Exogenous Auxin and Gibberellin on Fluoride Phytoremediation by Eichhornia crassipes
Abstract
1. Introduction
2. Materials and Methods
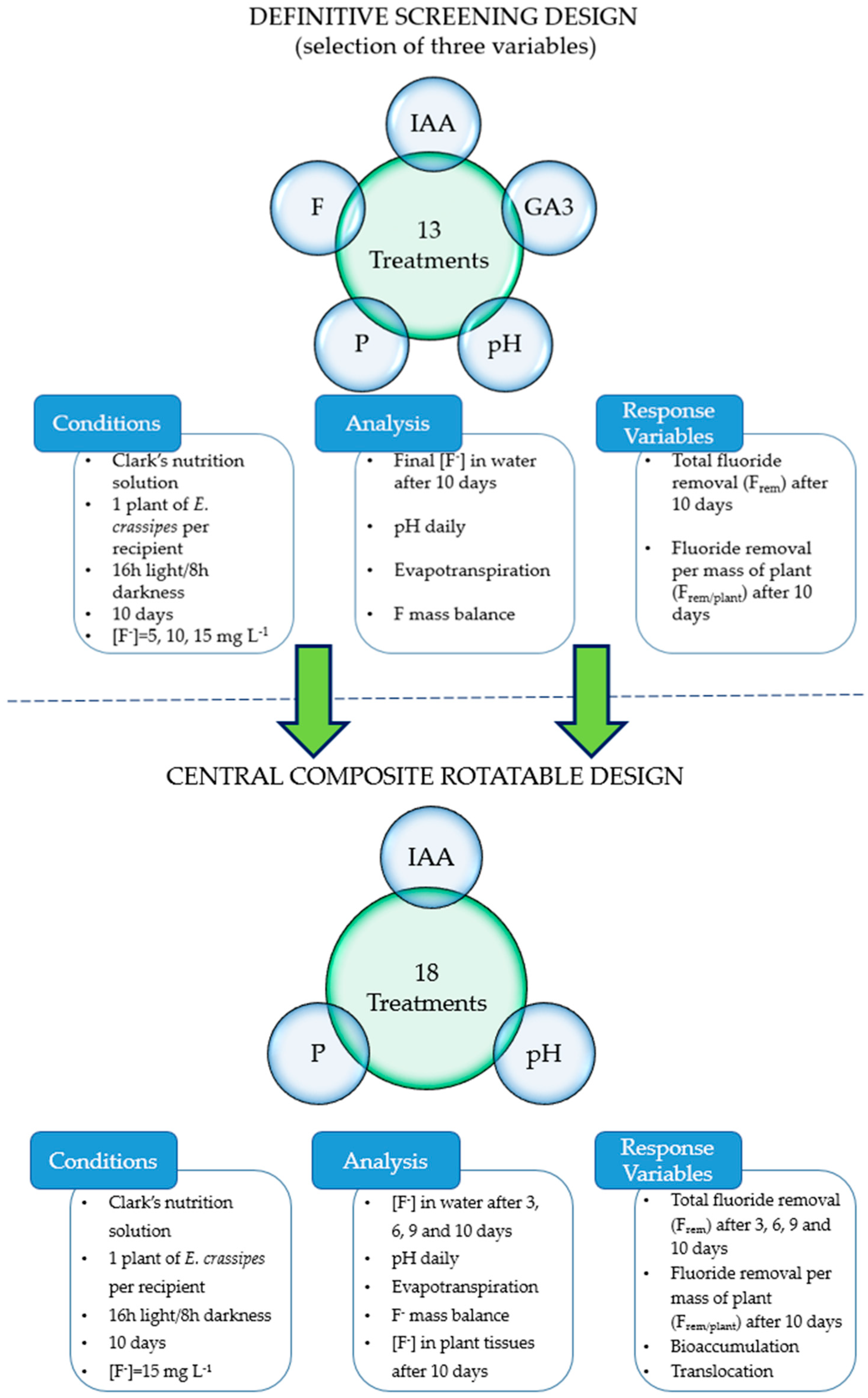
2.1. Definitive Screening Design Experiment
2.1.1. Treatments and Design
2.1.2. Collection and Acclimation of Eichhornia crassipes
2.1.3. Experimental Conditions
2.1.4. Analyses
2.2. Central Composite Rotatable Design Experiment
2.2.1. Treatments and Design
2.2.2. Experimental Conditions
2.2.3. Analyses
3. Results
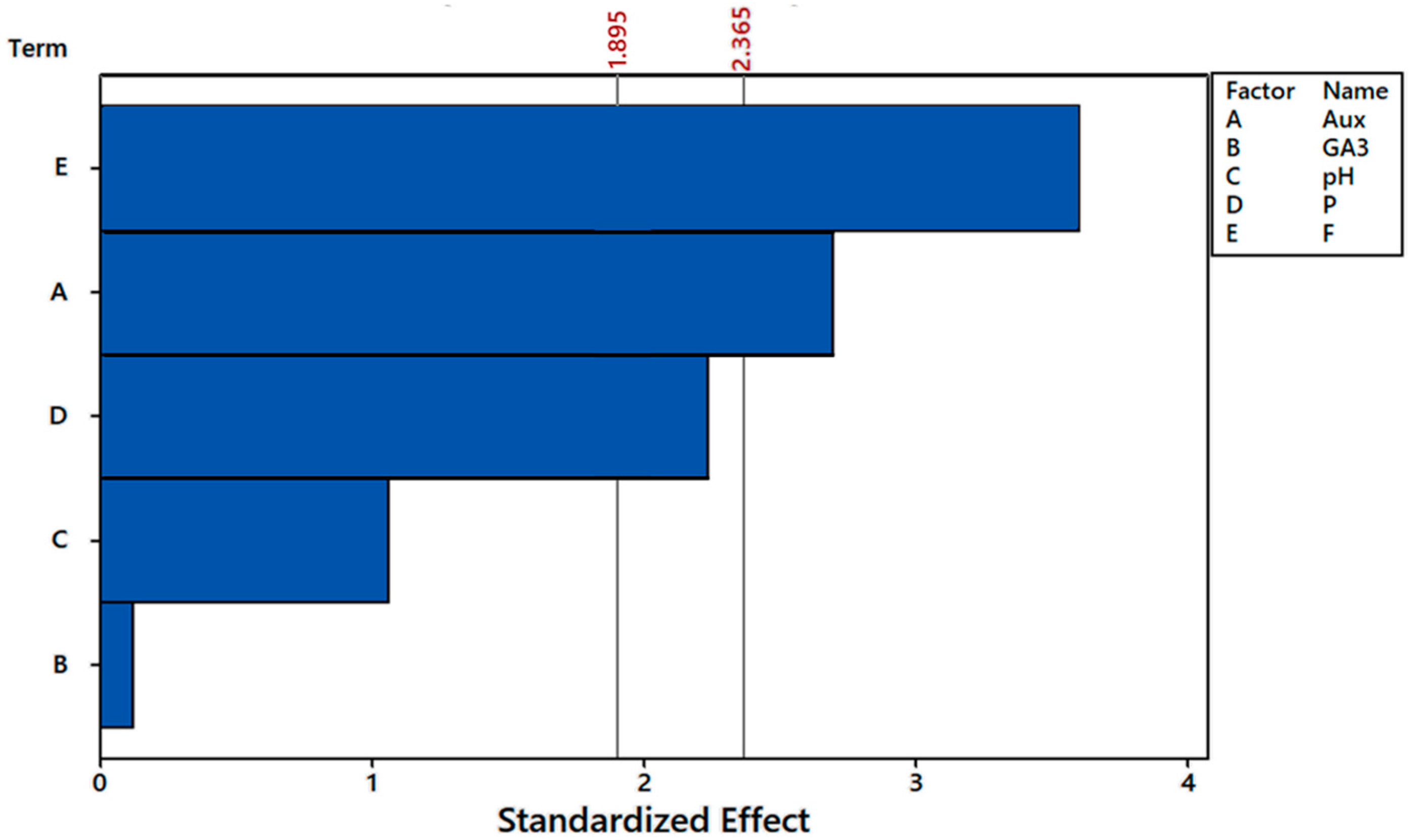
3.1. Definitive Screening Design
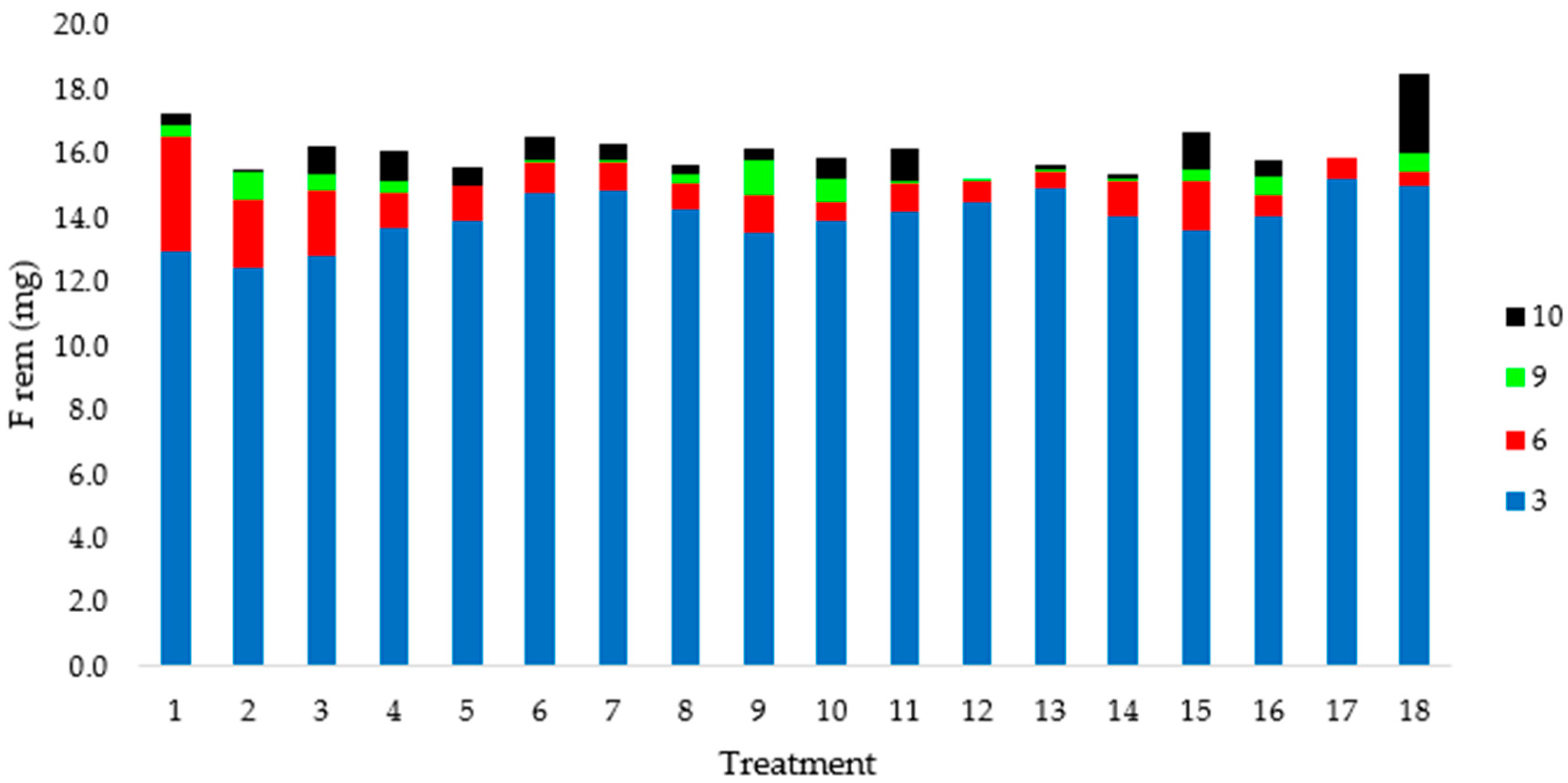
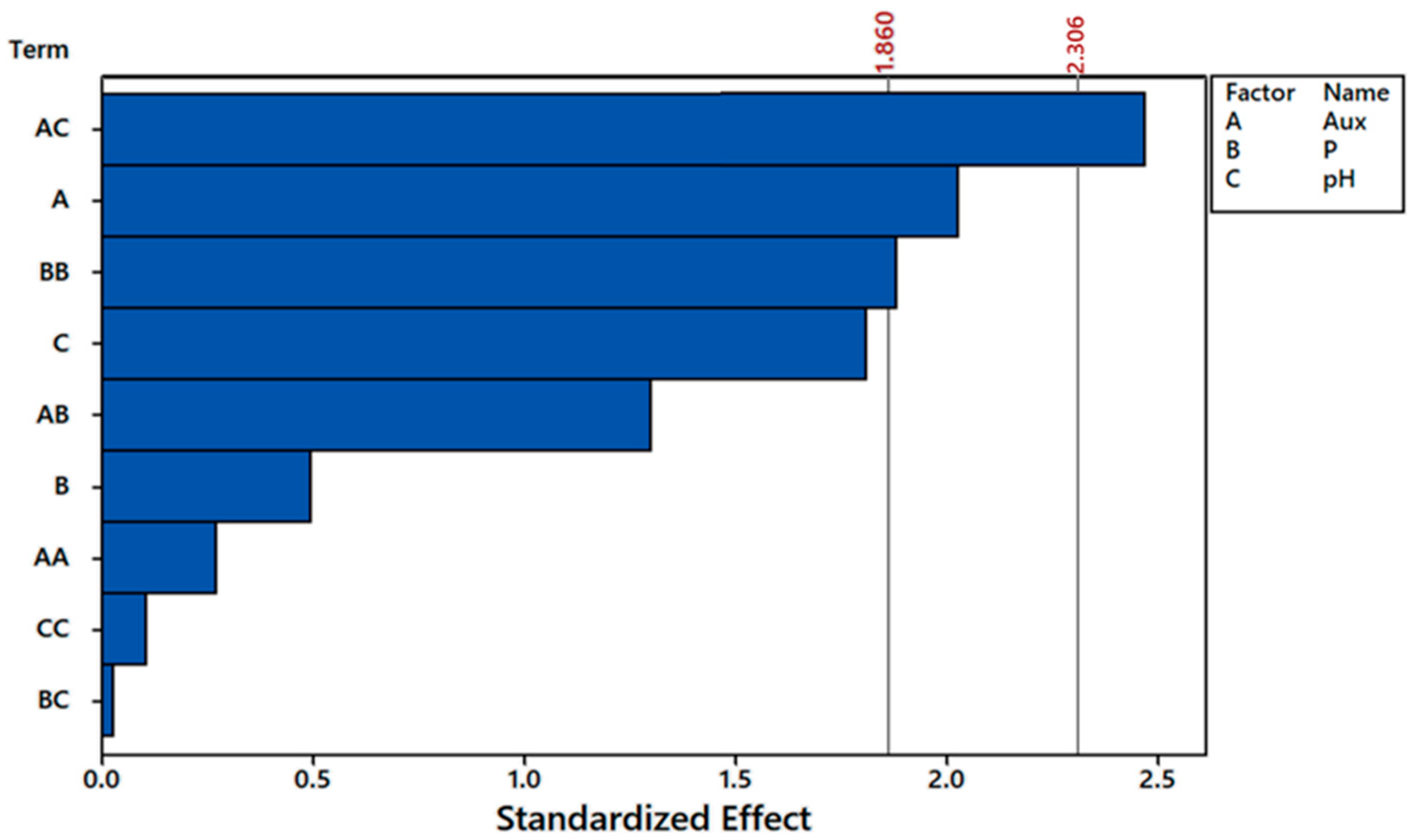
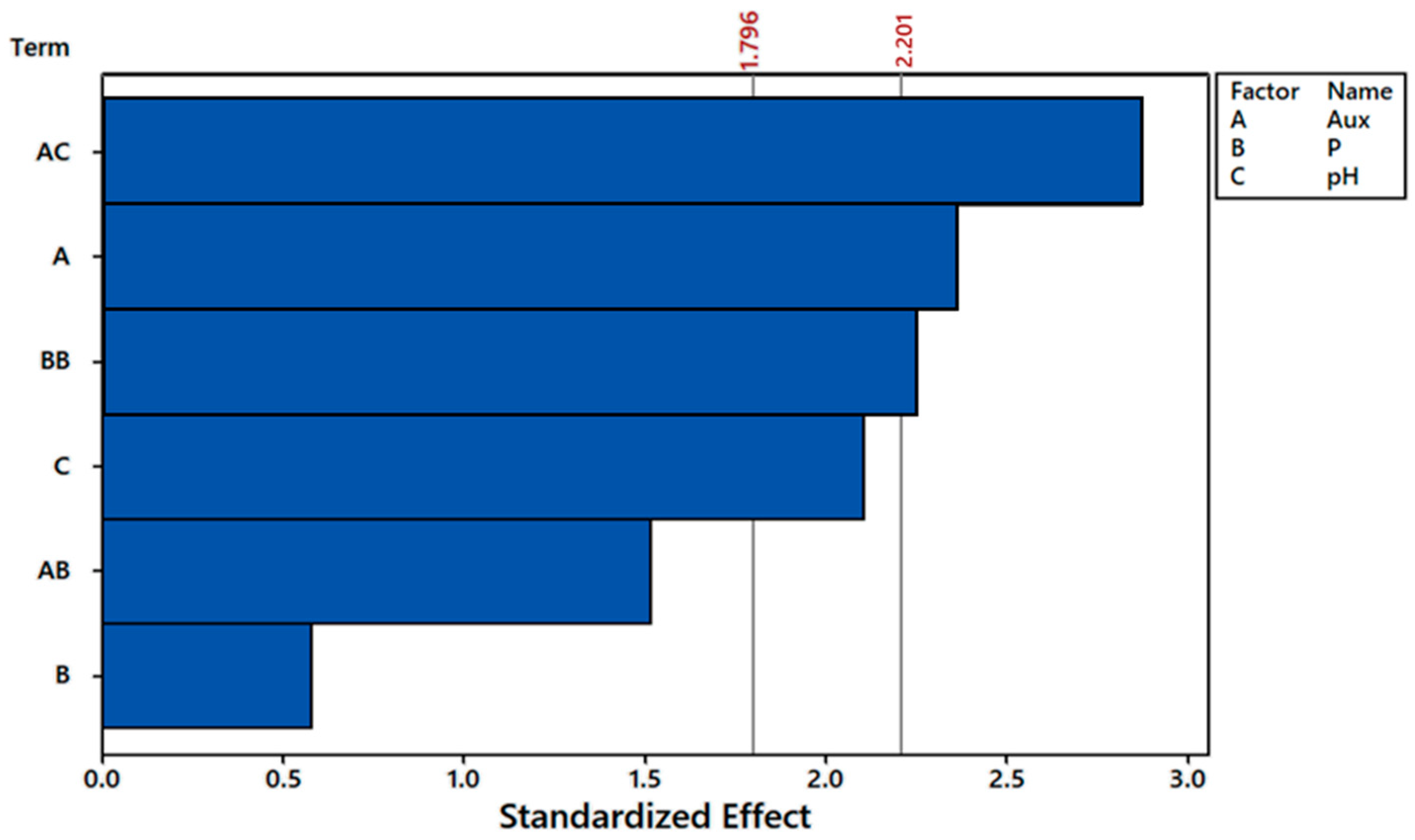
3.2. Central Composite Rotatable Design
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, N.E.; Death, C.E.; Coulson, G.; Newby, L.; Hufshmid, J. Interspecific variation in the diets of herbivores in an industrial environment: Implications for exposure to fluoride emissions. Environ. Sci. Pollut. Res. 2016, 23, 10165–10176. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.J.M.; Souza, P.R.; Silva, P.F.N.; Braga, R.O.; Guedes, E.M.S.; Lobato, A.K.S. Fluorine: Potentially toxic element for plants, animals and human beings. Rev. EDUCAmazônia-Educ. Soc. E Meio Ambiente 2013, 10, 78–92. (In Portuguese) [Google Scholar]
- Hem, J.D. The Study and Interpretation of the Chemical Characteristics of Natural Water, 3rd ed.; Paper 2254; U.S. Geological Survey Water-Supply: Alexandria, VA, USA, 1985; pp. 120–123. [Google Scholar]
- Oh, T.K.; Choi, B.; Shinogi, Y.; Chikushi, J. Effect of pH Conditions on Actual and Apparent Fluoride Adsorption by Biochar in Aqueous Phase. Water Air Soil Poll. 2012, 223, 3729–3738. [Google Scholar] [CrossRef]
- Cheng, K.K.; Chalmers, I.; Sheldon, T.A. Adding fluoride to water supplies. BMJ 2007, 335, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Rabb-Waytowich, D. Water Fluoridation in Canada: Past and Present. J. Can. Dent. Assoc. 2009, 75, 451–454. [Google Scholar] [PubMed]
- Abhilash, P.C.; Jamil, S.; Singh, N. Transgenic plants for enhanced biodegradation and phytoremediation of organic xenobiotics. Biotechnol. Adv. 2009, 27, 474–488. [Google Scholar] [CrossRef]
- Castillo-Valenzuela, J.; Martinez-Guerra, E.; Gude, V.G. Wetlands for Wastewater Treatment. Water Environ. Res. 2017, 89, 1063–1205. [Google Scholar] [CrossRef]
- Kaur, R.; Yadav, P.; Kohli, S.K.; Kumar, V.; Bakshi, P.; Mir, B.A.; Thukral, A.K.; Bhardwaj, R. Emerging trends and tools in transgenic plant technology for phytoremediation of toxic metals and metalloids. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids, 1st ed.; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 63–88. [Google Scholar]
