Nanoparticles-Based Delivery Systems for Salicylic Acid as Plant Growth Stimulator and Stress Alleviation
Abstract
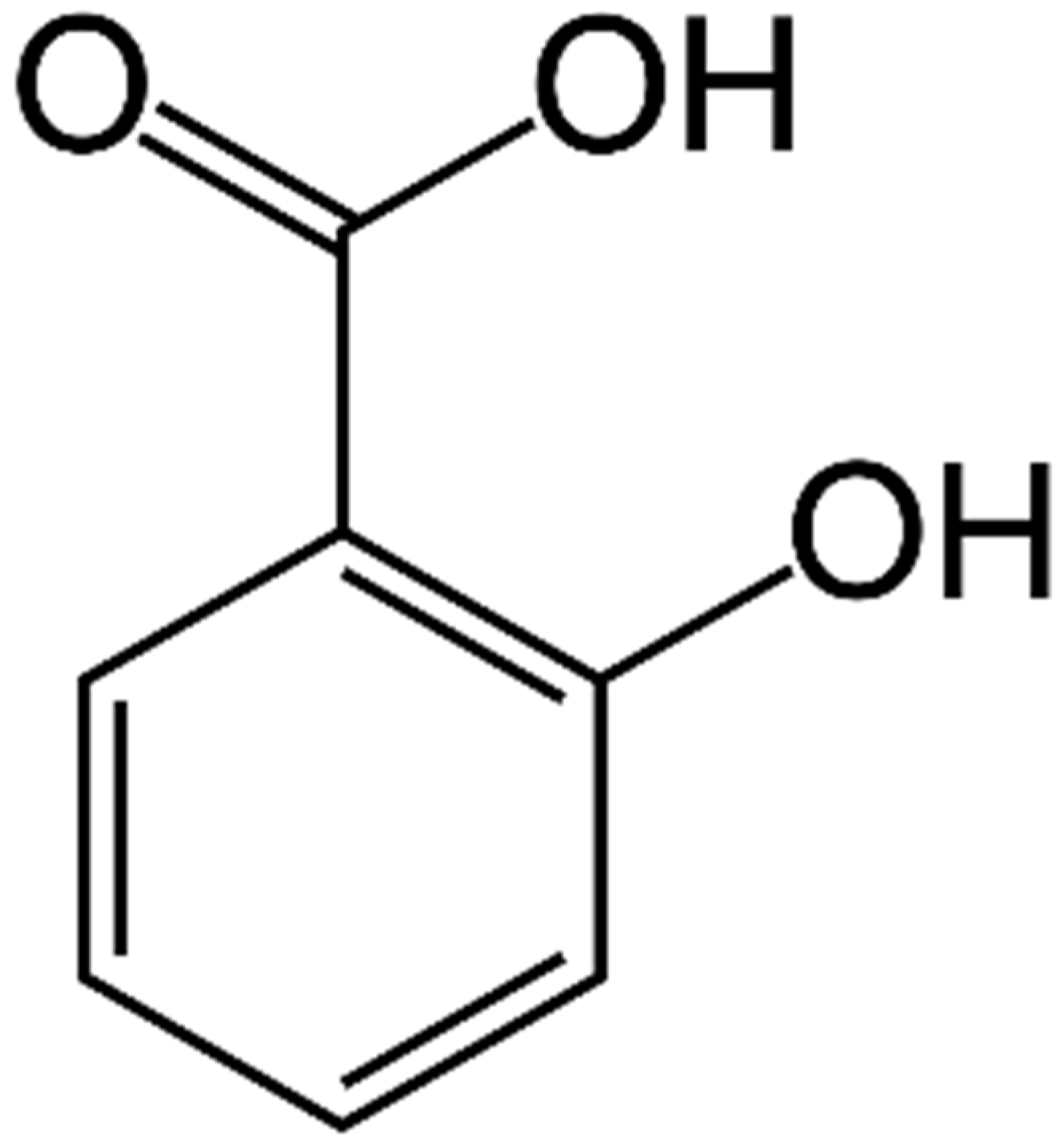
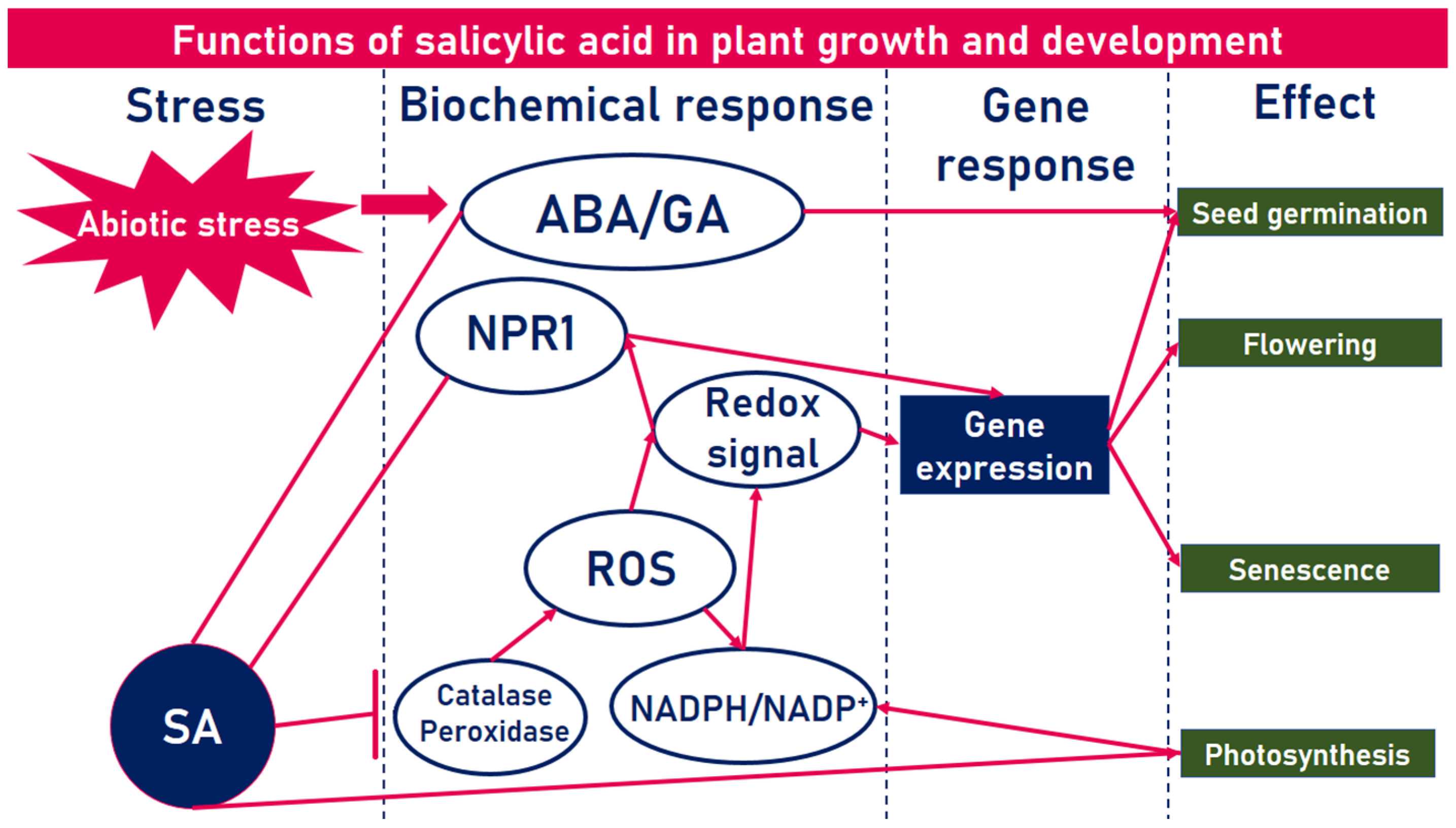
1. Introduction
2. Salicylic Acid Delivery Systems
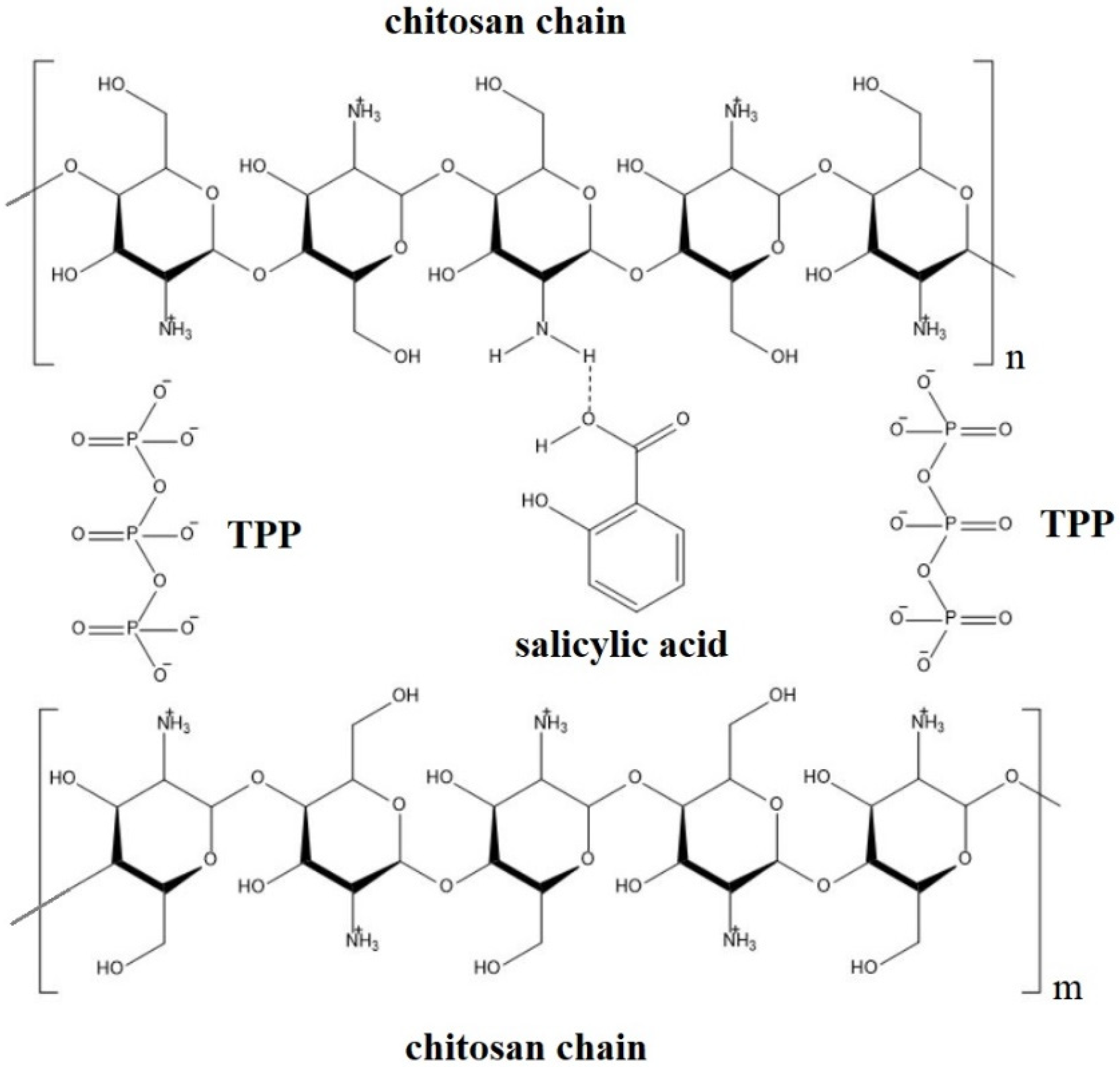
2.1. Chitosan-Based Delivery Systems
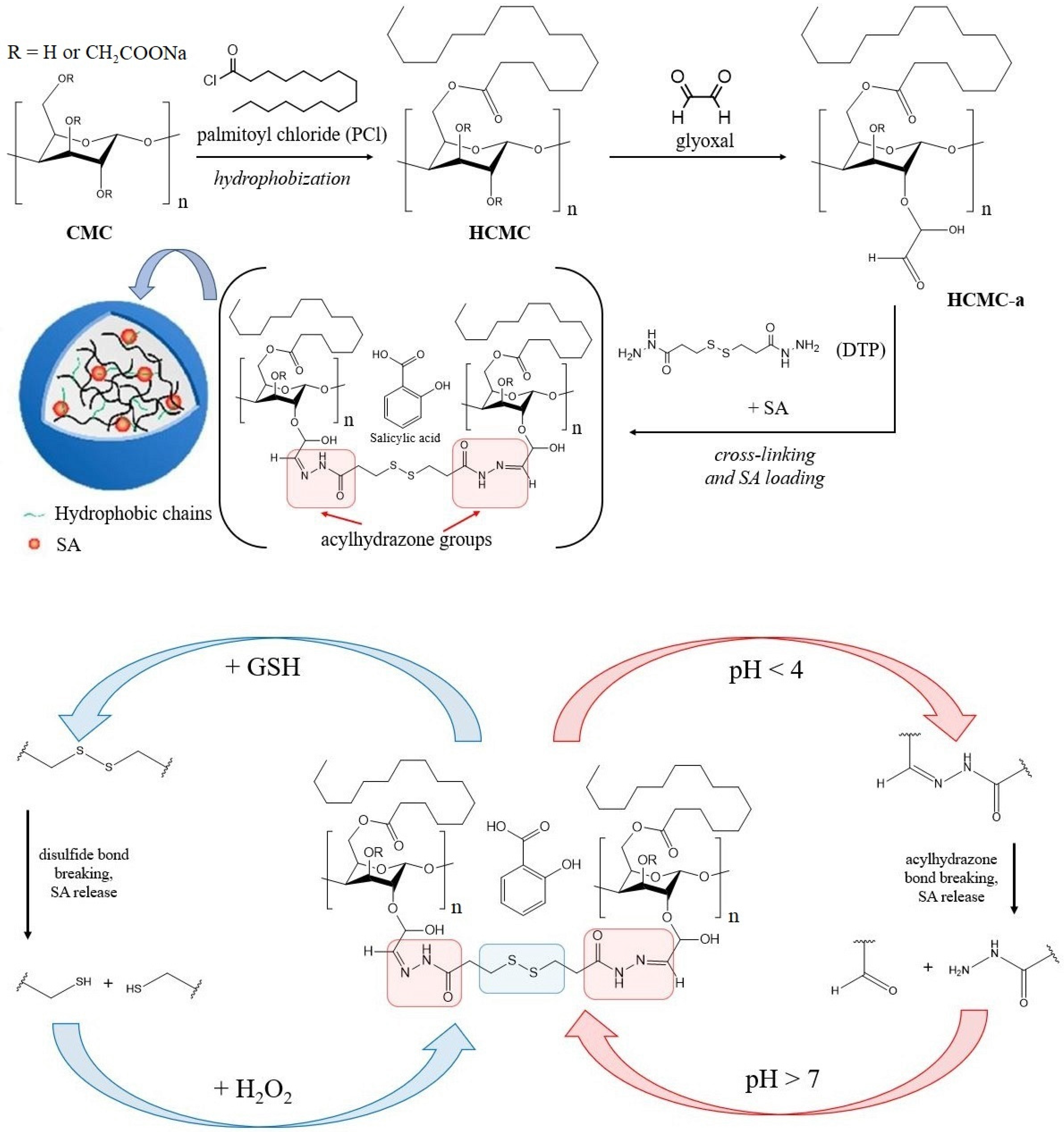
2.2. Cellulose-Based Delivery Systems
2.3. Silica-Based Delivery Systems
2.4. CeO2-Based Delivery Systems
2.5. SA-Based Delivery Systems
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | catalytic hydrolase-2; |
| CAT | catalase; |
| CMC | carboxymethyl cellulose; |
| CM-β-CD | carboxymethyl-β-cyclodextrin; |
| CS | chitosan; |
| CTAB | cetyltrimethylammonium bromide; |
| CYS | cystamine; |
| DD | degree of deacetylation; |
| DLS | dynamic light scattering; |
| DTP | 3,3′-dithiobis(propionohydrazide); |
| DTT | dithiothreitol; |
| EDC | 1-(3-(dimethylamino)propyl)-3-ethylcarbodiimide hydrochloride; |
| EE | encapsulation efficiency; |
| EN | entrapment efficiency; |
| FW | fresh weight; |
| GM | gentamicin; |
| GSH | glutathione; |
| HCMC | hydrophobic carboxymethyl cellulose; |
| HCMC-a | hydrophobic carboxymethyl cellulose with aldehyde groups; |
| LC | loading capacity; |
| LEA | late embryogenesis-abundant protein; |
| LOXD | lipoxygenase D; |
| MAPK | mitogen-activated protein kinase; |
| MDA | malondialdehyde; |
| MPs | microparticles; |
| MPTMS | 3-mercaptopropyltrimethoxysilane; |
| MSN | mesoporous silica nanoparticles; |
| NHS | N-hydroxysuccinimide; |
| NPs | nanoparticles; |
| PAL | phenylalanine ammonia-lyase; |
| PCl | palmitoyl chloride; |
| PFSR | post flowering stalk rot; |
| POD | peroxidase; |
| PPO | polyphenol oxidase; |
| PR | primary root; |
| PR-1 | pathogenesis-related protein 1; |
| RAP | ethylene-responsive transcription factor 3; |
| PINII | proteinase inhibitor II; |
| ROS | reactive oxygen species; |
| SA | salicylic acid; |
| SAR | systemic acquired resistance; |
| SOD | superoxide dismutase; |
| SVI | seedling vigor index; |
| TEM | transmission electron microscopy; |
| XET-2 | xyloglucan endotransglucosylase 2. |
References
- Steensland, A.; Thompson, T. Global Agricultural Productivity Report: Productivity Growth for Sustainable Diets, and More. GAP Rep. 2019. [Google Scholar]
- Rodrigues, S.M.; Demokritou, P.; Dokoozlian, N.; Hendren, C.O.; Karn, B.; Mauter, M.S.; Sadik, O.A.; Safarpour, M.; Unrine, J.M.; Viers, J.; et al. Nanotechnology for sustainable food production: Promising opportunities and scientific challenges. Environ. Sci. Nano 2017, 4, 767–781. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. In Biotic and Abiotic Stresses in Plants; Bosco de Oliveira, A., Ed.; IntechOpen: London, UK, 2019; pp. 1–6. [Google Scholar]
- Saharan, B.S.; Brar, B.; Duhan, J.S.; Kumar, R.; Marwaha, S.; Rajput, V.D.; Minkina, T. Molecular and Physiological Mechanisms to Mitigate Abiotic Stress Conditions in Plants. Life 2022, 12, 1634. [Google Scholar] [CrossRef] [PubMed]
