Trends in Harnessing Plant Endophytic Microbiome for Heavy Metal Mitigation in Plants: A Perspective
Abstract
1. Introduction
2. Heavy Metal Stress and Its Impact on Plant Endophytic Microbiome
3. Omics Biology in Deciphering Plant Microbiome and Alleviation of Heavy Metal Stress
3.1. Metagenomics
3.2. Metaproteomics
3.3. Metatranscriptomics
3.4. Metabolomics
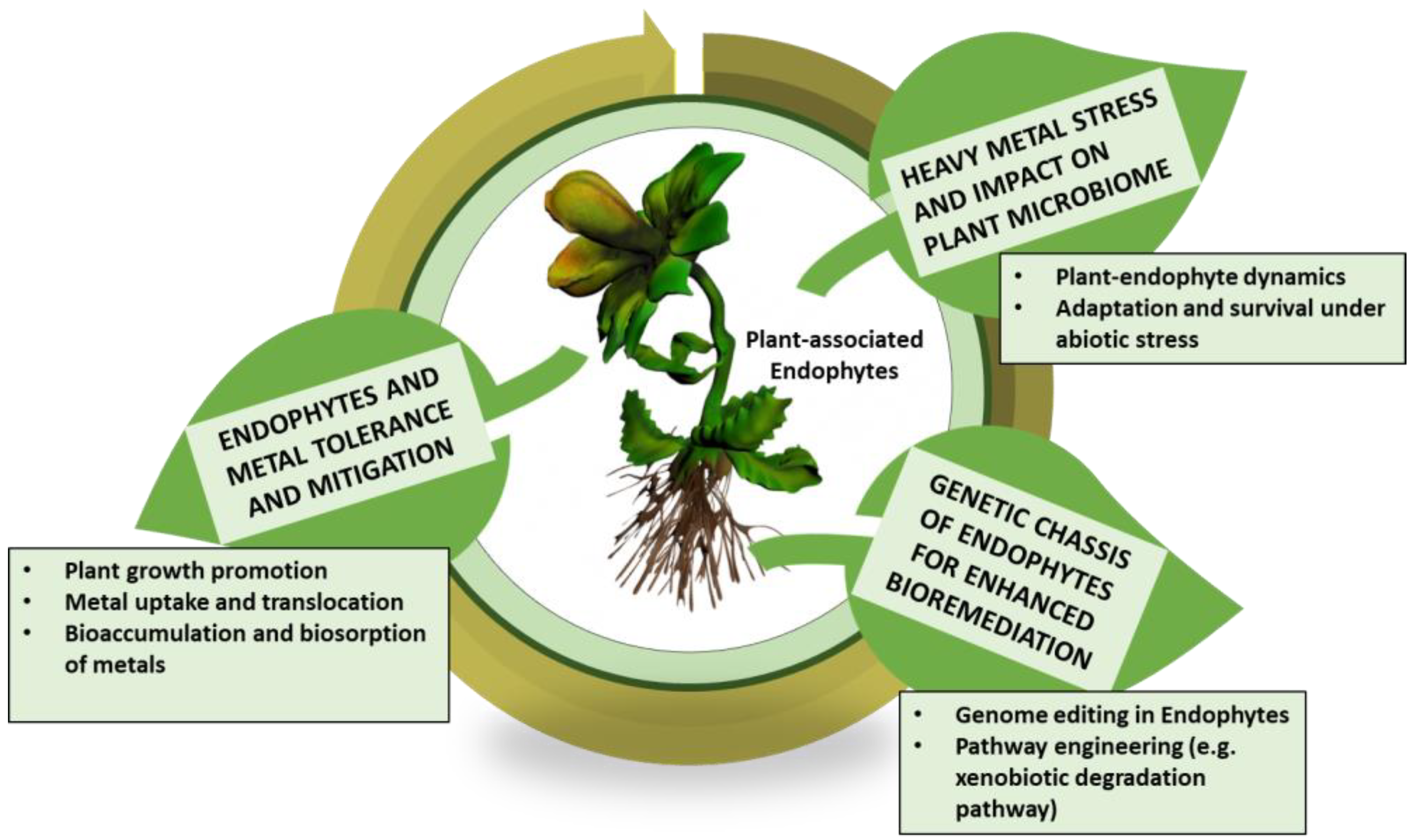
4. Harnessing Plant Endophytic Microbiome for Heavy Metal Tolerance and Mitigation
4.1. Characterization of Plant-Associated Endophytes and Their Dynamics
4.2. Endophytes and Molecular Mechanisms in Heavy Metal Tolerance
4.2.1. Direct and Indirect Promotion of Plant Growth
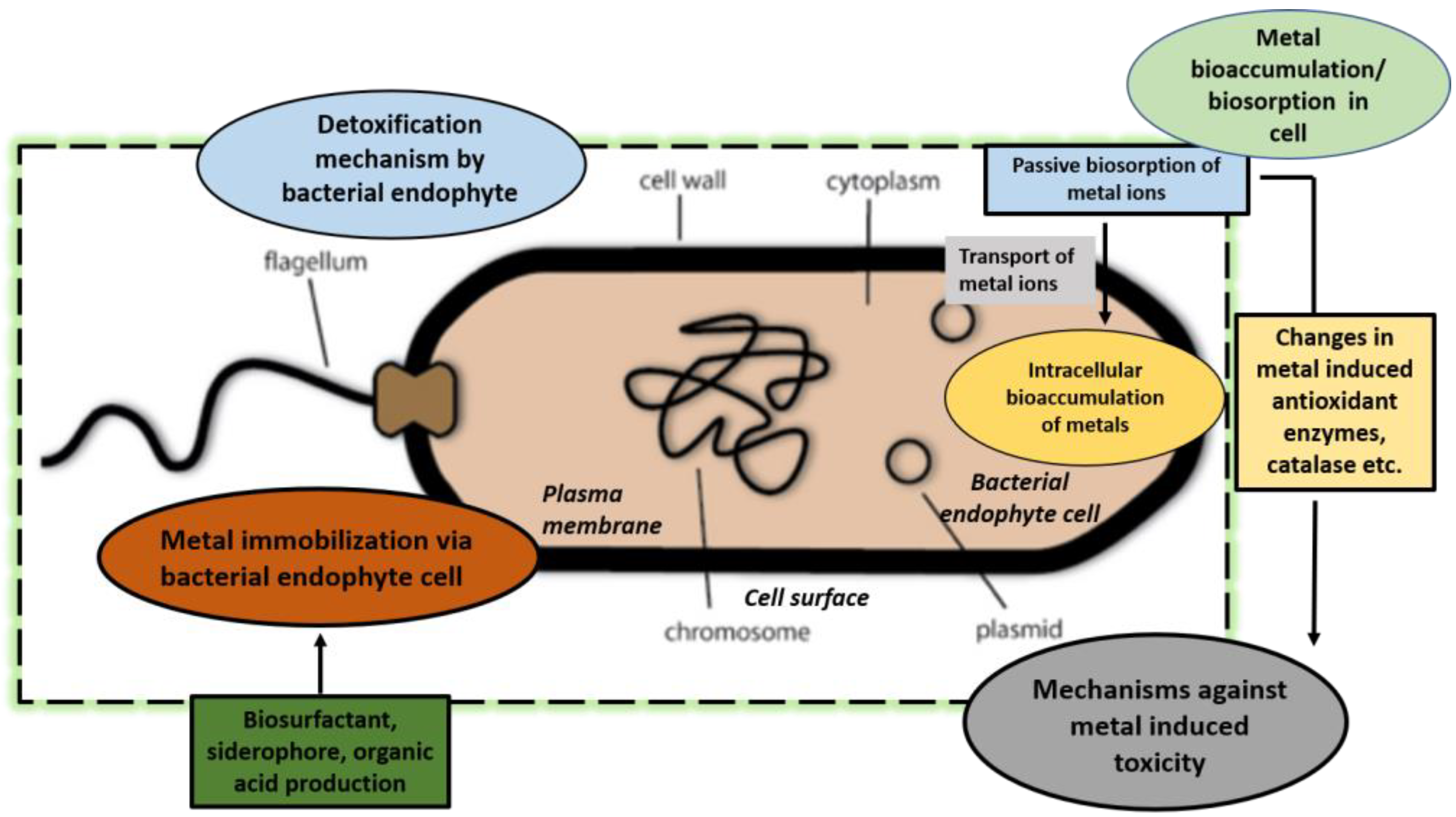
4.2.2. Plant Metal Uptake and Metal Stress Alleviation
4.2.3. Endophytes Enhance Metal Bioavailability
4.2.4. Uptake of Metals and Translocation by Endophytes
4.2.5. Bioaccumulation of Metals and Biosorption
4.3. Genetic Engineering-Mediated Chassis of Endophytes and Enhanced Tolerance to Heavy Metals
5. Perspectives and Future Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Oliveira, M.M. Mechanisms underlying plant resilience to water deficits: Prospects for water-saving agriculture. J. Exp. Bot. 2004, 55, 2365–2384. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P. Sustainable agriculture and nanotechnologies for food and nutraceutical production- an update. In Plant and Nanoparticles; Chen, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 978–981-19–2502-3. [Google Scholar]
- Timm, C.M.; Carter, K.R.; Carrell, A.A.; Jun, S.R.; Jawdy, S.S.; Vélez, J.M.; Gunter, L.E.; Yang, Z.; Nookaew, I.; Engle, N.L.; et al. Abiotic stresses shift belowground Populus-associated bacteria toward a core stress microbiome. mSystems 2018, 3, e00070-17. [Google Scholar] [CrossRef]
- Santos-Medellín, C.; Edwards, J.; Liechty, Z.; Nguyen, B.; Sundaresan, V. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. mBio 2017, 8, e00764-17. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Bajpai, M.; Singh, L.K.; Mishra, S.; Yadav, A.N. Phytohormones producing fungal communities: Metabolic engineering for abiotic stress tolerance in plants. In Agriculturally Important Fungi for Sustainable Agriculture; Gupta, V.K., Tuohy, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-mediated stress resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Bajpai, M.; Singh, L.K.; Yadav, A.; Bae, H. Portraying fungal mechanisms in stress tolerance: Perspective for sustainable agriculture. In Recent Trends in Mycological Research; Yadav, A., Ed.; Fungal Biology; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Liu, H.; Brettell, L.E. Plant defense by VOC-induced microbial priming. Trends Plant Sci. 2019, 24, 187–189. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; de Jonge, R.; Berendsen, R.L. The soil-borne legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Ruiz-Buck, D.; Mendes, L.W.; van Ijcken, W.F.J.; Gomez-Exposito, R.; Elsayed, S.S.; et al. Pathogen-induced activation of disease suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef]
- Tiwari, P.; Khare, T.; Shriram, V.; Bae, H.; Kumar, V. Exploring synthetic biology strategies for producing potent antimicrobial phytochemicals. Biotechnol. Adv. 2021, 48, 107729. [Google Scholar] [CrossRef]
- Tiwari, P.; Srivastava, Y.; Kumar, V. Antimicrobial peptides as effective agents against drug-resistant pathogens. In Antimicrobial Resistance; Kumar, V., Shriram, V., Paul, A., Thakur, M., Eds.; Springer: Singapore, 2022; pp. 289–322. [Google Scholar]
- Tiwari, P.; Mohd, A.; Bae, H. Endophyte-mediated bioremediation-an efficient biological strategy in ecological subsistence and agriculture. In Endophytic Fungi and Their Role in Sustainable Agriculture; Nova Publishers: New York, NY, USA, 2023. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Lu, T.; Ke, M.; Lavoie, M.; Jin, Y.; Fan, X.; Zhang, Z.; Fu, Z.; Sun, L.; Gillings, M.; Peñuelas, J.; et al. Rhizosphere microorganisms can influence the timing of plant flowering. Microbiome 2018, 6, 231. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Kang, S.; Bae, H. Plant-endophyte associations: Rich yet under-explored sources of novel bioactive molecules and applications. Microbiol. Res. 2023, 266, 127241. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Yang, N.; Wen, Z.; Sun, X.; Chai, Y.; Ma, Z. Wheat microbiome bacteria can reduce the virulence of a plant pathogenic fungus by altering histone acetylation. Nat. Commun. 2018, 9, 3429. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.J.; Kong, H.G.; Choi, K.; Kwon, S.K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslmam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–25. [Google Scholar]
- Singh, J.; Kalamdhad, A.S. Chemical speciation of heavy metals in compost and compost amended soil-a review. Int. J. Environ. Eng. Res. 2013, 2, 27–37. [Google Scholar]
- Tiwari, S.; Lata, C. Heavy metal stress, signaling, and tolerance due to plant-associated microbes: An overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef]
- Tiwari, P.; Mohd, A.; Basavegowda, N.; Chen, J. Plant-associated endophytes: Molecular mechanisms and significance in promoting sustainable agriculture. In Endophytes: Types, Potential Uses and Mechanisms of Action; Tiwari, P., Ed.; Nova Publishers: New York, NY, USA, 2022; ISBN 979-8-88697-045-6. [Google Scholar]
- Tiwari, P. Endophytes: Types, Potential Uses and Mechanisms of Action; Nova Publishers: New York, NY, USA, 2022; ISBN 979-8-88697-045-6. [Google Scholar]