- Pieterse, A.H.; Roorda, F.A.; Verhagen, L. Ten-fold enhancement of 2,4-D effect on water hyacinth by addition of gibberellic acid. Experientia 1979, 36, 650–651. [Google Scholar] [CrossRef]
- Trindade, C.R.T.; Pereira, S.A.; Albertoni, E.F.; Palma-Silva, C. Characterization and importance of aquatic macrophytes in limnic environments of Campus Carreiros—FURG, Rio Grande, RS. Cad. Ecol. Aquát. 2010, 5, 1–22. (In Portuguese) [Google Scholar]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2009. [Google Scholar]
- Boukhris, A.; Laffont-Schwob, I.; Mezghani, I.; Kadri, L.E.; Prudent, P.; Pricop, A.; Tatoni, T.; Chaieb, M. Screening biological traits and fluoride contents of native vegetations in arid environments to select efficiently fluoride-tolerant native plant species for in-situ phytoremediation. Chemosphere 2015, 119, 217–223. [Google Scholar] [CrossRef]
- Zavoda, J.; Cutright, T.; Szpak, J.; Fallon, E. Uptake, selectivity, and inhibition of hydroponic treatment of contaminants. J. Environ. Eng. 2001, 127, 502–508. [Google Scholar] [CrossRef]
- Karmakar, S.; Mukherjee, J.; Mukherjee, S. Removal of fluoride contamination in water by three aquatic plants. Int. J. Phytoremed. 2016, 18, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Khandare, R.V.; Desai, S.B.; Bhujbal, S.S.; Watharkar, A.D.; Biradar, S.P.; Pawar, P.; Govindwar, A.P. Phytoremediation of fluoride with garden ornamentals Nerium oleander, Portulaca oleracea, and Pogonatherum crinitum. Environ. Sci. Poll. Res. Int. 2017, 24, 6833–6839. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Nayak, A.K.; Sharma, Y.K. Fluoride toxicity effects in onion (Allium cepa L.) grown in contaminated soils. Chemosphere 2009, 76, 353–356. [Google Scholar] [CrossRef]
- Sant’anna-Santos, B.F.; Azevedo, A.A.; Alves, T.G.; Campos, N.V.; Oliva, M.A.; Valente, V.M.M. Effects of Emissions from an Aluminium Smelter in a Tree Tropical Species Sensitive to Fluoride. Water Air Soil Poll. 2014, 225, 1817. [Google Scholar] [CrossRef]
- Stevens, D.P.; Mclaughlin, M.J.; Alston, A.M. Phytotoxicity of the fluoride ion and its uptake from solution culture by Avena sativa and Lycopersicon esculentum. Plant Soil 1998, 200, 119–129. [Google Scholar] [CrossRef]
- Singh, T.P.; Bhatnagar, J.; Balomajumder, C. Defluoridation of industrial wastewater using Eichhornia crassipes. Int. J. Sci. Eng. Technol. 2015, 3, 753–756. [Google Scholar]
