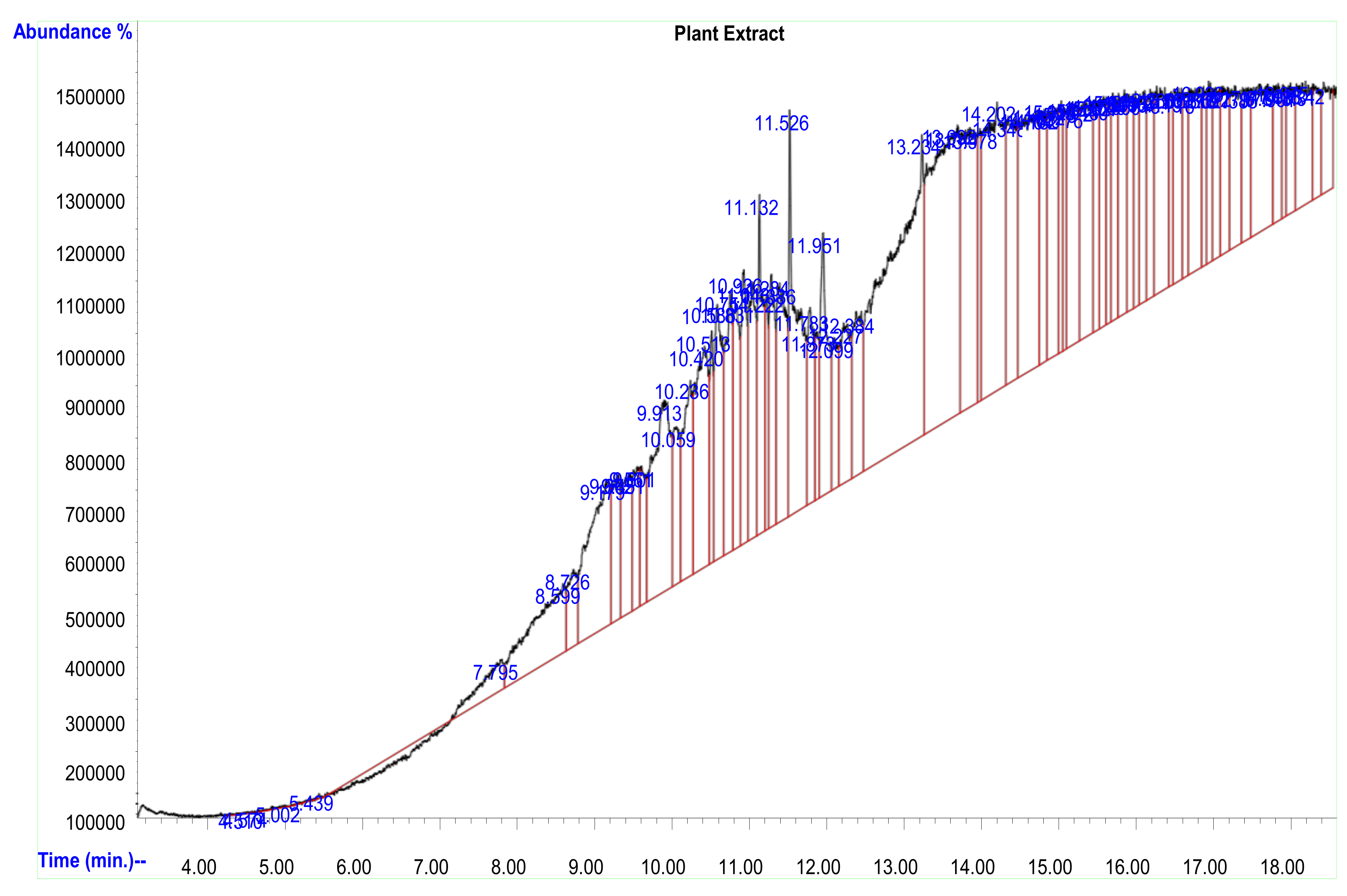
2.2. Gas Chromatography Mass Spectrometry (GC–MS) Analysis of the Plant Extract
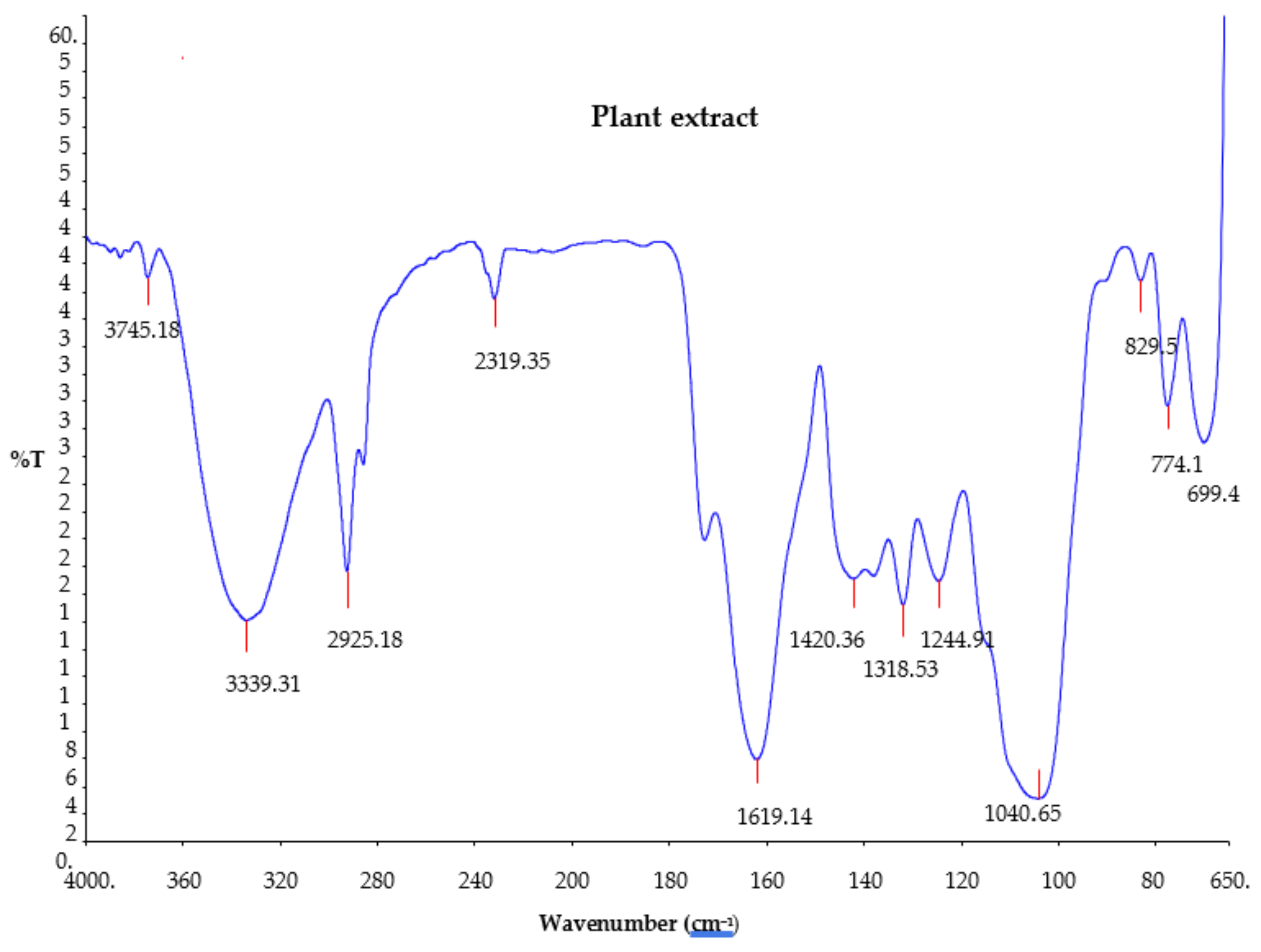
2.3. Fourier Transform Infrared Spectroscopy (FTIR) of Plant Extract
2.4. Biosynthesis of Silver Nanoparticles Using Citrus Aurantifolia Peel Extract
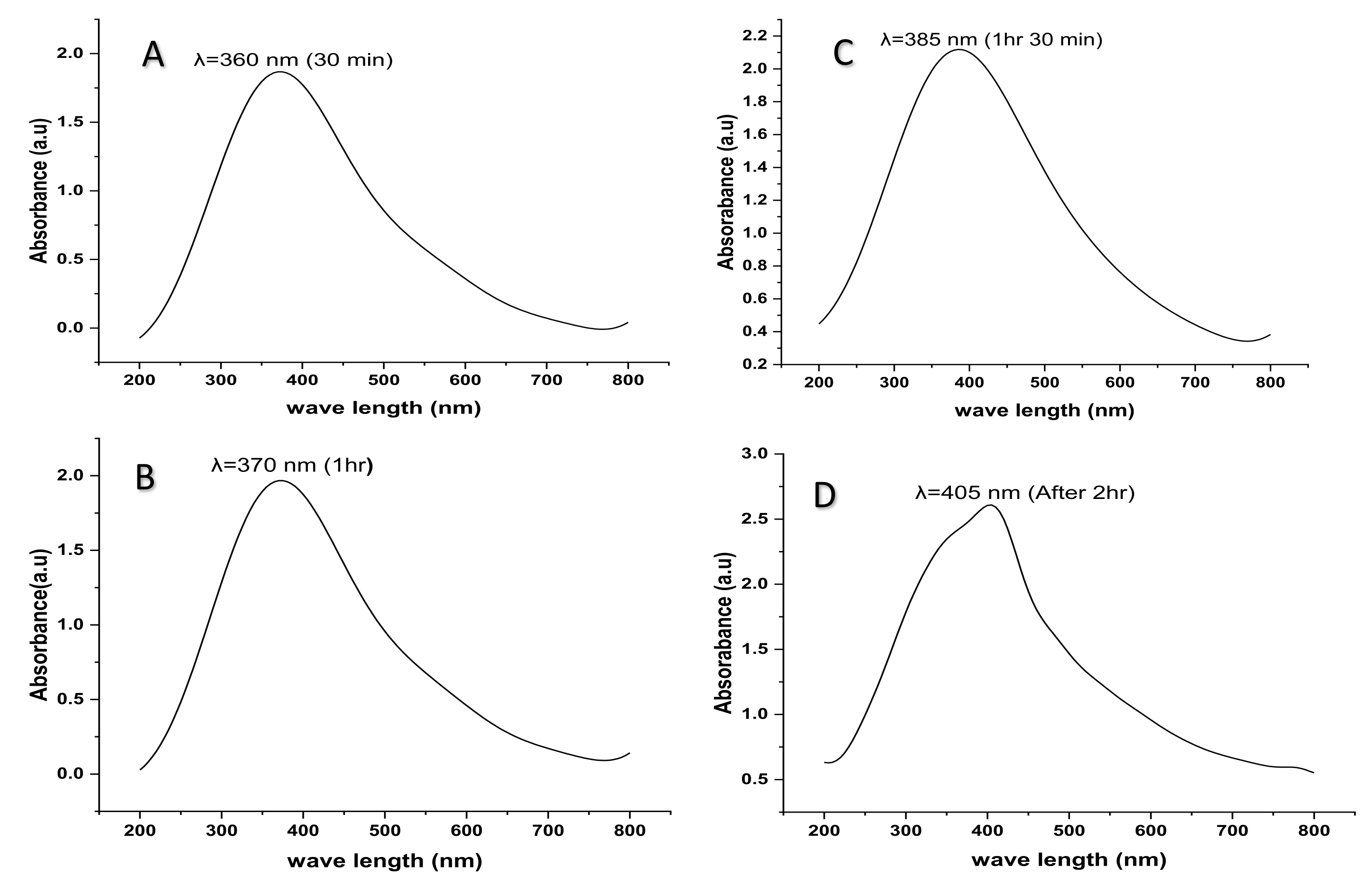
2.5. UV- Visible Spectroscopy
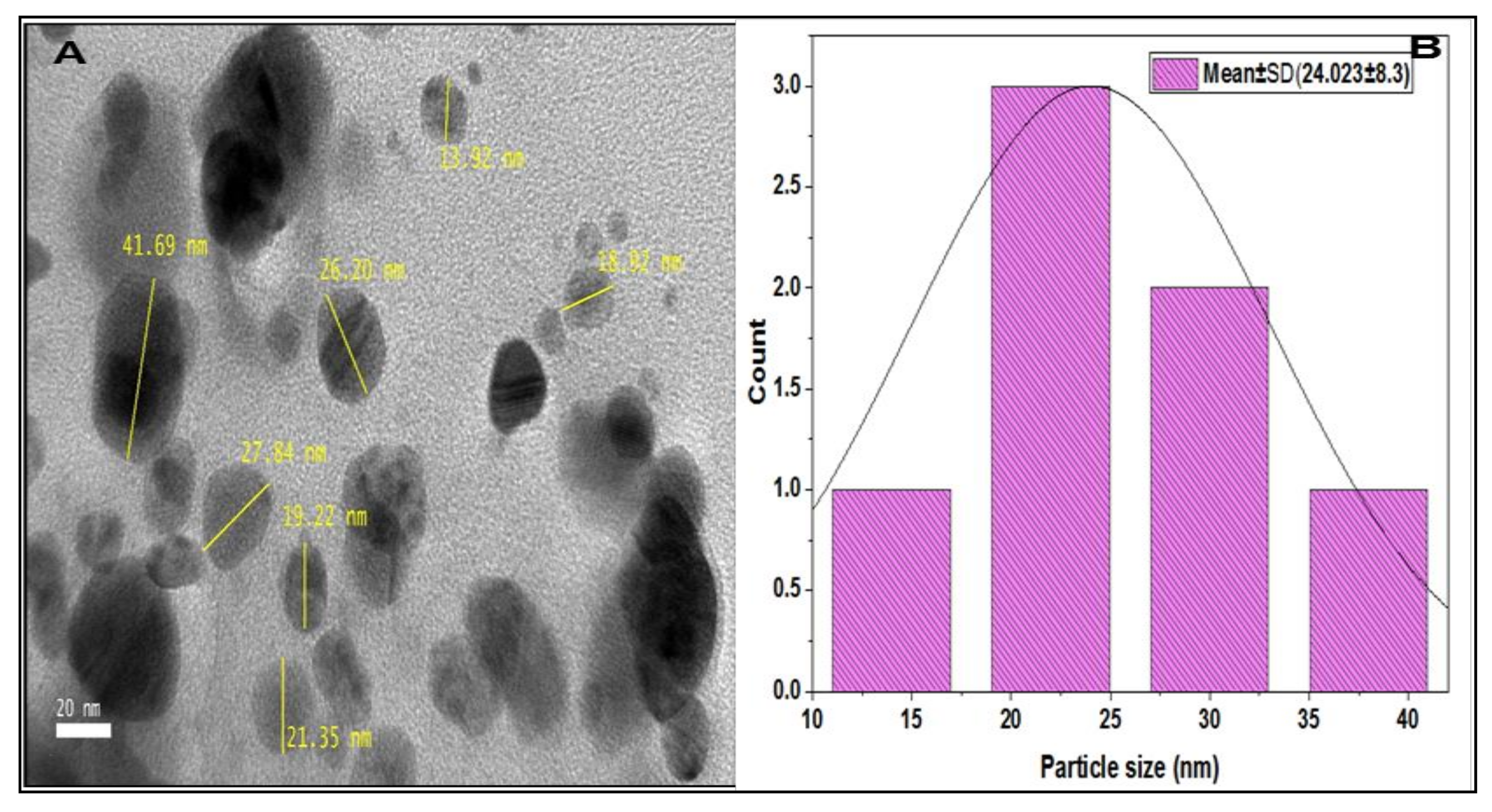
2.6. High Resolution Transmission Electron Microscope (HR-TEM)
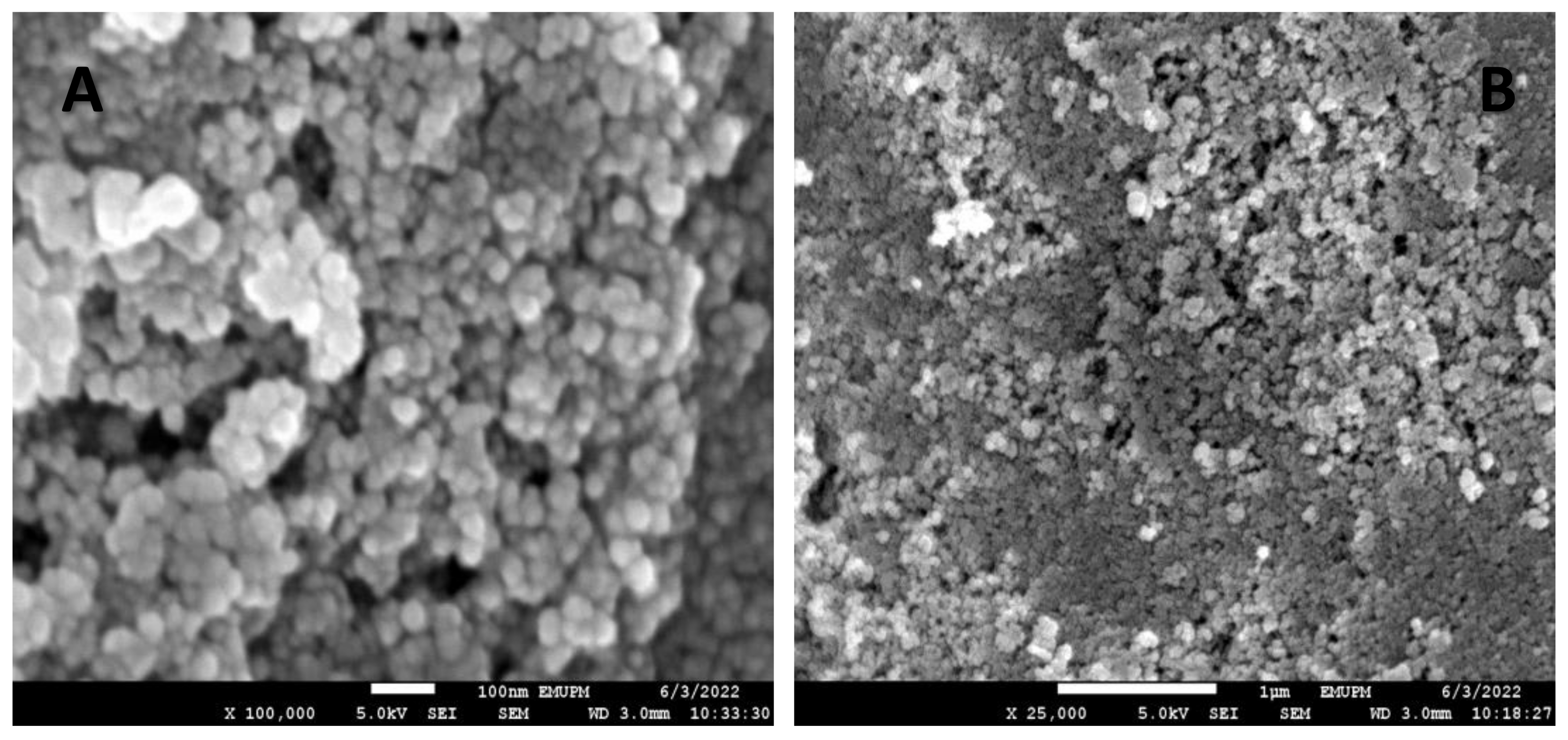
2.7. Field Emission Scanning Electron Microscope (FESEM)
2.8. Energy Dispersive X-ray Spectroscopy (EDX)
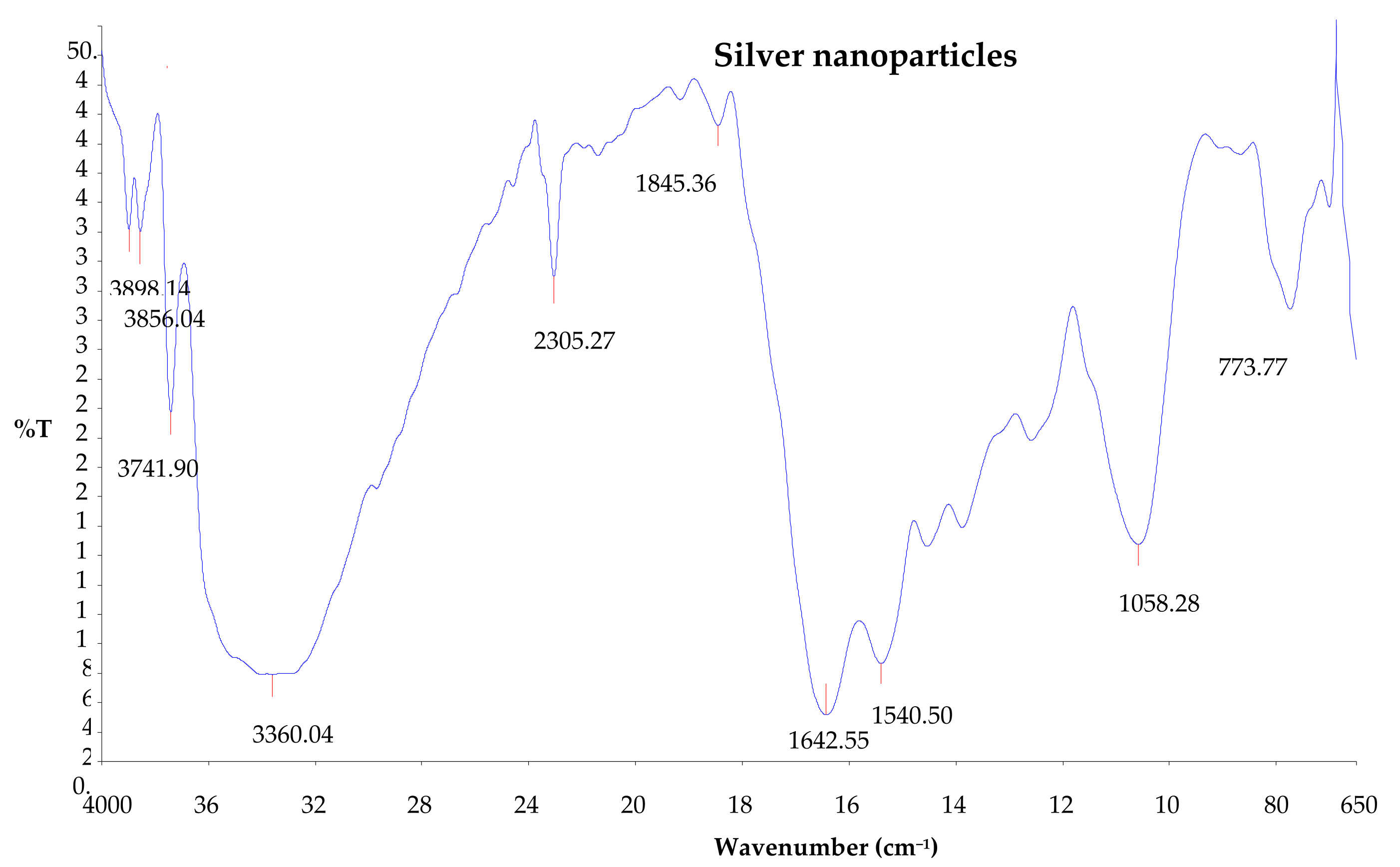
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
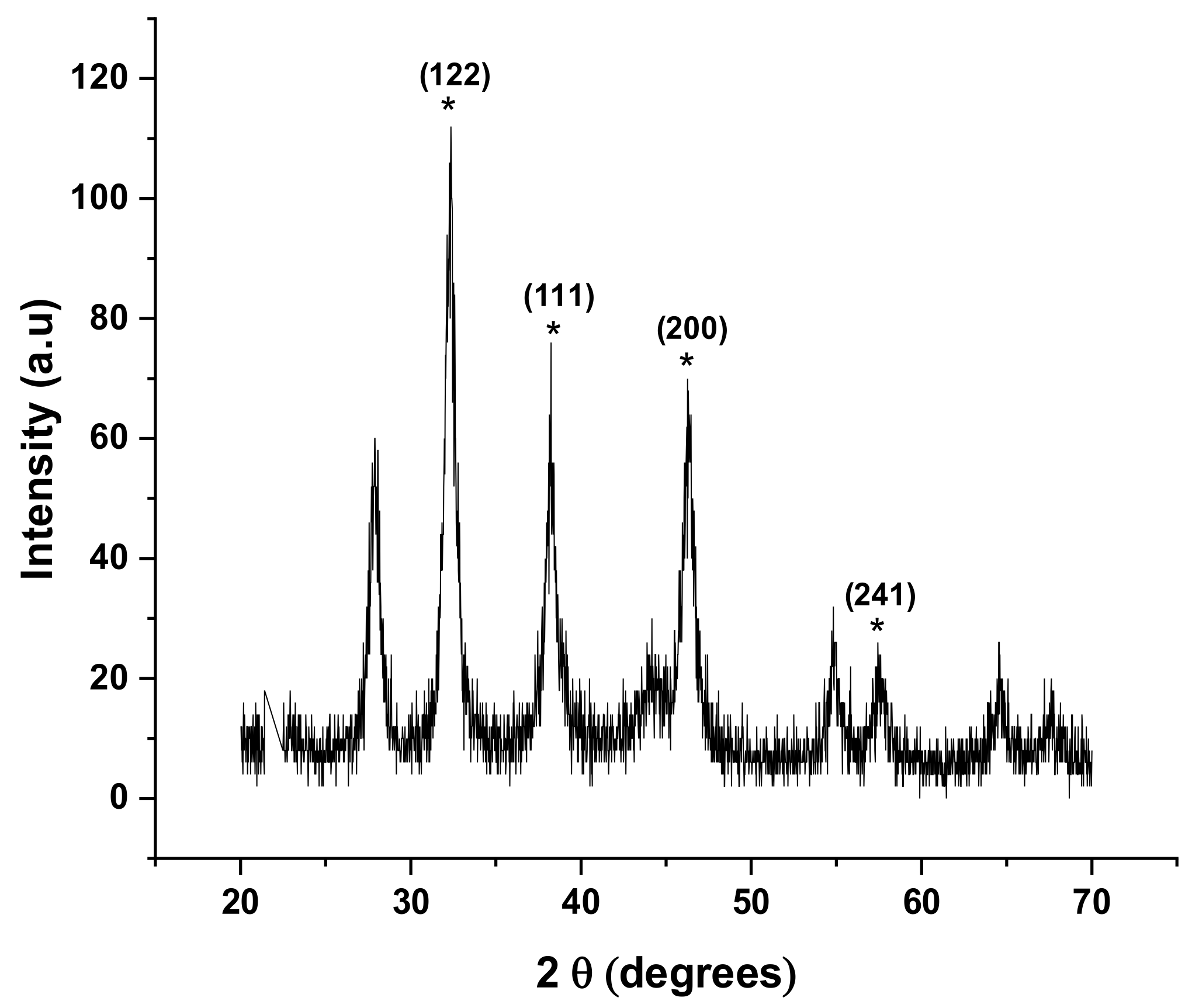
2.10. X-ray Diffraction Analysis (XRD)
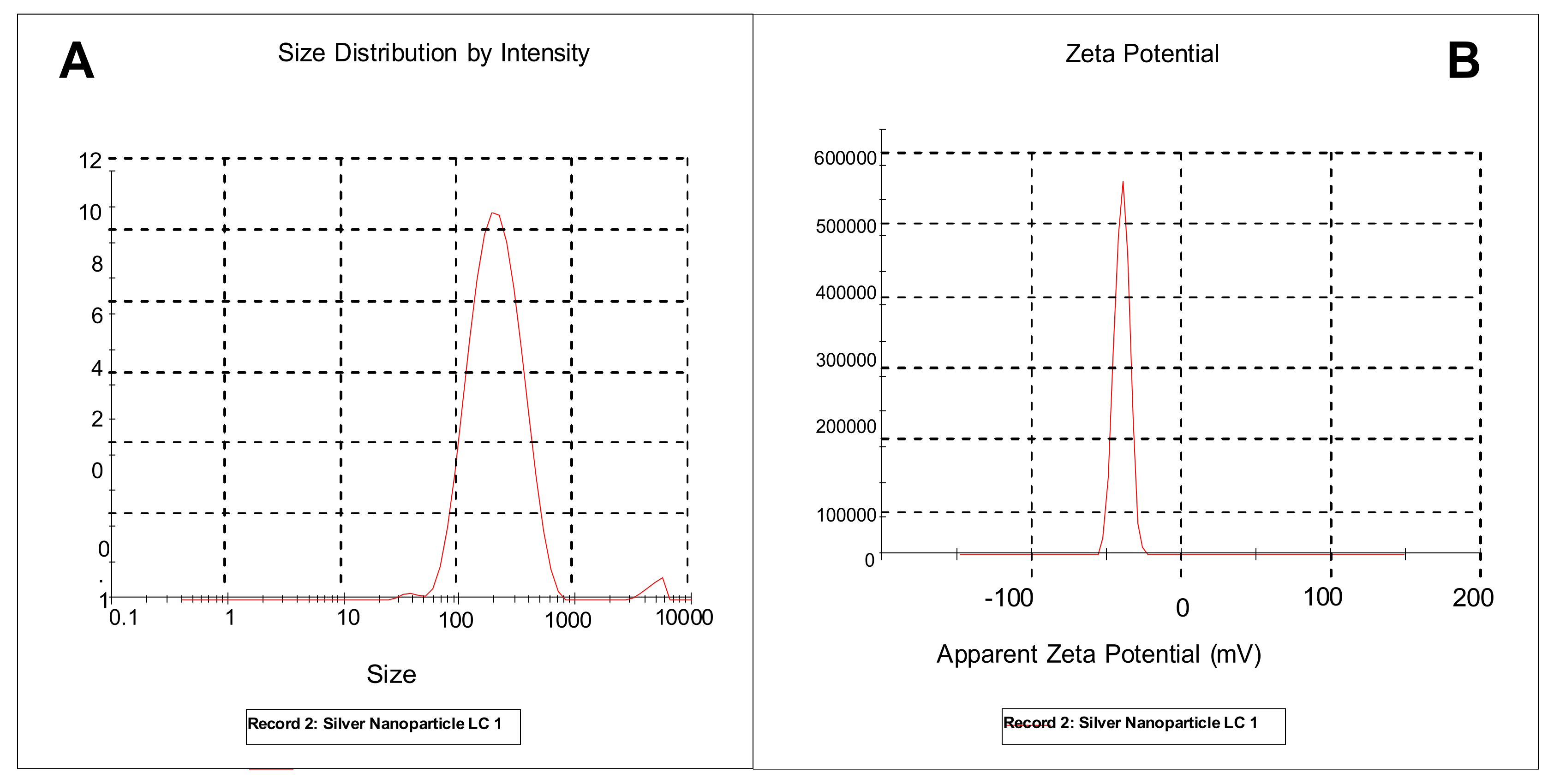
2.11. Dynamic Light Scattering (DLS) Analysis of Silver Nanoparticles
3. Discussion
4. Materials and Methods
4.1. Plant Material and Chemicals
4.2. Plant Extraction
4.3. Preliminary Phytochemical Analysis
4.3.1. Test for Alkaloids (Dragendorff’s Test)
4.3.2. Test for Saponins (Honeycomb Test)
4.3.3. Test for Tannins (Ferric Chloride Test)
