Shrub Invasion Overrides the Effect of Imposed Drought on the Photosynthetic Capacity and Physiological Responses of Mediterranean Cork Oak Trees
Abstract
1. Introduction
2. Results
2.1. Meteorological Conditions
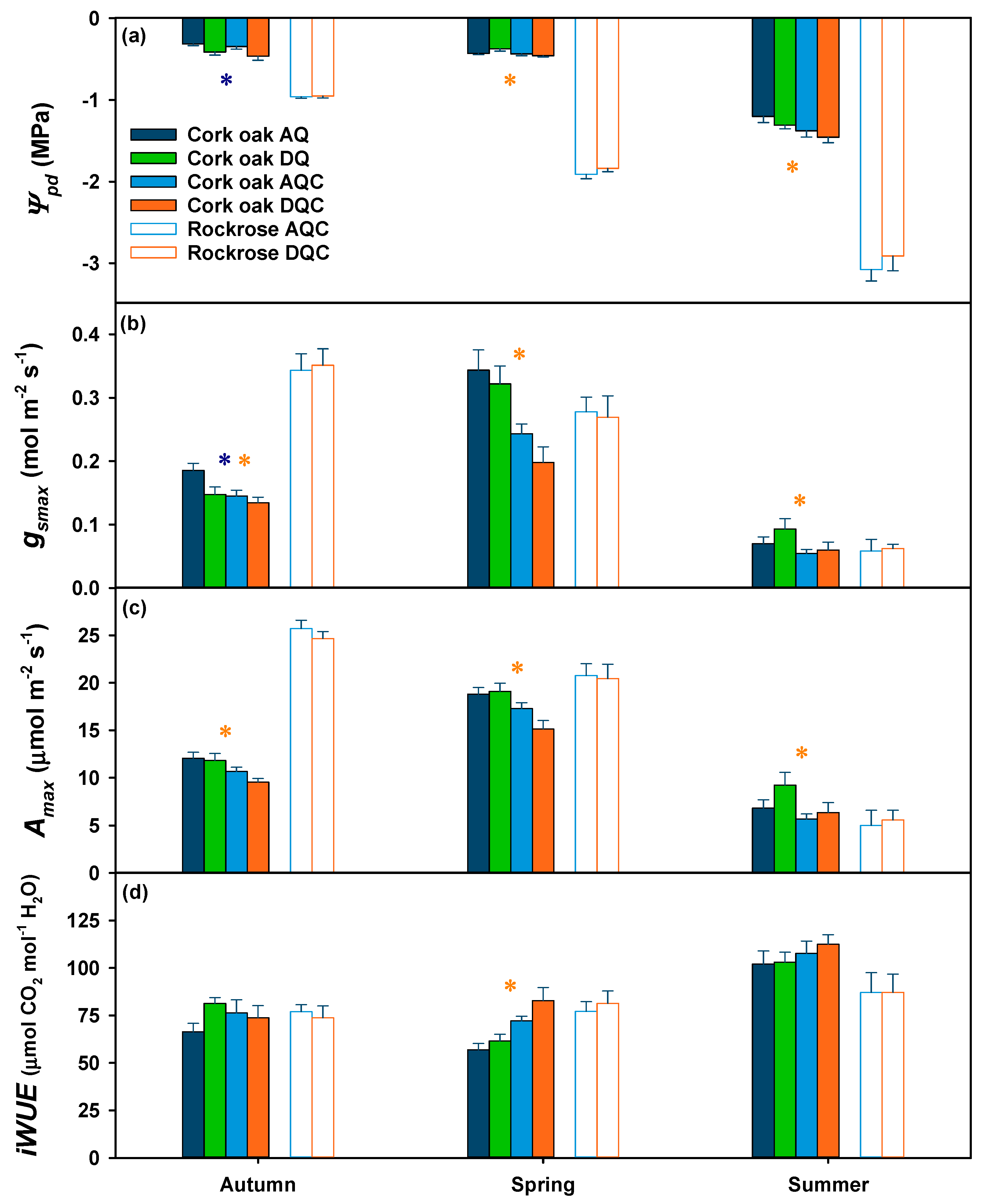
2.2. Effects of Imposed Drought and Shrub Encroachment on Cork Oak Physiological Responses
2.3. Effects of Imposed Drought on Gum Rockrose Physiological Responses
2.4. Photosynthetic Capacity (Vcmax and Jmax) of Cork Oak Trees and Gum Rockrose Shrubs
3. Discussion
4. Materials and Methods
4.1. Study Site and Experimental Set-Up
4.2. Environmental Monitoring
4.3. Leaf Water Potential
4.4. Leaf Gas Exchange
4.5. Leaf Nitrogen Concentration
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archer, S.R.; Andersen, E.M.; Predick, K.I.; Schwinning, S.; Steidl, R.J.; Woods, S.R. Woody Plant Encroachment: Causes and Consequences. In Rangeland Systems: Processes, Management and Challenges, Briske, D.D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 25–84. [Google Scholar]
- Rolo, V.; Moreno, G. Shrub encroachment and climate change increase the exposure to drought of Mediterranean wood-pastures. Sci. Total Environ. 2019, 660, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Bugalho, M.N.; Caldeira, M.C.; Pereira, J.S.; Aronson, J.; Pausas, J.G. Mediterranean cork oak savannas require human use to sustain biodiversity and ecosystem services. Front. Ecol. Environ. 2011, 9, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Keenan, T. Photosynthetic responses to stress in Mediterranean evergreens: Mechanisms and models. Environ. Exp. Bot. 2014, 103, 24–41. [Google Scholar] [CrossRef]
- Haberstroh, S.; Lobo-do-Vale, R.; Caldeira, M.C.; Dubbert, M.; Cuntz, M.; Werner, C. Plant invasion modifies isohydricity in Mediterranean tree species. Funct. Ecol. 2022, 36, 2384–2398. [Google Scholar] [CrossRef]
- Caldeira, M.C.; Lecomte, X.; David, T.S.; Pinto, J.G.; Bugalho, M.N.; Werner, C. Synergy of extreme drought and shrub invasion reduce ecosystem functioning and resilience in water-limited climates. Sci. Rep. 2015, 5, 15110. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Plant-soil interactions in Mediterranean forest and shrublands: Impacts of climatic change. Plant Soil 2013, 365, 1–33. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Acácio, V.; Holmgren, M.; Rego, F.; Moreira, F.; Mohren, G. Are drought and wildfires turning Mediterranean cork oak forests into persistent shrublands? Agrofor. Syst. 2009, 76, 389–400. [Google Scholar] [CrossRef]
- Costa, A.; Pereira, H.; Madeira, M. Analysis of spatial patterns of oak decline in cork oak woodlands in Mediterranean conditions. Ann. For. Sci. 2010, 67, 204. [Google Scholar] [CrossRef]
- Acácio, V.; Holmgren, M.; Jansen, P.A.; Schrotter, O. Multiple Recruitment Limitation Causes Arrested Succession in Mediterranean Cork Oak Systems. Ecosystems 2007, 10, 1220–1230. [Google Scholar] [CrossRef]
- Gallego, J.C.A.; Caro, J.G.; Campos, V.H.; Lobón, N.C. Effect of Leaf Litter from Cistus ladanifer L. on the Germination and Growth of Accompanying Shrubland Species. Plants 2020, 9, 593. [Google Scholar] [CrossRef]