- Cassina, L.; Tassi, E.; Pedron, F.; Petruzzelli, G.; Ambrosini, P.; Barbafieri, M. Using a plant hormone and a thioligand to improve phytoremediation of Hg-contaminated soil from a petrochemical plant. J. Hazard. Mater. 2012, 231–232, 36–42. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Vieira, E.L.; Souza, G.S.; Santos, A.R.; Silva, J.S. Plant Physiology Manual, 1st ed.; Edufma: São Luis, Brazil, 2010. [Google Scholar]
- Du, R.J.; He, E.K.; Tang, Y.T.; Hu, P.J.; Ying, R.R.; Morel, J.L.; Qiu, R.L. How Phytohormone IAA and Chelator EDTA Affect Lead Uptake by Zn/Cd Hyperaccumulator Picris Divaricata. Int. J. Phytoremed. 2011, 13, 1024–1036. [Google Scholar] [CrossRef]
- Fässler, E.; Evangelou, M.W.; Robinson, B.H.; Schulin, R. Effects of indole-3-acetic acid (IAA) on sunflower growth and heavy metal uptake in combination with ethylene diamine disuccinic acid (EDDS). Chemosphere 2010, 80, 901–907. [Google Scholar] [CrossRef] [PubMed]
- López, M.L.; Peralta-Videa, J.R.; Benitez, T.; Gardea-Torresdey, J.L. Enhancement of lead uptake by alfalfa (Medicago sativa) using EDTA and a plant growth promoter. Chemosphere 2005, 61, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Mahmood, S.; Khan, M.Y.; Aslam, A.; Hussain, M.B.; Asghar, H.N.; Akhtar, M.J. Phytoremediation of Cadmium Contaminated Soil by Auxin Assisted Bacterial Inoculation. Asian J. Agric. Biol. 2013, 1, 79–84. [Google Scholar]
- Khan, A.L.; Waqas, M.; Hussain, J.; Al-Harrasi, A.; Hamayun, M.; Lee, I.J. Phytohormones enabled endophytic fungal symbiosis improve aluminum phytoextraction in tolerant Solanum lycopersicum: An example of Penicillium janthinellum LK5 and comparison with exogenous GA3. J. Hazard. Mater. 2015, 295, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roychoudhury, A. Fluorine: A biohazardous agent for plants and phytoremediation strategies for its removal from the environment. Biol. Plant. 2019, 63, 104–112. [Google Scholar] [CrossRef]
- Clark, R.B. Characterization of phosphatase of intact maize roots. J. Agric. Food Chem. 1975, 23, 458–460. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Kostyshin, S.S.; Perepelitsa, O.O.; Smetanyuk, O.I. Peculiarities of Fluoride Accumulation in the Plants of Meadow Biotopes of Northern Bukovyna. Contemp. Probl. Ecol. 2011, 4, 621–625. [Google Scholar] [CrossRef]
- Ruan, J.; Ma, L.; Shi, Y.; Han, W. The Impact of pH and Calcium on the Uptake of Fluoride by Tea Plants (Camellia sinensis L.). Ann. Bot. 2004, 93, 97–105. [Google Scholar] [CrossRef]
- Zouari, M.; Ahmed, C.B.; Fourati, R.; Delmail, D.; Rouina, B.B.; Labrousse, P.; Abdallah, F.B. Soil fluoride spiking effects on olive trees (Olea europaea L. cv. Chemlali). Ecotoxicol. Environ. Saf. 2014, 108, 78–83. [Google Scholar] [CrossRef]