4.3.4. Test for Flavonoids (Shibita’s Test)
4.3.5. Test for Phenols (Ferric Chloride Test)
4.3.6. Test for Terpenoids (Sulphuric Test)
4.3.7. Test for Steroids (Test for Salkowski)
4.3.8. Test for Anthraquinones (Borntrager’s Test)
4.4. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
4.5. Fourier Transform Infrared Spectroscopy (FTIR) of Plant Extract
4.6. Silver Nanoparticles (AgNPs) Synthesis Using CAFP Extract
4.7. Characterization of Silver Nanoparticles (AgNPs)
4.7.1. Visual Observation
4.7.2. UV-Visible Spectrophotometry Analysis
4.7.3. High Resolution Transmission Electron Microscope (HR-TEM)
4.7.4. Field Emission Scanning Electron Microscope (FESEM)
4.7.5. Energy Dispersive X-ray Spectroscopy (EDX)
4.7.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.7.7. X-ray Diffraction Analysis (XRD)
4.7.8. Dynamic Light Scattering (DLS) and Particle Size
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pirtarighat, S.; Ghannadnia, M.; Baghshahi, S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostructure Chem. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Albrecht, M.A.; Evans, C.W.; Raston, C.L. Green chemistry and the health implications of nanoparticles. Green Chem. 2006, 8, 417–432. [Google Scholar] [CrossRef]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. Resource-Efficient Technologies Review article A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Technol. 2017, 3, 406–413. [Google Scholar]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A Review on Plants and Microorganisms Mediated Synthesis of Silver Nanoparticles, Role of Plants Metabolites and Applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.; Kim, J. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Govindarajan, M.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Alyahya, S.A.; Al-anbr, M.N.; Gopinath, K.; Sudha, A. Nanosilver crystals capped with Bauhinia acuminata phytochemicals as new antimicrobials and mosquito larvicides. J. Trace Elem. Med. Biol. 2018, 50, 146–153. [Google Scholar] [CrossRef]
- Ayodele, M.; Chikodiri, V.; Adebayo-tayo, B.C. Green synthesis and cream formulations of silver nanoparticles of Nauclea latifolia (African peach) fruit extracts and evaluation of antimicrobial and antioxidant activities. Sustain. Chem. Pharm. 2020, 15, 100197. [Google Scholar]
