Nootropic Herbs, Shrubs, and Trees as Potential Cognitive Enhancers
Abstract
1. Introduction
2. Cognitive Impairment and Dementia
3. Specific Nootropic Herb, Shrub, and Tree Species
3.1. Ashwagandha (Withania somnifera (L.) Dunal)
3.1.1. History
3.1.2. Plant Description
3.1.3. Occurrence
3.1.4. Chemical Composition
3.1.5. Uses and Specific Nootropic and Cognitive Effects of Ashwagandha
3.1.6. Side Effects and Contraindications
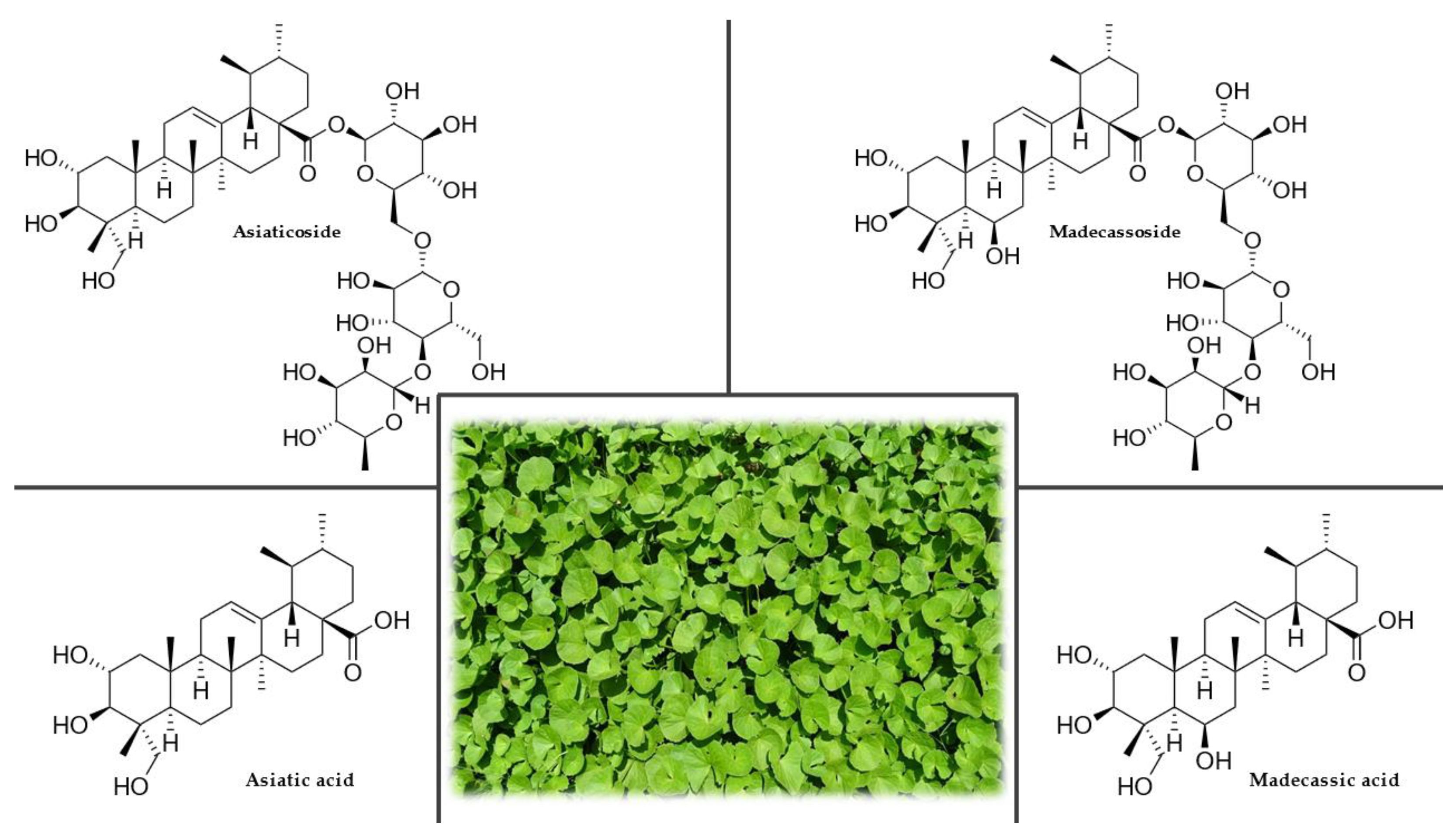
3.2. Asiatic Pennywort (Centella asiatica (L.) Urban)
3.2.1. History
3.2.2. Plant Description
3.2.3. Occurrence
3.2.4. Chemical Composition
3.2.5. Uses and Nootropic or Cognitive effects of Gotu Kola
3.2.6. Side Effects and Contraindications
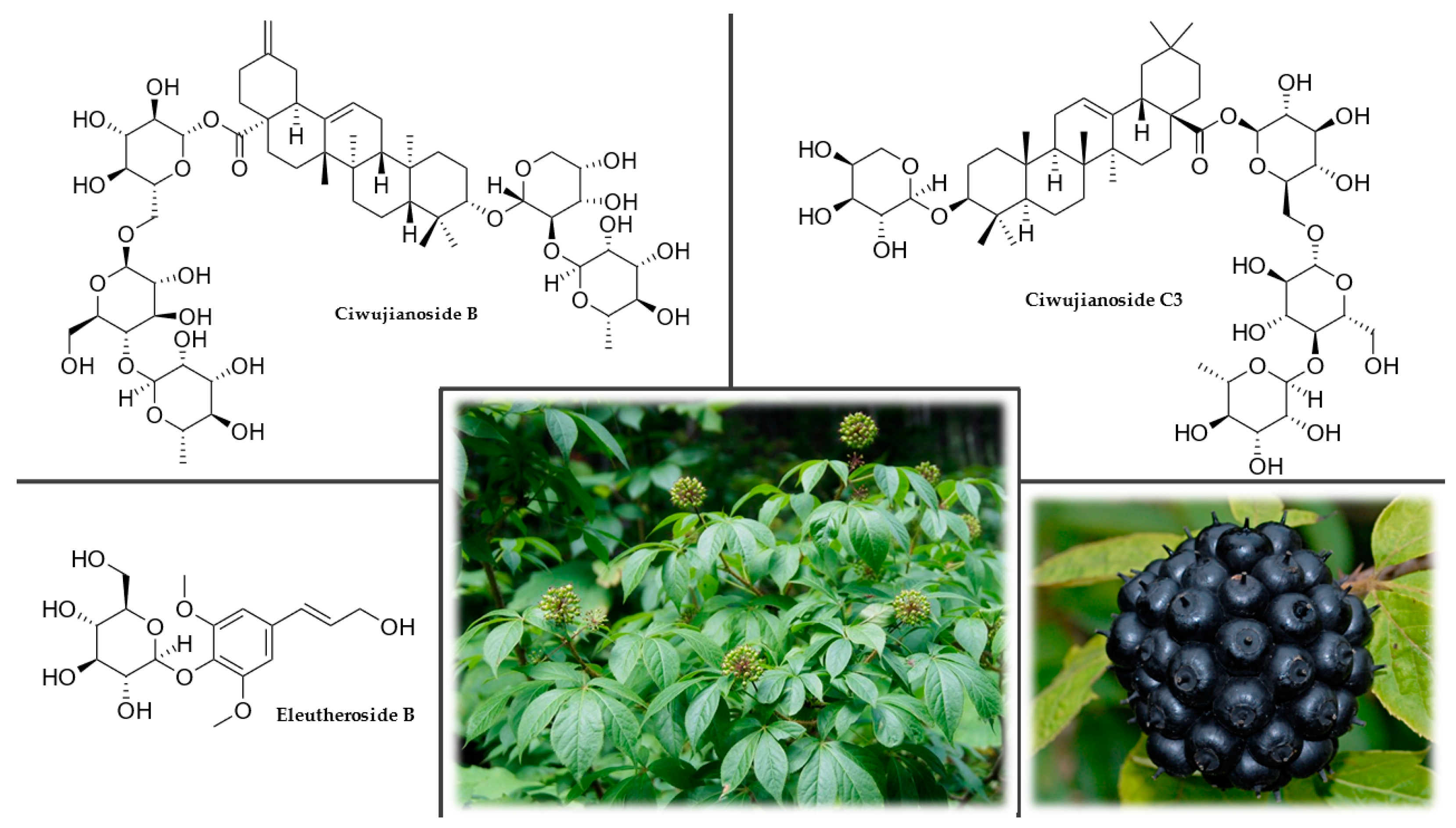
3.3. Eleuthero (Eleutherococcus senticosus (Rupr. & Maxim.) Maxim.)
3.3.1. History
3.3.2. Plant Description
3.3.3. Occurrence
3.3.4. Chemical Composition
3.3.5. Uses and Nootropic or Cognitive Effects of Eleutherococcus senticosus (Rupr. & Maxim.) Maxim.
3.3.6. Side Effects and Contraindications
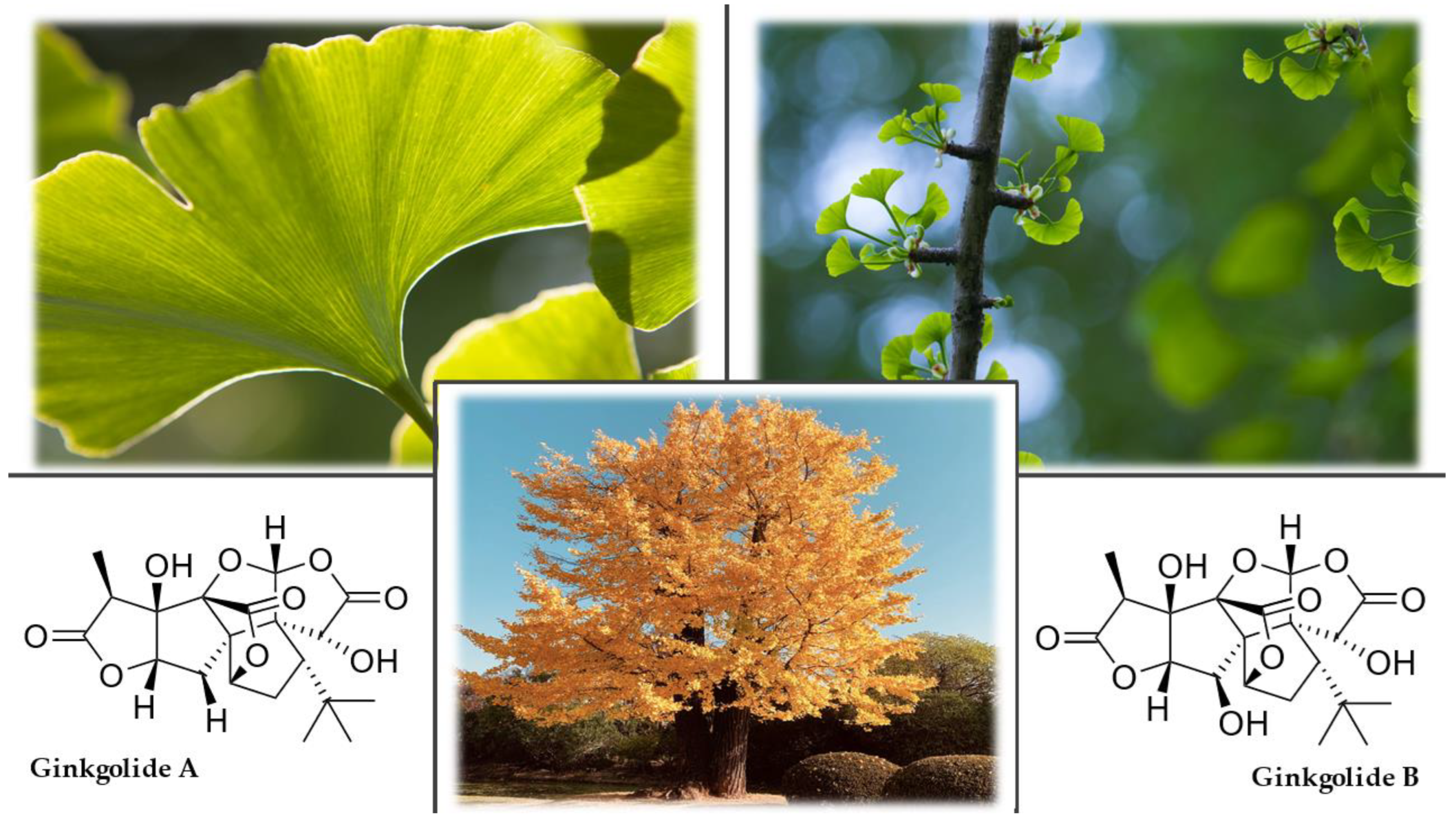
3.4. Ginkgo (Ginkgo biloba L.)
3.4.1. History
3.4.2. Plant Description
3.4.3. Occurrence
3.4.4. Chemical Composition
3.4.5. Uses and Nootropic and Cognitive Effects of Ginkgo biloba L.
3.4.6. Side Effects and Contraindications
3.5. Ginseng (Panax ginseng C.A. Meyer)
3.5.1. History
3.5.2. Plant Description
3.5.3. Occurrence
3.5.4. Chemical Composition
3.5.5. Uses and Nootropic or Cognitive Effects of Panax ginseng C.A. Meyer
3.5.6. Side Effects and Contraindications
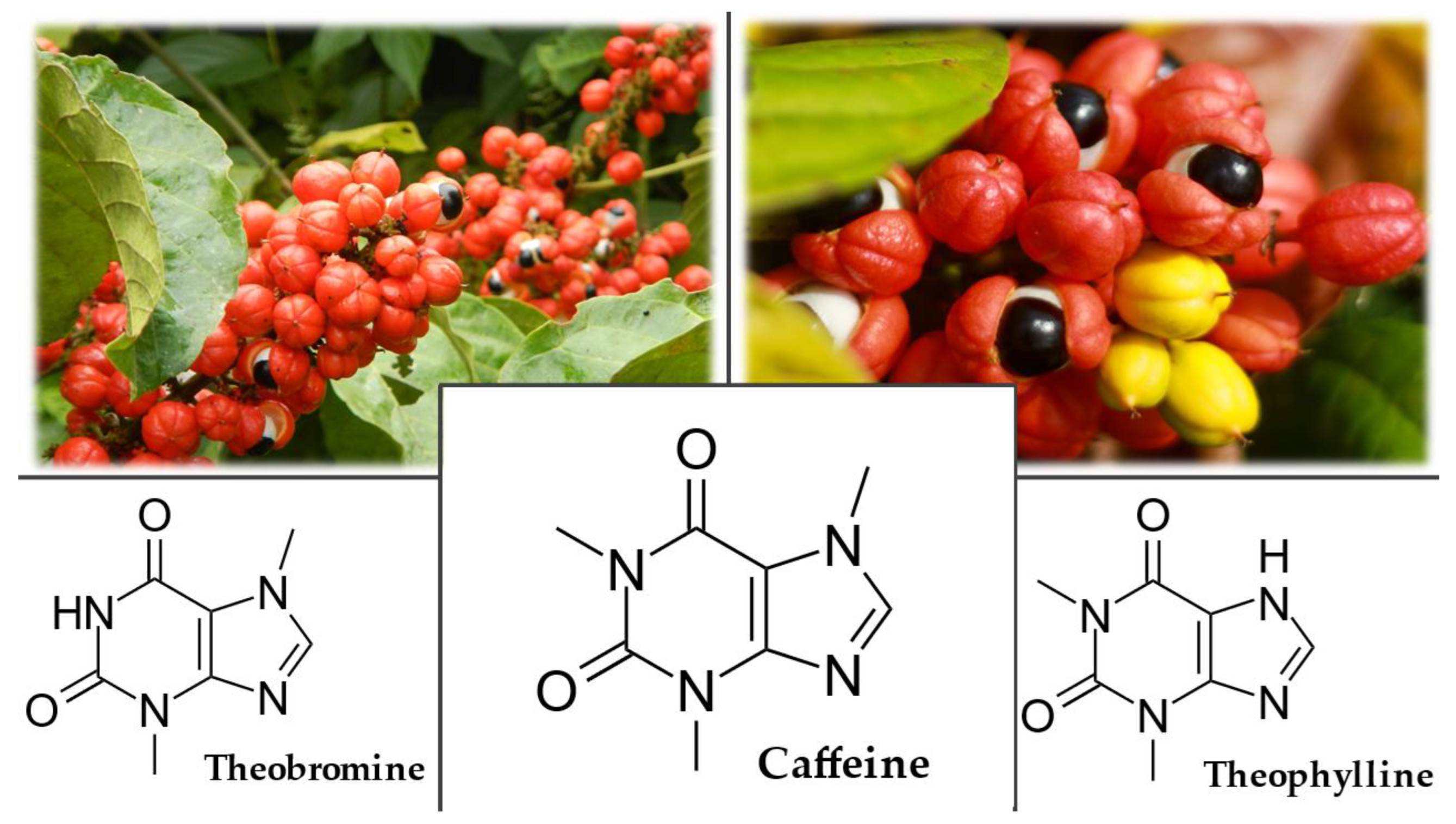
3.6. Guarana (Paullinia cupana Kunth)
3.6.1. History
3.6.2. Plant Description
3.6.3. Occurrence
3.6.4. Chemical Composition
3.6.5. Uses and Nootropic or Cognitive Effects of Paullinia cupana Kunth
3.6.6. Side Effects and Contraindications
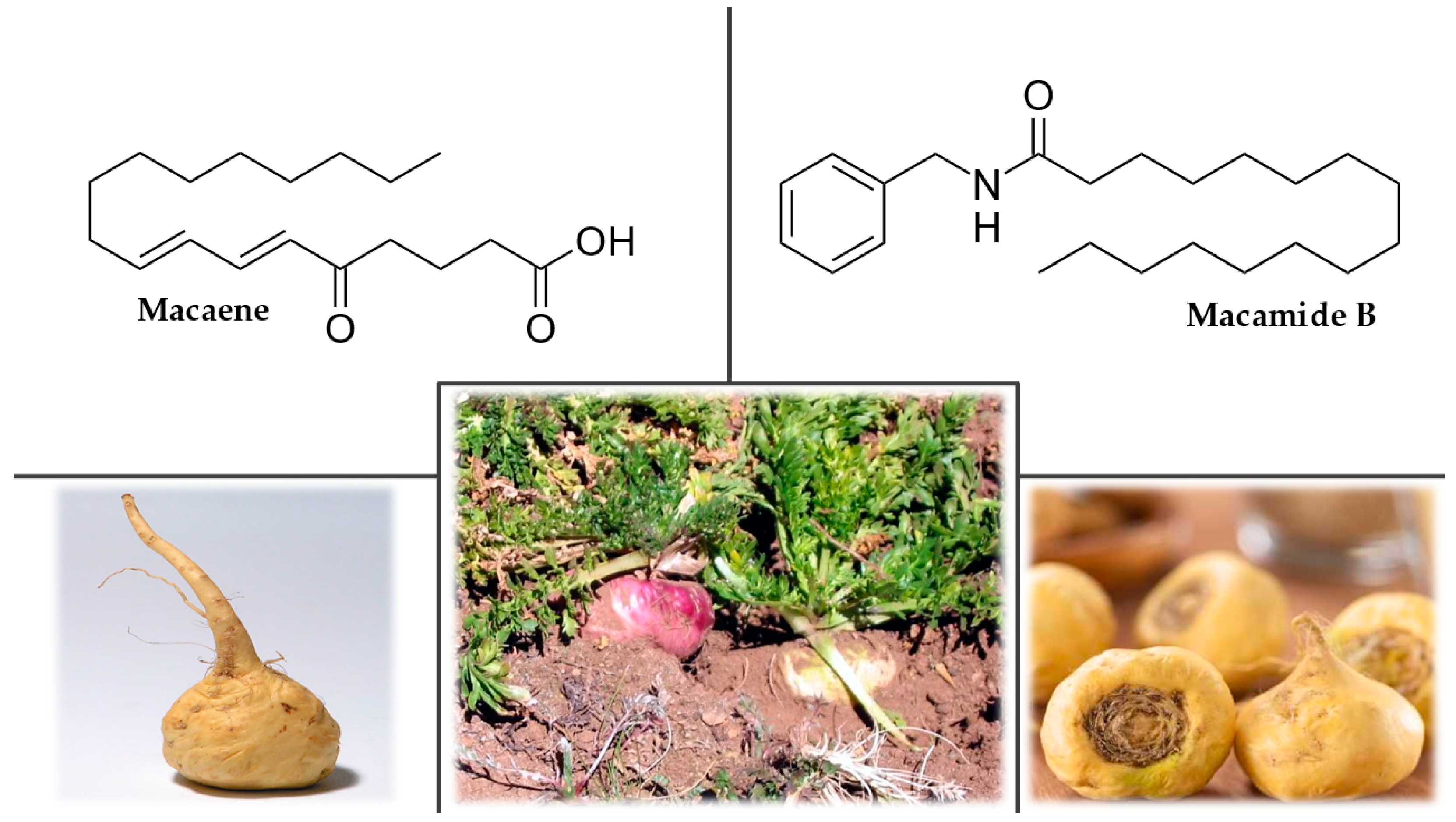
3.7. Maca (Lepidium meyenii Walp.)
3.7.1. History
3.7.2. Plant Description
3.7.3. Occurrence
3.7.4. Chemical Composition
3.7.5. Uses and Nootropic or Cognitive Effects of Lepidium meyenii Walp.
3.7.6. Side Effects and Contraindications
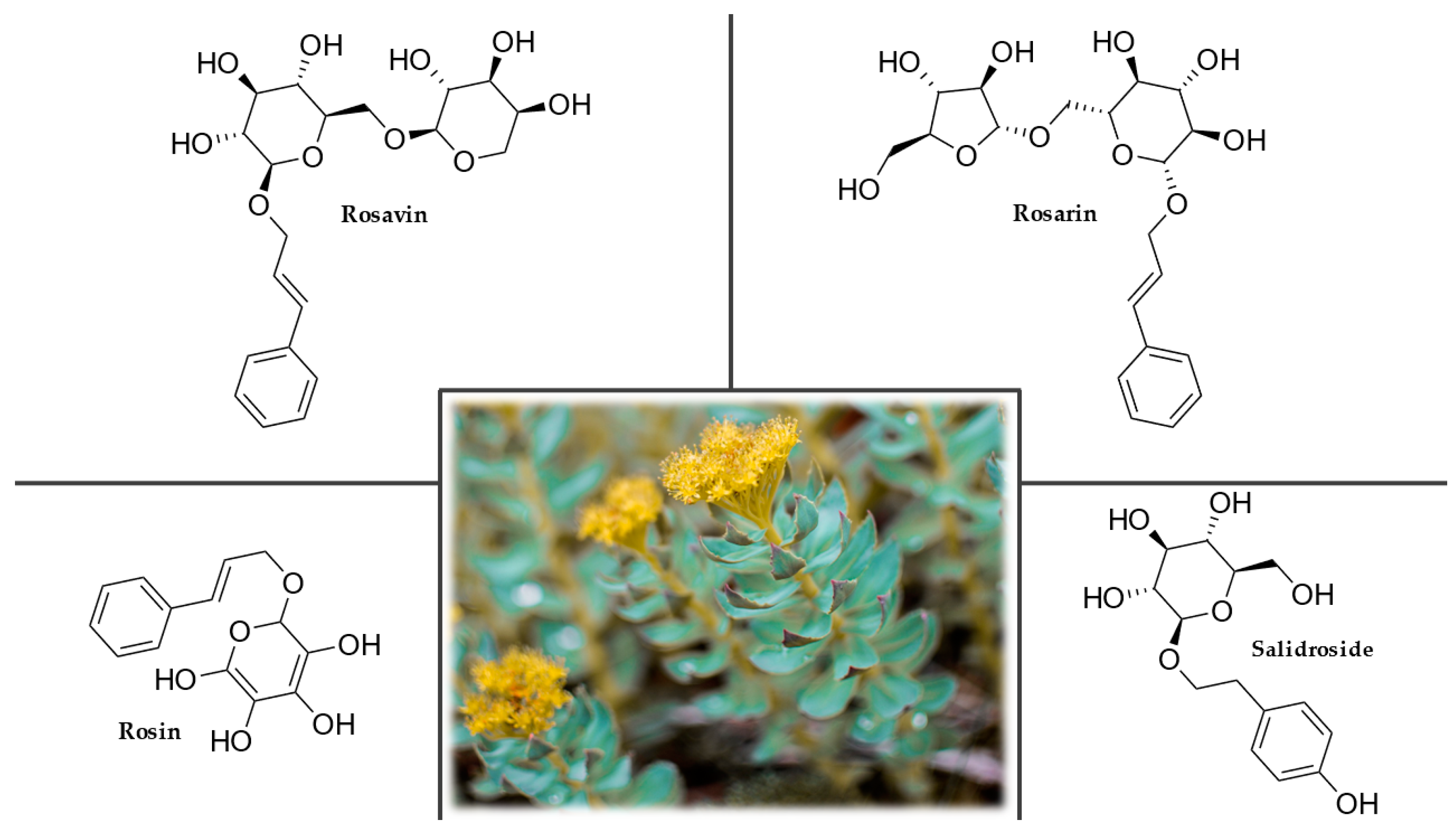
3.8. Rhodiola (Rhodiola rosea L.)
3.8.1. History
3.8.2. Plant Description
3.8.3. Occurrence
3.8.4. Chemical Composition
3.8.5. Uses and Nootropic or Cognitive Effects of Rhodiola rosea L.
3.8.6. Side effects and Contraindications
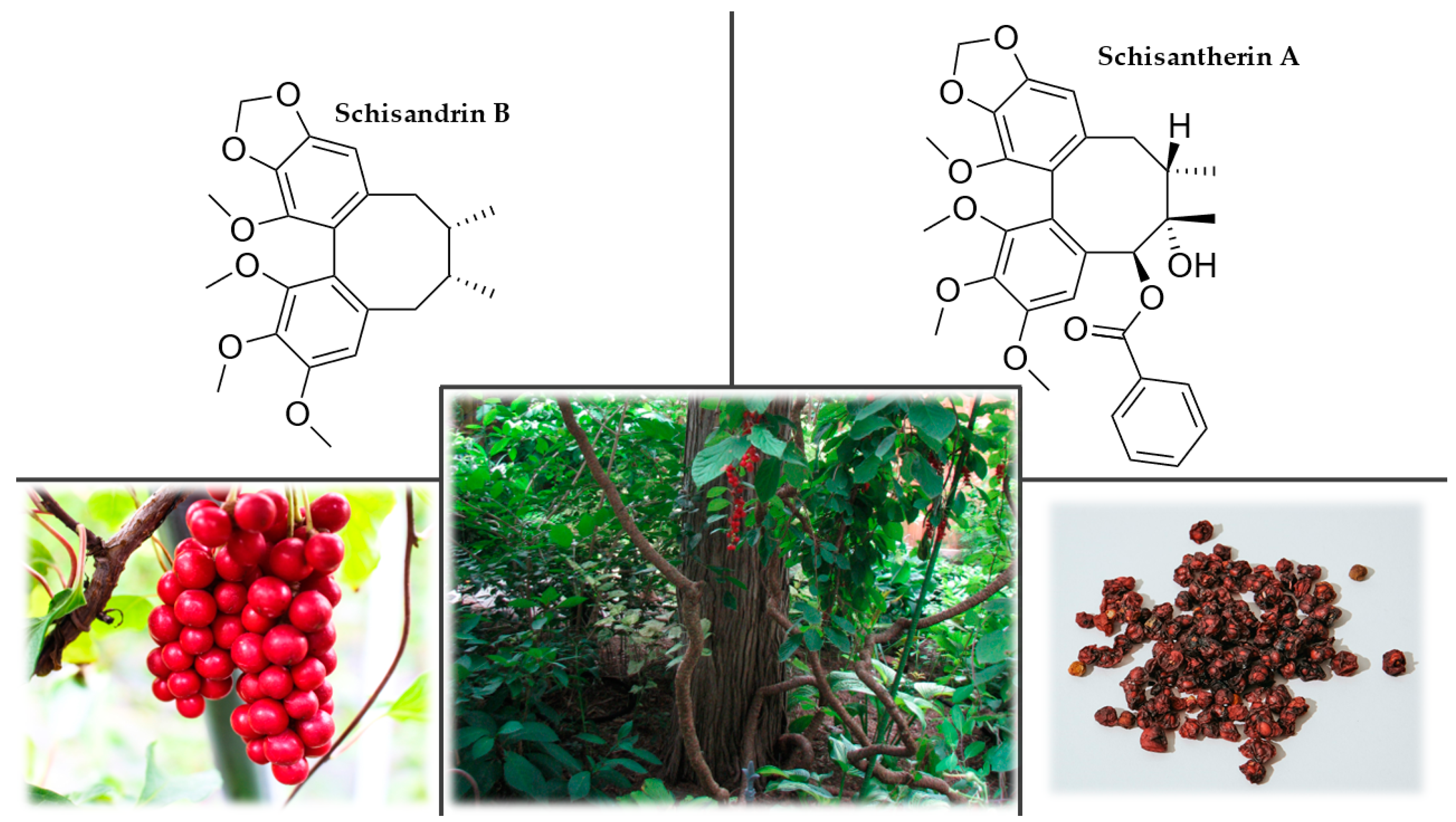
3.9. Schisandra (Schisandra chinensis Turcz. Baill.)
3.9.1. History
3.9.2. Plant Description
3.9.3. Occurrence
3.9.4. Chemical Composition
3.9.5. Uses and Nootropic or Cognitive Effects of Schisandra chinensis (Turcz.) Baill.
3.9.6. Side Effects and Contraindications
3.10. Water Hyssop, Brahmi (Bacopa monnieri (L.) Wettst.)
3.10.1. History
3.10.2. Plant Description
3.10.3. Occurrence