- Camarena-Rangel, N.; Velázquez, A.N.R.; del Socorro Santos-Díaz, M. Fluoride bioaccumulation by hydroponic cultures of camellia (Camellia japonica spp.) and sugar cane (Saccharum officinarum spp.). Chemosphere 2015, 136, 56–62. [Google Scholar] [CrossRef]
- Karmakar, S.; Mukherjee, J.; Mukherjee, S. Biosorption of fluoride by water lettuce (Pistia stratiotes) from contaminated water. Int. J. Environ. Sci. Technol. 2018, 15, 801–810. [Google Scholar] [CrossRef]
- López, M.L.; Peralta-Videa, J.R.; Parsons, J.G.; Benitez, T.; Gardea-Torresdey, J.L. Gibberellic acid, kinetin, and the mixture indole-3-acetic acid-kinetin assisted with EDTA-induced lead hyperaccumulation in alfalfa plants. Environ. Sci. Technol. 2007, 41, 8165–8170. [Google Scholar] [CrossRef]
- Farooq, H.; Asghar, H.N.; Khan, M.Y.; Saleem, M.; Zahir, Z.A. Auxin-mediated growth of rice in cadmium-contaminated soil. Turk. J. Agric. For. 2015, 39, 272–276. [Google Scholar] [CrossRef]
- Cabello-Conejo, M.I.; Centofanti, T.; Kidd, P.S.; Prieto-Fernández, A.; Chaney, R.L. Evaluation of plant growth regulators to increase nickel phytoextraction by Alyssum species. Int. J. Phytoremed. 2013, 15, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.J.N. Phytohormonal Roles in Plant Responses to Heavy Metal Stress: Implications for Using Macrophytes in Phytoremediation of Aquatic Ecosystems. Environ. Toxicol. Chem. 2021, 40, 7–22. [Google Scholar] [CrossRef]
- Noor, J.; Ullah, A.; Saleem, M.H.; Tariq, A.; Ullah, S.; Waheed, A.; Okla, M.K.; Al-Hashimi, A.; Chen, Y.; Ahmed, Z.; et al. Effect of Jasmonic Acid Foliar Spray on the Morpho-Physiological Mechanism of Salt Stress Tolerance in Two Soybean Varieties (Glycine max L.). Plants 2022, 11, 651. [Google Scholar] [CrossRef]
- Arnao, M.B.; Ruiz, J.H. Role of Melatonin to Enhance Phytoremediation Capacity. Appl. Sci. 2019, 9, 5293. [Google Scholar] [CrossRef]
- Solomon, C.B.; Bradley, K.W. Influence of application timings and sublethal rates of synthetic auxin herbicides on soybean. Weed Technol. 2014, 28, 454–464. [Google Scholar] [CrossRef]
- Elobeid, M.; Polle, A. Interference of heavy metal toxicity with auxin physiology. In Metal Toxicity in Plants: Perception, Signaling and Remediation, 1st ed.; Gupta, D., Sandalio, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 249–259. [Google Scholar]
- Ordin, L.; Garber, M.J.; Skoe, B.P.; Rollé, G. Role of auxin in growth of inhibitor-treated oat coleoptile tissue. Physiol. Plant. 1966, 19, 937–945. [Google Scholar] [CrossRef]
- Javed, M.T.; Stoltz, E.; Lindberg, S.; Greger, M. Changes in pH and organic acids in mucilage of Eriophorum angustifolium roots after exposure to elevated concentrations of toxic elements. Environ. Sci. Poll. Res. Int. 2013, 20, 1876–1880. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Ma, L.; Ruan, J. Characterization of fluoride uptake by roots of tea plants (Camellia sinensis (L.) O. Kuntze). Plant Soil 2013, 366, 659–669. [Google Scholar] [CrossRef]
