Anticancer Podophyllotoxin Recovery from Juniper Leaves at Atmospheric and High Pressure Using Eco-Friendly Solvents
Abstract
1. Introduction
2. Results
2.1. Extraction Optimization of PPT from Juniper Leaves at Atmospheric Pressure
2.1.1. Selection of Appropriate Solvent for PPT Extraction
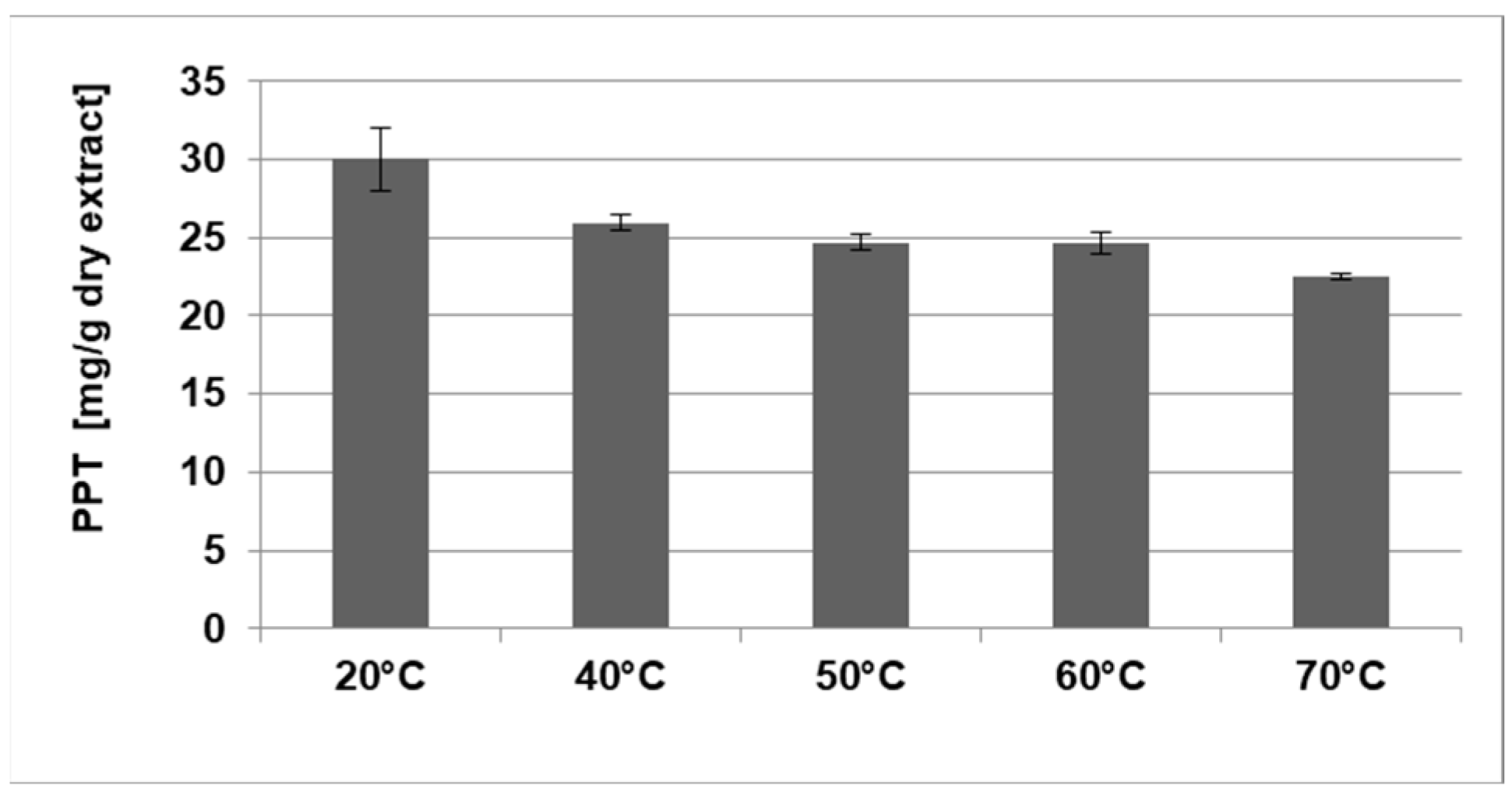
2.1.2. Determination of the Optimal PPT Extraction Temperature
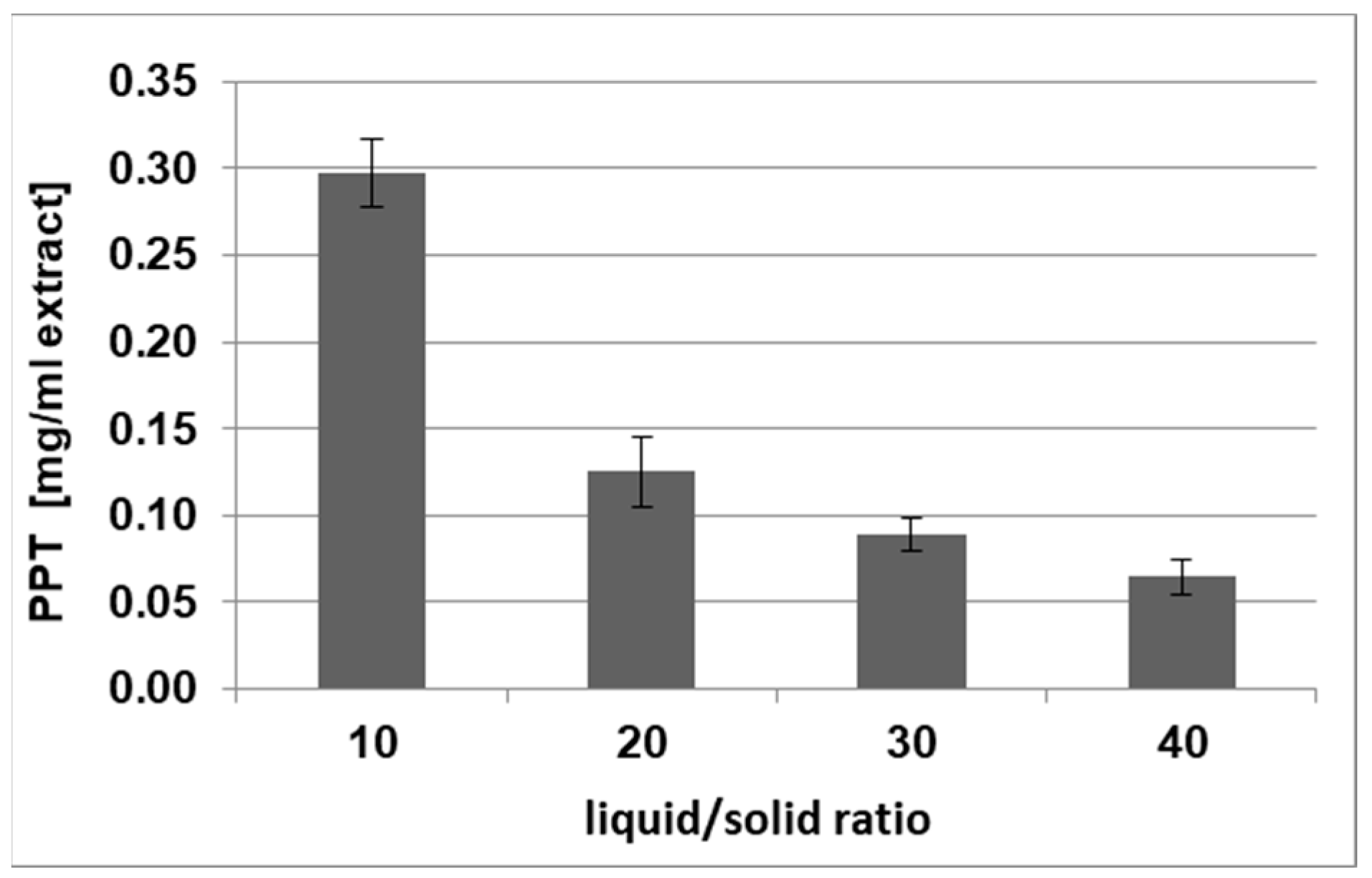
2.1.3. Determination of the Liquid-to-Solid Ratio (LSR) for PPT Extraction
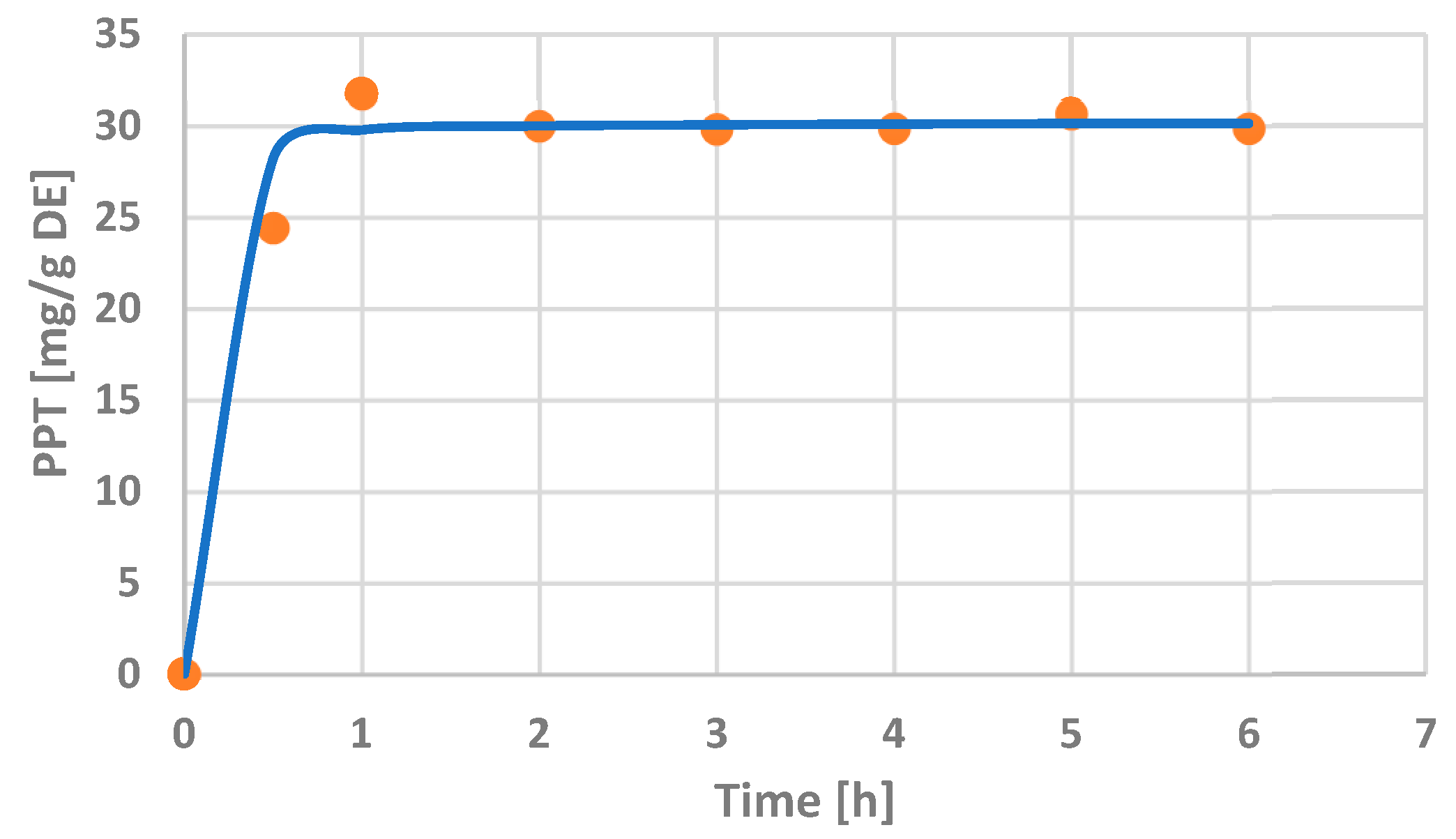
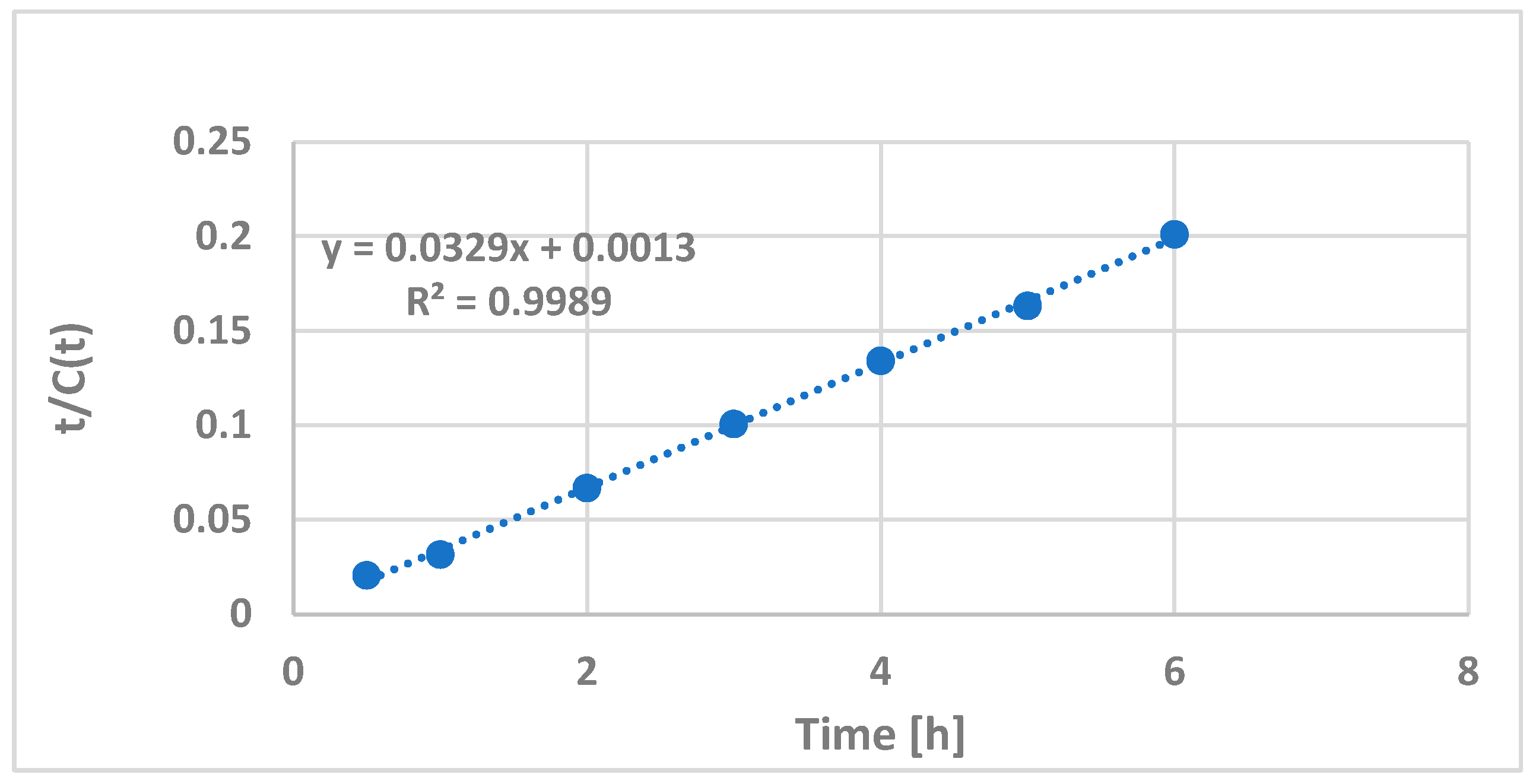
2.1.4. Optimization of the Extraction Time and Peleg’s Kinetic Modeling