3.10.4. Chemical Composition
3.10.5. Uses and Nootropic or Cognitive Effects of Bacopa monnieri (L.) Wettst.
3.10.6. Side Effects and Contraindications
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saran, P.L.; Singh, S.; Solanki, V.; Choudhary, R.; Manivel, P. Evaluation of Asparagus adscendens accessions for root yield and shatavarin IV content in India. Turk. J. Agric. For. 2021, 45, 475–483. [Google Scholar] [CrossRef]
- Tamer, C.E.; Temel, Ş.G.; Suna, S.; Karabacak, A.Ö.; Özcan, T.; Ersan, L.Y.; Kaya, B.T.; Çopur, Ö.U. Evaluation of bioaccessibility and functional properties of kombucha beverages fortified with different medicinal plant extracts. Turk. J. Agric. For. 2021, 45, 13–32. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Vyas, S.; Kothari, S.; Kachhwaha, S. Nootropic medicinal plants: Therapeutic alternatives for Alzheimer’s disease. J. Herb. Med. 2019, 17, 100291. [Google Scholar] [CrossRef]
- Giurgea, C. Pharmacology of integrative activity of the brain. Attempt at nootropic concept in psychopharmacology. Actual. Pharm. 1972, 25, 115–156. [Google Scholar]
- Giurgea, C. The “nootropic” approach to the pharmacology of the integrative activity of the brain 1, 2. Integr. Psychol. Behav. Sci. 1973, 8, 108–115. [Google Scholar] [CrossRef]
- Giurgea, C.; Salama, M. Nootropic drugs. Prog. Neuro-Psychopharmacol. 1977, 1, 235–247. [Google Scholar] [CrossRef]
- Dormehl, I.C.; Jordaan, B.; Oliver, D.W.; Croft, S. SPECT monitoring of improved cerebral blood flow during long-term treatment of elderly patients with nootropic drugs. Clin. Nucl. Med. 1999, 24, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Suliman, N.A.; Mat Taib, C.N.; Mohd Moklas, M.A.; Adenan, M.I.; Hidayat Baharuldin, M.T.; Basir, R. Establishing Natural Nootropics: Recent Molecular Enhancement Influenced by Natural Nootropic. Evid.-Based Complement. Altern. Med. 2016, 2016, 4391375. [Google Scholar] [CrossRef]
- Kulkarni, R.; Girish, K.J.; Kumar, A. Nootropic herbs (Medhya Rasayana) in Ayurveda: An update. Pharmacogn. Rev. 2012, 6, 147–153. [Google Scholar] [CrossRef]
- Malík, M.; Tlustoš, P. Nootropics as Cognitive Enhancers: Types, Dosage and Side Effects of Smart Drugs. Nutrients 2022, 14, 3367. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Solon-Biet, S.M.; Cogger, V.C.; Fontana, L.; Simpson, S.J.; Le Couteur, D.G.; Ribeiro, R.V. Aging, lifestyle and dementia. Neurobiol. Dis. 2019, 130, 104481. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.Y. The borderland between normal aging and dementia. Tzu. Chi. Med. J. 2017, 29, 65–71. [Google Scholar] [CrossRef]
- Jalbert, J.J.; Daiello, L.A.; Lapane, K.L. Dementia of the Alzheimer Type. Epidemiol. Rev. 2008, 30, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Emre, M. Dementia associated with Parkinson’s disease. Lancet Neurol. 2003, 2, 229–237. [Google Scholar] [CrossRef]