- Braga, A.F.; Borges, A.C.; Vaz, L.R.L.; Rosa, A. Phytoremediation of fluoride-contaminated water by Landoltia punctata. Eng. Agrícola 2021, 41, 171–180. [Google Scholar] [CrossRef]
- Stevens, D.P.; Mclaughlin, M.J.; Randall, P.J.; Keerthisinghe, G. Effect of fluoride supply on fluoride concentrations in five pasture species: Levels required to reach phytotoxic or potentially zootoxic concentrations in plant tissue. Plant Soil 2000, 227, 223–233. [Google Scholar] [CrossRef]
- Buwalda, F.; Warmenhoven, M. Growth-limiting phosphate nutrition suppresses nitrate accumulation in greenhouse lettuce. J. Exp. Bot. 1999, 50, 813–821. [Google Scholar] [CrossRef]
- Ren, B.; Wang, M.; Chen, Y.; Sun, G.; Li, Y.; Shen, Q.; Guo, S. Water absorption is affected by the nitrogen supply to rice plants. Plant Soil 2015, 396, 397–410. [Google Scholar] [CrossRef]
- Xian-Chen, Z.; Hong-Jian, G.; Zheng-Zhu, Z.; Xiao-Chun, W. Influences of different ion channel inhibitors on the absorption of fluoride in tea plants (Camellia sinesis L.). Plant Growth Regul. 2013, 69, 99–106. [Google Scholar] [CrossRef]
- Ekamparam, A.S.S.; Singh, A. Transformation of calcite to fluorapatite at room temperature: Impact of initial phosphate and fluoride levels. Geochim. Cosmochim. Acta 2020, 288, 16–35. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.; Zhang, Y.; Huang, K. Defluoridation efficiency assessment of spiny hierarchical-structured calcium hydroxyphosphate particles. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127219. [Google Scholar] [CrossRef]
- Yadav, M.; Kumari, N.; Sharma, V. Phytoremediation efficiency of Brassica juncea cultivars at vegetative and reproductive growth stages under individual and combined treatment of fluoride and aluminum. Int. J. Phytoremed. 2018, 20, 922–929. [Google Scholar] [CrossRef]
- Saini, P.; Khan, S.; Baunthiyal, M.; Sharma, V. Organ-wise accumulation of fluoride in Prosopis juliflora and its potential for phytoremediation of fluoride contaminated soil. Chemosphere 2012, 89, 633–635. [Google Scholar] [CrossRef]
- Sinha, S.; Saxena, R.; Singh, S. Fluoride Removal from Water by Hydrilla verticillata (l. f.) Royle and Its Toxic Effects. Bull. Environ. Contam. Toxicol. 2000, 65, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Gadi, B.R.; Kumar, R.; Goswami, B.; Rankawar, R.; Rao, S.R. Recent Developments in Understanding Fluoride Accumulation, Toxicity, and Tolerance Mechanisms in Plants: An Overview. J. Soil Sci. Plant Nutr. 2020, 21, 209–228. [Google Scholar] [CrossRef]