- Bamal, D.; Singh, A.; Chaudhary, G.; Kumar, M.; Singh, M.; Rani, N.; Mundlia, P.; Sehrawat, A.R. Silver nanoparticles biosynthesis, characterization, antimicrobial activities, applications, cytotoxicity and safety issues: An updated review. Nanomaterials 2021, 11, 2086. [Google Scholar] [CrossRef]
- Subramaniam, J.; Murugan, K.; Panneerselvam, C.; Kovendan, K.; Madhiyazhagan, P.; Mahesh Kumar, P.; Dinesh, D.; Chandramohan, B.; Suresh, U.; Nicoletti, M.; et al. Eco-friendly con- trol of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi synthesized silver nanoparticles: Towards an integrative approach? Env. Sci. Pollut Res. 2015, 23, 233–235. [Google Scholar]
- Kumar, P.; Kumar, D.; Kumar, V.; Chauhan, R.; Singh, H. Mosquito larvicidal potential of Solanum xanthocarpum leaf extract derived silver nanoparticles and its bio-toxicity on non-target aquatic organism. J. Vector Borne Dis. 2022, 59, 216–227. [Google Scholar]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.; Alam, M.; Kumar, R. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Al-zahrani, S.; Astudillo-calder, S.; Pintos, B.; Elena, P.; Manzanera, A.; Mart, L.; Gomez-garay, A. Role of Synthetic Plant Extracts on the Production of Nanoparticles, Silver-derived. Plants 2021, 10, 1671. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. AIP Conf. Proc. 2016, 1724, 020048. [Google Scholar]