- Knopman, D.S.; Boeve, B.F.; Petersen, R.C. Essentials of the Proper Diagnoses of Mild Cognitive Impairment, Dementia, and Major Subtypes of Dementia. Mayo Clin. Proc. 2003, 78, 1290–1308. [Google Scholar] [CrossRef]
- Szakiel, A.; Pączkowski, C.; Henry, M. Influence of environmental abiotic factors on the content of saponins in plants. Phytochem. Rev. 2011, 10, 471–491. [Google Scholar] [CrossRef]
- Pandey, A.; Savita, R. Harvesting and post-harvest processing of medicinal plants: Problems and prospects. J. Pharm. Innov. 2017, 6, 229–235. [Google Scholar]
- Tanko, H.; Carrier, D.J.; Duan, L.; Clausen, E. Pre- and post-harvest processing of medicinal plants. Plant Genet. Res. 2005, 3, 304–313. [Google Scholar] [CrossRef]
- Rocha, R.P.; Melo, E.C.; Radünz, L.L. Influence of drying process on the quality of medicinal plants: A review. J. Med. Plant Res. 2011, 5, 7076–7084. [Google Scholar] [CrossRef]
- Mohammad Azmin, S.N.H.; Abdul Manan, Z.; Wan Alwi, S.R.; Chua, L.S.; Mustaffa, A.A.; Yunus, N.A. Herbal Processing and Extraction Technologies. Sep. Purif. Rev. 2016, 45, 305–320. [Google Scholar] [CrossRef]
- Dwivedi, P.; Singh, R.; Malik, M.T.; Jawaid, T. A traditional approach to herbal nootropic agents: An overview. Int. J. Pharm. Sci. 2012, 3, 630. [Google Scholar]
- Lorca, C.; Mulet, M.; Arévalo-Caro, C.; Sanchez, M.Á.; Perez, A.; Perrino, M.; Bach-Faig, A.; Aguilar-Martínez, A.; Vilella, E.; Gallart-Palau, X.; et al. Plant-derived nootropics and human cognition: A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Mohiuddin, E.; Hannan, A.; Usmanghani, K. Withania somnifera (L.) Dunal(Pharmacology Activity). Pharmacogn. J. 2011, 2, 77–78. [Google Scholar] [CrossRef]
- Verma, N.; Gupta, S.K.; Tiwari, S.; Mishra, A.K. Safety of Ashwagandha Root Extract: A Randomized, Placebo-Controlled, study in Healthy Volunteers. Complement. Ther. Med. 2021, 57, 102642. [Google Scholar] [CrossRef] [PubMed]
- Santhanu, K.; Senthil, K. Therapeutic potential of Withania somnifera (Linn) Dunal (Ashwagandha) in historical perspective and pharmacological evidence. Ann. Ayurvedic Med. 2021, 10, 135. [Google Scholar] [CrossRef]
- Mir, B.A.; Khazir, J.; Mir, N.A.; Hasan, T.-u.; Koul, S. Botanical, chemical and pharmacological review of Withania somnifera (Indian ginseng): An ayurvedic medicinal plant. Indian J. Drug Dis. 2012, 1, 147–160. [Google Scholar]
- Rajeswara Rao, B.R.; Rajput, D.K.; Nagaraju, G.; Adinarayana, G. Opportunities and challenges in the cultivation of Ashwagandha {Withania somnifera (L.) DUNAL}. J. Pharmacogn. 2012, 3, 88–91. [Google Scholar]
- Kumar, M.; Patel, M.; Chauhan, R.; Tank, C.; Solanki, S.; Patel, P.; Bhadauria, H.; Gami, R.; Pachchigar, K.; Soni, N.; et al. Elucidation of genotype–environment interactions and genetic stability parameters for yield, quality and agromorphological traits in ashwagandha (Withania somnifera (L.) Dunal). J. Genet. 2020, 99, 59. [Google Scholar] [CrossRef]