- Agarwal, R.; Chauhan, S.S. Bioaccumulation of sodium fluoride toxicity in plant parts at different phases and its impact on crop Hordeum vulgare (Barley) Varity RD 2052. Int. J. Multidiscip. Res. Dev. 2015, 2, 16–21. [Google Scholar]
- Baunthiyal, M.; Sharma, V. Response of Three Semi-Arid Plant Species to Fluoride; Consequences for Chlorophyll Florescence. Int. J. Phytoremed. 2014, 16, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Patra, P.K.; Mondal, B. Uptake of fluoride by two paddy (Oryza sativa L.) varieties treated with fluoride-contaminated water. Paddy Water Environ. 2013, 11, 619–623. [Google Scholar] [CrossRef]
- Wang, W.X. Bioaccumulation and Biomonitoring. In Marine Ecotoxicology; Blasco, J., Chapman, P.M., Campana, O., Hampel, M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2016; pp. 99–119. [Google Scholar]
- Rizzu, M.; Tanda, A.; Cappai, C.; Roggero, P.P.; Seddaiu, G. Impacts of soil and water fluoride contamination on the safety and productivity of food and feed crops: A systematic review. Sci. Total Environ. 2021, 787, 147650. [Google Scholar] [CrossRef]
- Santos-Díaz, M.S.; Zamora-Pedraza, C. Fluoride removal from water by plant species that are tolerant and highly tolerant to hydrogen fluoride. Fluoride 2010, 43, 150–156. [Google Scholar]





| Coded Levels | |||
|---|---|---|---|
| Variable | −1 | 0 | +1 |
| IAA (µM) | 0 | 5 | 10 |
| GA3 (µM) | 0 | 25 | 50 |
| pH | 5 | 7 | 9 |
| P (mg L−1) | 2.14 | 4.28 | 6.42 |
| F (mg L−1) | 5 | 10 | 15 |
| Coded Values | Decoded Values * | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | IAA | GA3 | pH | P | F | IAA | GA3 | pH | P | F |
| 1 | 0 | 1 | 1 | 1 | 1 | 5 | 50 | 9 | 6.42 | 15 |
| 2 | 0 | −1 | −1 | −1 | −1 | 5 | 0 | 5 | 2.14 | 5 |
| 3 | 1 | 0 | 1 | −1 | −1 | 10 | 25 | 9 | 2.14 | 5 |
| 4 | −1 | 0 | −1 | 1 | 1 | 0 | 25 | 5 | 6.42 | 15 |
| 5 | 1 | 1 | 0 | 1 | −1 | 10 | 50 | 7 | 6.42 | 5 |
| 6 | −1 | −1 | 0 | −1 | 1 | 0 | 0 | 7 | 2.14 | 15 |
| 7 | 1 | −1 | 1 | 0 | 1 | 10 | 0 | 9 | 4.28 | 15 |
| 8 | −1 | 1 | −1 | 0 | −1 | 0 | 50 | 5 | 4.28 | 5 |
| 9 | 1 | −1 | −1 | 1 | 0 | 10 | 0 | 5 | 6.42 | 10 |
| 10 | −1 | 1 | 1 | −1 | 0 | 0 | 50 | 9 | 2.14 | 10 |
| 11 | 1 | 1 | −1 | −1 | 1 | 10 | 50 | 5 | 2.14 | 15 |
| 12 | −1 | −1 | 1 | 1 | −1 | 0 | 0 | 9 | 6.42 | 5 |
| 13 | 0 | 0 | 0 | 0 | 0 | 5 | 25 | 7 | 4.28 | 10 |
| Coded Levels | |||||
|---|---|---|---|---|---|
| Variable | −1 | 0 | 1 | ||
| IAA (µM) | 0 | 0.51 | 1.25 | 1.99 | 2.50 |
| pH | 5 | 5.8 | 7 | 8.2 | 9 |
| P (mg L−1) | 1.00 | 2.82 | 5.50 | 8.18 | 10.00 |
| Coded Values | Decoded Values * | |||||