- Kotrba, P.; Najmanova, J.; Macek, T.; Ruml, T.; Mackova, M. Genetically modified plants in phytoremediation of heavy metal and metalloid soil and sediment pollution. Biotechnol. Adv. 2009, 27, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Rajput, V.D.; Kumari, A.; Mandzhieva, S.S.; Sushkova, S.; Prazdnova, E.V.; Zargar, S.M.; Raza, A.; Minkina, T.; Chung, G. Nanobionics in Crop Production: An Emerging Approach to Modulate Plant Functionalities. Plants 2022, 11, 692. [Google Scholar] [CrossRef]
- Krasilnikov, P.; Taboada, M.A.; Amanullah. Fertilizer Use, Soil Health and Agricultural Sustainability. Agriculture 2022, 12, 462. [Google Scholar] [CrossRef]
- Aschonitis, V.G.; Wambacq, E.; Alloul, A.; Grunert, O.; Carrette, J.; Vermeir, P.; Spanoghe, J.; Sakarika, M.; Vlaeminck, S.E.; Haesaert, G. Aerobes and phototrophs as microbial organic fertilizers: Exploring mineralization, fertilization and plant protection features. PLoS ONE 2022, 17, e0262497. [Google Scholar]
- Chandran, V.; Shahena, S.; Rajan, M.; Mathew, L. Controlled Release of Plant Hormones for Modifying Crop Yield. In Controlled Release of Pesticides for Sustainable Agriculture; Springer: Cham, Switzerland, 2020; pp. 253–266. [Google Scholar]
- Hopkins, W.G. Introduction to Plant Physiology; Wiley: Hoboken, NJ, USA, 1997. [Google Scholar]
- Jiang, Z.; Li, J.; Qu, L.-J. Hormone Metabolism and Signaling in Plants. 2 Auxins 2017, 39–76. [Google Scholar]
- Sheteiwy, M.S.; Ulhassan, Z.; Qi, W.; Lu, H.; AbdElgawad, H.; Minkina, T.; Sushkova, S.; Rajput, V.D.; El-Keblawy, A.; Jośko, I.; et al. Association of jasmonic acid priming with multiple defense mechanisms in wheat plants under high salt stress. Front. Plant Sci. 2022, 13, 2614. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanoparticle Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, D.; Chu, J.Y.; Boyle, P.; Wang, Y.; Brindle, I.D.; De Luca, V.; Despres, C. The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep. 2012, 1, 639–647. [Google Scholar] [CrossRef] [PubMed]
- El-Garhy, H.A.S.; Abdel-Rahman, F.A.; Shams, A.S.; Osman, G.H.; Moustafa, M.M.A. Comparative Analyses of Four Chemicals Used to Control Black Mold Disease in Tomato and Its Effects on Defense Signaling Pathways, Productivity and Quality Traits. Plants 2020, 9, 808. [Google Scholar] [CrossRef]
- Muller, M.; Munne-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, X.; Yao, W.; Gao, Y.; Zhao, K.; Guo, Q.; Zhou, B.; Jiang, T. Genome-wide identification and expression analysis of the xyloglucan endotransglucosylase/hydrolase gene family in poplar. BMC Genom. 2021, 22, 804. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Kang, L.; Nagaraj, S.; Blancaflor, E.B.; Mysore, K.S.; Chapman, K.D. Mutations in Arabidopsis fatty acid amide hydrolase reveal that catalytic activity influences growth but not sensitivity to abscisic acid or pathogens. J. Biol. Chem. 2009, 284, 34065–34074. [Google Scholar] [CrossRef]
- Rehman, S.; Jorgensen, B.; Rasmussen, S.K.; Aziz, E.; Akhtar, W.; Mahmood, T. Expression analysis of proteinase inhibitor-II under OsRGLP2 promoter in response to wounding and signaling molecules in transgenic Nicotiana benthamiana. 3 Biotech 2018, 8, 51. [Google Scholar] [CrossRef]