- Venugopal, S.; Padma, M.; Raj Kumar, M.; Seenivasan, N.; Saidaiah, P.; Sathish, G. Genetic variability studies in ashwagandha (Withania somnifera L.) for yield and quality traits. Pharm. Innov. J. 2021, 10, 188–192. [Google Scholar]
- Srivastava, A.; Gupta, A.K.; Shanker, K.; Gupta, M.M.; Mishra, R.; Lal, R.K. Genetic variability, associations, and path analysis of chemical and morphological traits in Indian ginseng [Withania somnifera (L.) Dunal] for selection of higher yielding genotypes. J. Ginseng. Res. 2018, 42, 158–164. [Google Scholar] [CrossRef]
- Kumar, V.; Dey, A.; Hadimani, M.B.; Marcovic, T.; Emerald, M. Chemistry and pharmacology of Withania somnifera: An update. CellMed 2015, 5, 1.1–1.13. [Google Scholar] [CrossRef]
- Singh, N.; Bhalla, M.; de Jager, P.; Gilca, M. An overview on ashwagandha: A Rasayana (rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Bamola, N.; Verma, P.; Negi, C. A review on some traditional medicinal plants. Int. J. Life Sci. Res. 2018, 4, 1550–1556. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Dhir, A. Withania somnifera: An Indian ginseng. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Yenisetti, S.; Manjunath, M.J.; Muralidhara, M. Neuropharmacological properties of Withania somnifera-Indian Ginseng: An overview on experimental evidence with emphasis on Clinical trials and patents. Recent Pat. CNS Drug Discov. 2015, 10, 204–215. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; George, B.; Mathur, R. Neuroprotection by Withania somnifera root extract against lithium-pilocarpine-induced seizures. Indian Drugs 1998, 35, 208–215. [Google Scholar]
- Candelario, M.; Cuellar, E.; Reyes-Ruiz, J.M.; Darabedian, N.; Feimeng, Z.; Miledi, R.; Russo-Neustadt, A.; Limon, A. Direct evidence for GABAergic activity of Withania somnifera on mammalian ionotropic GABAA and GABAρ receptors. J. Ethnopharmacol. 2015, 171, 264–272. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Nawaz, S.A.; Zaheer-ul-Haq; Lodhi, M.A.; Ghayur, M.N.; Jalil, S.; Riaz, N.; Yousuf, S.; Malik, A.; Gilani, A.H.; et al. Withanolides, a new class of natural cholinesterase inhibitors with calcium antagonistic properties. Biochem. Biophys. Res. Commun. 2005, 334, 276–287. [Google Scholar] [CrossRef]
- Ziauddin, M.; Phansalkar, N.; Patki, P.; Diwanay, S.; Patwardhan, B. Studies on the immunomodulatory effects of Ashwagandha. J. Ethnopharmacol. 1996, 50, 69–76. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A. Possible Neuroprotective Effect of Withania somnifera Root Extract Against 3-Nitropropionic Acid-Induced Behavioral, Biochemical, and Mitochondrial Dysfunction in an Animal Model of Huntington’s Disease. J. Med. Food 2009, 12, 591–600. [Google Scholar] [CrossRef]