- Aronson, J.; Pereira, J.S.; Pausas, J.G. Cork Oak Woodlands on the Edge: Ecology, Adaptive Management and Restoration; IslandPress: Washington, DC, USA, 2009; p. 315. [Google Scholar]
- Besson, C.K.; Lobo-do-Vale, R.; Rodrigues, M.L.; Almeida, P.; Herd, A.; Grant, O.M.; David, T.S.; Schmidt, M.; Otieno, D.; Keenan, T.F.; et al. Cork oak physiological responses to manipulated water availability in a Mediterranean woodland. Agric. For. Meteorol. 2014, 184, 230–242. [Google Scholar] [CrossRef]
- Haberstroh, S.; Caldeira, M.C.; Lobo-do-Vale, R.; Martins, J.I.; Moemken, J.; Pinto, J.G.; Werner, C. Nonlinear plant-plant interactions modulate impact of extreme drought and recovery on a Mediterranean ecosystem. New Phytol. 2021, 231, 1784–1797. [Google Scholar] [CrossRef]
- Lecomte, X.; Paulo, J.A.; Tomé, M.; Veloso, S.; Firmino, P.N.; Faias, S.P.; Caldeira, M.C. Shrub understorey clearing and drought affects water status and growth of juvenile Quercus suber trees. For. Ecol. Manag. 2022, 503, 119760. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Grant, O.M.; Tronina, L.; Ramalho, J.C.; Besson, C.K.; Lobo-do-Vale, R.; Pereira, J.S.; Jones, H.G.; Chaves, M.M. The impact of drought on leaf physiology of Quercus suber L. trees: Comparison of an extreme drought event with chronic rainfall reduction. J. Exp. Bot. 2010, 61, 4361–4371. [Google Scholar] [CrossRef]
- Chaves, M.M. Effects of Water Deficits on Carbon Assimilation. J. Exp. Bot. 1991, 42, 1–16. [Google Scholar] [CrossRef]
- Lobo-do-Vale, R.; Kurz Besson, C.; Caldeira, M.C.; Chaves, M.M.; Pereira, J.S. Drought reduces tree growing season length but increases nitrogen resorption efficiency in a Mediterranean ecosystem. Biogeosciences 2019, 16, 1265–1279. [Google Scholar] [CrossRef]
- Ramírez, D.A.; Parra, A.; Resco de Dios, V.; Moreno, J.M. Differences in morpho-physiological leaf traits reflect the response of growth to drought in a seeder but not in a resprouter Mediterranean species. Funct. Plant Biol. 2012, 39, 332–341. [Google Scholar] [CrossRef]
- Correia, O.; Catarino, F.; Tenhunen, J.D.; Lange, O.L. Regulation of water use by four species of Cistus in the scrub vegetation of the Serra da Arrábida, Portugal. In Plant Response to Stress; Springer: Berlin/Heidelberg, Germany, 1987; pp. 247–258. [Google Scholar]
- Simões, M.P.; Madeira, M.; Gazarini, L. The role of phenology, growth and nutrient retention during leaf fall in the competitive potential of two species of mediterranean shrubs in the context of global climate changes. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 578–589. [Google Scholar] [CrossRef]
- Bongers, F.J.; Olmo, M.; Lopez-Iglesias, B.; Anten, N.P.R.; Villar, R. Drought responses, phenotypic plasticity and survival of Mediterranean species in two different microclimatic sites. Plant Biol. 2017, 19, 386–395. [Google Scholar] [CrossRef]
- Maroco, J.P.; Rodrigues, M.L.; Lopes, C.; Chaves, M.M. Limitations to leaf photosynthesis in field-grown grapevine under drought-metabolic and modelling approaches. Funct. Plant Biol. 2002, 29, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Diaz-Espejo, A.; Gago, J.; Gallé, A.; Galmés, J.; Gulías, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Environ. Exp. Bot. 2014, 103, 12–23. [Google Scholar] [CrossRef]