- Hyun, M.W.; Yun, Y.H.; Kim, J.Y.; Kim, S.H. Fungal and Plant Phenylalanine Ammonia-lyase. Mycobiology 2011, 39, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhai, Q.; Wei, J.; Li, S.; Wang, B.; Huang, T.; Du, M.; Sun, J.; Kang, L.; Li, C.B.; et al. Role of tomato lipoxygenase D in wound-induced jasmonate biosynthesis and plant immunity to insect herbivores. PLoS Genet 2013, 9, e1003964. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Tang, B.; Zheng, L.; Chen, H.; Cui, X.; Ge, F.; Liu, D. A Pathogenesis-Related Protein-Like Gene Is Involved in the Panax notoginseng Defense Response to the Root Rot Pathogen. Front. Plant Sci. 2020, 11, 610176. [Google Scholar] [CrossRef]
- Yamasaki, K.; Kigawa, T.; Inoue, M.; Watanabe, S.; Tateno, M.; Seki, M.; Shinozaki, K.; Yokoyama, S. Structures and evolutionary origins of plant-specific transcription factor DNA-binding domains. Plant Physiol. Biochem. 2008, 46, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Brader, G.n.; Palva, E.T. The WRKY70 Transcription Factor: A Node of Convergence for Jasmonate-Mediated and Salicylate-Mediated Signals in Plant Defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef]
- An, J.-P.; Zhang, X.-W.; You, C.-X.; Bi, S.-Q.; Wang, X.-F.; Hao, Y.-J. MdWRKY40 promotes wounding-induced anthocyanin biosynthesis in association with MdMYB1 and undergoes MdBT2-mediated degradation. New Phytol. 2019, 224, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar] [CrossRef] [PubMed]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Front. Plant Sci. 2018, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Sugiyama, N.; Takahashi, F.; Anderson, J.C.; Ishihama, Y.; Peck, S.C.; Shinozaki, K. Genetics and Phosphoproteomics Reveal a Protein Phosphorylation Network in the Abscisic Acid Signaling Pathway in Arabidopsis thaliana. Sci. Signal. 2013, 6, rs8. [Google Scholar] [CrossRef]
- Aduse Poku, S.; Nkachukwu Chukwurah, P.; Aung, H.H.; Nakamura, I. Over-Expression of a Melon Y3SK2-Type LEA Gene Confers Drought and Salt Tolerance in Transgenic Tobacco Plants. Plants 2020, 9, 1749. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Zhang, B.; Yi, J.; Yang, Y.; Kong, C.; Lei, C.; Gong, M. The Role of the Late Embryogenesis-Abundant (LEA) Protein Family in Development and the Abiotic Stress Response: A Comprehensive Expression Analysis of Potato (Solanum tuberosum). Genes 2019, 10, 148. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity, and seed yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Slaymaker, D.H.; Navarre, D.A.; Clark, D.; del Pozo, O.; Martin, G.B.; Klessig, D.F. The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 11640–11645. [Google Scholar] [CrossRef]
- Moore, A.L.; Albury, M.S.; Crichton, P.G.; Affourtit, C. Function of the alternative oxidase: Is it still a scavenger? Trends Plant Sci. 2002, 7, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Klessing, D.F. Salicylic Acid Is a Modulator of Tobacco and Mammalian Catalases. J. Biol. Chem. 1996, 271, 28492–28501. [Google Scholar] [CrossRef]
- Durner, J.; Klessing, D.F. Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. Proc. Natl. Acad. Sci. USA 1995, 92, 11312–11316. [Google Scholar] [CrossRef]