- Naidu, P.S.; Singh, A.; Kulkarni, S.K. Effect of Withania somnifera root extract on reserpine-induced orofacial dyskinesia and cognitive dysfunction. Phytother. Res. 2006, 20, 140–146. [Google Scholar] [CrossRef]
- Yadav, C.S.; Kumar, V.; Suke, S.G.; Ahmed, R.S.; Mediratta, P.K.; Banerjee, B.D. Propoxur-induced acetylcholine esterase inhibition and impairment of cognitive function: Attenuation by Withania somnifera. Indian J. Biochem. Biophys. 2010, 47, 117–120. [Google Scholar]
- Chengappa, K.N.R.; Bowie, C.R.; Schlicht, P.J.; Fleet, D.; Brar, J.S.; Jindal, R. Randomized placebo-controlled adjunctive study of an extract of Withania somnifera for cognitive dysfunction in bipolar disorder. J. Clin. Psychiatry 2013, 74, 16816. [Google Scholar] [CrossRef]
- Remenapp, A.; Coyle, K.; Orange, T.; Lynch, T.; Hooper, D.; Hooper, S.; Conway, K.; Hausenblas, H.A. Efficacy of Withania somnifera supplementation on adult’s cognition and mood. J. Ayurveda Integr. Med. 2022, 13, 100510. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Yoo, C.; Gonzalez, D.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Leonard, M.; Ko, J.; Faries, M.; Kephart, W.; et al. Effects of Acute Ashwagandha Ingestion on Cognitive Function. Int. J. Environ. Res. Public Health 2022, 19, 11852. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Aswath, A.; Chaturvedi, S.K.; Srinivasa, M.; Raguram, R. A double-blind, placebo-controlled evaluation of the anxiolytic efficacy ff an ethanolic extract of Withania somnifera. Indian J. Psychiatry 2000, 42, 295–301. [Google Scholar] [PubMed]
- Raut, A.A.; Rege, N.N.; Tadvi, F.M.; Solanki, P.V.; Kene, K.R.; Shirolkar, S.G.; Pandey, S.N.; Vaidya, R.A.; Vaidya, A.B. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J. Ayurveda Integr. Med. 2012, 3, 111–114. [Google Scholar] [CrossRef]
- Malhotra, C.L.; Mehta, V.L.; Das, P.K.; Dhalla, N.S. Studies on Withania-ashwagandha, Kaul. V. The effect of total alkaloids (ashwagandholine) on the central nervous system. Indian J. Physiol. Pharmacol. 1965, 9, 127–136. [Google Scholar] [PubMed]
- Aphale, A.A.; Chibba, A.D.; Kumbhakarna, N.R.; Mateenuddin, M.; Dahat, S.H. Subacute toxicity study of the combination of ginseng (Panax ginseng) and ashwagandha (Withania somnifera) in rats: A safety assessment. Indian J. Physiol. Pharmacol. 1998, 42, 299–302. [Google Scholar]
- Meher, S.K.; Das, B.; Panda, P.; Bhuyan, G.C.; Rao, M.M. Uses of Withania somnifera (Linn) Dunal (Ashwagandha) in Ayurveda and its pharmacological evidences. Res. J. Pharmacol. Pharmacodyn. 2016, 8, 23. [Google Scholar] [CrossRef]
- Sharma, A.K.; Basu, I.; Singh, S. Efficacy and Safety of Ashwagandha Root Extract in Subclinical Hypothyroid Patients: A Double-Blind, Randomized Placebo-Controlled Trial. J. Altern. Complement. Med. 2018, 24, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Franklyn, J.A.; Boelaert, K. Thyrotoxicosis. Lancet 2012, 379, 1155–1166. [Google Scholar] [CrossRef]