- khan Pathan, R.; Gali, P.R.; Pathan, P.; Gowtham, T.; Pasupuleti, S. In vitro Antimicrobial Activity of Citrus aurantifolia and its Phytochemical screening. Asian Pac. J. Trop. Dis. 2012, 2, S328–S331. [Google Scholar] [CrossRef]
- Nur, S.; Othman, A.; Hassan, M.A.; Nahar, L.; Basar, N.; Jamil, S.; Sarker, S.D. Essential Oils from the Malaysian Citrus (Rutaceae) Medicinal Plants. Medicines 2016, 3, 13. [Google Scholar]
- Jain, A.S.; Arora, P.; Popli, H. A comprehensive review on Citrus aurantifolia essential oil: Its phytochemistry and pharmacological aspects. Brazilian J. Nat. Sci. 2020, 3, 354–364. [Google Scholar] [CrossRef]
- Hemlata, M.N.R.; Singh, A.P.; Tejavath, K.K. Biosynthesis of Silver Nanoparticles Using Cucumis prophetarum Aqueous Leaf Extract and Their Antibacterial and Antiproliferative Activity against Cancer Cell Lines. ACS Omega 2020, 5, 5520–5528. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarepour, A.; Zarrabi, A. Green synthesis of silver nanoparticles at low temperature in a fast pace with unique DPPH radical scavenging and selective cytotoxicity against MCF-7 and BT-20 tumor cell lines. Biotechnol. Rep. 2019, 24, e00393. [Google Scholar] [CrossRef]
