Abstract
This study investigated the beneficial properties of prickly pear peel (PPP) extracts from Opuntia ficus-indica (L.) Mill. Extracts were obtained via the Soxhlet extraction method using methanol (P1), ethanol (P2) and ethanol-water (P3) as extraction solvents. Their total phenolic and flavonoid content (TPC and TFC, respectively) and their antioxidant activity (AA) were determined. The PPP extracts were characterized in detail using mass spectrometry techniques. Their cyto-genotoxic effect and antigenotoxic potential against mitomycin C were evaluated via the cytokinesis block micronucleus (CBMN) assay on human lymphocytes. Enhanced TPC, TFC and AA values were recorded for all the extracts. Moreover, P1 and P2 were cytotoxic only at the highest concentrations, whereas P3 was found to be cytotoxic in all cases. No significant micronucleus induction was observed in the tested extracts. The PPP extracts contain bioactive compounds such as flavonoids, carboxylic acids, alkaloids, fatty acids and minerals (mainly K, Si, Mg, Ca, P and Zn). The results showed that all three extracts exerted high antigenotoxic activity. Our findings confirm the beneficial and genoprotective properties of PPP extracts and further studies on the bioactive compounds of Opuntia ficus-indica (L.) Mill. are recommended, as it constitutes a promising plant in pharmaceutical applications.
1. Introduction
Plants have been characterized as an important source of pharmaceutical products with therapeutic or prophylactic properties which are associated with anti-inflammatory, antioxidant, antibacterial, antigenotoxic and antiproliferative activities of their bioactive compounds [1,2,3,4]. Due to their beneficial properties, their use for various therapeutic purposes has increased tremendously over the last decades worldwide. Presently, many plants are used as a source of ingredients to prevent and/or treat a plethora of diseases [5]. Among them, Opuntia ficus-indica (L.) Mill., also known as prickly pear or cactus, belonging to the Cactaceae family and Opuntia genus, is of great interest due to its bioactive compounds and their potential valuable attributes [6,7].
Opuntia ficus-indica grows mainly in Latin America and Mexico, as well as South Africa and Mediterranean countries such as Spain and Greece [7,8,9,10]. The plant consists of cladodes, flowers, fruit and seeds and contains many biologically active substances including minerals, phenolic acids and flavonoids, [7,10,11,12]. In recent years there has been great interest in the fruit (prickly pear) of Opuntia ficus-indica due to its increased consumption fresh or as a processed product (e.g., juices and jams), which gives it a higher commercial value. Additionally, prickly pear is widely used in cosmetics, biofuel production and animal nutrition as well as for medical purposes [13,14,15,16]. Prickly pear fruit peel comprises a high percentage of the fruit ~45% to 50% and consequently an annual high quantity of waste generated and discarded due to its consumption and processing is inevitable. However, this kind of waste constitutes a significant source of high-added value products with many potential applications [11]. Thus, Opuntia ficus-indica waste seeds, resulting from the extraction of oil have been used for the manufacturing of active carbon for dye removal [17], as a sustainable lignocellulosic source [18] and to partially replace phenol formaldehyde resins to produce plywood composites [19]. Moreover, Opuntia ficus-indica fruit peels are deemed an exceptional carbon source for the production of lactic acid [20].
It is well known that peels and pomace of fruits constitute primary waste of the agri-food industry with high content of phenolic compounds [21]. According to Lizárraga-Velázquez et al. [22], plant wastes such as prickly pear peel represent a significant and low-cost source of antioxidants (terpenes, phenolic compounds, phytosterols) with potential applications as pharmaceutical products due to the antidiabetic, antihypertensive, anticancer and antibacterial properties they may possess [23,24]. Cladode extract of Opuntia ficus-indica induced antimicrobial activity against Gram-negative and Gram-positive bacteria, in addition to exerting antibiofilm activity against Staphylococcus aureus [25]. Prickly pear seed oil had a similar action against bacteria while displaying antifungal activity against Saccharomyces cerevisiae and Candida albicans [26]. In addition, prickly pear peel extract was found to possess significant potential against pneumonia pathogens [11]. Chronic inflammation induced in mice was mediated by the anti-inflammatory activity exhibited by the methanolic extract of prickly pear stem and was ascribed mainly to β-sitosterol [27]. Moreover, inflammatory response caused by alcohol consumption was attenuated by prickly pear juice [28]. The hepatoprotective effect of Opuntia ficus-indica cladode extract against lithium poisoning in rats was demonstrated by a significant increase of the hepatic catalase, superoxide dismutase and glutathione peroxidase activities [29]. Similarly, polysaccharides from prickly pears showed a liver protective action against organophosphorus pesticides [30]. Opuntia ficus-indica f. inermis juice had a hepatoprotective effect which was attributed to increased antioxidant activity [31]. The cardioprotective potential of Opuntia ficus-indica powdered cladodes was demonstrated against the development of atherosclerotic lesions in apoE-KO mice by Garoby-Salom et al. [32]. A rapid increase of HDL cholesterol levels and simultaneous decrease of LDL and triglycerides in women with metabolic syndrome was reported after consumption of prickly pear dried leaves [33]. Different parts of prickly pear have been tested for their chemopreventive and antigenotoxic potential. Namely, Helal et al. [34] recorded a concentration-dependent inhibition of the proliferation of lung (A549) and colon (Caco2) cancer cells following treatment with prickly pear extract. A similar pattern was observed against liver cancer cells (HepG2), colorectal adenocarcinoma (Caco-2) and breast cancer cell line (MCF-7), after treatment with increasing concentrations of prickly pear extract [35]. Furthermore, methanol extract of Opuntia ficus-indica peel exerted antiproliferative activity against breast (MDA-MB-231) and liver (HepG2) cancer cell lines [36]. According to the results of several studies, the antigenotoxic activity of prickly pear parts is attributed to their antioxidant capacity, the inhibition of cell proliferation and induction of apoptosis, their pro-oxidant activity and lipid peroxidation modulation, among others ([37] and references therein).
Considering that prickly pear peel extracts could constitute a considerable natural product for medicinal applications, the present study focused on an investigation of the potential beneficial effects of Opuntia ficus-indica by determining the antioxidant, cytotoxic and antigenotoxic effects of three different extracts from the peel of prickly pear fruit. All the extracts were also fully characterized using a combination of mass spectrometry techniques. To the best of the authors’ knowledge, this is the first study assessing the cyto-genotoxic potential of prickly pear peel extracts against mitomycin C (MMC) in cultured human lymphocytes, via a CBMN assay.
2. Results
2.1. Extraction Yields
The overall Soxhlet yields were obtained over a 6 h period with different solvents (Table 1). The highest extraction yields were for the P1 and P3 extracts with percentages of 66.1 ± 1.4 and 61.2 ± 3.8 % wt. The P2 extract had the lowest yield with a value of 35.3 ± 0.8 % wt.

Table 1.
Soxhlet extraction yields of prickly pear peel using different solvents. The values designated by the different letters are significantly different (Bonferroni test, p < 0.05).
2.2. TPC, TFC and AA
2.2.1. TPC and TFC
The P3 extract showed the highest TPC value (27.46 ± 2.35 mg GAE g−1), followed by the P1 extract (22.68 ± 2.21 mg GAE g−1) (Table 2), whereas the P3 extract had the lowest TPC value (15.70 ± 0.80 mg GAE g−1). As far as TFC is concerned, the highest value was recorded for the P2 extract (2.73 ± 0.06 mg CE g−1), followed by the P3 (1.77 ± 0.17 mg CE g−1) and P1 (1.70 ± 0.07 mg CE g−1) extracts.

Table 2.
Total phenolic content (TPC) and total flavonoid content (TFC) of prickly pear peel extracts with different solvents. The values designated by the different letters are significantly different (Bonferroni test, p < 0.05).
