1. Introduction
Sacred lotus (
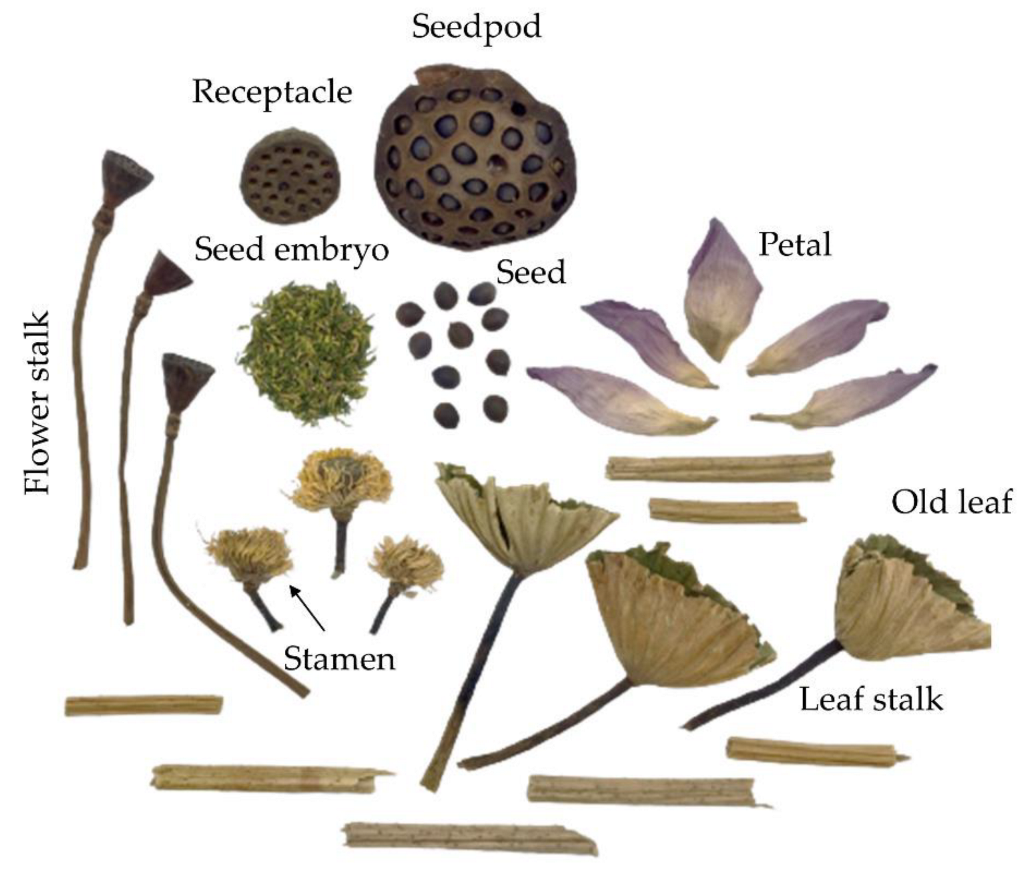
Nelumbo nucifera) in the Nelumbonaceae family is an aquatic rhizomatous plant, mainly distributed in Southeast Asia and used as a Buddhism spiritual symbol. In Thailand, sacred lotus parts are used as food ingredients and traditional herbs made from the flower, seed and rhizome. These sacred lotus parts are commonly consumed fresh, while the petal and leaf are steeped in hot water to make a tea beverage. Old leaf, stamen and stalk are rarely consumed due to their hard structure and unattractive appearance. Sacred lotus had been previously reported regarding their nutritional compositions [
1,
2]. Lotus seed was found to contain 61.3–70.1% carbohydrate, 16.2–28.2% protein and 0.2–3.7% fat [
2], while rhizome contained 16.03% carbohydrate, 2.60% protein and 0.10% fat [
1]. Temviriyanukul et al. (2020) reported that these plant parts could also be considered as functional ingredients due to their high phenolic contents [
3]. Old leaf and stamen exhibited higher total phenolic contents (TPCs) than petal, seed embryo, leaf stalk and flower stalk with flavonoids, especially naringenin, predominantly detected in all sacred lotus parts [
3]. Different levels of phenolic acids including
p-coumaric acid, gallic acid and ferulic acid were also detected. Other than flavonoids and phenolic acids, sacred lotus was also previously reported to contain alkaloids, tannins, saponins, terpenoids and coumarins [
4].