- Franco-Franklin, V.; Moreno-Riascos, S.; Ghneim-Herrera, T. Are endophytic bacteria an option for increasing heavy metal tolerance of plants? A meta-analysis of the effect size. Front. Environ. Sci. 2021, 8, 603668. [Google Scholar] [CrossRef]
- Kumar, A.; Asthana, M.; Tiwari, P. Some key case studies on Plant-associated endophytes. In Endophytes: Types, Potential Uses, and Mechanisms of Action; Tiwari, P., Ed.; Nova Publishers: New York, NY, USA, 2022; ISBN 979-8-88697-045-6. [Google Scholar]
- Nguyen, K.B.T.; Phan, T.H.T. Application of plant endophytic microorganisms (Endophytes) in the treatment of heavy metal pollution in Soils. In Advances in Research on Water Resources and Environmental Systems; Vo, P.L., Tran, D.A., Pham, T.L., Le Thi Thu, H., Nguyen Viet, N., Eds.; GTER 2022; Environmental Science and Engineering; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Persoh, D. Plant-associated fungal communities in the light of metaomics. Fungal Diver. 2015, 75, 1–25. [Google Scholar] [CrossRef]
- Soldi, E.; Casey, C.; Murphy, B.R.; Hodkinson, T.R. Fungal endophytes for grass-based bioremediation: An endophytic consortium isolated from Agrostis stolonifera stimulates the growth of Festuca arundinacea in lead-contaminated soil. J. Fungi 2020, 6, 254. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Nai, F.; Rajkumar, M.; Luo, Y.; Rocha, I.; Freitas, H. The hyperaccumulator Sedum plumbizincicola harbors metal-resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contamnated soil. J. Environ. Manag. 2015, 156, 62–69. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ye, Z.; Yang, D.; Yan, J.; Xiao, L.; Zhong, T.; Yuan, M.; Cai, X.; Fang, Z.; Jing, Y. Characterization of endophytic Rahnella sp. JN6 from Polygonum pubescens and its potential in promoting growth and Cd, Pb, Zn uptake by Brassica napus. Chemosphere 2013, 90, 1960–1965. [Google Scholar] [PubMed]
- Madhaiyan, M.; Poonguzhali, S.; Sa, T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 2007, 69, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, H.; Dong, Y.; He, Y.; Liu, C. Isolation of vanadium-resistance endophytic bacterium PRE01 from Pteris vittata in stone coal smelting district and characterization for potential use in phytoremediation. J. Hazard. Mater. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Toju, H.; Peay, K.G.; Yamamichi, M.; Narisawa, K.; Hiruma, K.; Naito, K.; Fukuda, S.; Ushio, M.; Nakaoka, S.; Onoda, Y.; et al. Publisher Correction: Core microbiomes for sustainable agroecosystems. Nature Plants 2018, 4, 733. [Google Scholar] [CrossRef]
- Tiwari, S.; Sarangi, B.K.; Thul, S.T. Identification of arsenic resistant endophytic bacteria from Pteris vittata roots and characterization for arsenic remediation application. J. Environ. Manag. 2016, 180, 359–365. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Sun, Y.; Zhao, K.; Xiang, Q.; Yu, X.; Zhang, X.; Chen, Q. Genetic diversity and characterization of arsenic-resistant endophytic bacteria isolated from Pteris vittata, an arsenic hyperaccumulator. BMC Microbiol. 2018, 18, 42. [Google Scholar] [CrossRef]
- Wang, L.; Lin, H.; Dong, Y.; Li, B.; He, Y. Effects of endophytes inoculation on rhizosphere and endosphere microecology of Indian mustard (Brassica juncea) grown in vanadium-contaminated soil and its enhancement on phytoremediation. Chemosphere 2020, 240, 124891. [Google Scholar] [CrossRef]
- Ahsan, M.T.; Najam-Ul-Haq, M.; Saeed, A.; Mustafa, T.; Afzal, M. Augmentation with potential endophytes enhances phytostabilization of Cr in contaminated soil. Environ. Sci. Pollut. Res. Int. 2018, 25, 7021–7032. [Google Scholar] [CrossRef]
- Ahsan, M.T.; Najam-Ul-Haq, M.; Idrees, M.; Ullah, I.; Afzal, M. Bacterial endophytes enhance phytostabilization in soils contaminated with uranium and lead. Int. J. Phytoremed. 2017, 19, 937–946. [Google Scholar] [CrossRef]
- Shahzad, R.; Bilal, S.; Imran, M.; Khan, A.L.; Alosaimi, A.A.; Abdullah Al-Shwyeh, H.; Almahasheer, H.; Rehman, S.; Lee, I.-J. Amelioration of heavy metal stress by endophytic Bacillus amyloliquefaciens RWL-1 in rice by regulating metabolic changes: Potential for bacterial bioremediation. Biochem. J. 2019, 476, 3385–3400. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.F.; Xia, J.J.; Jiang, C.Y.; He, L.Y.; Qian, M. Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ. Pollut. 2008, 156, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Gaiero, J.R.; McCall, C.A.; Thompson, K.A.; Day, N.J.; Best, A.S.; Dunfield, K.E. Inside the root microbiome: Bacterial root endophytes and plant growth promotion. Am. J. Bot. 2013, 100, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Leroy, C.; Jauneau, A.; Martinez, Y.; Cabin-Flaman, A.; Gibouin, D.; Orivel, J.; Sejalon- Delmas, N. Exploring fungus–plant N transfer in a tripartite ant–plant–fungus mutualism. Ann. Bot. 2017, 120, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ren, C.G.; Zhou, T.; Wei, Y.J.; Dai, C.C. A novel exopolysaccharide elicitor from endophytic fungus Gilmaniella sp. AL12 on volatile oils accumulation in Atractylodes lancea. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Babu, A.G.; Kim, J.D.; Oh, B.T. Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB-1. J. Hazard Mater. 2013, 250–251. [Google Scholar] [CrossRef]
- Wan, Y.; Luo, S.; Chen, J.; Xiao, X.; Chen, L.; Zeng, G.; Liu, C.; He, Y. Effect of endophyte-infection on growth parameters and Cd-induced phytotoxicity of Cd hyperaccumulator Solanum nigrum L. Chemosphere 2012, 89, 743–750. [Google Scholar] [CrossRef]
- He, L.Y.; Zhang, Y.F.; Ma, H.Y.; Su, L.N.; Chen, Z.Y.; Wang, Q.Y.; Qian, M.; Sheng, X.F. Characterization of copper-resistant bacteria and assessment of bacterial communities in rhizosphere soils of copper-tolerant plants. Appl. Soil Ecol. 2010, 44, 49–55. [Google Scholar] [CrossRef]