2.2.2. Antioxidant Activity (AA)
To identify the AA of all extracts, ABTS, DPPH and FRAP assays were used (Table 3). The P3 extract had the most pronounced AA in the ABTS assay. The P2 and P3 extracts demonstrated higher AA in DPPH and FRAP assays compared to P1, with the P2 extract showing the highest values.

Table 3.
Antioxidant activities (AA) of prickly pear peel extracts via ABTS, DPPH, and FRAP assays. The values designated by the different letters are significantly different (Bonferroni test, p < 0.05).
2.3. Characterization of Prickly Pear Peel Extracts by ICP-MS/MS, GC-MS and UHPLC-MS
In the present study, various minerals/elements (Mg, Al, Si, K, Ca, P, Mn, Fe, Cu and Zn) of prickly pear peel were determined and then quantified for all three extracts by ICP-MS/MS analysis. Table 4 shows the results of Mg, Al, Si, K, Ca, P, Mn, Fe, Cu and Zn contents determined by ICP-MS/MS in all extracts. In all cases K and Si had the highest concentrations, with values ranging from 11,423.07 to 25,071.58 μg g−1 (P1–P3) and 1337.33 to 1452.05 μg g−1 (P1–P3), respectively. In addition, high levels of Mg, Ca, P and Zn, but lower levels of Al, Mn, Fe and Cu were detected in all extracts.

Table 4.
Concentrations of minerals/elements in P1, P2 and P3 extracts.
Both GC-MS and UHPLC-MS analyses of the extracts (P1, P2 and P3) were performed and 11 compounds (Table 5) were identified in all cases.

Table 5.
Compounds identified by GC-MS and UHPLC-MS in P1, P2 and P3 extracts.
In the case of the GC-MS analysis, the compounds that were identified were (a) trans-cinnamic acid, (b) debrisoquine and (c) n-hexadecanoic acid (palmitic acid). The acquired spectra (Figure S1) were compared with the existing ones in the NIST library. With regard to the UHPLC-MS analysis, eight compounds were detected (tri-glycosylated kaempferol; tri-glycosylated methyl-quercetin derivative I; tri-glycosylated methyl-quercetin derivative II; tri-glycosylated quercetin I; tri-glycosylated quercetin II; di-glycosylated quercetin (rutin); di-glycosylated methyl-quercetin I; di-glycosylated methyl-quercetin I) and their structural assignment was conducted in accordance with the formation of molecular mass ions ([M-H]−) and their fragments (where available) following comparison to data already reported in past research [40]. Figure S2 depicts the mass spectra of the identified compounds identified by UHPLC-MS. According to the results, flavonoids, carboxylic acids, alkaloids, and fatty acids were mainly present.
2.4. CBMN Assay in Human Lymphocytes
The cyto-genotoxic potential of all prickly pear peel extracts was assessed at three concentrations (10, 100 and 200 μg mL−1). Afterwards, their capability to diminish MMC-mediated detrimental effects on human lymphocytes was investigated (Figure 1 and Figure 2). The cytotoxicity of the extracts in the presence and absence of MMC was determined through CBPI (Figure 1).
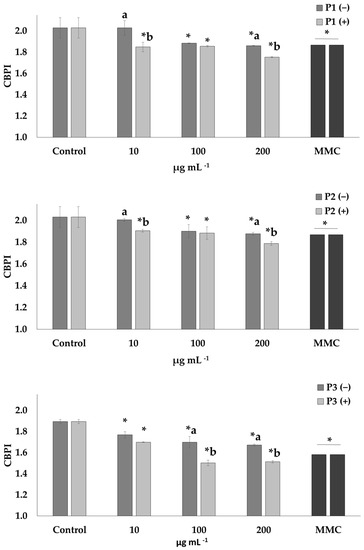
Figure 1.
Cytotoxic activity (CBPI values) of P1, P2 and P3 extracts of prickly pear peel in human lymphocytes in the presence (+) and absence (−) of mitomycin C (MMC, 0.5 μg mL−1). Values with asterisk (*) significantly differ from the control. The values designated by the different letters are significantly different (Mann–Whitney U-test, p < 0.05).
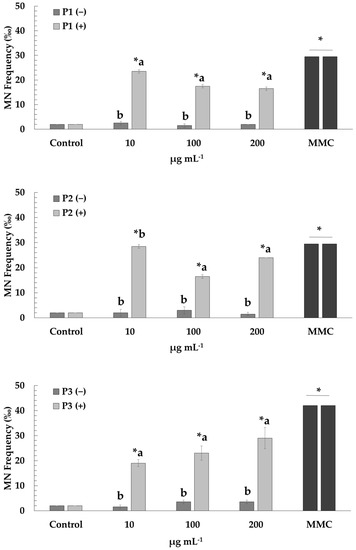
Figure 2.
Micronuclei induction in human lymphocytes after treatment with P1, P2 and P3 extracts of prickly pear peel in the presence (+) and absence (−) of mitomycin C (MMC, 0.5 μg mL−1). Values with asterisk (*) significantly differ from the control. The values designated by the different letters are significantly different (Mann–Whitney U-test, p < 0.05).
The P1 and P2 extracts were cytotoxic at the highest concentrations (100 and 200 μg mL−1), while for P3, higher rates of cytotoxicity were observed compared to the other two extracts at all tested concentrations (10–200 μg mL−1). Cells treated with MMC, in the presence and absence of the extracts, exhibited cytotoxic potential in all the concentrations (10–200 μg mL−1).
Regarding the genotoxic activity of the extracts (P1, P2 and P3), low frequencies of MN formation were observed at all tested concentrations (10–200 μg mL−1), compared to those that occurred in the control culture, thus indicating lack of genotoxicity. However, cells treated with MMC showed increased MN frequencies as expected, confirming its genotoxic action. Mixtures of MMC with P1 and P3 extracts led to the diminution of MMC-mediated genotoxic effects at all concentrations (10–200 μg mL−1). A similar pattern was reported for mixtures of MMC with P2 extract at the two highest concentrations (100 and 200 μg mL−1) (Figure 2).
3. Discussion
3.1. TPC, TFC and AA of Extracts
In the present study, extracts from prickly pear peel using three different solvents (methanol, ethanol, and ethanol-water; ratio 4:1) were prepared using the Soxhlet method. The evaluation of the yields of the extracts in relation to the used solvents, revealed that methanol and ethanol-water led to the highest yields compared to ethanol, and the results are in accordance with the yields observed for the same solvents in previous studies [41,42]. Other factors that affect the extraction yield and can be considered are geographical origin, harvest season and fruit ripeness [43,44,45].
All the studied extracts exhibited high TPC and TFC values. Regarding TPC content the following order P3 > P1 > P2 was observed. The observed values are in accordance with the reported values for the respective solvents as stated by Abou-Elella and Ali [42]. These findings indicate that phenolic compounds are often extracted in higher amounts in more polar solvents such as a mixture of water-ethanol compared with methanol or ethanol [46,47,48]. Our results regarding TFC contradict those observed by Abou-Elella and Ali [42] as a higher flavonoid content was found when EtOH was used as solvent (P2 > P3 > P1, EtOH > EtOH-W > MeOH, respectively).