- Horvath, E.; Szalai, G.; Janda, T. Induction of Abiotic Stress Tolerance by Salicylic Acid Signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Shirasu, K.; Nakajima, H.; Rajasekhar, V.K.; Dixon, R.A.; Lamb, C. Salicylic acid potentiates an agonist-dependent gain control that amplifies pathogen signals in the activation of defense mechanisms. The Plant Cell 1997, 9, 261–270. [Google Scholar]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef]
- Mateo, A.; Mühlenbock, P.; Rustérucci, C.; Chang, C.C.-C.; Miszalski, Z.; Karpinska, B.; Parker, J.E.; Mullineaux, P.M.; Karpinski, S. LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiol. 2004, 136, 2818–2830. [Google Scholar] [CrossRef]
- Ahmad, A.; Hayat, S.; Fariduddin, Q.; Ahmad, I. Photosynthetic Efficiency of Plants of Brassica Juncea, Treated with Chlorosubstituted Auxins. Photosynthetica 2001, 39, 565–568. [Google Scholar] [CrossRef]
- Alonso-Ramirez, A.; Rodriguez, D.; Reyes, D.; Jimenez, J.A.; Nicolas, G.; Lopez-Climent, M.; Gomez-Cadenas, A.; Nicolas, C. Evidence for a Role of Gibberellins in Salicylic Acid-Modulated Early Plant Responses to Abiotic Stress in Arabidopsis Seeds. Plant Physiol. 2009, 150, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Belghazi, M.; Huguet, R.; Robin, C.; Moreau, A.; Job, C.; Job, D. Proteomic Investigation of the Effect of Salicylic Acid on Arabidopsis Seed Germination and Establishment of Early Defense Mechanisms. Plant Physiol. 2006, 141, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Bhatt, R.K. Biochemical and Physiological Response to Salicylic Acid in Relation to the Systemic Acquired Resistance. Photosynthetica 1998, 35, 255–258. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P.; Murr, D.P.; Watkins, C.B. Influence of Salicylic Acid on H2O2 Production, Oxidative Stress, and H2O2-Metabolizing Enzymes. Plant Physiol. 1997, 115, 131–137+149. [Google Scholar] [CrossRef] [PubMed]
- La, V.H.; Lee, B.-R.; Zhang, Q.; Park, S.-H.; Islam, M.T.; Kim, T.-H. Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in Brassica rapa. Hortic. Environ. Biotechnol. 2018, 60, 31–40. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Raskin, I.; Skubatz, H.; Tang, W.; Meeuse, B.J. Salicylic Acid Levels in Thermogenic and Non-Thermogenic Plants. Ann. Bot. 1990, 66, 369–373. [Google Scholar] [CrossRef]
- Moharekar, S.T.; Lokhande(Moharekar), S.D.; Hara, T.; Tanaka, R.; Tanaka, A.; Chavan, P.D. Effect of Salicylic Acid on Chlorophyll and Carotenoid Contents of Wheat and Moong Seedlings. Photosynthetica 2003, 41, 315–317. [Google Scholar] [CrossRef]
- Pancheva, T.V.; Popova, L.P.; Uzunova, A.N. Effects of salicylic acid on growth and photosynthesis in barley plants. J. Plant Physiol. 1996, 149, 57–63. [Google Scholar] [CrossRef]
- Guan, L.; Scandalios, J.G. Developmentally related responses of maize catalase genes to salicylic acid. Proc. Natl. Acad. Sci. USA 1995, 92, 5930–5934. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, Z.-L.; Hanzlik, S.; Cook, E.; Shen, Q.J. Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Mol. Biol. 2007, 64, 293–303. [Google Scholar] [CrossRef]