- Ahn, E.Y.; Lee, Y.J.; Park, J.; Chun, P.; Park, Y. Antioxidant Potential of Artemisia capillaris, Portulaca oleracea, and Prunella vulgaris Extracts for Biofabrication of Gold Nanoparticles and Cytotoxicity Assessment. Nanoscale Res. Lett. 2018, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Al Namani, J.; Baqir, E.; Al Abri, A.; Al Hubaishi, T.; Husain, A.; Khan, S.A. Phytochemical screening, phenolic content and antioxidant activity of Citrus aurantifolia L. Leaves grown in two regions of oman. Iran. J. Pharm. Sci. 2018, 14, 27–34. [Google Scholar]
- El-Sayed, A.A.; Amr, A.; Kamel, O.M.H.M.; El-Saidi, M.M.T.; Abdelhamid, A.E. Eco-friendly fabric modification based on AgNPs@Moringa for mosquito repellent applications. Cellulose 2020, 27, 8429–8442. [Google Scholar] [CrossRef]
- Merck KGaA IR spectra Table 2022. Merck KGaA. IR Spectra Table. Germany: Sigma-Aldrich® Solutions. 2022. Available online: www.sigmaaldrich.com/MY/en/technical-documents/technical-article/analytical-chemistry/photometry (accessed on 14 February 2023).