In our efforts to evaluate the antioxidant activity (AA) of all the obtained extracts in this study we implemented the ABTS, DPPH and FRAP assays. The obtained results reveal that the highest AA values were observed in P2 and P3 extracts, followed by P1 extract, following the same pattern as the findings of Abou-Elella and Ali, [42]. In the case of ABTS, the highest antioxidant activity was induced by the P3 extract. However, P2 had the highest AA at the DPPH and FRAP assays. Τhis could be attributed to certain variations among the methods used such as the different absorbance of the radicals. The maximum absorbance of the ABTS radical is at 734 nm in an ethanolic medium, DPPH has an absorbance peak at 517 nm in a methanol solution and FRAP at 593 nm in a sodium acetate buffer solution [49,50,51]. The free radical scavenging activities of the extracts depend on the capacity of the antioxidant compounds that they contain, such as flavonoids and polyphenols [52]. Various studies in the literature report high TPC and TFC values as well as significant antioxidant activity of the different extracts of prickly pear [7,53,54,55,56,57].
The observed differences between TPC, TFC and AA values can be attributed to various factors related to the geographical origin of the plant as well as the microclimate and environmental conditions of the area. In addition, the experimental procedures and conditions applied during the extraction process in order to produce the extracts may affect the distribution of the TPC, TFC and AA values [41,58,59,60].
3.2. Cyto-Genotoxic Potential of the Extracts
The cytotoxic and genotoxic potential of prickly pear peel extracts (P1, P2 and P3) was evaluated via the CBMN assay.
The observed results on the potential cytotoxic activity of the studied extracts showed a slight decrease in CBPI values at the highest concentrations (100 and 200 μg mL−1) of P1 and P2 extracts, a fact that demonstrates a mild cytotoxic effect. In contrast, a high decrease in CBPI values was observed at all tested concentrations (10–200 μg mL−1) of P3. Despite the absence of data on the cytotoxicity of prickly pear peel, the high cytotoxicity could be explained by the individual compounds of extracts such as total phenolics, tannins and betalains (i.e., betacyanins and betaxanthins). According to Abou-Elella and Ali [42], the ethanol, methanol and ethanol-water (80:20) extracts of prickly pear peel are rich in polyphenols, tannins and betalains (betacyanins and betaxanthins) and the highest amounts of tannins and betalains were found in the ethanol-water extract. Studies on different cell lines such as a human chronic myeloid leukemia cell line (K562) and HepG2 cells demonstrated that betalains can induce apoptotic and cytotoxic effects, respectively [61,62]. Furthermore, tannins demonstrated cytotoxic effects in human lymphocytes [63] and extracts with high quantities of polyphenols have been reported to induce cytotoxic activity on human and mouse tumor cell lines [64,65].
Our data clearly indicate that treatment with different concentrations of prickly pear peel extracts (P1, P2 and P3) does not induce genotoxic effects (in terms of MN formation) in cultured human lymphocytes. Similar results in in vivo experiments in mice and rats, regarding the absence of genotoxicity, have been reported for extracts from other parts (cladodes and stems) of the Opuntia ficus-indica plant which is in line with our findings [6,66,67]. In addition, Madrigal-Santillán et al. [68] reported that treatment with prickly pear juice did not show any genotoxic potential in mice (strain NIH).
3.3. Cytoprotective and Antigenotoxic Effects of Prickly Pear Peel Extracts against Mitomycin C (MMC)
Considering that the compounds contained in plant extracts may reduce the damage caused by mutagens, the possible antigenotoxic/antimutagenic effects of prickly pear peel extracts against mitomycin C (MMC) were examined in human lymphocytes.
When applying a CBMN assay, MMC is recommended as a positive control by OECD protocol [69]. Mitomycin C can influence DNA synthesis by binding complementary helices found within it [70,71]. Furthermore, free radicals formation and the ability of MMC to alkylate guanine residues, induce MN formation [72,73], the presence of which is closely linked to tumor progression and carcinogenesis [74].
A significant increase in MN formation was induced by MMC as compared to the control, and the observed results are in accordance with previous studies [41,70,75,76,77]. A significant decrease of the genotoxic impact of MMC is reported for the first time, in the presence of P1 (concentrations, 10–200 μg mL−1), P2 (concentrations, 100 and 200 μg mL−1) and P3 (concentrations, 10–200 μg mL−1) prickly pear peel extracts, with the highest antigenotoxic rates observed in P1 and P3 extracts. The effectiveness of the specific extracts against MMC-mediated genotoxicity could be due to the presence and action of various beneficial compounds, such as flavonoids, phenolic acid and minerals, among others. In addition, the higher TPC values exhibited by the P1 and P3 extracts could partially explain their higher antigenotoxic activity as the beneficial properties of the extracts are linked to the presence of secondary metabolites. Despite the absence of data on the antigenotoxicity of prickly pear peel, there are few studies on the potential antigenotoxic activities of extracts from different parts of Opuntia ficus-indica against well-known genotoxic and/or mutagenic agents. Siriwardhana et al. [78] applying the comet assay, showed that a prickly pear fruit extract reduced the H2O2-induced DNA damage in human peripheral lymphocytes and the observed reduction could be associated with the extract constituents (mainly flavonoids and betalains). In vivo experiments in mice clearly demonstrated the protective and antigenotoxic activity of Opuntia ficus-indica cladode extracts against Aflatoxin B1 and the mycotoxin Zearalenone, probably due to their ability to promote the antioxidant defense system and inhibit the oxidative process induced by the aforementioned genotoxic/mutagenic agents [66,67].
This pattern could be associated with the chemical composition of the extracts and mainly the identified phenolic compounds and flavonoids. The main components were found to be n-hexadecanoic acid (palmitic acid), trans-cinnamic acid, debrisoquine, tri-glycosylated kaempferol, tri-glycosylated methyl-quercetin derivative I, tri-glycosylated methyl-quercetin derivative II, tri-glycosylated quercetin I, tri-glycosylated quercetin II, di-glycosylated quercetin (rutin), di-glycosylated methyl-quercetin I, and di-glycosylated methyl-quercetin II. Phenolic compounds and flavonoids have been reported to possess antioxidant, anti-inflammatory, anticancer and hepatoprotective activities [79].
Barcelos et al. [80] demonstrated that flavonoid quercetin and its derivative rutin cannot induce genotoxic effects in the examined concentrations. On the other hand, both flavonoids reduced DNA damage induced by genotoxic/mutagenic agents in HepG2 cells using the comet assay. The previous study confirmed the findings by Ramos et al. [81] about the antiproliferative activity of quercetin in the same cell line against tert-butyl hydroperoxide (t-BHP). Kaempferol was able to modulate and decrease the cytotoxic and genotoxic/clastogenic effect of zeocin in human lymphocytes [82] and to exert inhibitory activity against genotoxic/mutagenic agents via the SOS chromotest bacterial assay system in the presence of Escherichia coli PQ37 strain [83]. Prickly pear peel extracts also contain palmitic acid known for its antimutagenic/anticlastogenic properties [84].
Micronutrients are critical for DNA stability as well as in cellular processes. Certain micronutrients such as the minerals which were detected in the studied extracts (i.e., Mg, Si, Al, K, Ca, P, Mn, Fe, Cu and Zn) have been reported to play a key role in cellular processes. Moreover, the interactions and synergistic effects among the various components that coexist in natural products play a fundamental role to their potential positive impact ([41,70,77,85,86] and references therein).
4. Materials and Methods
Prickly pear peel extracts were primarily prepared using different extraction solvents (methanol, ethanol, and ethanol-water ratio 4:1) by the Soxhlet method, widely used for the extraction of bioactive compounds from natural products, as it leads to high extraction yields and bioactivity of the extracted molecules [87,88]. The DPPH, FRAP and ABTS tests were used to determine the total phenolic and flavonoid content (TPC and TFC) and antioxidant activity (AA) of the obtained extracts. Afterwards, the cyto-genotoxic potential of each extract, in addition to the cyto/genoprotective activity against mitomycin C (MMC) were investigated in cultured human lymphocytes, via the cytokinesis block micronucleus (CBMN) assay, which is a broadly applied and reliable method for the assessment of the cyto-genotoxic profile of chemicals [41,75,76,77,89]. The MMC is isolated from Streptomyces caespitosus and has been used as a chemotherapeutic agent as well as a genotoxic inducer in a plethora of antigenotoxicity studies [41,69,75,76,77,85].