- Kovácik, J.; Grúz, J.; Backor, M.; Strnad, M.; Repcák, M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009, 28, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Salicylic acid functionalized chitosan nanoparticle: A sustainable biostimulant for plant. Int. J. Biol. Macromol. 2019, 123, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Martin-Saldaña, S.; Chevalier, M.T.; Iglesias, M.J.; Colman, S.L.; Casalongué, C.A.; Álvarez, V.A.; Chevalier, A.A. Salicylic acid loaded chitosan microparticles applied to lettuce seedlings: Recycling shrimp fishing industry waste. Carbohydr. Polym. 2018, 200, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Kadam, P.M.; Prajapati, D.; Kumaraswamy, R.V.; Kumari, S.; Devi, K.A.; Pal, A.; Harish; Sharma, S.K.; Saharan, V. Physio-biochemical responses of wheat plant towards salicylic acid-chitosan nanoparticles. Plant Physiol. Biochem. 2021, 162, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Pan, Y.; Xiao, H.; Liu, J. Controlled Release of Agrochemicals Using pH and Redox Dual-Responsive Cellulose Nanogels. J. Agric. Food Chem. 2019, 67, 6700–6707. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Li, B.; Wang, L.; Xiao, H. Dual-Functional Redox-Responsive Nanocarriers for Loading Phytohormone and Complexation with Heavy Metal Ions. J. Agric. Food Chem. 2020, 68, 5076–5085. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Hussain, H.I.; Feng, C.; Sun, D.; She, F.; Rookes, J.E.; Cahill, D.M.; Kong, L. Functionalized Mesoporous Silica Nanoparticles with Redox-Responsive Short-Chain Gatekeepers for Agrochemical Delivery. ACS Appl. Mater. Interfaces 2015, 7, 9937–9946. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, D.; Rookes, J.E.; Kong, L.; Zhang, X.; Cahill, D.M. Nanoapplication of a Resistance Inducer to Reduce Phytophthora Disease in Pineapple (Ananas comosus L.). Front. Plant Sci. 2019, 10, 1238. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Vojodi Mehrabani, L.; Badali, R.; Aazami, M.A.; Rasouli, F.; KaKaei, K.; Szczepanek, M. Cerium Oxide Salicylic Acid Nanoparticles’ (CeO2: SA-NPs) Foliar Application and In-Soil Animal Manure Use Influence the Growth and Physiological Responses of Aloe vera L. Agronomy 2022, 12, 731. [Google Scholar] [CrossRef]
- Salem, D.; El-Garhy, H.A.S.; Ismail, I.A.; Dessoky, E.S.; Samra, B.N.; Shoala, T. Nanobiotechnological Approaches to Enhance Drought Tolerance in Catharanthus roseus Plants Using Salicylic Acid in Bulk and Nanoform. Molecules 2022, 27, 5112. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Suryadi, Y.; Priyatno, T.P.; Samudra, M.; Susilowati, D.; Sriharyani, T.S.; Syaefudin. Control of Anthracnose Disease (Colletotrichum gloeosporioides) Using Nano Chitosan Hydrolyzed by Chitinase Derived from Burkholderia cepacia Isolate E76. J. AgroBiogen 2017, 13, 111–122. [Google Scholar] [CrossRef]
- Popova, E.V.; Zorin, I.M.; Domnina, N.S.; Novikova, I.I.; Krasnobaeva, I.L. Chitosan–Tripolyphosphate Nanoparticles: Synthesis by the Ionic Gelation Method, Properties, and Biological Activity. Russ. J. Gen. Chem. 2020, 90, 1304–1311. [Google Scholar] [CrossRef]