- Castagneri, D.; Vacchiano, G.; Hacket-Pain, A.; DeRose, R.J.; Klein, T.; Bottero, A. Meta-analysis Reveals Different Competition Effects on Tree Growth Resistance and Resilience to Drought. Ecosystems 2022, 25, 30–43. [Google Scholar] [CrossRef]
- IPMA. Climate Normals. Available online: www.ipma.pt (accessed on 15 January 2018).
- Frazão, D.F.; Raimundo, J.R.; Domingues, J.L.; Quintela-Sabarís, C.; Gonçalves, J.C.; Delgado, F. Cistus ladanifer (Cistaceae): A natural resource in Mediterranean-type ecosystems. Planta 2018, 247, 289–300. [Google Scholar] [CrossRef]
- Quero, J.L.; Sterck, F.J.; Martínez-Vilalta, J.; Villar, R. Water-use strategies of six co-existing Mediterranean woody species during a summer drought. Oecologia 2011, 166, 45–57. [Google Scholar] [CrossRef]
- Rolo, V.; Moreno, G. Shrub species affect distinctively the functioning of scattered Quercus ilex trees in Mediterranean open woodlands. For. Ecol. Manag. 2011, 261, 1750–1759. [Google Scholar] [CrossRef]
- Otieno, D.O.; Schmidt, M.W.T.; Kurz-Besson, C.; Lobo-do-Vale, R.; Pereira, J.S.; Tenhunen, J.D. Regulation of transpirational water loss in Quercus suber trees in a Mediterranean-type ecosystem. Tree Physiol. 2007, 27, 1179–1187. [Google Scholar] [CrossRef]
- David, T.S.; Henriques, M.O.; Kurz-Besson, C.; Nunes, J.; Valente, F.; Vaz, M.; Pereira, J.S.; Siegwolf, R.; Chaves, M.M.; Gazarini, L.C.; et al. Water-use strategies in two co-occurring Mediterranean evergreen oaks: Surviving the summer drought. Tree Physiol. 2007, 27, 793–803. [Google Scholar] [CrossRef]
- Kurz-Besson, C.; Otieno, D.; Lobo-do-Vale, R.; Siegwolf, R.; Schmidt, M.; Herd, A.; Nogueira, C.; David, T.S.; David, J.S.; Tenhunen, J.; et al. Hydraulic lift in cork oak trees in a savannah-type Mediterranean ecosystem and its contribution to the local water balance. Plant Soil 2006, 282, 361–378. [Google Scholar] [CrossRef]
- Piayda, A.; Dubbert, M.; Rebmann, C.; Kolle, O.; Costa e Silva, F.; Correia, A.; Pereira, J.S.; Werner, C.; Cuntz, M. Drought impact on carbon and water cycling in a Mediterranean Quercus suber L. woodland during the extreme drought event in 2012. Biogeosciences 2014, 11, 7159–7178. [Google Scholar] [CrossRef]
- Vaz, M.; Pereira, J.S.; Gazarini, L.C.; David, T.S.; David, J.S.; Rodrigues, A.; Maroco, J.; Chaves, M.M. Drought-induced photosynthetic inhibition and autumn recovery in two Mediterranean oak species (Quercus ilex and Quercus suber). Tree Physiology 2010, 30, 946–956. [Google Scholar] [CrossRef]
- Zhou, S.; Duursma, R.A.; Medlyn, B.E.; Kelly, J.W.G.; Prentice, I.C. How should we model plant responses to drought? An analysis of stomatal and non-stomatal responses to water stress. Agric. For. Meteorol. 2013, 182–183, 204–214. [Google Scholar] [CrossRef]
- Turnbull, M.H.; Whitehead, D.; Tissue, D.T.; Schuster, W.S.F.; Brown, K.J.; Engel, V.C.; Griffin, K.L. Photosynthetic characteristics in canopies of Quercus rubra, Quercus prinus and Acer rubrum differ in response to soil water availability. Oecologia 2002, 130, 515–524. [Google Scholar] [CrossRef]