4.1. Chemicals and Reagents
Ham’s F-10 medium, foetal bovine serum (FBS), phytohemagglutinin (PHA) and L-glutamine were purchased from Gibco, (Fisher Scientific, Loughborough, Leicestershire, UK Ltd.). Mitomycin C (MMC) and Giemsa were commercially procured from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) and cytochalasin-B (Cyt-B) from Santa Cruz Biotechnology (Heidelberg, Germany). The remaining solvents and chemicals used were of the highest grade commercially available. All stocks of the compounds and solutions were stored at 4 °C until use. The primary stocks of the tested compounds were utilized for the treatments.
4.2. Sample Preparation
The harvest of the Opuntia ficus-indica fruit took place at the Aitoloakarnania region, Western Greece (coordinates: 38°24′2″ N 21°50′8″ E) in September 2020, where the ambient temperature was between 30 to 35 °C and the fruit was at physiological maturity. After harvesting, the fresh fruits were transferred to the laboratory and plant identification carried out. The Department of Biology Herbarium at the University of Patras possesses the voucher specimen of Opuntia ficus-indica (39988 UPA-Herbarium) which was deposited there for reference purposes.
The fruits were washed under tap water with a brush for 2 min and the peels from the fruits were removed manually [7,90]. The peels were cut into small pieces and dried in a forced air oven at 60 °C for 24 h [91,92]. After drying, peel was ground in a domestic food processor for 10 s to determine the average particle size. This was accomplished in a vertical vibrating sieve shaker using Tyler series sieves with apertures of 8, 10, 20, 28, 32 and 35 mesh [93]. The average particle size (PS) obtained was 0.57 ± 0.02 mm. The raw material was packed in plastic bags and stored at −20 °C until use.
Soxhlet Extraction
Soxhlet extraction was applied according to the method 920.39 of AOAC [94] and the procedure followed has been previously described [41] (see Section S4.2)
4.3. Determination of TPC, TFC, and AA
4.3.1. Preparation of Samples
A volume of 0.8 mL of methanol:H2O (80:20 v/v) was added to 0.2 mL of each extract (P1–P3) and the solutions were vigorously agitated for 5 min before centrifugation (3000 rpm, 10 min). The supernatant was used to determine the total phenolic content (TPC), total flavonoid content (TFC) and the antioxidant activity (AA). All samples were prepared and analyzed in triplicate.
4.3.2. Total Phenolic and Flavonoid Content (TPC and TFC) and Antioxidant Capacity (AA) Determination
The experimental process for the determination of total phenolic and flavonoid content in all extracts (P1–P3) was performed according to Dormousoglou et al. [41] (see Sections S4.3.1 and S4.3.2). Accordingly, the antioxidant activity (AA) was performed through three methods, using the extract solubilized in a methanol-water solution (80% v/v) as previously described [41] (see Section S4.3.3).
4.4. Characterization of Prickly Pear Peel Extracts by ICP-MS/MS, GC-MS and UHPLC-MS
A thorough characterization of all the extracts (P1–P3) was conducted using mass spectrometry techniques, i.e., ICP-MS/MS, GC-MS and UHPLC-MS.
4.4.1. ICP-MS/MS Analysis
An Agilent 8900 Triple Quadrupole ICP-MS/MS (Agilent Technologies, Tokyo, Japan) was used for the detection of the minerals/elements of P1, P2 and P3 extracts according to the testing protocols of ISO 17294 [95,96]. Their quantification was based on calibration curves (R2 > 0.999) prepared by an analysis of calibration standards. Before analysis, preparation of the samples took place in an acid matrix (2.5% v/v HNO3 and 0.5% v/v HCl). Minerals concentration was calculated and expressed as dry weight in μg g−1 [97].
4.4.2. GC-MS Analysis
A volume of 2 μL of each extract was injected in splitless mode and analyzed by GC-MS (Agilent 5975B MS coupled to an Agilent 6890N GC) using an Agilent 19091S-433 HP-5MS (5-% phenyl methyl siloxane) of 30 m length, 250 μm diameter and 0.25 μm film thickness analytical column. The appropriate parameters were implemented (see Section S4.4.1).
4.4.3. UHPLC/MS Analysis
An Ultimate 3000 RSLC System (Thermo Fisher Scientific, Waltham, MA, USA) coupled to an amaZon SL ion trap mass spectrometer (Bruker, Bremen, Germany) with an ESI source was employed for further analysis of the extracts, with an injection volume of 5 μL. Appropriate parameters were applied (see Section S4.4.2).
4.5. CBMN Assay in Human Lymphocytes In Vitro
4.5.1. Ethics Statement
The specific research received approval from the Research Ethics Committee (REC) of the University of Patras (UPAT) (Ref. No. 11584/6 March 2018). Blood samples were acquired from two healthy non-smoking male donors (20 and 25 years old), who declared that they had not been recently exposed to radiation, drug treatment or any viral infection. Thereafter, samples were handled and treated appropriately for conducting the CBMN assay.
4.5.2. CBMN Assay Application
The cytotoxic, genotoxic and antigenotoxic potential of prickly pear peel extracts (P1–P3) in human lymphocytes was determined by the in vitro cytokinesis block micronucleus (CBMN) assay using cytochalasin-B (see Section S4.5.1, Figure S3), according to standard procedures [69].
Cytotoxicity was determined using the cytokinesis block proliferation index (CBPI), by counting 1000 cells for each experimental point and using the equation below:
where N1, N2, N3 and N4 represent the numbers of cells with one, two, three and four nuclei, while N is the total number of cells [98].
CBPI = [N1 + N2 + 3(N3 + N4)]/N,
4.6. Statistical Analysis
All data are expressed as mean ± standard deviation of three independent experiments in each case. The data sets of the extraction yields TPC, TFC, ABTS, DPPH, FRAP, observed in each extract (P1–P3), as well as the CBPI and MN frequency values observed in challenged cells after exposure to different concentrations of each extract (P1–P3), were checked for homogeneity of variance (Levene’s test of equality of error variances) and assumptions of normality (Shapiro-Wilk W Test) via the SPSS 25 (IBM Inc., Armonk, NY, USA, 2019) software package. Thereafter, a one-way ANOVA was performed to determine statistical variance among groups for each chemical parameter tested, followed by a post hoc analysis (Bonferroni test) to estimate statistically significant differences among extracts in each case. Regarding the CBPI and MN frequency values observed in challenged cells, statistical variance among groups (MMC-treated and MMC-free cells, treated with different concentrations of P1–P3) were assessed non-parametrically via the Kruskal-Wallis test, while statistically significant differences among MMC-treated and MMC-free cells were evaluated by the use of the Mann–Whitney U-test. Significant levels were established as p < 0.05 in all cases.