|---|---|---|---|---|---|---|
| Treatment | IAA | P | pH | IAA | P | pH |
| 1 | −1 | −1 | −1 | 0.51 | 2.82 | 5.81 |
| 2 | 1 | −1 | −1 | 1.99 | 2.82 | 5.81 |
| 3 | −1 | 1 | −1 | 0.51 | 8.18 | 5.81 |
| 4 | 1 | 1 | −1 | 1.99 | 8.18 | 5.81 |
| 5 | −1 | −1 | 1 | 0.51 | 2.82 | 8.19 |
| 6 | 1 | −1 | 1 | 1.99 | 2.82 | 8.19 |
| 7 | −1 | 1 | 1 | 0.51 | 8.18 | 8.19 |
| 8 | 1 | 1 | 1 | 1.99 | 8.18 | 8.19 |
| 9 | 0 | 0 | 0.00 | 5.50 | 7.00 | |
| 10 | 0 | 0 | 2.50 | 5.50 | 7.00 | |
| 11 | 0 | 0 | 1.25 | 1.00 | 7.00 | |
| 12 | 0 | 0 | 1.25 | 10.00 | 7.00 | |
| 13 | 0 | 0 | 1.25 | 5.50 | 5.00 | |
| 14 | 0 | 0 | 1.25 | 5.50 | 9.00 | |
| 15 | 0 | 0 | 0 | 1.25 | 5.50 | 7.00 |
| 16 | 0 | 0 | 0 | 1.25 | 5.50 | 7.00 |
| 17 | 0 | 0 | 0 | 1.25 | 5.50 | 7.00 |
| 18 | 0 | 0 | 0 | 1.25 | 5.50 | 7.00 |
| Treat. | IAA | GA3 | pH | P | F | (mg) | (%) | (mg g−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 50 | 9 | 6.42 | 15 | 16.47 (±0.96) * | 54.9 | 12.45 (±0.59) * |
| 2 | 5 | 0 | 5 | 2.14 | 5 | 5.73 (±0.24) | 57.3 | 1.96 (±0.07) |
| 3 | 10 | 25 | 9 | 2.14 | 5 | 5.73 (±0.63) | 57.2 | 0.98 (±0.05) |
| 4 | 0 | 25 | 5 | 6.42 | 15 | 16.49 (±1.40) | 54.9 | 13.86 (±0.96) |
| 5 | 10 | 50 | 7 | 6.42 | 5 | 5.88 (±0.40) | 58.8 | 1.20 (±0.06) |
| 6 | 0 | 0 | 7 | 2.14 | 15 | 18.90 (±1.20) | 63.0 | 6.72 (±0.35) |
| 7 | 10 | 0 | 9 | 4.28 | 15 | 18.44 (±0.85) | 61.5 | 5.54 (±0.21) |
| 8 | 0 | 50 | 5 | 4.28 | 5 | 6.17 (±0.48) | 61.7 | 1.74 (±0.11) |
| 9 | 10 | 0 | 5 | 6.42 | 10 | 12.37 (±0.31) | 61.8 | 5.79 (±0.15) |
| 10 | 0 | 50 | 9 | 2.14 | 10 | 12.51 (±0.83) | 62.5 | 9.27 (±0.50) |
| 11 | 10 | 50 | 5 | 2.14 | 15 | 18.40 (±0.88) | 61.3 | 5.22 (±0.29) |
| 12 | 0 | 0 | 9 | 6.42 | 5 | 5.95 (±0.80) | 59.5 | 8.88 (±0.39) |
| 13 | 5 | 25 | 7 | 4.28 | 10 | 11.89 (±0.29) | 59.4 | 2.73 (±0.08) |
| Treat. | IAA | pH | P | (mg) | (%) | (mg g−1) |
|---|---|---|---|---|---|---|
| 1 | 0.51 | 5.8 | 2.82 | 17.24 (±1.22) * | 57.5 | 4.03 (±0.28) * |
| 2 | 1.99 | 5.8 | 2.82 | 15.27 (±0.31) | 50.9 | 6.42 (±0.10) |
| 3 | 0.51 | 5.8 | 8.18 | 16.24 (±0.38) | 54.1 | 4.46 (±0.09) |
| 4 | 1.99 | 5.8 | 8.18 | 16.07 (±0.67) | 53.6 | 5.12 (±0.41) |
| 5 | 0.51 | 8.2 | 2.82 | 15.62 (±0.33) | 52.1 | 5.14 (±0.12) |
| 6 | 1.99 | 8.2 | 2.82 | 16.51 (±0.81) | 55.0 | 4.86 (±0.24) |
| 7 | 0.51 | 8.2 | 8.18 | 16.35 (±0.35) | 54.5 | 4.91 (±0.10) |
| 8 | 1.99 | 8.2 | 8.18 | 15.68 (±0.69) | 52.3 | 4.26 (±0.29) |
| 9 | 0.00 | 7.0 | 5.50 | 16.17 (±0.71) | 53.9 | 4.89 (±0.21) |
| 10 | 2.50 | 7.0 | 5.50 | 15.91 (±0.51) | 53.0 | 6.17 (±0.20) |
| 11 | 1.25 | 7.0 | 1.00 | 16.21 (±0.53) | 54.0 | 4.60 (±0.32) |
| 12 | 1.25 | 7.0 | 10.00 | 14.82 (±0.48) | 49.4 | 4.99 (±0.26) |
| 13 | 1.25 | 5.0 | 5.50 | 15.65 (±0.50) | 52.2 | 6.58 (±0.21) |