- Oh, J.-W.; Chun, S.C.; Chandrasekaran, M. Preparation and In Vitro Characterization of Chitosan Nanoparticles and Their Broad-Spectrum Antifungal Action Compared to Antibacterial Activities against Phytopathogens of Tomato. Agronomy 2019, 9, 21. [Google Scholar] [CrossRef]
- Abdel-Aliem, H.A.; Gibriel, A.Y.; Rasmy, N.M.H.; Sahab, A.F.; El-Nekeety, A.A.; Abdel-Wahhab, M.A. Antifungal efficacy of chitosan nanoparticles against phytopathogenic fungi and inhibition of zearalenone production by Fusarium graminearum. Comun. Sci. 2019, 10, 338–345. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Chitosan nanoparticle induced defense responses in fingermillet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohydr. Polym. 2016, 154, 241–246. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016, 151, 321–325. [Google Scholar] [CrossRef]
- Saharan, V.; Mehrotra, A.; Khatik, R.; Rawal, P.; Sharma, S.S.; Pal, A. Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol. 2013, 62, 677–683. [Google Scholar] [CrossRef]
- Boruah, S.; Dutta, P. Fungus mediated biogenic synthesis and characterization of chitosan nanoparticles and its combine effect with Trichoderma asperellum against Fusarium oxysporum, Sclerotium rolfsii and Rhizoctonia solani. Indian Phytopathol. 2020, 74, 81–93. [Google Scholar] [CrossRef]
- Hernandez-Lauzardo, A.N.; Bautista-Banos, S.; Velazquez-Del Valle, M.G.; Mendez-Montealvo, M.G.; Sanchez-Rivera, M.M.; Bello-Perez, L.A. Antifungal effects of chitosan with different molecular weights on in vitro development of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Carbohydr. Polym. 2008, 73, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, V.A.; Bolla, P.K.; Kalhapure, R.S.; Boddu, S.H.S.; Neupane, R.; Franco, J.; Renukuntla, J. Preparation and Characterization of Furosemide-Silver Complex Loaded Chitosan Nanoparticles. Processes 2019, 7, 206. [Google Scholar] [CrossRef]
- Ji, J.; Hao, S.; Wu, D.; Huang, R.; Xu, Y. Preparation, characterization and in vitro release of chitosan nanoparticles loaded with gentamicin and salicylic acid. Carbohydr. Polym. 2011, 85, 803–808. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z. Chitosan-Based Agronanochemicals as a Sustainable Alternative in Crop Protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent Applications of Chitin- and Chitosan-Based Polymers in Plants. Polymers 2019, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Mano, J.F. Chitosan-based particles as controlled drug delivery systems. Drug Deliv. 2005, 12, 41–57. [Google Scholar] [CrossRef]
- Morales, M.; Munne-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Kubala, S.; Wojtyla, Ł.; Quinet, M.; Lechowska, K.; Lutts, S.; Garnczarska, M. Enhanced expression of the proline synthesis gene P5CSA in relation to seed osmopriming improvement of Brassica napus germination under salinity stress. J. Plant Physiol. 2015, 183, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.R.; Jin, Y.L.; Avice, J.C.; Cliquet, J.B.; Ourry, A.; Kim, T.H. Increased proline loading to phloem and its effects on nitrogen uptake and assimilation in water-stressed white clover (Trifolium repens). New Phytol. 2009, 182, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Venekamp, J.H. Regulation of cytosoi acidity in plants under conditions of drought. Physiol. Plant. 1989, 76, 112–117. [Google Scholar] [CrossRef]