- Tanya Morocho, M.; Leiva-Mora, M. Microorganismos eficientes, propiedades funcionales y aplicaciones agrícolas. Cent. Agrícola 2019, 46, 93–103. [Google Scholar]
- Tiwari, P.; Bae, H. Endophytic fungi: Key insights, emerging prospects, and challenges in natural product drug discovery. Microorganisms 2022, 10, 360. [Google Scholar] [CrossRef]
- Bose, S.K.; Das, J.; Bajpai, M.; Gautam, A.; Tiwari, P. Endophytic actinomycetes: Overview, Distribution, and multi-faceted Applications. In Endophytes: Types, Potential Uses and Mechanisms of Action; Tiwari, P., Ed.; Nova Publishers: New York, NY, USA, 2022; ISBN 979-8-88697-045-6. [Google Scholar]
- Zhai, Y.; Chen, Z.; Malik, K.; Wei, X.; Li, C. Effect of fungal endophyte Epichloë bromicola infection on Cd tolerance in Wild Barley (Hordeum brevisubulatum). J. Fungi 2022, 8, 366. [Google Scholar] [CrossRef] [PubMed]
- Wiewióra, B.; Żurek, G. The response of the associations of grass and Epichloë endophytes to the increased content of heavy metals in the soil. Plants 2021, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mistry, V.; Kumar, V.; Tiwari, P. Production of effective phyto antimicrobials via metabolic engineering strategies. Curr. Top. Med. Chem. 2022, 22, 1068–1092. [Google Scholar] [PubMed]
- Germaine, K.J.; Keogh, E.; Ryan, D.; Dowling, D.N. Bacterial endophyte-mediated naphthalene phytoprotection and phytoremediation. FEMS Microbiol. Lett. 2009, 296, 226–234. [Google Scholar] [CrossRef]
- Feng, N.-X.; Yua, J.; Zhao, H.-M.; Cheng, Y.-T.; Moa, C.-H.; Ca, Q.-Y.; Li, Y.-W.; Li, H.; Wong, M.-H. Efficient phytoremediation of organic contaminants in soils using plant–endophyte partnerships. Sci. Total Environ. 2017, 583, 352–368. [Google Scholar] [CrossRef]
- Phurailatpam, L.; Mishra, S. Role of plant endophytes in conferring abiotic stress tolerance. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II; Hasanuzzaman, M.M., Ed.; Springer: Singapore, 2020; pp. 603–628. [Google Scholar]
- Yang, Y.; Ding, J.; Chi, Y.; Yuan, J. Characterization of bacterial communities associated with the exotic and heavy metal tolerant wetland plant Spartina alterniflora. Sci. Rep. 2020, 10, 17985. [Google Scholar] [CrossRef]
- Rajkumar, M.; Sandhya, S.; Prasad, M.N.V.; Freitas, H. Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol. Adv. 2012, 30, 1562–1574. [Google Scholar] [CrossRef]
- Hauser, M.T. Molecular basis of natural variation and environmental control of trichome patterning. Front. Plant Sci. 2014, 5, 320. [Google Scholar] [CrossRef]
- Boiteau, R.M.; Shaw, J.B.; Pasa-Tolic, L.; Koppenaal, D.W.; Jansson, J.K. Micronutrient metal speciation is controlled by competitive organic chelation in grassland soils. Soil Biol. Biochem. 2018, 120, 283–291. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 1–12. [Google Scholar] [CrossRef]
- Borymski, S.; Cycon, M.; Beckmann, M.; Mur, L.A.; Piotrowska-Seget, Z. Plant species and heavy metals affect biodiversity of microbial communities associated with metal-tolerant plants in metalliferous soils. Front. Microbiol. 2018, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Phurailatpam, L.; Dalal, V.K.; Singh, N.; Mishra, S. Heavy metal stress alleviation through omics analysis of soil and plant microbiome. Front. Sustain. Food Syst. 2022, 5, 817932. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S. Bioremediation of heavy metals from industrial effluents by endophytes and their metabolic activity: Recent advances. Bioresour. Technol. 2021, 339, 125589. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Sharma, T.; Dhar, M. “Omics” tools for better understanding the plant–endophyte interactions. Front. Plant Sci. 2016, 7, 955. [Google Scholar] [CrossRef]
- Salam, L.B.; Obayori, O.S.; Ilori, M.O.; Amund, O.O. Effects of cadmium perturbation on the microbial community structure and heavy metal resistome of a tropical agricultural soil. Bioresour. Bioprocess. 2020, 7, 1–19. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Huang, H.; Mou, L.; Ru, J.; Zhao, J.; Xiao, S. Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci. Total Environ. 2018, 637, 1400–1412. [Google Scholar] [CrossRef]
- Photolo, M.M.; Sitole, L.; Mavumengwana, V.; Tlou, M.G. Genomic and physiological investigation of heavy metal resistance from plant endophytic Methylobacterium radiotolerans MAMP 4754, isolated from Combretum erythrophyllum. Int. J. Environ. Res. Public Health 2021, 18, 997. [Google Scholar] [CrossRef]
- Feng, G.; Xie, T.; Wang, X.; Bai, J.; Tang, L.; Zhao, H.; Wei, W.; Wang, M.; Zhao, Y. Metagenomic analysis of microbial community and function involved in cd-contaminated soil. BMC Microbiol. 2018, 18, 11. [Google Scholar] [CrossRef]
- Lehembre, F.; Doillon, D.; David, E.; Perrotto, S.; Baude, J.; Foulon, J.; Harfouche, L.; Vallon, L.; Poulain, J.; Da Silva, V.; et al. Soil metatranscriptomics for mining eukaryotic heavy metal resistance genes. Environ. Microbiol. 2013, 15, 2829–2840. [Google Scholar] [CrossRef]
- Yu, Z.; Pei, Y.; Zhao, S. Metatranscriptomic analysis reveals active microbes and genes responded to short-term Cr (VI) stress. Ecotoxicology 2021, 30, 1527–1537. [Google Scholar] [CrossRef]
- Pei, Y.; Tao, C.; Ling, Z.; Yu, Z.; Ji, J.; Khan, A.; Mamtimin, T.; Liu, P.; Li, X. Exploring novel Cr (VI) remediation genes for Cr (VI)-contaminated industrial wastewater treatment by comparative metatranscriptomics and metagenomics. Sci. Total Environ. 2020, 742, 140435. [Google Scholar] [CrossRef] [PubMed]