5. Conclusions
Τhe results of the present study reveal significant findings on the antioxidant, cytotoxic, genotoxic and antigenotoxic profile, from three extracts of prickly pear peel. According to the results, all extracts (P1, P2 and P3) exhibited satisfactory and relatively high content of phenolics and flavonoids, while the highest AA values were observed in P2 and P3 extracts, followed by P1 extract. The significant antigenotoxic potential demonstrated by all the studied extracts (P1, P2 and P3) was verified for the first time. All the extracts induced cytotoxic effects with P3 inducing the highest cytotoxicity. The chemical composition of the extracts and mostly the presence of phenolic compounds and flavonoids could potentially lead to the observed properties. Simultaneously, the valorization of prickly pear’s peel for the formation of products with beneficial properties is highlighted. Considering the antioxidant potential and the remarkable protective effects of prickly pear peel extracts against the mutagenic agent MMC, the opportunity arises for their further assessment and implementation in pharmaceutical products and medicinal applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12071537/s1, Figure S1: Mass spectra obtained by GC-MS analysis of (a) trans-cinnamic acid, (b) debrisoquine, (c) n-hexadecanoic acid (palmitic acid). Figure S2: Mass spectra obtained by UHPLC-MS in negative ionization mode of (a) tri-glycosylated kaempferol (b) tri-glycosylated methyl-quercetin derivative I (c) tri-glycosylated methyl-quercetin derivative II (d)tri-glycosylated quercetin I (e)tri-glycosylated quercetin II (f) di-glycosylated quercetin (rutin), (g) di-glycosylated methyl-quercetin I, (h) di-glycosylated methyl-quercetin I. Figure S3: Schematic representation of CBMN assay. Refs. [49,50,51,99,100] cited in Supplementary Materials.
Author Contributions
Conceptualization, D.V., M.A. and S.D.; Methodology, D.V., S.D., M.A., M.D., I.E. and G.H.; Software, M.D. and S.D.; Validation, D.V., S.D. and M.A.; Formal Analysis, D.V., S.D. and M.A.; Investigation, M.D., I.E. and G.H.; Resources, D.V. and M.A. Data Curation, D.V., M.A. and S.D.; Writing—Original Draft Preparation, I.E., M.D., D.V., M.A. and S.D.; Writing—Review and Editing, D.V., M.A., S.D. and I.E.; Visualization, D.V., M.A. and S.D.; Supervision, D.V. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The experimental usage of human lymphocytes was carried out in accordance with international bioethics criteria, after permission/approval by the Research Ethics Committee of the University of Patras (Ref. No. 11584/6 March 2018).
Informed Consent Statement
After obtaining written informed consent, two healthy non-smoking male individuals (younger than 30 years) who were not exposed to radiation, not under any drug treatment and had no viral infections in the recent past, were used as blood donors to establish whole blood lymphocyte cultures.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The present work was supported in part by the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No. 778168. Margarita Dormousoglou was supported by a PhD scholarship from the Andreas Mentzelopoulos Foundation. The authors would like to thank the Laboratory of Instrumental Analysis of the University of Patras for GC-MS and UHPLC-MS analysis and Akrokeramos Sewerage Laboratory of Athens Water Supply and Sewerage Company (EYDAP SA) for ICP-MS/MS analysis. The authors would like to thank in particular the partners of the European Union’s Horizon 2020 research and innovation programme: Maria Papadaki, Marcos L. Corazza and Samir Santzouk for their productive cooperation. M.D. and I.E. would like to thank (a) Marcos L. Corazza for his kind hospitality in his lab at Federal University of Parana in Brazil and (b) Maria Papadaki for the total support and the opportunity given to visit the Federal University of Parana in Brazil.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Majolo, F.; Bitencourt, S.; Monteiro, B.W.; Haute, G.V.; Alves, C.; Silva, J.; Pinteus, S.; Santos, R.C.V.; Torquato, H.F.V.; Paredes-Gamero, E.J.; et al. Antimicrobial and antileukemic effects: In vitro activity of Calyptranthes grandifolia aqueous leaf extract. J. Toxicol. Environ. Health Part A 2020, 83, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Nunes, H.L.; Tuttis, K.; Serpeloni, J.M.; Nascimento, J.R.D.; da Rocha, C.Q.; Silva, V.A.O.; Lengert, A.V.H.; Reis, R.M.; de Syllos Cólus, I.M. Characterization of the in vitro cytotoxic effects of brachydins isolated from Fridericia platyphylla in a prostate cancer cell line. J. Toxicol. Environ. Health Part A 2020, 83, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Freire, J.; Dos Santos Fernandes, B.C.; da Silva, J.; da Silva Araújo, J.R.; de Almeida, P.M.; da Costa Júnior, J.S.; da Silva, J.N.; de Freitas, S.; Martins, F.A. Phytochemical and antioxidant characterization, cytogenotoxicity and antigenotoxicity of the fractions of the ethanolic extract of in Poincianella bracteosa (Tul.) L.P. Queiroz. J. Toxicol. Environ. Health Part A 2020, 83, 730–747. [Google Scholar] [CrossRef]
- WHO, World Health Organization. Traditional medicine growing needs and potential. WHO Policy Perspect. Med. 2002, 2, 1–6. [Google Scholar]
- Tognolini, M.; Barocelli, E.; Ballabeni, V.; Bruni, R.; Bianchi, A.; Chiavarini, M.; Impicciatore, M. Comparative screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006, 78, 1419–1432. [Google Scholar] [CrossRef]
- Han, E.H.; Lim, M.K.; Lee, S.H.; Rahman, M.M.; Lim, Y.H. An oral toxicity test in rats and a genotoxicity study of extracts from the stems of Opuntia ficus-indica var. saboten. BMC Complement. Altern. Med. 2019, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Andreu, L.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Antioxidant properties and chemical characterization of Spanish Opuntia ficus-indica Mill. cladodes and fruits. J. Sci. Food Agric. 2018, 98, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Santzouk, G.; Santzouk, S.; Gerodimou, I.; Tsaoulidis, D.; Dormousoglou, M. Opuntia ficus indica (Prickly pear): Extraction and characterization of products with anti-age and antioxidant activity. Bulg. Chem. Commun. 2019, 51, 052–055. [Google Scholar]
- Elkady, W.M.; Bishr, M.M.; Abdel-Aziz, M.M.; Salama, O.M. Identification and isolation of anti-pneumonia bioactive compounds from Opuntia ficus-indica fruit waste peels. Food Funct. 2020, 11, 5275–5283. [Google Scholar] [CrossRef]