- Khokhar, M.K.; Hooda, K.S.; Sharma, S.S.; Singh, V. Post Flowering Stalk Rot Complex of Maize—Present Status and Future Prospects. Maydica 2014, 59, 226–242. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Ko, J.A.; Park, H.J.; Hwang, S.J.; Park, J.B.; Lee, J.S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 2002, 249, 165–174. [Google Scholar] [CrossRef]
- Helbling, I.M.; Busatto, C.A.; Fioramonti, S.A.; Pesoa, J.I.; Santiago, L.; Estenoz, D.A.; Luna, J.A. Preparation of TPP-crosslinked chitosan microparticles by spray drying for the controlled delivery of progesterone intended for estrus synchronization in cattle. Pharm. Res. 2018, 35, 66. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Preparation of cross-linked chitosan microspheres by spray drying: Effect of cross-linking agent on the properties of spray dried microspheres. J. Microencapsul. 2005, 22, 377–395. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Fundamentals and Applications of Controlled Release Drug Delivery. Swelling Controlled Drug Delivery Systems. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: Boston, MA, USA, 1983; pp. 153–170. [Google Scholar]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Young, D.H.; Köhle, H.; Kauss, H. Effect of Chitosan on Membrane Permeability of Suspension-Cultured Glycine max and Phaseolus vulgaris Cells. Plant Physiol. 1982, 70, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D.; Teutonico, R.A. Chitosan immobilization and permeabilization of Amaranthus tricolor cells. J. Agric. Food Chem. 1986, 34, 96–97. [Google Scholar] [CrossRef]
- Brodelius, P.; Funk, C.; Häner, A.; Villegas, M. A procedure for the determination of optimal chitosan concentrations for elicitation of cultured plant cells. Phytochemistry 1989, 28, 2651–2654. [Google Scholar] [CrossRef]
- Sudisha, J.; Sharathchandra, R.G.; Amruthesh, K.N.; Kumar, A.; Shetty, H.S. Pathogenesis Related Proteins in Plant Defense Response. In Plant Defence: Biological Control; Springer: Dordrecht, The Netherlands, 2012; pp. 379–403. [Google Scholar]
- Wang, A.; Wang, Y.; Sun, C.; Wang, C.; Cui, B.; Zhao, X.; Zeng, Z.; Yao, J.; Yang, D.; Liu, G.; et al. Fabrication, Characterization, and Biological Activity of Avermectin Nano-delivery Systems with Different Particle Sizes. Nanoscale Res. Lett. 2018, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Mullineaux, P.M.; Rausch, T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynth. Res. 2005, 86, 459–474. [Google Scholar] [CrossRef]
- Jahani, S.; Saadatmand, S.; Mahmoodzadeh, H.; Khavari-Nejad, R.A. Effect of foliar application of cerium oxide nanoparticles on growth, photosynthetic pigments, electrolyte leakage, compatible osmolytes and antioxidant enzymes activities of Calendula officinalis L. Biologia 2019, 74, 1063–1075. [Google Scholar] [CrossRef]