- Mattarozzi, M.; Manfredi, M.; Montanini, B.; Gosetti, F.; Sanangelantoni, A.M.; Marengo, E.; Careri, M.; Visioli, G. A metaproteomic approach dissecting major bacterial functions in the rhizosphere of plants living in serpentine soil. Anal. Bioanal. Chem. 2017, 409, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Farinati, S.; DalCorso, G.; Panigati, M.; Furini, A. Interaction between selected bacterial strains and Arabidopsis halleri modulates shoot proteome and cadmium and zinc accumulation. J. Exp. Bot. 2011, 62, 3433–3447. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, L.; Liu, Q.; Vestergård, M.; Topalovic, O.; Wang, Q.; Zhou, Q.; Huang, L.; Yang, X.; Feng, Y. The plant-growth-promoting bacteria promote cadmium uptake by inducing a hormonal crosstalk and lateral root formation in a hyperaccumulator plant Sedum alfredii. J. Hazard. Mater. 2020, 395, 122661. [Google Scholar] [CrossRef] [PubMed]
- Kalu, C.M.; Ogola, H.J.O.; Selvarajan, R.; Tekere, M.; Ntushelo, K. Fungal and metabolome diversity of the rhizosphere and endosphere of Phragmites australis in an AMD-polluted environment. Heliyon 2021, 7, e06399. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.Y.; Wang, S.K.; Zhou, J.; Di, D.L.; Sun, P.; Huang, D.Z. Inoculation with indigenous rhizosphere microbes enhances aboveground accumulation of lead in Salix integra Thunb. by improving transport coefficients. Front. Microbiol. 2021, 12, 686812. [Google Scholar] [CrossRef]
- Han, H.; Zhang, H.; Qin, S.; Zhang, J.; Yao, L.; Chen, Z.; Yang, J. Mechanisms of Enterobacter bugandensis TJ6 immobilization of heavy metals and inhibition of Cd and Pb uptake by wheat based on metabolomics and proteomics. Chemosphere 2021, 276, 130–157. [Google Scholar] [CrossRef]
- Sahu, P.K.; Mishra, S. Effect of hybridization on endophytes: The endo-microbiome dynamics. Symbiosis 2021, 84, 369–377. [Google Scholar] [CrossRef]
- Akinsanya, M.A.; Goh, J.K.; Lim, S.P.; Ting, A.S.Y. Metagenomics study of endophytic bacteria in Aloe vera using next-generation technology. Genom. Data 2015, 6, 159–163. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Fan, M.; Xiao, X.; Guo, Y.; Zhang, J.; Wang, E.; Chen, W.; Lin, H.; Wei, G. Enhanced phytoremediation of Robinia pseudoacacia in heavy metal-contaminated soils with rhizobia and the associated bacterial community structure and function. Chemosphere 2018, 197, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Remenár, M.; Harichová, J.; Zámocký, M.; Pangallo, D.; Szemes, T.; Budiš, J.; Soltys, K.; Ferianc, P. Metagenomics of a nickel-resistant bacterial community in an anthropogenic nickel contaminated soil in southwest Slovakia. Biol. Plant. 2017, 72, 971–981. [Google Scholar] [CrossRef]
- Gutleben, J.; Chaib De Mares, M.; Van Elsas, J.D.; Smidt, H.; Overmann, J.; Sipkema, D. The multi-omics promise in context: From sequence to microbial isolate. Crit. Rev. Microbiol. 2018, 44, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, S.; Shi, S.; Ren, L.; Yan, W.; Zhao, H. Microbial diversity and metaproteomic analysis of activated sludge responses to naphthalene and anthracene exposure. RSC Adv. 2019, 9, 22841–22852. [Google Scholar] [CrossRef]
- Mengoni, A.; Barzanti, R.; Gonnelli, C.; Gabbrielli, R.; Bazzicalupo, M. Characterization of nickel-resistant bacteria isolated from serpentine soil. Environ. Microbiol. 2001, 3, 691–698. [Google Scholar] [CrossRef]
- Simon, C.; Daniel, R. Metagenomic analyses: Past and future trends. Appl. Environ. Microbiol. 2011, 77, 1153–1161. [Google Scholar] [CrossRef]
- White, R.A.; Borkum, M.I.; Rivas-Ubach, A.; Bilbao, A.; Wendler, J.P.; Colby, S.M.; Köberl, M.; Jansson, C. From data to knowledge: The future of multi-omics data analysis for the rhizosphere. Rhizosphere 2017, 3, 222–229. [Google Scholar] [CrossRef]
- Thakur, B.; Yadav, R.; Fraissinet-Tachet, L.; Marmeisse, R.; Reddy, M.S. Isolation of multi-metal tolerant ubiquitin fusion protein from metal polluted soil by metatranscriptomic approach. J. Microbiol. Methods 2018, 152, 119–125. [Google Scholar] [CrossRef]
- Mukherjee, A.; Reddy, M.S. Metatranscriptomics: An approach for retrieving novel eukaryotic genes from polluted and related environments. 3 Biotech 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Mukherjee, A.; Yadav, R.; Marmeisse, R.; Fraissinet-Tachet, L.; Reddy, M.S. Heavy metal hypertolerant eukaryotic aldehyde dehydrogenase isolated from metal contaminated soil by metatranscriptomics approach. Biochimie 2019, 160, 183–192. [Google Scholar] [CrossRef]
- Grim, C.M.; Luu, G.T.; Sanchez, L.M. Staring into the void: Demystifying microbial metabolomics. FEMS Microbiol. Lett. 2019, 366, fnz135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Huang, Z.; He, L.Y.; Sheng, X.F. Assessment of bacterial communities and characterization of lead-resistant bacteria in the rhizosphere soils of metal-tolerant Chenopodium ambrosioides grown on lead–zinc mine tailings. Chemosphere 2012, 87, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed your friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Toyama, T.; Furukawa, T.; Maeda, N.; Inoue, D.; Sei, K.; Mori, K.; Kikuchi, S.; Ike, M. Accelerated biodegradation of pyrene and benzo [a] pyrene in the Phragmites australis rhizosphere by bacteria–root exudate interactions. Water Res. 2011, 45, 1629–1638. [Google Scholar] [CrossRef]
- Neal, A.L.; Ahmad, S.; Gordon-Weeks, R.; Ton, J. Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS ONE 2012, 7, e35498. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Kumar, S.; Trivedi, P.K. Heavy metal stress signaling in plants. In Plant Metal Interaction- Emerging Remediation Techniques; Ahmad, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 585–603. [Google Scholar]