- Slimen, I.B.; Mabrouk, M.; Hanène, C.; Najar, T.; Abderrabba, M. LC-MS analysis of phenolic acids, flavonoids and betanin from spineless Opuntia ficus-indica fruits. Cell Biol. 2017, 5, 17–28. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; Lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, chemical, and biochemical characterization of cladodes from prickly pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, L.; Vernon-Carter, E.J.; Gallegos-Infante, J.A.; Rocha-Guzman, N.E.; Herrera-Valencia, E.E.; Calderas, F.; Jiménez-Alvarado, R. Study of the antioxidant properties of extracts obtained from nopal cactus (Opuntia ficus-indica) cladodes after convective drying. J. Sci. Food Agric. 2011, 91, 1001–1005. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.; Nagaty, M.A.; Salman, M.S.; Bazaid, S.A. Phytochemicals, nutritionals and antioxidant properties of two prickly pear cactus cultivars (Opuntia ficus indica Mill.) growing in Taif, KSA. Food Chem. 2014, 160, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Tassotti, M.; Andreu, L.; Nuncio-Jáuregui, N.; Legua, P.; Del Rio, D.; Hernández, F. Phytochemical characterization of different prickly pear (Opuntia ficus-indica (L.) Mill.) cultivars and botanical parts: UHPLC-ESI-MSn metabolomics profiles and their chemometric analysis. Food Res. Int. 2018, 108, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Ouhammou, M.; Lahnine, L.; Mghazli, S.; Hidar, N.; Bouchdoug, M.; Jaouad, A.; Mandi, L.; Mahrouz, M. Valorisation of cellulosic waste basic cactus to prepare activated carbon. J. Saudi Soc. Agric. Sci. 2017, 18, 133–140. [Google Scholar] [CrossRef]
- Ait Benhamou, A.; Kassab, Z.; Boussetta, A.; Salim, M.H.; Ablouh, E.H.; Nadifiyine, M.; Qaiss, A.E.K.; Moubarik, A.; El Achaby, M. Beneficiation of cactus fruit waste seeds for the production of cellulose nanostructures: Extraction and properties. Int. J. Biol. Macromol. 2022, 203, 302–311. [Google Scholar] [CrossRef]
- Ait Benhamou, A.; Boussetta, A.; Kassab, Z.; Nadifiyine, M.; Salim, M.H.; Grimi, N.; El Achaby, M.; Moubarik, A. Investigating the characteristics of cactus seeds by-product and their use as new filler in phenol formaldehyde wood adhesive. Int. J. Adhes. 2021, 110, 102940. [Google Scholar] [CrossRef]
- Derabli, B.; Nancib, A.; Nancib, N.; Aníbal, J.; Raposo, S.; Rodrigues, B.; Boudrant, J. Opuntia ficus indica waste as a cost effective carbon source for lactic acid production by Lactobacillus plantarum. Food Chem. 2022, 370, 131005. [Google Scholar] [CrossRef]
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive phenolic compounds from agri-food wastes: An update on green and sustainable extraction methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Lizárraga-Velázquez, C.E.; Leyva-López, N.; Hernández, C.; Gutiérrez-Grijalva, E.P.; Salazar-Leyva, J.A.; Osuna-Ruíz, I.; Martínez-Montaño, E.; Arrizon, J.; Guerrero, A.; Benitez-Hernández, A.; et al. Antioxidant molecules from plant waste: Extraction techniques and biological properties. Processes 2020, 8, 1566. [Google Scholar] [CrossRef]
- Dang, Y.; Zhou, T.; Hao, L.; Cao, J.; Sun, Y.; Pan, D. In vitro and in vivo studies on the angiotensin-converting enzyme inhibitory activity peptides isolated from broccoli protein hydrolysate. J. Agric. Food Chem. 2019, 67, 6757–6764. [Google Scholar] [CrossRef] [PubMed]
- Colantuono, A.; Ferracane, R.; Vitaglione, P. In vitro bioaccessibility and functional properties of polyphenols from pomegranate peels and pomegranate peels-enriched cookies. Food Funct. 2016, 7, 4247–4258. [Google Scholar] [CrossRef]
- Blando, F.; Russo, R.; Negro, C.; De Bellis, L.; Frassinetti, S. Antimicrobial and antibiofilm activity against Staphylococcus aureus of Opuntia ficus-indica (L.) Mill. cladode polyphenolic extracts. Antioxidants 2019, 8, 117. [Google Scholar] [CrossRef]
- Ramírez-Moreno, E.; Cariño-Cortés, R.; Cruz-Cansino, N.d.S.; Delgado-Olivares, L.; Ariza-Ortega, J.A.; Montañez-Izquierdo, V.Y.; Hernández-Herrero, M.M.; Filardo-Kerstupp, T. Antioxidant and antimicrobial properties of cactus pear (Opuntia) seed oils. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Park, E.H.; Kahng, J.H.; Lee, S.H.; Shin, K.H. An antiinflammatory principle from cactus. Fitoterapia 2001, 72, 288–290. [Google Scholar] [CrossRef]
- Wiese, J.; McPherson, S.; Odden, M.C.; Shlipak, M.G. Effect of Opuntia ficus indica on symptoms of the alcohol hangover. Arch. Intern. Med. 2004, 164, 1334–1340. [Google Scholar] [CrossRef]
- Ben Saad, A.; Dalel, B.; Rjeibi, I.; Smida, A.; Ncib, S.; Zouari, N.; Zourgui, L. Phytochemical, antioxidant and protective effect of cactus cladodes extract against lithium-induced liver injury in rats. Pharm. Biol. 2017, 55, 516–525. [Google Scholar] [CrossRef]
- Ncibi, S.; Othman, M.B.; Akacha, A.; Krifi, M.N.; Zourgui, L. Opuntia ficus indica extract protects against chlorpyrifos-induced damage on mice liver. Food Chem. Toxicol. 2008, 46, 797–802. [Google Scholar] [CrossRef]
- Alimi, H.; Hfaeidh, N.; Bouoni, Z.; Sakly, M.; Ben Rhouma, K. Protective effect of Opuntia ficus indica f. inermis prickly pear juice upon ethanol-induced damages in rat erythrocytes. Alcohol 2012, 46, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Garoby-Salom, S.; Guéraud, F.; Camaré, C.; de la Rosa, A.P.B.; Rossignol, M.; Díaz, M.d.S.S.; Salvayre, R.; Negre-Salvayre, A. Dietary cladode powder from wild type and domesticated Opuntia species reduces atherogenesis in apoE knock-out mice. J. Physiol. Biochem. 2016, 72, 59–70. [Google Scholar] [CrossRef]
- Linarès, E.; Thimonier, C.; Degre, M. The effect of neopuntia® on blood lipid parameters—Risk factors for the metabolic syndrome (Syndrome χ). Adv. Ther. 2007, 24, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Helal, F.A.; Galal, M.; Mohamed, A.F. Evaluation of anti-cancer potential of proteinacious euphorbia tirucalli and Opuntia ficus-indica cactus extracts: In vitro study. Paripex Indian J. Res. 2019, 8, 1–4. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Elmelegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical screening, antimicrobial, antiaxidant, anticancer activities and nutritional values of cactus (Opuntia ficus indicia) pulp and peel. Fresenius Environ. Bull. 2019, 28, 1534–1551. Available online: https://www.scopus.com/record/display.uri?eid=2-s2.0-85062410808&origin=inward&txGid=6a40dc3925cd900e52849de543b1912b (accessed on 22 July 2022).