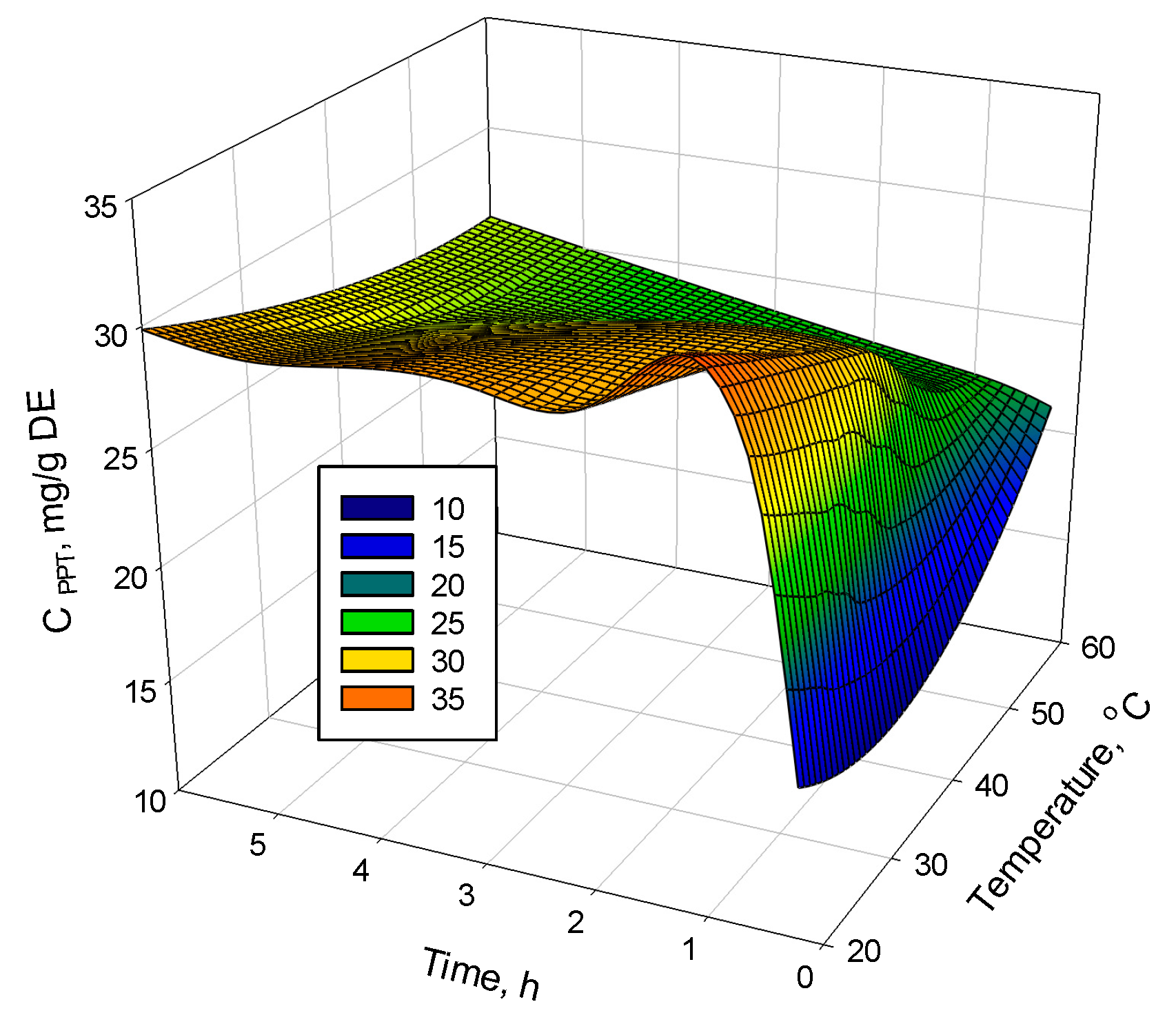
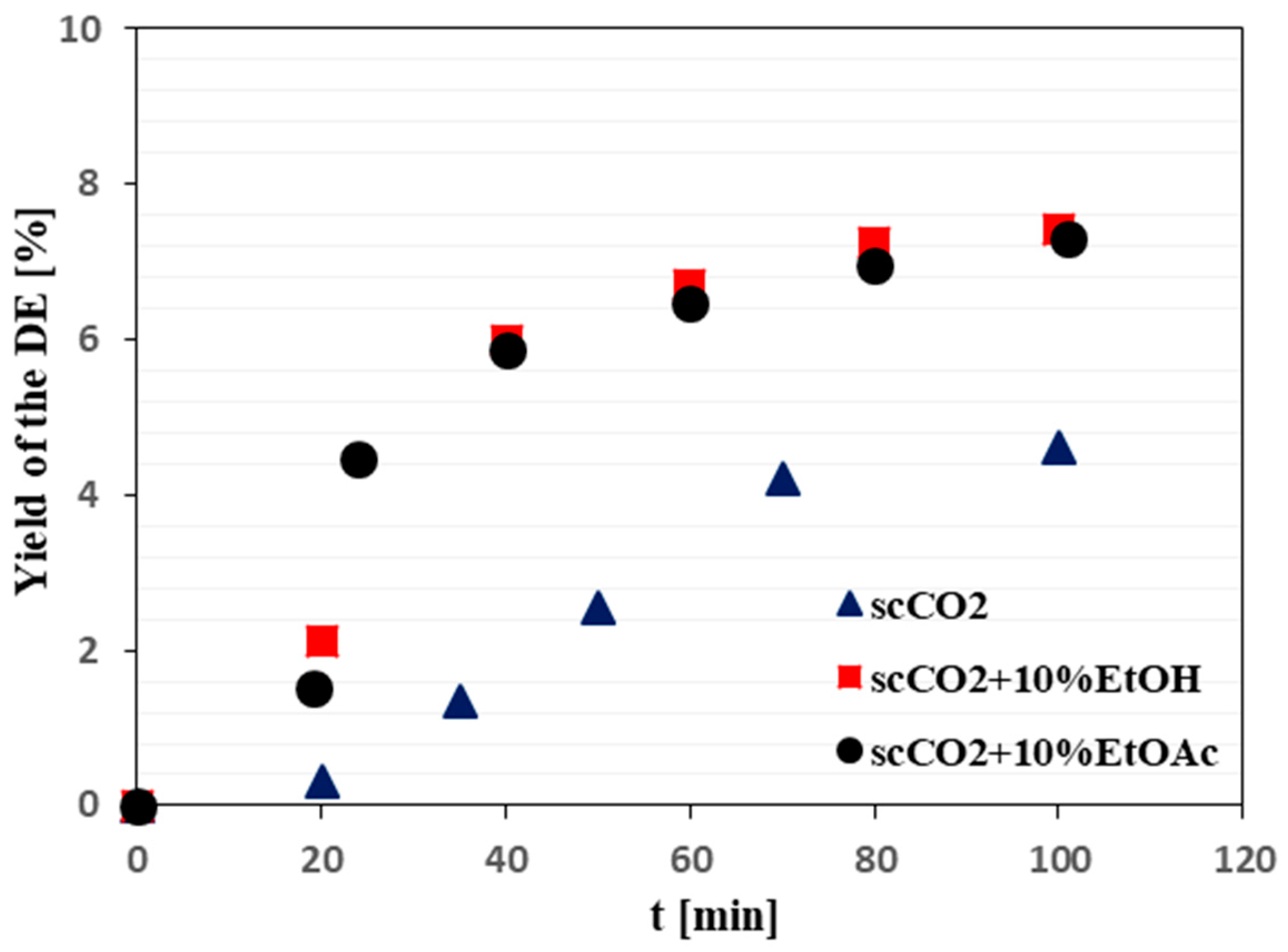
2.2. Supercritical Fluid Extraction of PPT from Juniper Leaves
2.3. Accelerated Solvent Extraction of PPT from Juniper Leaves
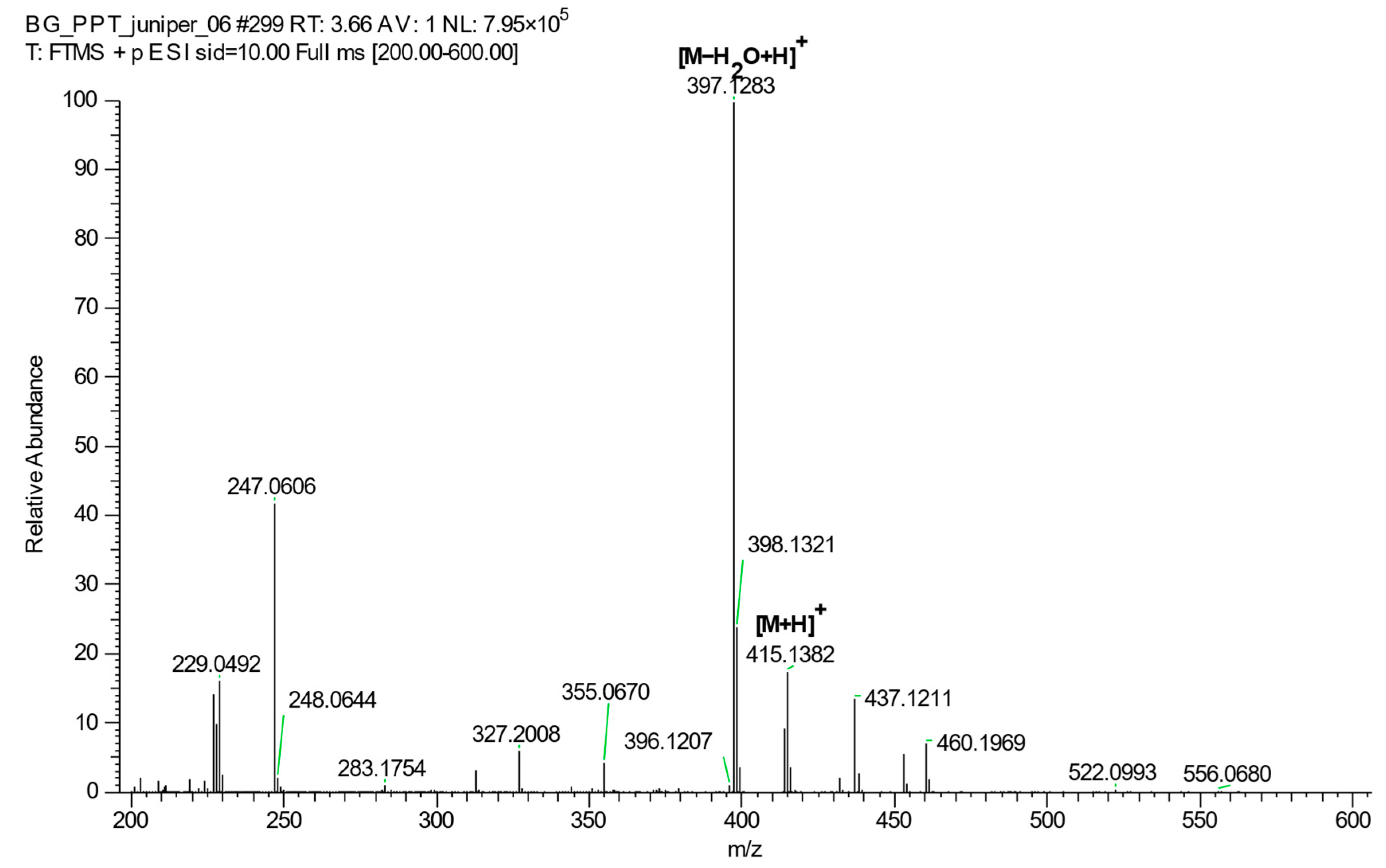
2.4. The UHPLC-HRMS Method for PPT Quantification in Juniper Leaf Extracts and Its Validation
2.5. Seasonal and Storage Stability of the PPT in Juniper Leaves
- Seasonal stability of the PPT in juniper leaves
- Storage stability of the PPT content in juniper leaves
3. Discussion
3.1. Influence of the Process Parameters on the PPT Recovery from Juniper Leaves by Extraction at Atmospheric Pressure