- Perez, C.A.; Perez, C.C.; Chamorro, A.L. Diversidad de bacterias endofitas asociadas a cultivo de arroz en el departamento de cordoba-colombia. Estudio preliminar. Rev. Colombiana Cienc. Anim. 2013, 5, 83–92. [Google Scholar] [CrossRef]
- Ahemad, M.; Khan, M.S. Alleviation of fungicide-induced phytotoxicity in greengram [Vigna radiata (L.) Wilczek using fungicide tolerant and plant growth promoting Pseudomonas strain. Saudi J. Biol. Sci. 2012, 19, 451–459. [Google Scholar] [CrossRef]
- Srivastava, S.; Verma, P.C.; Chaudhry, V.; Singh, N.; Abhilash, P.C.; Kumar, K.V.; Sharma, N.; Singh, N. Influence of inoculation of arsenic-resistant Staphylococcus arlettae on growth and arsenic uptake in Brassica juncea (L.) czern. Var. R-46. J. Hazard Mater. 2013, 262, 1039–1047. [Google Scholar] [CrossRef]
- Zhang, Y.F.; He, L.Y.; Chen, Z.J.; Zhang, W.H.; Wang, Q.Y.; Qian, M.; Sheng, X.F. Characterization of lead-resistant and ACC deaminase-producing endophytic bacteria and their potential in promoting lead accumulation of rape. J. Hazard Mater. 2011, 186, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.; Hsieh, M.; Kandel, S.L.; Cantillo, J.; Doty, S.L.; Kim, S.H. Do endophytes promote growth of host plants under stress? A meta-analysis on plant stress mitigation by endophytes. Microb. Ecol. 2018, 75, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Ham, B.-K.; Chen, J.; Yan, Y.; Lucas, W.J. Insights into plant phosphate sensing and signaling. Curr. Opin. Biotechnol. 2018, 49, 1–9. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Copeland, J.; Wang, P.W.; Guttman, D.S.; Kotanen, O.M.; Johnson, M.T.J. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc. Natl. Acad. Sci. USA. 2018, 115, E1157–E1165. [Google Scholar] [CrossRef]
- Durán, P.; Thiergart, T.; Garrido-Oter, R.; Agler, M.; Kemen, E.; Schulze-Lefert, P.; Hacquard, S. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell 2018, 175, 973–983. [Google Scholar] [CrossRef]
- Castrillo, G.; Teixeira, P.J.P.L.; Paredes, S.H.; Law, T.F.; de Lorenzo, L.; Feltcher, M.E.; Finkel, O.M.; Breakfield, N.W.; Mieczkowski, P.; Jones, C.D.; et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 2017, 543, 513–518. [Google Scholar] [CrossRef]
- Teixeira, P.J.P.L.; Colaianni, N.R.; Fitzpatrick, C.R.; Dangl, J.L. Beyond pathogens: Microbiota interactions with the plant immune system. Curr. Opin. Microbiol. 2019, 49, 7–17. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef]
- Mercado-Blanco, J. Life of microbes inside the plant. In Principles of Plant-Microbe Interactions; Lugtenberg, B.J.J., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 25–32. [Google Scholar]
- Bacon, C.W.; White, J. Microbial Endophytes; Marcel Dekker, CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780824788315. [Google Scholar]
- Tiwari, P.; Srivastava, Y.; Bae, H. Trends of pharmaceutical design of Endophytes as anti-infective. Curr. Top. Med. Chem. 2021, 21, 1572–1586. [Google Scholar] [CrossRef]
- De Bary, A. Morphologic und Physiologie der Plize, Flechten, und Myxomyceten; Hofmeister’s Hand Book of Physiological Botany; Wilhelm Engelmann: Leipzig, German, 1866; Volume 2. [Google Scholar]
- Wilson, D. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Backman, P.A.; Sikora, R.A. Endophytes: An emerging tool for biological control. Biolog. Control 2008, 46, 1–3. [Google Scholar] [CrossRef]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F., Jr. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.; Msimbira, L.A.; Nazari, M.; Antar, M.; Pagé, A.; Shah, A.; Monjezi, N.; Zajonc, J.; Tanney, C.A.S.; Backer, R.; et al. The coevolution of plants and microbes underpins sustainable agriculture. Microorganisms 2021, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Prasad, M.N.V.; Sandhya, S.; Freitas, H. Climate change is driven by plant-metal-microbe interactions. Environ. Int. 2013, 53, 74–86. [Google Scholar] [CrossRef]
- Chen, L.; Luo, S.L.; Li, X.J.; Wan, Y.; Chen, J.L.; Liu, C.B. Interaction of Cd hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biol. Biochem. 2014, 68, 300–308. [Google Scholar] [CrossRef]
- Zhu, L.J.; Guan, D.X.; Luo, J.; Rathinasabapathi, B.; Ma, L.Q. Characterization of arsenic-resistant endophytic bacteria from hyperaccumulators Pteris vittata and Pteris multifida. Chemosphere 2014, 113, 9–16. [Google Scholar] [CrossRef]
- Arantza, S.J.; Hiram, M.R.; Erika, K.; Chávez-Avilés, M.N.; Valiente-Banuet, J.I.; Fierros-Romero, G. Bio- and phytoremediation: Plants and microbes to the rescue of heavy metal polluted soils. SN Appl. Sci. 2022, 4, 59. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, S.K.; Singh, V.K.; Singh, M.K.; Modi, A.; Zhimo, V.Y.; Singh, A.V.; Kumar, A. Endophytic microbe approaches in bioremediation of organic pollutants. In Microbial Endophytes; Woodhead Publishing: Sawston, Cambridgeshire, UK, 2020; pp. 157–174. [Google Scholar]
- Rajkumar, M.; Ae, N.; Freitas, H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 2009, 77, 153–160. [Google Scholar] [CrossRef]
- Pereira, S.I.A.; Castro, P.M.L. Diversity and characterization of culturable bacterial endophytes from Zea mays and their potential as plant growth promoting agents in metal-degraded soils. Environ. Sci. Pollut. Res. 2014, 21, 14110–14123. [Google Scholar] [CrossRef]
- Hurek, T.; Reinhold-Hurek, B. Azoarcus sp. strain BH72 as a model for nitrogen-fixing grass endophytes. J. Biotechnol. 2003, 106, 169–178. [Google Scholar] [CrossRef]
- Doty, S.L.; Oakley, B.; Xin, G.; Kang, J.W.; Singleton, G.; Khan, Z.; Vajzovic, A.; Staley, J.T. Diazotrophic endophytes of native black cottonwood and willow. Symbiosis 2009, 47, 23–33. [Google Scholar] [CrossRef]