Sacred lotus contains a high amount of functional bioactive compounds and is commonly used as a green medicine with pharmaceutical properties such as anti-inflammation, anti-obesity, anti-microbial and anti-Alzheimer’s disease activities depending on the plant parts used [
3,
4,
5]. The fruit and seed have been widely studied regarding their health properties such as the anti-ischemic effect in a rat model [
6], hepatoprotective activity in carbon tetrachloride (CCl
4)- and aflatoxin B1-induced hepatotoxic cell culture [
7] and antiproliferative effect in peripheral blood mononuclear cells in human [
8]. Leaf was previously reported for its anti-diabetic effect both in vitro and in vivo through inhibition of lipase and α-amylase [
9] as well as reducing free cholesterol, total cholesterol and phospholipids in high fat-induced obesity mice [
10]. The flowers inhibited hypoglycemic activity in rabbit model [
11] and possessed hepatoprotective activity in paracetamol and CCl
4-induced hepatotoxicity in a rat model [
12]. The stamen exhibited high anti-Alzheimer’s disease activities through the inhibition of neurotransmitter degrading enzymes (cholinesterases) and β-amyloid formation enzyme (β-secretase) [
3]. The lotus stalk (it not indicated whether this was leaf stalk or flower stalk) also exhibited an anti-pyretic effect in a normal body temperature and yeast-induced pyrexia rat model [
13], while the flower stalk was traditionally used to ameliorate bleeding gastric ulcers, post-partum hemorrhage and excessive menstruation [
14]. Therefore, unpopular sacred lotus parts with potential health-promoting properties should be further investigated to increase fundamental knowledge on plant consumption and product development.
Sacred lotus is not farmed throughout the year in Thailand because the plant enters a resting stage during the dry season (November–March). Most sacred lotus products are harvested from May to October as early (May–June), middle (July–August) and late (September–October) rainy season. Sacred lotus products collected during different harvesting periods contain varied amounts of bioactive compounds with diverse biochemical activities. Therefore, sample quality control in the sacred lotus industry is difficult, impacting future product development. This study investigated TPCs, antioxidant activities and in vitro health properties of sacred lotus plant parts (seed embryo, petal, stamen, old leaf, leaf stalk and flower stalk) collected at different time periods (beginning, middle and end of the rainy season). In vitro health properties could reduce the risk of non-communicable diseases (NCDs) through inhibition of the key enzymes relevant to Alzheimer’s disease (β-secretase, acetylcholinesterase and butyrylcholinesterase), hypertension (angiotensin-converting enzyme), obesity (lipase) and diabetes (α-glucosidase). Information gained from this study can be used to support agricultural management, consumption and potential product development from different parts of the sacred lotus plant.
3. Discussion