- Wullschleger, S.D. Biochemical limitations to carbon assimilation in C3 plants—A retrospective analysis of the A/Ci curves from 109 species. J. Exp. Bot. 1993, 44, 907–920. [Google Scholar] [CrossRef]
- Dias, T.; Crous, C.J.; Liberati, D.; Munzi, S.; Gouveia, C.; Ulm, F.; Afonso, A.C.; Ochoa-Hueso, R.; Manrique, E.; Sheppard, L.; et al. Alleviating Nitrogen Limitation in Mediterranean Maquis Vegetation Leads to Ecological Degradation. Land Degrad. Dev. 2017, 28, 2482–2492. [Google Scholar] [CrossRef]
- Galle, A.; Florez-Sarasa, I.; Aououad, H.E.; Flexas, J. The Mediterranean evergreen Quercus ilex and the semi-deciduous Cistus albidus differ in their leaf gas exchange regulation and acclimation to repeated drought and re-watering cycles. J. Exp. Bot. 2011, 62, 5207–5216. [Google Scholar] [CrossRef]
- Westerband, A.C.; Wright, I.J.; Maire, V.; Paillassa, J.; Prentice, I.C.; Atkin, O.K.; Bloomfield, K.J.; Cernusak, L.A.; Dong, N.; Gleason, S.M.; et al. Coordination of photosynthetic traits across soil and climate gradients. Glob. Chang. Biol. 2023, 29, 856–873. [Google Scholar] [CrossRef]
- Smith, N.G.; Keenan, T.F. Mechanisms underlying leaf photosynthetic acclimation to warming and elevated CO2 as inferred from least-cost optimality theory. Glob. Chang. Biol. 2020, 26, 5202–5216. [Google Scholar] [CrossRef]
- Dong, N.; Prentice, I.C.; Wright, I.J.; Wang, H.; Atkin, O.K.; Bloomfield, K.J.; Domingues, T.F.; Gleason, S.M.; Maire, V.; Onoda, Y.; et al. Leaf nitrogen from the perspective of optimal plant function. J. Ecol. 2022, 110, 2585–2602. [Google Scholar] [CrossRef]
- Ali, A.A.; Xu, C.; Rogers, A.; McDowell, N.G.; Medlyn, B.E.; Fisher, R.A.; Wullschleger, S.D.; Reich, P.B.; Vrugt, J.A.; Bauerle, W.L.; et al. Global scale environmental control of plant photosynthetic capacity. Ecol. Appl. 2015, 25, 2349–2365. [Google Scholar] [CrossRef]
- Walker, A.P.; Beckerman, A.P.; Gu, L.H.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The relationship of leaf photosynthetic traits-V-cmax and J(max)-to leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Wang, Q.; Jin, J. Exploring the instability of the relationship between maximum potential electron transport rate and maximum carboxylation rate in cool-temperate deciduous forests. Agric. For. Meteorol. 2021, 308–309, 108614. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Caemmerer, S.v.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 carbon pathway species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Tezara, W.; Mitchell, V.J.; Driscoll, S.D.; Lawlor, D.W. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 1999, 401, 914–917. [Google Scholar] [CrossRef]
- Bota, J.; Medrano, H.; Flexas, J. Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol. 2004, 162, 671–681. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sisó, S.; Flexas, J.; Galmés, J.; García-Nogales, A.; Niinemets, Ü.; Sancho-Knapik, D.; Saz, M.Á.; Gil-Pelegrín, E. Cell-level anatomical characteristics explain high mesophyll conductance and photosynthetic capacity in sclerophyllous Mediterranean oaks. New Phytol. 2017, 214, 585–596. [Google Scholar] [CrossRef]