3.1.1. Determination of Optimal Conditions for Conventional PPT Extraction from Juniper Leaves at Atmospheric Pressure
3.1.2. Correlation of Peleg’s Kinetic Modeling with PPT Extraction Duration
3.2. Effects of the Extraction Conditions on the PPT Recovery from Juniper Leaves by Supercritical Fluid Extraction (SFE)
3.3. Effect of the Process Conditions on the PPT Recovery from Juniper Leaves by Accelerated Solvent Extraction
3.4. Seasonal and Storage Stability of PPT in Juniper Leaves
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Extraction Procedures
4.3.1. Extraction at Atmospheric Pressure
4.3.2. Supercritical Fluid Extraction (SFE)
4.3.3. Accelerated Solvent Extraction (ASE)
4.4. Ultra-High-Performance Liquid Chromatography Coupled to High-Resolution Mass Spectrometry (UHPLC-HRMS) Analysis
4.5. Sample Preparation Prior to UHPLC-HRMS Analysis
4.6. Validation of the UHPLC-HRMS Method for PPT Quantification in Juniper Leaf Extracts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, Z.; Gohar, U.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-Ui-Haq, M.; Toma, S.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, recent advances and future prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef] [PubMed]
- Stähelin, H.F.; Von Wartburg, A. The chemical and biological route from podophyllotoxin glucoside to etoposide: Memorial award lecture. Cancer Res. 1991, 51, 5–15. [Google Scholar]
- Motyka, S.; Jafernik, K.; Ekiert, H.; Sharifi-Rad, J.; Calina, D.; Al-Omari, B.; Szopa, A. Podophyllotoxin and its derivatives: Potential anticancer agents of natural origin in cancer chemotherapy. Biomed. Pharmacother. 2023, 158, 114145. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trial. Etoposide in Patients with COVID-19 Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT04356690 (accessed on 8 February 2023).