Several sacred lotus parts have well-recognized pharmacological activities, resulting from their contained phytochemicals. Some sacred lotus parts, i.e., seed, leaf and root have been widely studied and used as both general foods and green medicine, while other parts, i.e., petal, stamen and stalk are underutilized due to limited information on their bioactive compounds and health-promoting activities [
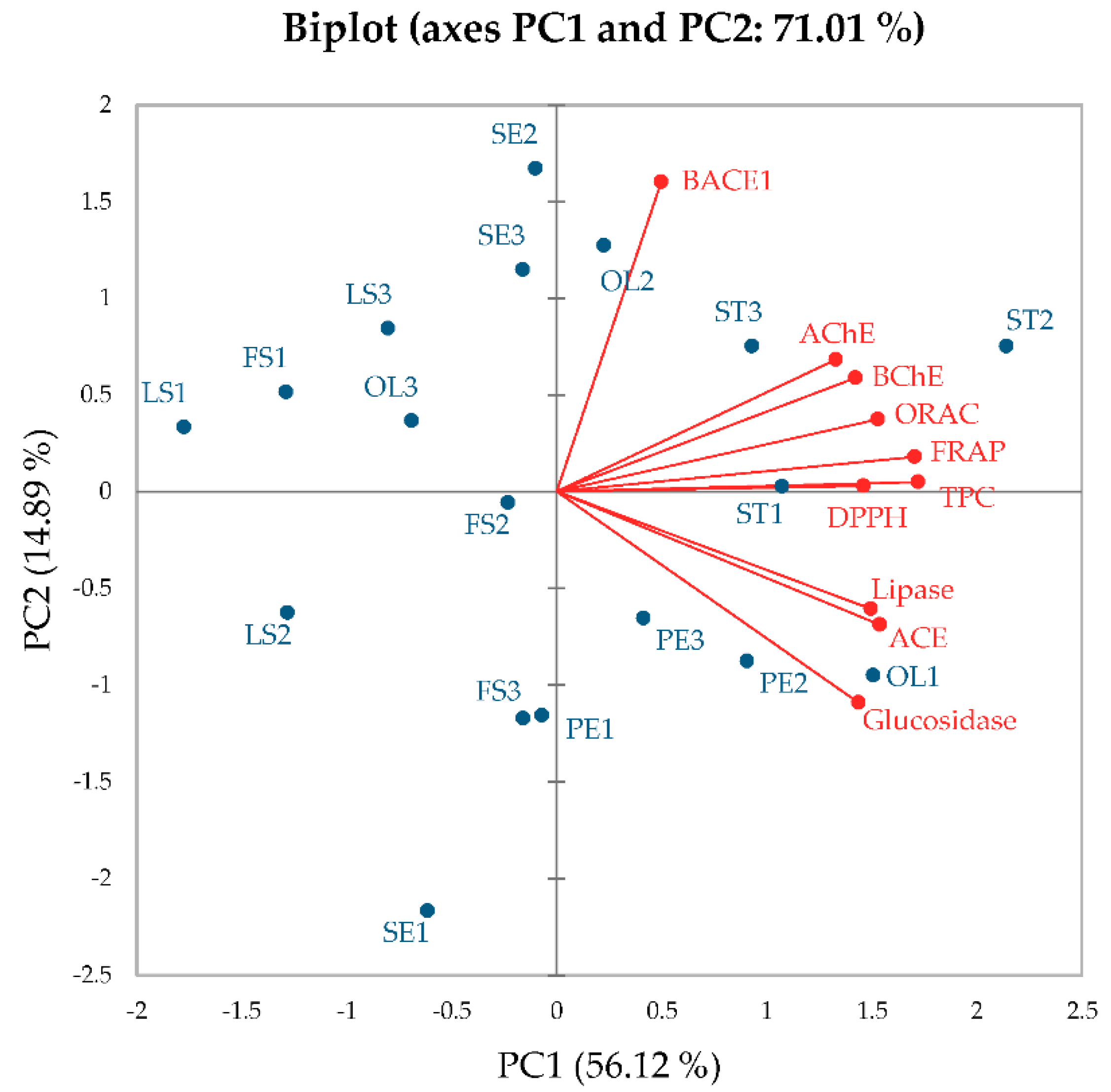
5]. Sacred lotus harvested at different time periods has variegated biological properties due to diversity of the bioactive compounds. In this study, different parts of sacred lotus including seed embryo, petal, stamen, old leaf, leaf stalk and flower stalk collected at different time periods were subjected to aqueous-based extraction and investigated regarding their TPCs, antioxidant activities and key enzyme inhibitions relevant to AD (BACE-1, AChE and BChE), hypertension (ACE), diabetes (α-glucosidase) and obesity (lipase). The results indicated that (i) stamen exhibited potentially high TPCs, leading to high antioxidant activities and most enzyme inhibitions (BACE-1, AChE, BChE, ACE and α-glucosidase), (ii) petal and old leaf exhibited high lipase inhibition despite having low TPCs and (iii) these activities were superior in stamen collected during time period 2 (or at the beginning of the rainy season) than the other time periods.
The previous study had identified phenolics in different sacred lotus parts by using high-performance liquid chromatography (HPLC) [
3]. It was found that naringenin, cyanidin and delphinidin were predominantly detected in seed embryo and petal, while flower stalk, leaf stalk and stamen exhibited exceptionally high naringenin contents [
3]. Besides, old leaf also contained high naringenin, quercetin and cyanidin [
3]. Among sacred lotus parts, we found that stamen exhibited relatively high TPCs, corresponding to the previous findings of high TPCs in both stamen and old leaf [
3], leading to high antioxidant activities. In this study, stamen collected during every harvesting period exhibited highest FRAP activities, while ORAC activities varied according to sacred lotus part and harvesting time periods. A previous study indicated that even though stamen and old leaf exhibited similar TPCs (7% variation), stamen exhibited 1.2-fold higher FRAP activities, while old leaf exhibited 1.8-fold higher ORAC activities [
3]. This information suggested that antioxidative agents in stamen preferred the SET-based mechanism (FRAP assay), while those of old leaf preferred the HAT-based mechanism (ORAC assay). Stamen contained
p-coumaric acid, myricetin, quercetin, naringenin, kaempferol, isorhamnetin, cyanidin and delphinidin, with naringenin being the most abundant phenolic detected by (HPLC [
3]. The density functional theory determined that naringenin in water phase preferred a sequential proton loss electron transfer (SPLET) owing to its 7−OH moiety (the hydroxyl group attached to the position of C7 in naringenin structure) to HAT- and SET-based mechanisms [
15]. This SPLET mechanism included two steps of proton transfer from the antioxidant after electron donation from the anion.