- Bevege, L. Centella asiatica: A review. Aust. J. Herb. Med. 2004, 16, 15–27. [Google Scholar] [CrossRef]
- Mala, A.; Tulika, T. Therapeutic efficacy of Centella asiatica (L.) and Momordica charantia: As traditional medicinal plant. J. Plant Sci. 2015, 3, 1–9. [Google Scholar] [CrossRef]
- Torbati, F.A.; Ramezani, M.; Dehghan, R.; Amiri, M.S.; Moghadam, A.T.; Shakour, N.; Elyasi, S.; Sahebkar, A.; Emami, S.A. Ethnobotany, Phytochemistry and Pharmacological Features of Centella asiatica: A Comprehensive Review. In Pharmacological Properties of Plant-Derived Natural Products and Implications for Human Health; Barreto, G.E., Sahebkar, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 451–499. [Google Scholar] [CrossRef]
- Chachai, N.; Pensuriya, B.; Pinsuntiae, T.; Pratubkong, P.; Mungngam, J.; Nitmee, P.; Kaewsri, P.; Wongsatchanan, S.; Jindajia, R.; Triboun, P.; et al. Variability of Morphological and Agronomical Characteristics of Centella asiatica in Thailand. Trends Sci. 2021, 18, 502. [Google Scholar] [CrossRef]
- Devkota, A.; Jha, P.K. Phenotypic plasticity of Centella asiatica (L.) Urb. growing in different habitats of Nepal. Trop. Plant Res. 2019, 6, 01–07. [Google Scholar] [CrossRef]
- Devkota, A.; Jha, P.K. Variation in growth of Centella asiatica along different soil composition. Bot. Res. Int. 2009, 2, 55–60. [Google Scholar]
- Devkota, A.; Jha, P.K. Growth performance and Nutrient status of Centella asiatica (L.) Urban in different landuses of Kathmandu valley, Nepal. Int. J. Ecol. Environ. Sci. 2008, 34, 269–275. [Google Scholar]
- Rohini, M.R.; Smitha, G.R. Studying the effect of morphotype and harvest season on yield and quality of Indian genotypes of Centella asiatica: A potential medicinal herb cum underutilized green leafy vegetable. S. Afr. J. Bot. 2022, 145, 275–283. [Google Scholar] [CrossRef]
- Yousaf, S.; Hanif, M.A.; Rehman, R.; Azeem, M.W.; Racoti, A. Chapter 32—Indian Pennywort. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 423–437. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Aslam, H.; Ali, S.T.; Khan, S.; Begum, S. Chemical constituents of Centella asiatica. J. Asian Nat. Prod. Res. 2007, 9, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Zainol, N.; Voo, S.; Sarmidi, M.; Aziz, R. Profiling of Centella asiatica (L.) Urban extract. Malaysian J. Anal. Sci. 2008, 12, 322–327. [Google Scholar]
- Seevaratnam, V.; Banumathi, P.; Premalatha, M.; Sundaram, S.; Arumugam, T. Functional properties of Centella asiatica (L.): A review. Int. J. Pharm. Pharm. Sci. 2012, 4, 8–14. [Google Scholar]