- Podwyssotzki, V. Pharmakologische Studien über Podophyllum peltatum. Arch. Exp. Pathol. Pharmakol. 1880, 13, 29–52. [Google Scholar] [CrossRef]
- Borsche, W.; Niemann, J. Über Podophyllin. Eur. J. Org. Chem. 1932, 494, 126–142. [Google Scholar] [CrossRef]
- Cushman, K.E.; Maqbool, M.; Lata, H.; Bedir, E.; Khan, I.A.; Moraes, R.M. Podophyllotoxin content and yield of American Mayapple leaves in sun and shade. HortScience 2005, 40, 60–63. [Google Scholar] [CrossRef]
- Pandey, H.; Nandi, S.K.; Kumar, A.; Palni, U.T.; Palni, L.M.S. Podophyllotoxin content in Podophyllum hexandrum Royle plants of known age of seed origin and grown at a lower altitude. Acta Physiol. Plant. 2007, 29, 121–126. [Google Scholar] [CrossRef]
- Gupta, D.K.; Verma, M.K.; Lal, S.; Anand, R.; Khajuria, R.K.; Kitchlu, S.; Koul, S. Extraction studies of Podophyllum hexandrum using conventional and nonconventional methods by HPLC-UV-DAD. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 259–273. [Google Scholar] [CrossRef]
- Yu, X.; Che, Z.P.; Xu, H. Recent advances in the chemistry and biology of podophyllotoxins. Chem. Eur. J. 2016, 10, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Ardalani, H.; Avan, A.; Ghayour-Mobarhan, M. Podophyllotoxin: A novel potential natural anticancer agent. Avicenna J. Phytomed. 2017, 7, 285–294. [Google Scholar]
- Slanina, J.; Glatz, Z. Analytical Technologies in the Biomedical and Life Sciences. Separation procedures applicable to lignan analysis. J. Chromatogr. B 2004, 812, 215–229. [Google Scholar] [CrossRef]
- Hartwell, J.L.; Johnson, J.M.; Fitzgerald, D.B.; Belkin, M. Podophyllotoxin from Juniperus species; Savinin. J. Am. Chem. Soc. 1953, 75, 235–236. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Donega, M.A.; Astatkie, T.; Heidel, B. Podophyllotoxin concentration in junipers in the Big Horn Mountains in Wyoming. HortScience 2012, 47, 1696–1697. [Google Scholar] [CrossRef]
- Och, M.; Och, A.; Ciesla, L.; Kubrak, T.; Pecio, Ł.; Stochmal, A.; Kocki, J.; Bogucka-Kocka, A. Study of cytotoxic activity, podophyllotoxin, and deoxypodophyllotoxin content in selected Juniperus species cultivated in Poland. Pharm. Biol. 2015, 53, 831–837. [Google Scholar] [CrossRef]
- Ivanova, D.I.; Nedialkov, P.T.; Tashev, A.N.; Olech, M.; Nowak, R.; Ilieva, Y.E.; Kokanova-Nedialkova, Z.K.; Atanasova, T.N.; Angelov, G.; Najdenski, H.M. Junipers of various origins as potential sources of the anticancer drug precursor podophyllotoxin. Molecules 2021, 26, 5179. [Google Scholar] [CrossRef]
- Renouard, S.; Lopez, T.; Hendrawati, O.; Dupré, P.; Doussot, J.; Falguieres, A.; Ferroud, C.; Hagège, D.; Lamblin, F.; Lainé, E.; et al. Podophyllotoxin and deoxypodophyllotoxin in Juniperus bermudiana and 12 other Juniperus species: Optimization of extraction, method validation, and quantification. J. Agric. Food Chem. 2011, 59, 8101–8107. [Google Scholar] [CrossRef] [PubMed]
- Eller, F.J.; Clausen, C.A.; Green, F.; Taylor, S.L. Critical fluid extraction of Juniperus virginiana L. and bioactivity of extracts against subterranean termites and wood-rot fungi. Ind. Crops Prod. 2010, 32, 481–485. [Google Scholar] [CrossRef]
- Eller, F.J.; King, J.W. Supercritical carbon dioxide extraction of cedarwood oil: A study of extraction parameters and oil characteristics. Phytochem. Anal. 2000, 11, 226–231. [Google Scholar] [CrossRef]
- Eller, F.J.; Taylor, S.L. Pressurized fluids for extraction of cedarwood oil from Juniperus virginiana. J. Agric. Food Chem. 2004, 52, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Tumen, I.; Süntar, I.; Eller, F.J.; Keleş, H.; Akkol, E.K. Topical wound-healing effects and phytochemical composition of heartwood essential oils of Juniperus virginiana L., Juniperus occidentalis Hook., and Juniperus ashei J. Buchholz. J. Med. Food 2013, 16, 48–55. [Google Scholar] [CrossRef]
- Peleg, M. An empirical model for the description of moisture sorption curves. J. Food Sci. 1988, 53, 1216–1217. [Google Scholar] [CrossRef]
- Charpe, T.W.; Rathod, V.K. Kinetics of ultrasound assisted extraction of wedelolactone from Eclipta alba. Braz. J. Chem. Eng. 2016, 33, 1003–1010. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Xue, H.; Jin, Y.; Yang, M.; Leng, F. Comparative analysis of three kinds of extraction kinetic models of crude polysaccharides from Codonopsis pilosula and evaluate the characteristics of crude polysaccharides. Biomass Convers. Biorefin. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yan, G.; Ge, T. A fragmentation study of podophyllotoxin and its 4′-demethyl-4β-substituted derivatives by electrospray ionization ion-trap time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2843–2852. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Liu, S.; Tian, P.; Li, D. Juniperus sabina L. as a source of Podophyllotoxins: Extraction optimization and anticholinesterase activities. Int. J. Mol. Sci. 2022, 23, 10205. [Google Scholar] [CrossRef] [PubMed]
- Vale, A. Methanol. Medicine 2007, 35, 633–644. [Google Scholar] [CrossRef]
- Pozzani, U.C.; Carpenter, C.P.; Palm, P.E.; Weil, C.S.; Nair, J.H. An investigation of the mammalian toxicity of acetonitrile. J. Occup. Med. 1959, 1, 634–642. [Google Scholar] [CrossRef]
- Twenty-Fifth Commission Directive 2000/11/EC of 10 March 2000 adapting to technical progress Annex II to Council Directive 76/768/EEC on the approximation of laws of the Member States relating to cosmetic products. Off. J. Eur. Communities 2000, L65, 22–25.