| 14 | 1.25 | 9.0 | 5.50 | 15.37 (±0.79) | 51.2 | 4.82 (±0.25) |
| 15 | 1.25 | 7.0 | 5.50 | 16.70 (±1.07) | 55.7 | 5.49 (±0.55) |
| 16 | 1.25 | 7.0 | 5.50 | 15.78 (±0.59) | 52.6 | 5.89 (±0.22) |
| 17 | 1.25 | 7.0 | 5.50 | 15.79 (±0.21) | 52.6 | 4.67 (±0.12) |
| 18 | 1.25 | 7.0 | 5.50 | 18.54 (±0.38) | 61.8 | 5.47 (±0.30) |
| Treatment | Aux | pH | P | BF | TF | ||
|---|---|---|---|---|---|---|---|
| 1 | 0.51 | 5.8 | 2.82 | 0.624 (±0.06) * | 0.498 (±0.02) * | 0.201 | 1.252 |
| 2 | 1.99 | 5.8 | 2.82 | 0.235 (±0.02) | 0.359 (±0.02) | 0.042 | 0.655 |
| 3 | 0.51 | 5.8 | 8.18 | 0.421 (±0.05) | 0.374 (±0.01) | 0.109 | 1.125 |
| 4 | 1.99 | 5.8 | 8.18 | 0.523 (±0.02) | 0.327 (±0.01) | 0.113 | 1.599 |
| 5 | 0.51 | 8.2 | 2.82 | 0.490 (±0.02) | 0.347 (±0.02) | 0.099 | 1.411 |
| 6 | 1.99 | 8.2 | 2.82 | 0.796 (±0.04) | 0.423 (±0.03) | 0.187 | 1.882 |
| 7 | 0.51 | 8.2 | 8.18 | 0.538 (±0.04) | 0.306 (±(0.01) | 0.120 | 1.756 |
| 8 | 1.99 | 8.2 | 8.18 | 0.326 (±0.03) | 0.344 (±0.02) | 0.085 | 0.946 |
| 9 | 0.00 | 7.0 | 5.50 | 0.513 (±0.04) | 0.415 (±0.02) | 0.119 | 1.237 |
| 10 | 2.50 | 7.0 | 5.50 | 0.529 (±0.08) | 0.374 (±0.01) | 0.091 | 1.413 |
| 11 | 1.25 | 7.0 | 1.00 | 0.518 (±0.06) | 0.479 (±0.01) | 0.130 | 1.080 |
| 12 | 1.25 | 7.0 | 10.00 | 0.377 (±0.05) | 0.396 (±0.02) | 0.074 | 0.952 |
| 13 | 1.25 | 5.0 | 5.50 | 0.775 (±0.04) | 0.326 (±0.02) | 0.116 | 2.374 |
| 14 | 1.25 | 9.0 | 5.50 | 0.521 (0.02) | 0.353 (±0.01) | 0.109 | 1.473 |
| 15 | 1.25 | 7.0 | 5.50 | 0.635 (±0.05) | 0.375 (±0.02) | 0.138 | 1.696 |
| 16 | 1.25 | 7.0 | 5.50 | 0.379 (±0.04) | 0.413 (±0.04) | 0.072 | 0.917 |
| 17 | 1.25 | 7.0 | 5.50 | 0.613 (±0.02) | 0.321 (±0.01) | 0.136 | 1.911 |
| 18 | 1.25 | 7.0 | 5.50 | 0.386 (±0.05) | 0.451 (±0.05) | 0.118 | 0.857 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaz, L.R.L.; Borges, A.C.; Ribeiro, D.M. Exogenous Auxin and Gibberellin on Fluoride Phytoremediation by Eichhornia crassipes. Plants 2023, 12, 1624. https://doi.org/10.3390/plants12081624
Vaz LRL, Borges AC, Ribeiro DM. Exogenous Auxin and Gibberellin on Fluoride Phytoremediation by Eichhornia crassipes. Plants. 2023; 12(8):1624. https://doi.org/10.3390/plants12081624
Chicago/Turabian StyleVaz, Lucas Rafael Lommez, Alisson Carraro Borges, and Dimas Mendes Ribeiro. 2023. "Exogenous Auxin and Gibberellin on Fluoride Phytoremediation by Eichhornia crassipes" Plants 12, no. 8: 1624. https://doi.org/10.3390/plants12081624
APA StyleVaz, L. R. L., Borges, A. C., & Ribeiro, D. M. (2023). Exogenous Auxin and Gibberellin on Fluoride Phytoremediation by Eichhornia crassipes. Plants, 12(8), 1624. https://doi.org/10.3390/plants12081624