- Jayasinghe, M.; Senadheera, S.; Wijesekara, I.; Ranaweera, K. Determination of macronutrient compositions in selected, frequently consumed leafy vegetables, prepared according to common culinary methods in Sri Lanka. Vidyodaya J. Sci. 2019, 22, 1–6. [Google Scholar] [CrossRef]
- Cox, D.N.; Rajasuriya, S.V.; Soysa, P.E.; Gladwin, J.; Ashworth, A. Problems encountered in the community-based production of leaf concentrate as a supplement for pre-school children in Sri Lanka. Int. J. Food Sci. Nutr. 1993, 44, 123–132. [Google Scholar] [CrossRef]
- Chen, C.-L.; Tsai, W.-H.; Chen, C.-J.; Pan, T.-M. Centella asiatica extract protects against amyloid β1–40-induced neurotoxicity in neuronal cells by activating the antioxidative defence system. J. Tradit. Complement. Med. 2016, 6, 362–369. [Google Scholar] [CrossRef]
- Veerendra Kumar, M.H.; Gupta, Y.K. Effect of different extracts of Centella asiatica on cognition and markers of oxidative stress in rats. J. Ethnopharmacol. 2002, 79, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, G.; Kurup Muraleedhara, G.; Sudarslal, S.; Jacob, V.B. Anti-oxidant activity of Centella asiatica on lymphoma-bearing mice. Fitoterapia 2003, 74, 431–434. [Google Scholar] [CrossRef]
- Zainol, M.K.; Abd-Hamid, A.; Yusof, S.; Muse, R. Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban. Food Chem. 2003, 81, 575–581. [Google Scholar] [CrossRef]
- Subathra, M.; Shila, S.; Devi, M.A.; Panneerselvam, C. Emerging role of Centella asiatica in improving age-related neurological antioxidant status. Exp. Gerontol. 2005, 40, 707–715. [Google Scholar] [CrossRef]
- Chanana, P.; Kumar, A. Possible Involvement of Nitric Oxide Modulatory Mechanisms in the Neuroprotective Effect of Centella asiatica Against Sleep Deprivation Induced Anxiety Like Behaviour, Oxidative Damage and Neuroinflammation. Phytother. Res. 2016, 30, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Soumyanath, A.; Zhong, Y.-P.; Yu, X.; Bourdette, D.; Koop, D.R.; Gold, S.A.; Gold, B.G. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in-vitro. J. Pharm. Pharmacol. 2005, 57, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.B.; Chetana, M.; Uma Devi, P. Centella asiatica treatment during postnatal period enhances learning and memory in mice. Physiol. Behav. 2005, 86, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Wijeweera, P.; Arnason, J.T.; Koszycki, D.; Merali, Z. Evaluation of anxiolytic properties of Gotukola—(Centella asiatica) extracts and asiaticoside in rat behavioral models. Phytomedicine 2006, 13, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Jana, U.; Sur, T.K.; Maity, L.N.; Debnath, P.K.; Bhattacharyya, D. A clinical study on the management of generalized anxiety disorder with Centella asiatica. Nepal. Med. Coll. J. 2010, 12, 8–11. [Google Scholar]