- Seegers, C.L.C.; Tepper, P.G.; Setroikromo, R.; Quax, W.J. Cytotoxic Deoxypodophyllotoxin Can Be Extracted in High Purity from Anthriscus sylvestris Roots by Supercritical Carbon Dioxide. Planta Med. 2018, 84, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Hendrawati, O.; Woerdenbag, H.J.; Hille, J.; Quax, W.J.; Kayser, O. Seasonal variations in the deoxypodophyllotoxin content and yield of Anthriscus sylvestris L. (Hoffm.) grown in the field and under controlled conditions. J. Agric. Food Chem. 2011, 59, 8132–8139. [Google Scholar] [CrossRef] [PubMed]
- Boyadzhieva, S.; Coelho, J.A.P.; Errico, M.; Reynel-Avilla, H.E.; Yankov, D.S.; Bonilla-Petriciolet, A.; Stateva, R.P. Assessment of Gnaphalium viscosum (Kunth) valorization prospects: Sustainable recovery of antioxidants by different techniques. Antioxidants 2022, 11, 2495. [Google Scholar] [CrossRef] [PubMed]
- ICH [International Council of Harmonisation, Expert Working Group]. Validation of Analytical Procedures: Text and Methodology Q2(R1). 2005. Available online: https://www.gmp-compliance.org/files/guidemgr/Q2(R1).pdf (accessed on 8 February 2023).
- Kazusaki, M.; Ueda, S.; Takeuchi, N.; Ohgami, Y. Validation of analytical procedures by high-performance liquid chromatography for pharmaceutical analysis. Chromatography 2012, 33, 65–73. [Google Scholar] [CrossRef]
- Borman, P.; Elder, D. Q2 (R1) validation of analytical procedures. In ICH Quality Guidelines: An Implementation Guide, 1st ed.; Teasdale, A., Elder, D., Nims, R.W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 127–166. ISBN 978-1-11897-114-7. [Google Scholar] [CrossRef]
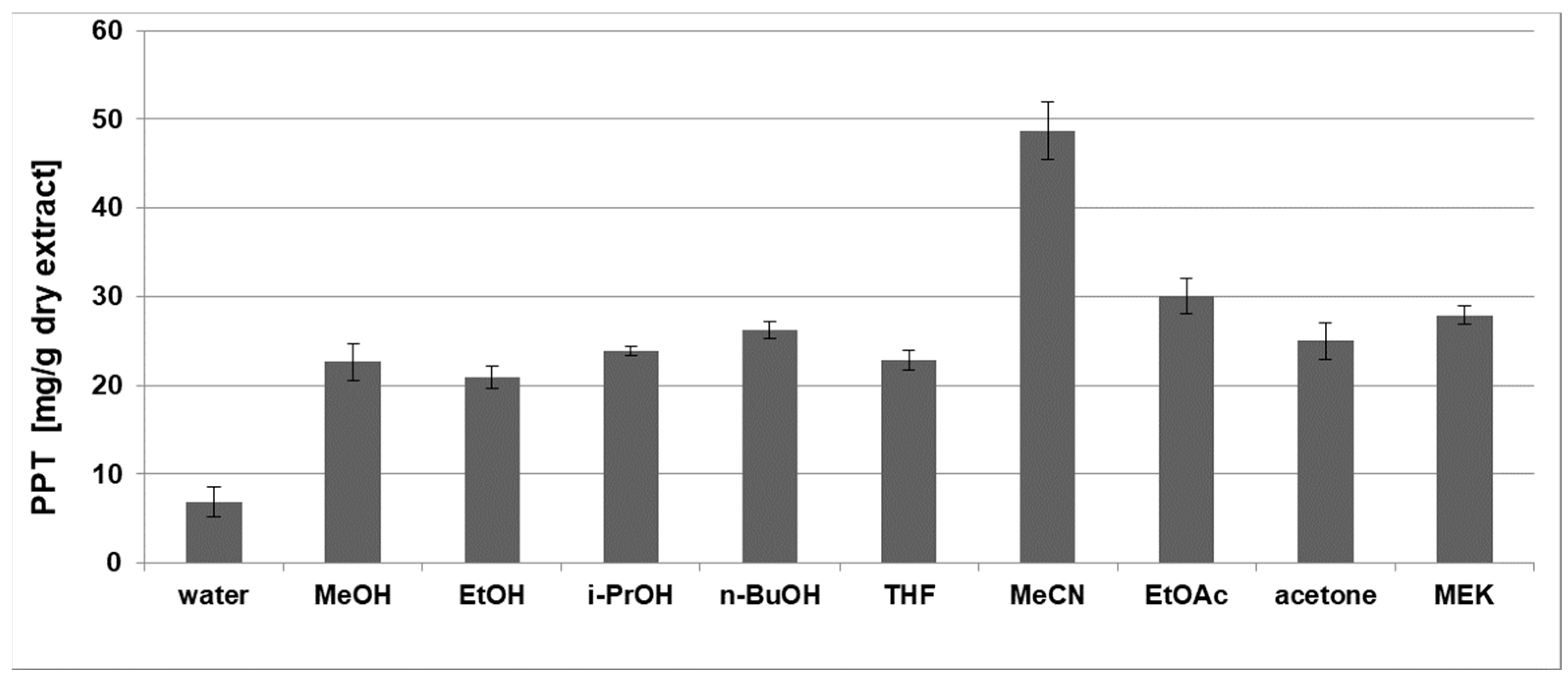
| Solvent | Water | MeOH | EtOH | i-PrOH | n-BuOH | THF | MeCN | EtOAc | Acetone | MEK |
|---|---|---|---|---|---|---|---|---|---|---|
| PPT (mg/g DW) | 0.8 ± 0.2 | 4.1 ± 0.2 | 2.9 ± 0.2 | 1.9 ± 0.2 | 2.1 ± 0.1 | 2.7 ± 0.1 | 2.4 ± 0.3 | 3.5 ± 0.1 | 2.2 ± 0.2 | 2.0 ± 0.3 |
| Temperature | 20 °C | 40 °C | 50 °C | 60 °C | 70 °C |
|---|---|---|---|---|---|
| DE yield (%) | 11.0 ± 0.7 | 12.0 ± 0.6 | 13.0 ± 0.9 | 14.0 ± 0.6 | 15.2 ± 0.5 |
| PPT (mg/g DW) | 3.3 ± 0.2 | 3.1 ± 0.2 | 3.2 ± 0.2 | 3.4 ± 0.1 | 3.4 ± 0.1 |
| LSR (v/w) | 10 | 20 | 30 | 40 |
|---|---|---|---|---|
| PPT (mg/g DE) | 30 ± 3 | 24 ± 2 | 25 ± 1 | 25 ± 1 |
| PPT (mg/g DW) | 3.1 ± 0.2 | 2.6 ± 0.1 | 2.7 ± 0.1 | 2.7 ± 0.1 |
| SFE Solvent | scCO2 | scCO2 + 10% EtOH | scCO2 + 10% EtOAc |
|---|---|---|---|
| PPT (mg/g DE) | 24 ± 1 | 42 ± 1 | 42 ± 1 |
| PPT (mg/g DW) | 1.1 ± 0.1 | 3.1 ± 0.1 | 3.1 ± 0.1 |
| Conditions | EtOH 20 °C | EtOH 40 °C | EtOH 60 °C | EtOH 80 °C | EtOAc 20 °C | EtOAc 40 °C | EtOAc 60 °C | EtOAc 80 °C | |
|---|---|---|---|---|---|---|---|---|---|