- Khader, S.Z.A.; Syed Zameer Ahmed, S.; Sathyan, J.; Mahboob, M.R.; PVenkatesh, K.; Ramesh, K. A comparative study on larvicidal potential of selected medicinal plants over green synthesized silver nano particles. Egypt. J. Basic Appl. Sci. 2018, 5, 54–62. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret ftir spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Malarkodi, C.; Rajeshkumar, S.; Paulkumar, K.; Gnanajobitha, G.; Vanaja, M.; Annadurai, G. Bacterial synthesis of silver nanoparti- cles by using optimized biomass growth of Bacillus sp. J. Nanosci. Nanotechnol. 2013, 3, 26–32. [Google Scholar]
- Mayasari, O.D.; Lestari, W.W.; Saraswatic, T.E. Infrared absorption spectra analysis of amine-modified carbon-based nanoparticles synthesised using submerged arc discharge method with gas injection. AIP Conf. Proc. 2020, 2296, 020071. [Google Scholar]
- Jalani, N.S.; Michell, W.; Lin, W.E.; Hanani, S.Z.; Hashim, U.; Abdullah, R. Biosynthesis of silver nanoparticles using Citrus grandis. Malaysian J. Anal. Sci. 2018, 22, 676–683. [Google Scholar]
- Shah, R.K.; Yadav, R.N.S. Qualitative Phytochemical Analysis and Estimation of Total Phenols and Flavonoids in Leaf Extract of Sarcochlamys Pulcherrima Wedd. Glob. J. Bio-Sci. Biotechnol. 2015, 4, 81–84. [Google Scholar]
- Abdullahi, S.A.; Unyah, N.Z.; Nordin, N.; Basir, R.; Nasir, W.M.; Alapid, A.A.; Hassan, Y.; Mustapha, T.; Majid, R.A. Phytochemicals and Potential Therapeutic Targets on Toxoplasma gondii Parasite. Mini-Rev. Med. Chem. 2019, 20, 739–753. [Google Scholar] [CrossRef]
- Kumaresan, M.; Kannan, M.; Sankari, A.; Chandrasekhar, C.N. Phytochemical screening and antioxidant activity of Jasminum multiflorum (pink Kakada) leaves and flowers. J. Pharmacogn. Phytochem. 2019, 8, 1168–1173. [Google Scholar]
- Agustina, T.E.; Handayani, W.; Imawan, C. The UV-VIS Spectrum Analysis From Silver Nanoparticles Synthesized Using Diospyros maritima Blume. Leaves Extract. Adv. Biol. Res. 2021, 14, 411–419. [Google Scholar]
- Nata’ala, M.; Dalhat, M.; Omoye, B.; Isah, A.; Kabiru, S.; Bashiru, I.; Umar, F. Phytochemical Screening and Antibacterial Activity of Citrus sinensis (L.) Osbeck [Orange] and Citrus aurantifolia (Cristm.) Swingle [Lime] Stem from Bacteria Associated with Dental Caries. J. Adv. Microbiol. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Firoozi, S.; Jamzad, M.; Yari, M. Biologically synthesized silver nanoparticles by aqueous extract of Satureja intermedia C.A. Mey and the evaluation of total phenolic and flavonoid contents and antioxidant activity. J. Nanostructure Chem. 2016, 6, 357–364. [Google Scholar] [CrossRef]
- Ahmed, R.H.M.D.E. Green Synthesis of Silver Nanoparticles Mediated by Traditionally Used Medicinal Plants in Sudan. Int. Nano Lett. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Kapoor, S.; Lawless, D.; Kennepohl, P.; Meisel, D.; Serpone, N. Reduction and Aggregation of Silver Ions in Aqueous Gelatin Solutions. Langmuir 1994, 10, 3018–3022. [Google Scholar] [CrossRef]
- Ahmad, B.; Ali, J.; Bashir, S. Optimization and effects of different reaction conditions for the bioinspired synthesis of silver nanoparticles using hippophae rhamnoides linn. leaves aqueous extract. World Appl. Sci. J. 2013, 22, 836–843. [Google Scholar]
- Amin, M.A.; Ismail, M.A.; Badawy, A.A.; Awad, M.A.; Hamza, M.F.; Awad, M.F.; Fouda, A. The potency of fungal-fabricated selenium nanoparticles to improve the growth performance of Helianthus annuus L. and control of cutworm Agrotis ipsilon. Catalysts 2021, 11, 1551. [Google Scholar] [CrossRef]
- Carvalho, P.M.; Felício, M.R.; Santos, N.C.; Gonçalves, S.; Domingues, M.M. Application of light scattering techniques to nanoparticle characterization and development. Front. Chem. 2018, 6, 1–17. [Google Scholar] [CrossRef]
- Majeed, S.; Khanday, M. Green Synthesis of Silver Nanoparticles using Bark Extract of Salix Alba and its Antimicrobial Effect Against Bacteria Isolated from Dental Plaque. Orient. J. Chem. 2016, 32, 1611–1618. [Google Scholar] [CrossRef]
- Daskum, A.M.; Godly, C.; Qadeer, M.A. Effect of Senna occidentalis (Fabaceae) leaves extract on the formation of β-hematin and evaluation of in vitro antimalarial activity. Int. J. Herb. Med. 2019, 7, 46–51. [Google Scholar]