- Önem, E.; Kendir, G.; Akkoç, S.; Erzurumlu, Y.; Muhammed, M.T.; Özaydın, A.G. Biochemical contents and antiquorum sensing, antiproliferative activities of Opuntia ficus-indica (L.) Mill. peel extract. S. Afr. J. Bot. 2022, 150, 296–304. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; Portillo-Reyes, J.; Madrigal-Bujaidar, E.; Sánchez-Gutiérrez, M.; Mercado-Gonzalez, P.E.; Izquierdo-Vega, J.A.; Vargas-Mendoza, N.; Álvarez-González, I.; Fregoso-Aguilar, T.; Delgado-Olivares, L.; et al. Opuntia genus in Human Health: A Comprehensive Summary on Its Pharmacological, Therapeutic and Preventive Properties. Part 1. Horticulturae 2022, 8, 88. [Google Scholar] [CrossRef]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; pp. 389–469. [Google Scholar]
- Guedes, A.R.; de Souza, A.R.C.; Barbi, R.C.T.; Escobar, E.L.N.; Zanoello, E.F.; Corazza, M.L. Extraction of Synadenium grantii Hook f. using conventional solvents and supercritical CO2 + ethanol. J. Supercrit. Fluids 2020, 160, 8–10. [Google Scholar] [CrossRef]
- Santos, C.; Campestrini, L.H.; Vieira, D.L.; Pritsch, I.; Yamassaki, F.T.; Zawadzki-Baggio, S.F.; Maurer, J.; Molento, M.B. Chemical Characterization of Opuntia ficus-indica (L.) Mill. Hydroalcoholic Extract and Its Efficiency against Gastrointestinal Nematodes of Sheep. Vet. Sci. 2018, 5, 80. [Google Scholar] [CrossRef]
- Dormousoglou, M.; Efthimiou, I.; Antonopoulou, M.; Fetzer, D.L.; Hamerski, F.; Corazza, M.L.; Papadaki, M.; Santzouk, S.; Dailianis, S.; Vlastos, D. Investigation of the Genotoxic, Antigenotoxic and Antioxidant Profile of Different Extracts from Equisetum arvense L. Antioxidants 2022, 11, 1393. [Google Scholar] [CrossRef]
- Abou-Elella, F.M.; Ali, R.F.M. Antioxidant and anticancer activities of different constituents extracted from Egyptian prickly pear Cactus (Opuntia Ficus-Indica) Peel. Biochem. Anal. Biochem. 2014, 3, 2161-1009.1000158. [Google Scholar] [CrossRef]
- El Mannoubi, I.; Barrek, S.; Skanji, T.; Casabianca, H.; Zarrouk, H. Characterization of Opuntia ficus indica seed oil from Tunisia. Chem. Nat. Compd. 2009, 45, 616–620. [Google Scholar] [CrossRef]
- Ortega-Ortega, M.D.; Cruz-Cansino, N.D.; Alanís-García, E.; Delgado-Olivares, L.; Ariza-Ortega, J.A.; Ramírez-Moreno, E.; Manríquez-Torres, J.D. Optimization of ultrasound extraction of cactus pear (Opuntia ficus indica) seed oil based on antioxidant activity and evaluation of its antimicrobial activity. J. Food Qual. 2017, 2017, 9315360. [Google Scholar] [CrossRef]
- Al-Naqeb, G.; Fiori, L.; Ciolli, M.; Aprea, E. Prickly Pear Seed Oil Extraction, Chemical Characterization and Potential Health Benefits. Molecules 2021, 26, 5018. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, C.Z. Comparison of Techniques for the Extraction of Flavonoids from Cultured Cells of Saussurea medusa Maxim. World J. Microbiol. Biotechnol. 2005, 21, 1461–1463. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/ technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Shabir, G.; Anwar, F.; Sultana, B.; Khalid, Z.M.; Afzal, M.; Khan, Q.M.; Ashrafuzzaman, M. Antioxidant and Antimicrobial Attributes and Phenolics of different Solvent Extracts from Leaves, Flowers and Bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf. Molecules 2011, 16, 7302–7319. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, L.; Kolay, E.; Agilonu, Y.; Aslan, Z.; Kargioglu, M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J. Biol. Sci. 2013, 20, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Coelho, M.C.; Ozcan, K.; Pinto, C.A.; Teixeira, J.A.; Saraiva, J.A.; Pintado, M. Emergent Technologies for the Extraction of Antioxidants from Prickly Pear Peel and Their Antimicrobial Activity. Foods 2021, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Yeddes, N.; Cherif, J.K.; Guyot, S.; Sotin, H.; Ayadi, M.T. Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of the peel and pulp of three tunisian Opuntia forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.J.; Heliodoro, D.; Alejandro, Z.C.; Ruth, B.C.; Noé, A.C. The optimization of phenolic compounds extraction from cactus pear (Opuntia ficus-indica) skin in a reflux system using response surface methodology. Asian Pac. J. Trop. Biomed. 2013, 3, 436–442. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betalian and indicaxanthin. J. Agri. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef]
- Gentile, C.; Tesoriere, L.; Allegra, M.; Livrea, M.A.; Alession, P. Antioxidant betallians from cactus prea (Opuntia ficus Indica) inhibit endothelial ICAM-1expression. Ann. N. Y. Acad. Sci. 2004, 1028, 481–486. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.J.; Jiang, F.; Yang, Y.; Wang, X.X.; Zhang, Z.; Li, Z.; Li, L. Caffeic acid improves cell viability and protects against DNA damage: Involvement of reactive oxygen species and extracellular signal-regulated kinase. Braz. J. Med. Biol. Res. 2015, 48, 502–508. [Google Scholar] [CrossRef]
- Du, G.; Zhao, H.Y.; Song, Y.L.; Zhang, Q.W.; Wang, Y.T. Rapid simultaneous determination of isoflavones in Radix puerariae using high-performance liquid chromatography-triple quadrupole mass spectrometry with novel shell-type column. J. Sep. Sci. 2011, 34, 2576–2585. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, Q.W.; Li, S.L.; Wang, Y.; Ye, W.C.; Zhao, J.; Wang, Y.T. Simultaneous quantification of major flavonoids in “Bawanghua”, the edible flower of Hylocereus undatus using pressurised liquid extraction and high performance liquid chromatography. Food Chem. 2012, 135, 528–533. [Google Scholar] [CrossRef]
- Sreekanth, D.; Arunasree, M.K.; Roy, K.R.; Chandramohan Reddy, T.; Reddy, G.V.; Reddanna, P. Betanin a betacyanin pigment purified from fruits of Opuntia ficus-indica induces apoptosis in human chronic myeloid leukemia Cell line-K562. Phytomedicine 2007, 14, 739–746. [Google Scholar] [CrossRef]
- Khan, M.I.; Harsha, P.S.; Giridhar, P.S.C.P.; Ravishankar, G.A. Pigment identification, nutritional composition, bioactivity, and in vitro cancer cell cytotoxicity of Rivina humilis L. berries, potential source of betalains. LWT 2012, 47, 315–323. [Google Scholar] [CrossRef]
- Buyukleyla, M.; Azirak, S.; Rencuzogullari, E.; Kocaman, A.Y.; Ila, H.B.; Topaktas, M.; Darici, C. The genotoxic and antigenotoxic effects of tannic acid in human lymphocytes. Drug Chem. Toxicol. 2011, 35, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004, 52, 2512–2517. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Yáñez, J.; Vicente, V.; Alcaraz, M.; Benavente-García, O.; Castillo, J.; Lorente, J.; Lozano, J.A. Effects of several flavonoids on the growth of B16F10 and SK- MEL-1 melanoma cell lines: Relationship between structure and activity. Melanoma Res. 2002, 12, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zorgui, L.; Ayed-Boussema, I.; Ayed, Y.; Bacha, H.; Hassen, W. The antigenotoxic activities of cactus (Opuntia ficus-indica) cladodes against the mycotoxin zearalenone in Balb/c mice: Prevention of micronuclei, chromosome aberrations and DNA fragmentation. Food Chem. Toxicol. 2009, 47, 662–667. [Google Scholar] [CrossRef]
- Brahmi, D.; Bouaziz, C.; Ayed, Y.; Ben Mansour, H.; Zourgui, L.; Bacha, H. Chemopreventive effect of cactus Opuntia ficus indica on oxidative stress and genotoxicity of aflatoxin B1. Nutr. Metab. 2011, 8, 73. [Google Scholar] [CrossRef]
- Madrigal-Santillán, E.; García-Melo, F.; Morales-González, J.A.; Vázquez-Alvarado, P.; Muñoz-Juárez, S.; Zuñiga-Pérez, C.; Sumaya-Martínez, M.T.; Madrigal-Bujaidar, E.; Hernández-Ceruelos, A. Antioxidant and anticlastogenic capacity of prickly pear juice. Nutrients 2013, 5, 4145–4458. [Google Scholar] [CrossRef]
- OECD. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris. 2016. Available online: https://www.oecd-ilibrary.org/environment/test-no-487-in-vitro-mammalian-cell-micronucleus-test_9789264264861-en (accessed on 22 July 2022).