- Nautiyal, C.S.; Bhadauria, S.; Kumar, P.; Lal, H.; Mondal, R.; Verma, D. Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol. Lett. 2000, 182, 291–296. [Google Scholar] [CrossRef]
- van der Hiejden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.W.; Hinton, D.M. Endophytic and biological control potential of Bacillus mojavensis and related species. Biol. Control. 2002, 23, 274–284. [Google Scholar] [CrossRef]
- Viterbo, A.; Landau, U.; Kim, S.; Chernin, L.; Chet, I. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiol. Lett. 2010, 305, 42–48. [Google Scholar] [CrossRef]
- Jiang, M.; Cao, L.; Zhang, R. Effects of Acacia (Acacia auriculaeformis A. Cunn) associated fungi on mustard (Brassica juncea (L.) Coss. var. foliosa Bailey) growth in Cd and Ni-contaminated soils. Lett. Appl. Microbiol. 2008, 47, 561–565. [Google Scholar] [CrossRef]
- Deng, Z.; Cao, L.; Zhang, R.; Wang, W.; Shi, Y.; Tan, H.; Wang, Z.; Cao, L. Enhanced phytoremediation of multi-metal contaminated soils by interspecific fusion between the protoplasts of endophytic Mucor sp. CBRF59 and Fusarium sp. CBRF14. Soil Biol. Biochem. 2014, 77, 31–40. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Beneficial role of bacterial endophytes in heavy metal phytoremediation. J. Environ. Manag. 2016, 174, 14–25. [Google Scholar] [CrossRef]
- Sheoran, N.; Valiya Nadakkakath, A.; Munjal, V.; Kundu, A.; Subaharan, K.; Venugopal, V.; Rajamma, S.; Eapen, S.J.; Kumar, A. Genetic analysis of plant endophytic Pseudomonas putida BP25 and chemo-profiling of its antimicrobial volatile organic compounds. Microbiol. Res. 2015, 173, 66–78. [Google Scholar] [CrossRef]
- Aravind, R.; Eapen, S.J.; Kumar, A.; Ramana, K.V. Screening of endophytic bacteria and evaluation of selected isolates for suppression of burrowing nematode (Radopholus similis Thorne) using three varieties of black pepper (Piper nigrum L.). Crop. Prot. 2010, 29, 318–324. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Beauchamp, C.J. A review of issues related to measuring colonization of plant roots by bacteria. Can. J. Microbiol. 1992, 38, 1219–1232. [Google Scholar] [CrossRef]
- Sturz, A.V.; Matheson, B.G. Populations of endophytic bacteria which influence host-resistance to Erwinia-induced bacterial soft rot in potato tubers. Plan. Soil 1996, 184, 265–271. [Google Scholar] [CrossRef]
- Gechev, T.S.; van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Parmar, S.; Tang, W.; Hu, H.; White, J.F., Jr.; Li, H. Effects of fungal seed endophyte FXZ2 on Dysphania ambrosioides Zn/Cd tolerance and accumulation. Front. Microbiol. 2022, 13, 995830. [Google Scholar] [CrossRef]
- Shin, M.; Shim, J.; You, Y.; Myung, H.; Bang, K.S.; Cho, M.; Kamala-Kannan, S.; Oh, B.T. Characterization of lead resistant endophytic Bacillus sp. MN3-4 and its potential for promoting lead accumulation in metal hyperaccumulator Alnus firma. J. Hazard. Mater. 2012, 199, 314–320. [Google Scholar] [CrossRef]
- Babu, A.G.; Shea, P.J.; Sudhakar, D.; Jung, I.B.; Oh, B.T. Potential use of Pseudomonas koreensis AGB-1 in association with Miscanthus sinensis to remediate heavy metal(loid)-contaminated mining site soil. J. Environ. Manag. 2015, 151, 160–166. [Google Scholar] [CrossRef]
- Guo, H.J.; Luo, S.L.; Chen, L.; Xiao, X.; Xi, Q.; Wei, W.Z.; Zeng, G.; Liu, C.; Wan, Y.; Chen, J.; et al. Bioremediation of heavy metals by growing hyperaccumulator endophytic bacterium Bacillus sp. L14. Bioresour. Technol. 2010, 101, 8599–8605. [Google Scholar] [CrossRef]
- Tiwari, P.; Bae, H. Horizontal gene transfer and endophytes: An implication for the acquisition of novel traits. Plants 2020, 9, 305. [Google Scholar] [CrossRef]
- Zhang, X.X.; Li, C.J.; Nan, Z.B. Effects of cadmium stress on growth and antioxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. J. Hazard. Mater. 2010, 175, 703–709. [Google Scholar] [CrossRef]
- Parmar, S.; Sharma, V.K.; Li, T.; Tang, W.; Li, H. Fungal seed endophyte FZT214 improves Dysphania ambrosioides Cd tolerance throughout different developmental stages. Front. Microbiol. 2022, 12, 783475. [Google Scholar] [CrossRef]
- Cursino, L.; Mattos, S.V.; Azevedo, V.; Galarza, F.; Bucker, D.H.; Chartone-Souza, E.; Nascimento, A. Capacity of mercury volatilization by mer (from Escherichia coli) and glutathione S-transferase (from Schistosoma mansoni) genes cloned in Escherichia coli. Sci. Total Environ. 2000, 261, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.L.; Stoyanov, J.V.; Kidd, S.P.; Hobman, J.L. The merR family of transcriptional regulators. FEMS Microbiol. Rev. 2003, 27, 145–163. [Google Scholar] [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Luo, S.L.; Chen, L.; Chen, J.L.; Xiao, X.; Xu, T.Y.; Wan, Y.; Rao, C.; Liu, C.B.; Liu, Y.T.; Lai, C.; et al. Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere 2011, 85, 1130–1138. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Luo, Y.M.; Freitas, H. Inoculation of endophytic bacteria on the host and non-host plants effects on plant growth and Ni uptake. J. Hazard. Mater. 2011, 195, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, H.; Dong, Y.; Li, B. Increase of P and Cd bioavailability in the rhizosphere by endophytes promoted phytoremediation efficiency of Phytolacca acinosa. J Hazard. Mater. 2022, 431, 128546. [Google Scholar] [CrossRef]
- Luo, S.; Xu, T.; Chen, L.; Chen, J.; Rao, C.; Xiao, X.; Wan, Y.; Zeng, G.; Long, F.; Liu, C.; et al. Endophyte-assisted promotion of biomass production and metal uptake of energy crop sweet sorghum by plant-growth-promoting endophyte Bacillus sp. SLS18. Appl. Microbiol. Biotechnol. 2012, 93, 1745–1753. [Google Scholar] [CrossRef]