- Smith, M.D.; Knapp, A.K.; Collins, S.L. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 2009, 90, 3279–3289. [Google Scholar] [CrossRef]
- Chaves, N.; Sosa, T.; Alias, J.C.; Escudero, J.C. Germination inhibition of herbs in Cistus ladanifer L. soils: Possible involvement of allelochemicals. Allelopath. J. 2003, 11, 31–42. [Google Scholar]
- FAO. World Reference Base for Soil Resources 2006, First Update 2007; IUSS Working Group WRB: Rome, Italy, 2007. [Google Scholar]
- Caldeira, M.C.; Ibáñez, I.; Nogueira, C.; Bugalho, M.N.; Lecomte, X.; Moreira, A.; Pereira, J.S. Direct and indirect effects of tree canopy facilitation in the recruitment of Mediterranean oaks. J. Appl. Ecol. 2014, 51, 349–358. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Duursma, R.A. Plantecophys--An R Package for Analysing and Modelling Leaf Gas Exchange Data. PLoS ONE 2015, 10, e0143346. [Google Scholar] [CrossRef]
- Rowland, L.; Lobo-do-Vale, R.; Christoffersen, B.O.; Melém, E.A.; Kruijt, B.; Vasconcelos, S.S.; Domingues, T.; Binks, O.J.; Oliveira, A.A.R.; Metcalfe, D.; et al. After more than a decade of soil moisture deficit, tropical rainforest trees maintain photosynthetic capacity, despite increased leaf respiration. Glob. Chang. Biol. 2015, 21, 4662–4672. [Google Scholar] [CrossRef]
- Zhou, S.; Medlyn, B.; Sabaté, S.; Sperlich, D.; Prentice, I.C.; Whitehead, D. Short-term water stress impacts on stomatal, mesophyll and biochemical limitations to photosynthesis differ consistently among tree species from contrasting climates. Tree Physiol. 2014, 34, 1035–1046. [Google Scholar] [CrossRef]
- Medlyn, B.E.; Dreyer, E.; Ellsworth, D.; Forstreuter, M.; Harley, P.C.; Kirschbaum, M.U.F.; Le Roux, X.; Montpied, P.; Strassemeyer, J.; Walcroft, A.; et al. Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ. 2002, 25, 1167–1179. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo-do-Vale, R.; Rafael, T.; Haberstroh, S.; Werner, C.; Caldeira, M.C. Shrub Invasion Overrides the Effect of Imposed Drought on the Photosynthetic Capacity and Physiological Responses of Mediterranean Cork Oak Trees. Plants 2023, 12, 1636. https://doi.org/10.3390/plants12081636
Lobo-do-Vale R, Rafael T, Haberstroh S, Werner C, Caldeira MC. Shrub Invasion Overrides the Effect of Imposed Drought on the Photosynthetic Capacity and Physiological Responses of Mediterranean Cork Oak Trees. Plants. 2023; 12(8):1636. https://doi.org/10.3390/plants12081636
Chicago/Turabian StyleLobo-do-Vale, Raquel, Teresa Rafael, Simon Haberstroh, Christiane Werner, and Maria Conceição Caldeira. 2023. "Shrub Invasion Overrides the Effect of Imposed Drought on the Photosynthetic Capacity and Physiological Responses of Mediterranean Cork Oak Trees" Plants 12, no. 8: 1636. https://doi.org/10.3390/plants12081636
APA StyleLobo-do-Vale, R., Rafael, T., Haberstroh, S., Werner, C., & Caldeira, M. C. (2023). Shrub Invasion Overrides the Effect of Imposed Drought on the Photosynthetic Capacity and Physiological Responses of Mediterranean Cork Oak Trees. Plants, 12(8), 1636. https://doi.org/10.3390/plants12081636