High TPCs in stamen also led to high BACE-1, AChE, BChE, ACE and α-glucosidase inhibitory activities. These results concurred with a previous study, indicating the lowest half maximal inhibitory concentration (IC
50) of stamen against AChE and BChE among all six sacred lotus parts, while BACE-1 inhibition showed 6.5% variation, with highest activities detected in flower stalk and leaf stalk [
3]. Naringenin inhibited BACE-1 with IC
50 of 30.31 µM, AChE with IC
50 of 42.66 µM and BChE with IC
50 of >100 µM [
16], showing lower effectiveness than the synthetic drug, donepezil (IC
50 values of 1.31, 3.12 and 2.14 µM against BACE-1, AChE and BChE, respectively) [
17]. Naringenin (500 µM) also inhibited ACE at 22.3% [
18]. Interestingly, a previous in vivo experiment indicated that naringenin (50 mg/kg) downregulated ACE and mineralocorticoid receptor (indicators of hypertension) and also alleviated neuronal oxidative stress (indicator of neurological damage) in L-N
G-nitro arginine methyl ester (L-NAME)-induced hypertensive rat [
19]. These results indicated that naringenin possessed neuroprotective and hypertensive inhibitory effects even though the in vitro enzyme inhibitory investigation suggested that naringenin was not a good inhibitor. Naringenin was also an effective inhibitor against α-glucosidase (IC
50 of 0.79 mM) comparing to acarbose, an anti-diabetic drug (IC
50 of 2.03 mM) [
20]. Naringenin (25 mg/kg) could lower postprandial serum glucose levels in streptozotocin (STZ)-induced diabetic rats [
21]. These data suggested that stamen with potentially high naringenin content showed promise as an excellent provider for AD, hypertensive and diabetic inhibitory agents.
Despite having low TPCs, petal and old leaf exhibited high lipase inhibition. Sacred lotus leaf has a long history of use as an anti-obesity agent in both traditional and modern medicines [
4]. Two quercetin derivatives, quercetin-3-
O-
β-D-arabinopyranosyl-(1→2)-
β-D-galactopyranoside and quercetin-3-
O-
β-D-glucuronide in sacred lotus leaf inhibited lipase with the IC
50 of 88.8 and 35.8 µM, respectively [
22]. However, compared to orlistat, a commercially available lipase inhibitor with an IC
50 value of 1.53 µM [
22], these two flavonoids were less effective. Nevertheless, leaf extract (140–560 mg/kg) could reduce total cholesterol (TC) and triglycerides (TG) in egg yolk-induced acute hyperlipidemia rat [
8], while it also lowered TC, TG and low-density lipoprotein cholesterol (LDL-C) levels, and increased high-density lipoprotein cholesterol (HDL-C) level in high fat diet-induced hyperlipidemia rat [
8]. Previous research mainly focused on sacred lotus leaf, with scant information on petal. It was previously reported that petal exhibited inhibitory effect on lipid storage in adipocytes and promoted lipolysis by inhibiting lipase with the IC
50 of 47 µg/mL [
23].
Variation in harvesting time also affected TPCs and biochemical activities. Stamen, collected at the beginning of the rainy season exhibited higher TPCs, antioxidant activities and enzyme inhibitions than stamen collected from the other two time periods. Our results concurred with a report on
Adenia viridiflora Craib., indicating that higher TPCs were obtained in plants collected during March and April, after the plant resting stage [
24]. Low rainfall is experienced in March in Thailand, with increased precipitation during the late April monsoon season. Thus, high phenolic biosynthesis occurs after the plant resting stage or at the beginning of the rainy season. Phenolics with numerous biological functions (i.e., anti-microorganism and antioxidant activities) may also benefit plant growth and survival [
24,
25].