- Visioli, G.; D’Egidio, S.; Vamerali, T.; Mattarozzi, M.; Sanangelantoni, A.M. Culturable endophytic bacteria enhance Ni translocation in the hyperaccumulator Noccaea caerulescens. Chemosphere 2014, 117, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.N.; Zhang, Y.F.; He, L.Y.; Chen, Z.J.; Wang, Q.Y.; Qian, M.; Sheng, X.F. Genetic diversity and characterization of heavy metal-resistant-endophytic bacteria from two copper-tolerant plant species on copper mine wasteland. Bioresour. Technol. 2010, 101, 501–509. [Google Scholar] [CrossRef]
- Luo, S.L.; Wan, Y.; Xiao, X.; Guo, H.; Chen, L.; Xi, Q.; Zeng, G.; Liu, C.; Chen, J. Isolation and characterization of endophytic bacterium LRE07 from cadmium hyperaccumulator Solanum nigrum L. and its potential for remediation. Appl. Microbiol. Biotechnol. 2011, 89, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–262. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B.; Schiewer, S. Biosorption of metals. In Encyclopedia of Bioprocess Technology; Flickinger, M., Drew, S.W., Eds.; Wiley: New York, NY, USA, 1999; pp. 433–453. [Google Scholar]
- Yang, Y.; Liu, X.; Cai, J.; Chen, Y.; Li, B.; Guo, Z.; Huang, G. Genomic characteristics and comparative genomics analysis of the endophytic fungus Sarocladium brachiariae. BMC Genom. 2019, 20, 782. [Google Scholar] [CrossRef]
- He, X.; Yuan, Z. Near-Chromosome-Level genome assembly of the Dark septate endophyte Laburnicola rhizohalophila: A model for investigating root-fungus symbiosis. Genome Biol. Evol. 2021, 13, evab026. [Google Scholar] [CrossRef]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar]
- Siciliano, S.D.; Fortin, N.; Mihoc, A.; Wisse, G.; Labelle, S.; Beaumier, D.; Ouellette, D.; Roy, R.; Whyte, L.G.; Banks; et al. Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl. Environ. Microbiol. 2001, 67, 2469–2475. [Google Scholar] [CrossRef]
- Van Aken, B.; Yoon, J.M.; Schnoor, J.L. Biodegradation of nitro-substituted explosives TNT, RDX, and HMX by a phytosymbiotic Methylobacterium sp. associated with Populus (Populus deltoides × nigra DN34). Appl. Environ. Microbiol. 2004, 70, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Barac, T.; Taghavi, S.; Borremans, B.; Provoost, A.; Oeyen, L.; Colpaert, J.V.; Vangronsveld, J.; Lelie, D.V. Engineered endophytic bacteria improve phytoremediation of water-soluble, volatile, organic pollutants. Nat. Biotechnol. 2004, 22, 583–588. [Google Scholar] [CrossRef]
- Newman, L.A.; Reynolds, C.M. Bacteria and phytoremediation: New uses for endophytic bacteria in plants. Trends Biotechnol. 2005, 23, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Gao, Y.; Li, Q.; Mao, W.; Jiang, Y.; Li, H. Effects of Endophytes on Dysphania ambrosioides heavy metal tolerance and accumulation under multi-heavy metal stress. Chiang Mai J. Sci. 2022, 49, 312–324. [Google Scholar] [CrossRef]
- Bibi, S.; Hussain, A.; Hamayun, M.; Rahman, H.; Iqbal, A.; Shah, M.; Irshad, M.; Qasim, M.; Islam, B. Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere 2018, 211, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M.; Irshad, M.; Rahman, H.; Qasim, M.; Afridi, S.G.; Qadir, M.; Hussain, A. Alleviation of heavy metal toxicity and phytostimulation of Brassica campestris L. by endophytic Mucor sp. MHR-7. Ecotoxicol. Environ. Saf. 2017, 142, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Doty, S.L. Enhancing phytoremediation through the use of transgenics and endophytes. New Phytol. 2008, 179, 318–333. [Google Scholar] [CrossRef]
- Abhilash, P.C.; Jamil, S.; Singh, N. Transgenic plants for enhanced biodegradation and phytoremediation of organic xenobiotics. Biotechnol. Adv. 2009, 27, 474–488. [Google Scholar] [CrossRef]
- Taghavi, S.; Weyens, N.; Vangronsveld, J.; van der Lelie, D. Improved phytoremediation of organic contaminants through engineering of bacterial endophytes of trees. In Endophytes of Forest Trees; Springer: Amsterdam, The Netherlands, 2011; pp. 205–216. [Google Scholar]
- Ijaz, A.; Imran, A.; Haq, M.A.; Khan, Q.M.; Afzal, M. Phytoremediation: Recent advances in plant-endophytic synergistic interactions. Plant Soil 2016, 405, 179–195. [Google Scholar] [CrossRef]
- Taghavi, S.; Barac, T.; Greenberg, B.; Borremans, B.; Vangronsveld, J.; van der Lelie, D. Horizontal gene transfer to endogenous endophytic bacteria from Poplar improves phytoremediation of toluene. Appl. Environ. Microbiol. 2005, 71, 8500–8505. [Google Scholar] [CrossRef]
- Weyens, N.; Croes, S.; Dupae, J.; Newman, L.; van der Lelie, D.; Carleer, R.; Vangronsveld, J. Endophytic bacteria improve phytoremediation of Ni and TCE co-contamination. Environ. Pollut. 2010, 158, 2422–2427. [Google Scholar] [CrossRef]
- Oliveira, V.; Gomes, N.C.M.; Almeida, A.; Silva, A.M.S.; Simões, M.M.Q.; Smalla, K.; Cunha, Â. Hydrocarbon contamination and plant species determine the phylogenetic and functional diversity of endophytic-degrading bacteria. Mol. Ecol. 2014, 23, 1392–1404. [Google Scholar] [CrossRef]
- Chowdhary, K.; Arora, H.; Sharma, S. CRISPR/Cas9-Based genome editing as a way ahead for inducing the production of bioactive metabolites in Endophytes. Natl. Acad. Sci. Lett. 2022, 45, 275–280. [Google Scholar] [CrossRef]
- Wen-jia, T.; Gui-ming, D.; Sha, W.; Jing-ya, S.; Yu-chao, M. Establishment of a CRISPR/Cas9-mediated gene cluster-knockout system in the endophytic Streptomyces SAT1. Biotechnol. Bull. 2019, 35, 1–8. [Google Scholar]
- El-Sayed, A.S.A.; Abdel-Ghany, S.E.; Ali, G.S. Genome editing approaches: Manipulating of lovastatin and taxol synthesis of filamentous fungi by CRISPR/Cas9 system. Appl. Microbiol. Biotechnol. 2017, 101, 3953–3976. [Google Scholar] [CrossRef] [PubMed]