| Yields | |||||||||
| DE Yield (%) | 17.5 ± 0.2 | 21.7 ± 0.2 | 25.0 ± 1.0 | 28.5 ± 1.1 | 6.5 ± 0.2 | 8.4 ± 0.1 | 10.6 ± 0.1 | 12.3 ± 0.7 | |
| PPT (mg/g DE) | 16.3 ± 0.2 | 15.4 ± 0.8 | 15.0 ± 1.6 | 12.8 ± 0.3 | 45.5 ± 0.8 | 39.5 ± 1.9 | 32.9 ± 0.8 | 30.0 ± 0.2 | |
| PPT (mg/g DW) | 2.9 ± 0.1 | 3.3 ± 0.1 | 3.7 ± 0.2 | 3.6 ± 0.2 | 3.0 ± 0.1 | 3.3 ± 0.2 | 3.5 ± 0.1 | 3.7 ± 0.2 | |
| External Standard | Linear Range (ng/mL) | Regression Equation | R2 | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|
| podophyllotoxin | 12.25–392 | Y = 228,429 + 198,179 × X | 0.9996 | 0.32 | 0.97 |
| External Standard | Added (ng/mL) | Found 1 (ng/mL) | Recovery 1 (%) | RSD (%) |
|---|---|---|---|---|
| 49.00 | 49.41 ± 0.52 | 100.83 ± 1.06 | 1.05 | |
| podophyllotoxin | 98.00 | 97.73 ± 1.12 | 99.73 ± 1.15 | 1.15 |
| 147.00 | 149.64 ± 1.69 | 101.79 ± 1.15 | 1.13 |
| Precision Type | RT ± SD (min) | RSD (%) | Recovery ± SD (%) | RSD (%) |
|---|---|---|---|---|
| Intra-day | 3.63 ± 0.006 | 0.17 | 101.98 ± 1.59 | 1.56 |
| Inter-day | 3.62 ± 0.005 | 0.14 | 101.83 ± 1.04 | 1.02 |
| Month | Jan | Feb | Mar | Apr | May | June | July | Aug | Sep | Oct | Nov | Dec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPT | 22 ± 1 | 20 ± 2 | 19 ± 3 | 23 ± 1 | 17 ± 1 | 16 ± 1 | 17 ± 2 | 16 ± 3 | 16 ± 1 | 17 ± 1 | 18 ± 2 | 18 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, D.; Nedialkov, P.; Tashev, A.; Kokanova-Nedialkova, Z.; Olech, M.; Nowak, R.; Boyadzhieva, S.; Angelov, G.; Yankov, D. Anticancer Podophyllotoxin Recovery from Juniper Leaves at Atmospheric and High Pressure Using Eco-Friendly Solvents. Plants 2023, 12, 1526. https://doi.org/10.3390/plants12071526
Ivanova D, Nedialkov P, Tashev A, Kokanova-Nedialkova Z, Olech M, Nowak R, Boyadzhieva S, Angelov G, Yankov D. Anticancer Podophyllotoxin Recovery from Juniper Leaves at Atmospheric and High Pressure Using Eco-Friendly Solvents. Plants. 2023; 12(7):1526. https://doi.org/10.3390/plants12071526
Chicago/Turabian StyleIvanova, Diana, Paraskev Nedialkov, Alexander Tashev, Zlatina Kokanova-Nedialkova, Marta Olech, Renata Nowak, Stanislava Boyadzhieva, George Angelov, and Dragomir Yankov. 2023. "Anticancer Podophyllotoxin Recovery from Juniper Leaves at Atmospheric and High Pressure Using Eco-Friendly Solvents" Plants 12, no. 7: 1526. https://doi.org/10.3390/plants12071526
APA StyleIvanova, D., Nedialkov, P., Tashev, A., Kokanova-Nedialkova, Z., Olech, M., Nowak, R., Boyadzhieva, S., Angelov, G., & Yankov, D. (2023). Anticancer Podophyllotoxin Recovery from Juniper Leaves at Atmospheric and High Pressure Using Eco-Friendly Solvents. Plants, 12(7), 1526. https://doi.org/10.3390/plants12071526