- Efthimiou, I.; Vlastos, D.; Triantafyllidis, V.; Eleftherianos, A.; Antonopoulou, M. Investigation of the Genotoxicological Profile of Aqueous Betula pendula Extracts. Plants 2022, 11, 2673. [Google Scholar] [CrossRef]
- Iyer, V.N.; Szybalski, W. Mitomycins and porfiromycin: Chemical mechanism of activation and cross-linking of DNA. Science 1964, 145, 55–58. [Google Scholar] [CrossRef]
- Liao, P.H.; Lin, R.H.; Yang, M.L.; Li, Y.C.; Kuan, Y.H. Evaluation of differential representative values between Chinese hamster cells and human lymphocytes in mitomycin C-induced cytogenetic assays and caspase-3 activity. Toxicol. Ind. Health 2012, 28, 174–180. [Google Scholar] [CrossRef]
- Rencuzogullari, E.; Yildiz, A.M.; Buyukleyla, M. The genotoxic and anti-genotoxic effects of Stachys petrokosmos leaf extract in human lymphocytes using microsomal fractions. Cytotechnology 2012, 64, 83–94. [Google Scholar] [CrossRef]
- Bonassi, S.; El-Zein, R.; Bolognesi, C.; Fenech, M. Micronuclei frequency in peripheral blood lymphocytes and cancer risk: Evidence from human studies. Mutagenesis 2011, 26, 93–100. [Google Scholar] [CrossRef]
- Vlastos, D.; Mademtzoglou, D.; Drosopoulou, E.; Efthimiou, I.; Chartomatsidou, T.; Pandelidou, C.; Astyrakaki, M.; Chalatsi, E.; Mavragani-Tsipidou, P. Evaluation of the genotoxic and antigenotoxic effects of Chios mastic water by the in vitro micronucleus test on human lymphocytes and the in vivo wing somatic test on Drosophila. PLoS ONE 2013, 8, e69494. [Google Scholar] [CrossRef]
- Vlastos, D.; Drosopoulou, E.; Efthimiou, I.; Gavriilidis, M.; Panagaki, D.; Mpatziou, K.; Kalamara, P.; Mademtzoglou, D.; Mavragani-Tsipidou, P. Genotoxic and antigenotoxic assessment of Chios mastic oil by the in vitro micronucleus test on human lymphocytes and the in vivo wing somatic test on Drosophila. PLoS ONE 2015, 10, e0130498. [Google Scholar] [CrossRef] [PubMed]
- Drosopoulou, E.; Vlastos, D.; Efthimiou, I.; Kyrizaki, P.; Tsamadou, S.; Anagnostopoulou, M.; Kofidou, D.; Gavriilidis, M.; Mademtzoglou, D.; Mavragani-Tsipidou, P. In vitro and in vivo evaluation of the genotoxic and antigenotoxic potential of the major Chios mastic water constituents. Sci. Rep. 2018, 8, 12200. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, N.; Shahidi, F.; Jeon, Y.J. Potential antioxidative effects of cactus pear fruit (Opuntia ficus-indica) extract on radical scavenging and DNA damage reduction in human peripheral lymphocytes. J. Food Lipids. 2006, 13, 445–458. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, G.R.; Grotto, D.; Angeli, J.P.; Serpeloni, J.M.; Rocha, B.A.; Bastos, J.K.; Barbosa, F., Jr. Evaluation of antigenotoxic effects of plant flavonoids quercetin and rutin on HepG2 cells. Phytother. Res. 2011, 25, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.A.; Lima, C.F.; Pereira, M.L.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Antigenotoxic effects of quercetin, rutin and ursolic acid on HepG2 cells: Evaluation by the comet assay. Toxicol. Lett. 2008, 177, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Jovtchev, G.; Gateva, S.; Stankov, A. Lilium compounds kaempferol and jatropham can modulate cytotoxic and genotoxic effects of radiomimetic zeocin in plants and human lymphocytes in vitro. Environ. Toxicol. 2016, 31, 751–764. [Google Scholar] [CrossRef]
- Bhouri, W.; Sghaier, M.B.; Kilani, S.; Bouhlel, I.; Dijoux-Franca, M.G.; Ghedira, K.; Ghedira, L.C. Evaluation of antioxidant and antigenotoxic activity of two flavonoids from Rhamnus alaternus L. (Rhamnaceae): Kaempferol 3-O-β-isorhamninoside and rhamnocitrin 3-O-β-isorhamninoside. Food Chem. Toxicol. 2011, 49, 1167–1173. [Google Scholar] [CrossRef]
- Harada, H.; Yamashita, U.; Kurihara, H.; Fukushi, E.; Kawabata, J.; Kamei, Y. Antitumor activity of palmitic acid found as a selective cytotoxic substance in a marine red alga. Anticancer Res. 2002, 22, 2587–2590. [Google Scholar] [PubMed]
- Dormousoglou, M.; Boti, V.; Hela, D.; Vlastos, D.; Antonopoulou, M.; Chondrogiannis, C.; Petropoulou, Y.; Dailianis, S. Beneficial properties of Drimia numidica leaf methanolic extract against the cytogenotoxic effects of mitomycin C on human lymphocytes. Food Chem. Toxicol. 2023, 173, 113626. [Google Scholar] [CrossRef] [PubMed]
- Arigony, A.L.; de Oliveira, I.M.; Machado, M.; Bordin, D.L.; Bergter, L.; Prá, D.; Henriques, J.A. The influence of micronutrients in cell culture: A reflection on viability and genomic stability. Biomed. Res. Int. 2013, 2013, 597282. [Google Scholar] [CrossRef] [PubMed]
- Imbimbo, P.; D’Elia, L.; Liberti, D.; Olivieri, G.; Monti, D.M. Towards green extraction methods from microalgae learning from the classics. Appl. Microbiol. Biotechnol. 2020, 104, 9067–9077. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength, and limitation. Med. Aromat. Plants. 2015, 4, 1–6. [Google Scholar] [CrossRef]
- Grujičić, D.; Marković, A.; Tubić Vukajlović, J.; Stanković, M.; Jakovljević, M.R.; Ćirić, A.; Djordjević, K.; Planojević, N.; Milutinović, M.; Milošević-Djordjević, O. Genotoxic and cytotoxic properties of two medical plants (Teucrium arduini L. and Teucrium flavum L.) in relation to their polyphenolic contents. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2020, 852, 503168. [Google Scholar] [CrossRef]
- Akacha, A.; Rebai, T.; Zourgui, L.; Amri, M. Preventive effect of ethanolic extract of cactus (Opuntia ficus-indica) cladodes on methotrexate-induced oxidative damage of the small intestine in Wistar rats. J. Cancer Res. Ther. 2018, 14, 779. [Google Scholar] [CrossRef]
- Cai, W.; Gu, X.; Tang, J. Extraction, purification, and characterisation of the flavonoids from Opuntia milpa alta skin. Czech J. Food Sci. 2010, 28, 108–116. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Rivadeneyra-Mata, M.; Ramos-Cassellis, M.E.; Aparicio-Fernández, X.; Navarro-Cruz, A.R.; Ávila-Sosa, R.; Ochoa-Velasco, C.E. Characterization of red prickly pear peel (Opuntia ficus-indica L.) and its mucilage obtained by traditional and novel methodologies. J. Food Meas. Charact. 2019, 13, 1111–1119. [Google Scholar] [CrossRef]
- Gomide, R. Operações com Sistemas Sólidos Granulares; Catalogação da Câmara Brasileira de Publicação de Livros: São Paulo, Brazil, 1983; Volume 1, pp. 27–30. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; Methods 920.39, 925.09; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- (ISO)17294; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 1: General Guidelines. International Organization for Standardization: Geneva, Switzerland, 2004. Available online: https://www.iso.org/standard/32957.html (accessed on 15 October 2020).
- (ISO)17294; Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. International Organization for Standardization: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/62962.html (accessed on 15 October 2020).
- Yaldiz, G.; Koca Caliskan, U.; Aka, C. In vitro screening of natural drug potentials for mass production. Not. Bot. Horti Agrobot. Cluj Napoca. 2017, 45, 292–300. [Google Scholar] [CrossRef]
- Surrallés, J.; Xamena, N.; Creus, A.; Catalan, J.; Norppa, H.; Marcos, R. Induction of micronuclei by five pyrethroid insecticides in whole-blood and isolated human lymphocyte cultures. Mutat. Res. 1995, 341, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).