| Endophyte Strain | Plant Host | Pollutants/Contaminants | Mechanisms | Reference |
|---|---|---|---|---|
| Enterobacter sp. CBSB1 (genetically engineered strain) | Brassica juncea | Cd and Pb | Metal-tolerance and phytoremediation | [29] |
| Endophyte consortia | Agrostis stolonifera | Pb | Higher Pb tolerance, plant growth | [30] |
| Bacillus pumilus E2S2 | Sedum plumbizincicola | HM | HM phytoextraction from the soil | [31] |
| Rahnella sp. JN6 | Polygonum pubescens | Cd, Pb, and Zn | Metal-tolerance and alleviation | [32] |
| Methylobacterium oryzae CBMB20, Burkholderia sp. | Lycopersicon esculentum | Ni, Cd | Biosorption of Cd and Ni, Phytohormone synthesis, ACC deaminase activity | [33] |
| Bacterial endophyte PRE01 | Pteris vittata | V, Cd, Cr | Production of siderophore, ACC deaminase, indoleacetic acid (IAA), HM detoxification, metal uptake | [34] |
| Pseudomonas azotoformans ASS1 | Trifolium arvense | Zn, Ni, Cu | Phytoremediation of Ni, Zn, Cu | [35] |
| Bacillus, Stenotrophomonas, Enterobacter | Pteris vittata | As | Plant growth promotion, high As tolerance, phytoextraction of metal | [36] |
| Bacterial endophytes | P. vittata | As | High As tolerance, IAA production enhanced phytoremediation | [37] |
| Serratia PRE01, Arthrobacter PRE05 | B. juncea | V | Improved endosphere and rhizosphere micrology, enhanced phytoremediation | [38] |
| Pantoea stewartii ASI11, Enterobacter sp. HU38, Microbacterium arborescens HU33 | Brachiaria mutica, Leptochloa fusca | Cr | Plant growth promotion, increased plant biomass, Cr uptake and translocation, phytostabilization of Cr | [39] |
| Microbacterium arborescens HU33, Pantoea stewartii ASI11 | L. fusca (L.) Kunth | Pb, U | Plant growth, enhanced phytoremediation, phytostabilization of U- and Pb-contaminated soils | [40] |
| Bacillus amyloliquefaciens RWL-1 | Oryza sativa | Cu | Plant growth, reduces the metal accumulation | [41] |
| Microbacterium sp. G16, Pseudomonas fluorescens G10 | Brassica napus | Pb | Phytoextraction of Pb | [42] |
| Pseudomonas sp., Microbacterium sp. | Rumex acetosa | Mixed HM | Enhanced phytoremediation of HM | [43] |
| Bacterial endophyte consortia | Lupinus luteus | Metals and organic pollutants | Phytoremediation of metals and organic pollutants in contaminated site | [44] |
| Penicillium sp. CBRF65, Fusarium sp. CBRF44, Alternaria sp. CBSF68 | B. napus | Cd and Pb | Detoxification and remediation of HM | [45] |
| Bacillus thuringiensis GDB-1 | Pinus sylvestris | HM | Enhanced remediation of HM | [46] |
| Serratia nematodiphila LRE07 | S. nigrum | Cd | Plant growth, phytoremediation of Cd | [47] |
| Microbacterium lactium YJ7 | B. napus | Cu | Cu uptake and phytoextraction | [48] |
| Techniques Used | Biological Resource/Sample | Key Genes, Proteins and Metabolites and Their Function | Reference |
|---|---|---|---|
| Metagenomics | Microbial communities in agricultural soil | czcA, czcD and czrA in detoxification and efflux of cadmium | [67] |
| 16S rRNA gene sequencing, comparative metagenomics, and quantitative PCR | Bacterial microbiomes | Identification of metal-resistant genes | [68] |
| Genome sequencing | Methylobacterium radiotolerans MAMP 4754 (endophyte) | Genes involved in the tolerance of Ni, Cu, and Zn metals were identified | [69] |
| Metagenomics | Soil | Genes encoding for ABC transporters, detoxification process | [70] |
| Functional metatranscriptomics | Soil microbiota | Novel proteins, BolA proteins, saccaropine dehydrogenase (for Zn tolerance), and the C-terminal of aldehyde dehydrogenase (ADH) for Cd tolerance were identified | [71] |
| Metagenomics, metatranscriptomics | Soil microbiota | Genes involved in Cr resistance, transport, and reduction | [72] |
| Metagenomics, metatranscriptomics | Cr-contaminated soil | Identification of six novel genes having Cr tolerance (including gsr and mcr) | [73] |
| LC-HRMS-based metaproteomics and 16S rRNA gene sequencing | Noccaea caerulescens (Ni hyperaccumulator), Biscutella laevigata (HM-tolerant) | Proteins involved in metal transport (Ni, Cr, Co) and response to stimuli were identified | [74] |
| Proteomics | Arabidopsis halleri | Upregulation of stress-related proteins (superoxide dismutase, rubisco, and malate dehydrogenase) and decrease in defense-related proteins | [75] |
| Metabolomics, Transcriptomics | Sedum alfredii | Higher Cd phytoremediation via lateral root formation. | [76] |
| Metabolomics | P. australis | Spatial metabolite secretion due to different solutes, pH, and presence of different metals | [77] |
| Metabolomics | Salix integra | Identification of 401 metabolites (carbohydrates, organic acids, and amino acids) | [78] |
| Proteomics and metabolomics | Enterobacter bugandensis TJ6 | Betaine, arginine, and IAA secretion | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, P.; Bae, H. Trends in Harnessing Plant Endophytic Microbiome for Heavy Metal Mitigation in Plants: A Perspective. Plants 2023, 12, 1515. https://doi.org/10.3390/plants12071515
Tiwari P, Bae H. Trends in Harnessing Plant Endophytic Microbiome for Heavy Metal Mitigation in Plants: A Perspective. Plants. 2023; 12(7):1515. https://doi.org/10.3390/plants12071515
Chicago/Turabian StyleTiwari, Pragya, and Hanhong Bae. 2023. "Trends in Harnessing Plant Endophytic Microbiome for Heavy Metal Mitigation in Plants: A Perspective" Plants 12, no. 7: 1515. https://doi.org/10.3390/plants12071515
APA StyleTiwari, P., & Bae, H. (2023). Trends in Harnessing Plant Endophytic Microbiome for Heavy Metal Mitigation in Plants: A Perspective. Plants, 12(7), 1515. https://doi.org/10.3390/plants12071515