- Ezeonu, C.S.; Ejikeme, C.M. Qualitative and Quantitative Determination of Phytochemical Contents of Indigenous Nigerian Softwoods. New J. Sci. 2016, 2016, 5601327. [Google Scholar] [CrossRef]
- Joshi, A.; Bhobe, M.; Sattarkar, A. Phytochemical investigation of the roots of Grewia microcos Linn. J. Chem. Pharm. Res. 2013, 5, 80–87. [Google Scholar]
- Auwal, M.S.; Saka, S.; Mairiga, I.A.; Sanda, K.A.; Shuaibu, A.; Ibrahim, A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Vet. Res. Forum 2014, 5, 95–100. [Google Scholar]
- Das, B.K.; Al-Amin, M.M.; Russel, S.M.; Kabir, S.; Bhattacherjee, R.; Hannan, J.M.A. Phytochemical screening and evaluation of analgesic activity of Oroxylum indicum. Indian J. Pharm. Sci. 2014, 76, 571–575. [Google Scholar]
- Abubaker, M.A.; Huo, G.; Shi, J.; Farah, A.A.M.; Zhang, J. Gas Chromatography-Mass spectrum and Infra-Red spectral analysis of Fixed Oil from Sudanese Adansonia digitata Seeds. Chem. Methodol. J. 2021, 5, 240–249. [Google Scholar]
- Department of Chemistry and Biochemistry. FT-IR Sample Preparation; Michael Faraday Laboratory: Dekalb, IL, USA, 2010; pp. 4–6. [Google Scholar]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. Saudi J. Chem. Assoc. 2011, 15, 113–120. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S. Green Synthesis of Silver Nanoparticles Using Extracts of Ananas comosus. Green Sustain. Chem. 2012, 2, 141–147. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 2–7. [Google Scholar] [CrossRef]
- Erdogan, O.; Abbak, M.; Demirbolat, M.; Id, F.B. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef]

| Plant Species | Phytochemicals | Status |
|---|---|---|
| Citrus aurantifolia | Flavonoids | + |
| Alkaloids | + | |
| Tannins | + | |
| Saponins | + | |
| Phenols | + | |
| Terpenoids | + | |
| Steroids | + | |
| Anthraquinones | − |
| Peak | RT Time (min) | Compound Names | Peak Area % | MW (Da) |
|---|---|---|---|---|
| 67 | 17.630 | Tris (tert-butyldimethylsilyloxy) arsane | 2.017 | 342.4 |
| 46 | 15.343 | Cyclotrisiloxane, hexamethyl- | 2.10 | 468.7 |
| 39 | 10.584 | 7-Pentadecyne | 2.16 | 210.4 |
| 16 | 10.237 | 2-Pyridinamine, N-(4,5-dihydro-5-m ethyl-2-thiazolyl)-3-methy | 2.68 | 94.11 |
| 13 | 11.526 | Eicosane, 2-methyl- | 2.63 | 408.4 |
| 27 | 14.704 | 4-Methyl-2-trimethylsilyloxy-acetophenome | 3.55 | 222.3 |
| 40 | 14.203 | 1,2,4-Benzenetricarboxylic acid | 4.42 | 210.1 |
| 38 | 13.691 | 1,1,1,3,5,5,5-Heptamethyltrisiloxane | 6.48 | 468.7 |
| 35 | 9.449 | Fumaric acid, nonyl pentadecyl | 2.81 | 116.0 |
| 10 | 13.234 | Cyclotrisiloxane, hexamethyl | 8.16 | 282.5 |
| Spectrum | C | N | O | Al | S | Cl | Ag | Total % |
|---|---|---|---|---|---|---|---|---|
| Spectrum 1 | 36.11 | 15.04 | 0.47 | 0.50 | 7.46 | 40.42 | 100.00 | |
| Spectrum 2 | 33.54 | 5.94 | 11.37 | 0.48 | 0.57 | 7.67 | 40.44 | 100.00 |
| Spectrum 3 | 29.34 | 14.34 | 0.39 | 0.58 | 7.61 | 47.74 | 100.00 | |
| Maximum | 36.11 | 5.94 | 15.04 | 0.48 | 0.58 | 7.67 | 47.74 | |
| Minimum | 29.34 | 5.94 | 11.37 | 0.39 | 0.50 | 7.46 | 40.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustapha, T.; Ithnin, N.R.; Othman, H.; Abu Hasan, Z.-’I.; Misni, N. Bio-Fabrication of Silver Nanoparticles Using Citrus aurantifolia Fruit Peel Extract (CAFPE) and the Role of Plant Extract in the Synthesis. Plants 2023, 12, 1648. https://doi.org/10.3390/plants12081648
Mustapha T, Ithnin NR, Othman H, Abu Hasan Z-’I, Misni N. Bio-Fabrication of Silver Nanoparticles Using Citrus aurantifolia Fruit Peel Extract (CAFPE) and the Role of Plant Extract in the Synthesis. Plants. 2023; 12(8):1648. https://doi.org/10.3390/plants12081648
Chicago/Turabian StyleMustapha, Tijjani, Nur Raihana Ithnin, Hidayatulfathi Othman, Zatul-’Iffah Abu Hasan, and Norashiqin Misni. 2023. "Bio-Fabrication of Silver Nanoparticles Using Citrus aurantifolia Fruit Peel Extract (CAFPE) and the Role of Plant Extract in the Synthesis" Plants 12, no. 8: 1648. https://doi.org/10.3390/plants12081648
APA StyleMustapha, T., Ithnin, N. R., Othman, H., Abu Hasan, Z.-’I., & Misni, N. (2023). Bio-Fabrication of Silver Nanoparticles Using Citrus aurantifolia Fruit Peel Extract (CAFPE) and the Role of Plant Extract in the Synthesis. Plants, 12(8), 1648. https://doi.org/10.3390/plants12081648