- Prakash, V.; Peralta-Videa, J.; Tripathi, D.K.; Ma, X.; Sharma, S. Recent insights into the impact, fate and transport of cerium oxide nanoparticles in the plant-soil continuum. Ecotoxicol. Environ. Saf. 2021, 221, 112403. [Google Scholar] [CrossRef] [PubMed]
- Barrios, A.C.; Rico, C.M.; Trujillo-Reyes, J.; Medina-Velo, I.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of uncoated and citric acid coated cerium oxide nanoparticles, bulk cerium oxide, cerium acetate, and citric acid on tomato plants. Sci. Total Environ. 2016, 563–564, 956–964. [Google Scholar] [CrossRef]
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic Cerium Oxide Nanoparticles Protect Plant Photosynthesis from Abiotic Stress by Scavenging Reactive Oxygen Species. ACS Nano 2017, 11, 11283–11297. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ. Pollut. 2016, 219, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Rossi, L.; Stowers, C.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ. Sci. Pollut. Res. 2017, 25, 930–939. [Google Scholar] [CrossRef] [PubMed]
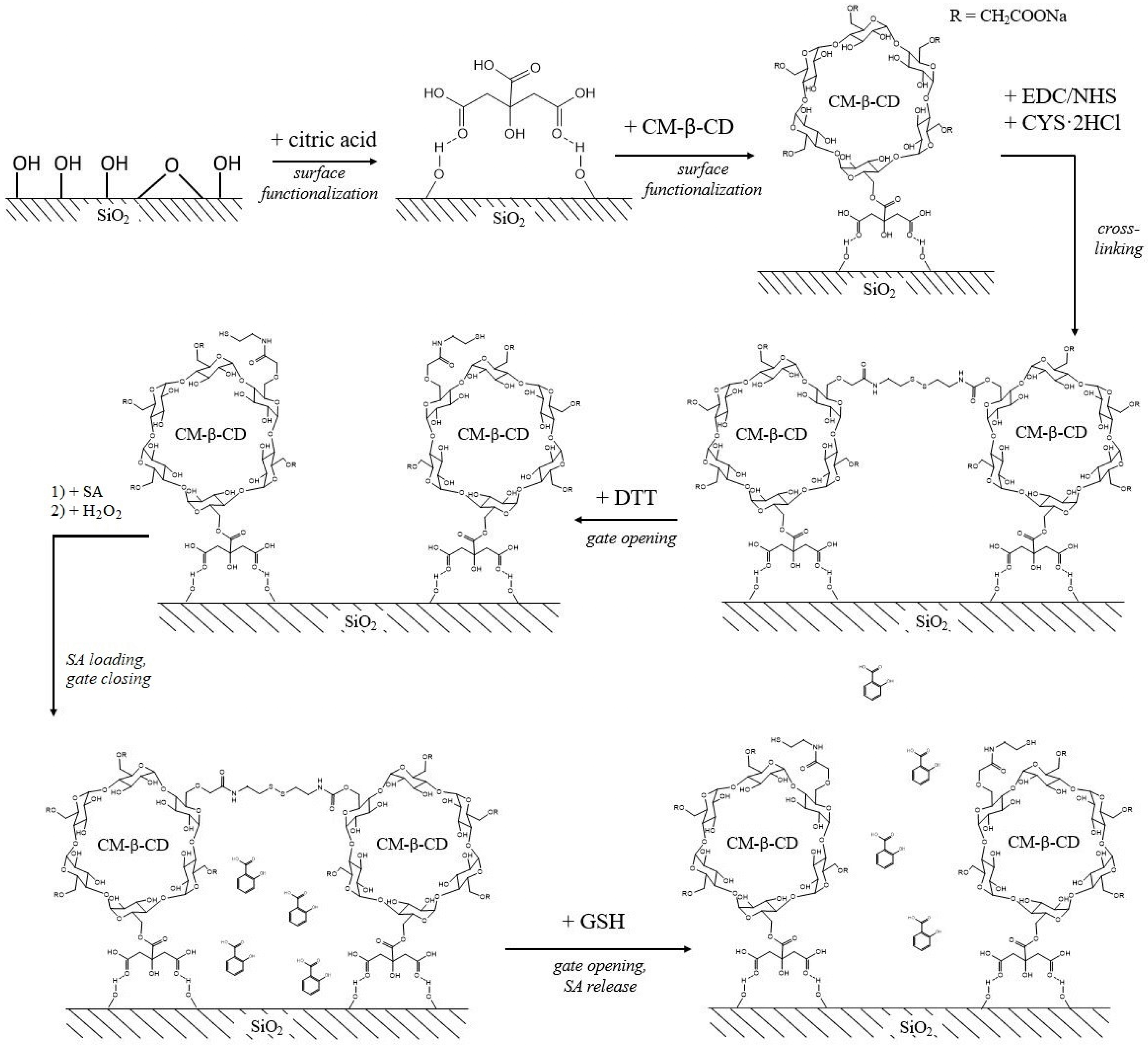
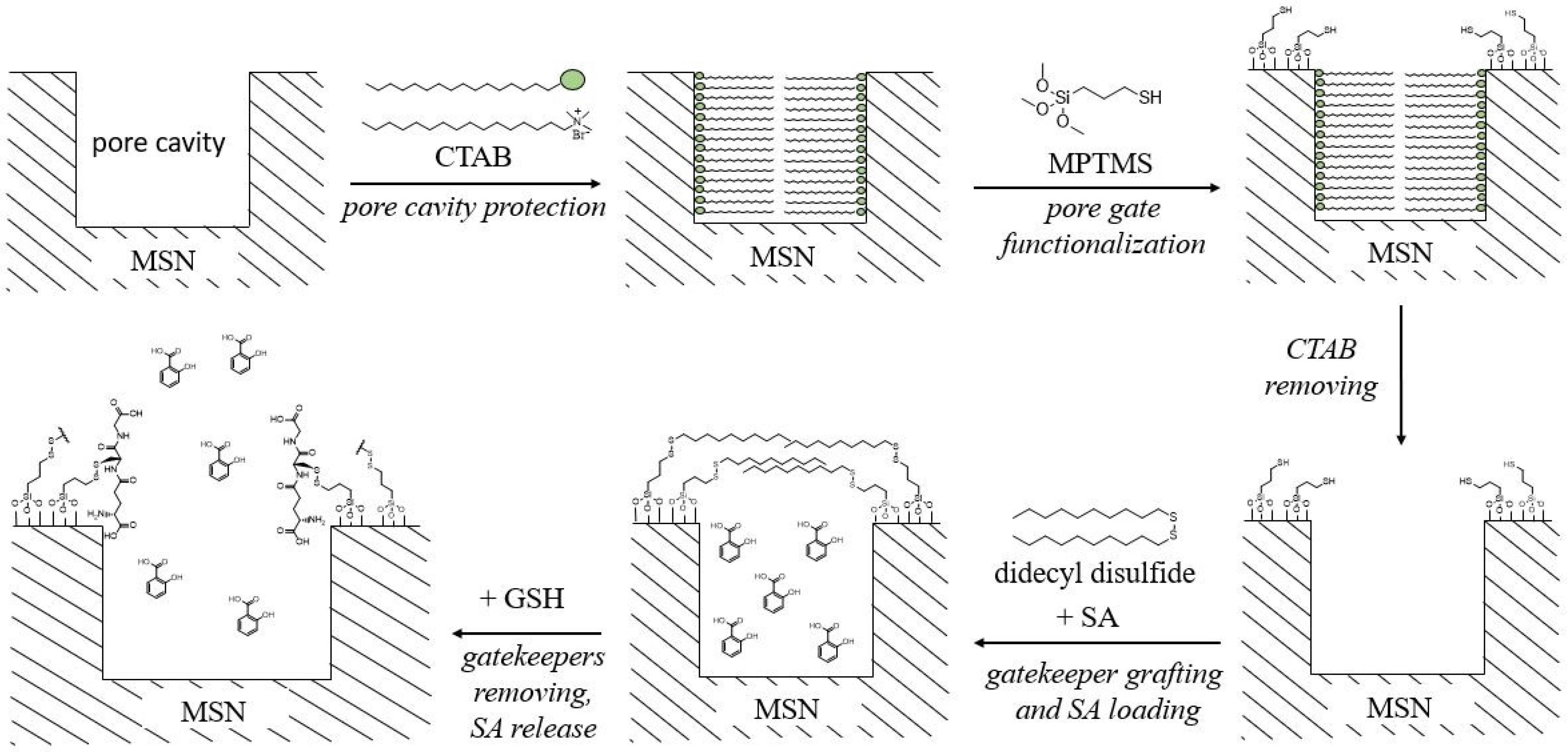
| Carrier | Size, nm/Shape/ | Plant | Effect | Ref. |
|---|---|---|---|---|
| Chitosan | ~368.7 by dynamic light scattering (DLS) 60–70 by transmission electron microscopy (TEM) /spherical/ | Maize [Zea mays] | inhibits the:
| [55] |
| 1570–3350 (TEM) /collapsed vesicular/ | Butterhead lettuce [Lactuca sativa cv. Reina de Mayo] | inhibits the:
| [56] | |
| ~368.7 (DLS) 60–70 (TEM) /spherical/ | Wheat [Triticum aestivum] | increase in the:
| [57] | |
| Cellulose nanogels | ~116 (TEM) /spherical/ | - |
| [58] |
| Thiol-CM-β-CD-modified SiO2 NPs | ~20 (TEM) /spherical/ | - |
| [59] |
| Decanethiol-modified SiO2 NPs | ~20 (TEM) /spherical/ | Arabidopsis thaliana |
| [60] |
| Decanethiol-modified SiO2 NPs | 20–30 (TEM) /spherical/ | Ananas comosus |
| [61] |
| CeO2 | 30–80 (TEM, DLS) /octahedral/ | Aloe vera | increase in the:
| [62] |
| SA NPs | 5.17–17.3 (TEM) /spherical/ | Catharanthus roseus | increase in the:
| [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polyakov, V.; Bauer, T.; Butova, V.; Minkina, T.; Rajput, V.D. Nanoparticles-Based Delivery Systems for Salicylic Acid as Plant Growth Stimulator and Stress Alleviation. Plants 2023, 12, 1637. https://doi.org/10.3390/plants12081637
Polyakov V, Bauer T, Butova V, Minkina T, Rajput VD. Nanoparticles-Based Delivery Systems for Salicylic Acid as Plant Growth Stimulator and Stress Alleviation. Plants. 2023; 12(8):1637. https://doi.org/10.3390/plants12081637
Chicago/Turabian StylePolyakov, Vladimir, Tatiana Bauer, Vera Butova, Tatiana Minkina, and Vishnu D. Rajput. 2023. "Nanoparticles-Based Delivery Systems for Salicylic Acid as Plant Growth Stimulator and Stress Alleviation" Plants 12, no. 8: 1637. https://doi.org/10.3390/plants12081637
APA StylePolyakov, V., Bauer, T., Butova, V., Minkina, T., & Rajput, V. D. (2023). Nanoparticles-Based Delivery Systems for Salicylic Acid as Plant Growth Stimulator and Stress Alleviation. Plants, 12(8), 1637. https://doi.org/10.3390/plants12081637