- Wattanathorn, J.; Mator, L.; Muchimapura, S.; Tongun, T.; Pasuriwong, O.; Piyawatkul, N.; Yimtae, K.; Sripanidkulchai, B.; Singkhoraard, J. Positive modulation of cognition and mood in the healthy elderly volunteer following the administration of Centella asiatica. J. Ethnopharmacol. 2008, 116, 325–332. [Google Scholar] [CrossRef]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological Review on Centella asiatica: A Potential Herbal Cure-all. Indian J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef]
- Anukunwithaya, T.; Tantisira, M.H.; Tantisira, B.; Khemawoot, P. Pharmacokinetics of a standardized extract of Centella asiatica ECa 233 in rats. Planta Med. 2017, 83, 710–717. [Google Scholar] [CrossRef]
- Brinkhaus, B.; Lindner, M.; Schuppan, D.; Hahn, E.G. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella aslatica. Phytomedicine 2000, 7, 427–448. [Google Scholar] [CrossRef]
- Izu, R.; Aguirre, A.; Gil, N.; Diaz-Perez, J.L. Allergic contact dermatitis from a cream containing Centella asiatica extract. Contact Derm. 1992, 26, 192–193. [Google Scholar] [CrossRef]
- Chivapat, S.; Chavalittumrong, P.; Tantisira, M.H. Acute and sub-chronic toxicity studies of a standardized extract of Centella asiatica ECa 233. Thai J. Pharm. Sci. 2011, 35, 55–64. [Google Scholar]
- Davydov, M.; Krikorian, A.D. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: A closer look. J. Ethnopharmacol. 2000, 72, 345–393. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens—History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef] [PubMed]
- Sonnenbora, U.; Hänsel, R. Eleutherococcus senticosus. In Adverse Effects of Herbal Drugs 2; De Smet, P.A.G.M., Keller, K., Hänsel, R., Chandler, R.F., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 159–169. [Google Scholar] [CrossRef]
- Bączek, K.; Pawełczak, A.; Przybył, J.L.; Kosakowska, O.; Węglarz, Z. Secondary Metabolites of Various Eleuthero (Eleutherococcus senticosus/Rupr. et Maxim./Maxim) Organs Derived from Plants Obtained by Somatic Embryogenesis. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 433–466. [Google Scholar] [CrossRef]
- An, C. In Vitro propagation of commonly used medicinal trees in Korea. J. For. Environ. Sci. 2019, 35, 272–280. [Google Scholar] [CrossRef]
- Załuski, D.; Olech, M.; Galanty, A.; Verpoorte, R.; Kuźniewski, R.; Nowak, R.; Bogucka-Kocka, A. Phytochemical Content and Pharma-Nutrition Study on Eleutherococcus senticosus Fruits Intractum. Oxid. Med. Cell Longev. 2016, 2016, 9270691. [Google Scholar] [CrossRef]
- Yan-Lin, S.; Lin-De, L.; Soon-Kwan, H. Eleutherococcus senticosus as a crude medicine: Review of biological and pharmacological effects. J. Med. Plant Res. 2011, 5, 5946–5952. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Ding, W.-L.; Li, X.-T.; Cai, M.-T.; Li, H.-L.; Yang, Z.-Y.; Piao, X.-H.; Zhu, S.; Tohda, C.; Komatsu, K.; et al. Memory enhancement effect of saponins from Eleutherococcus senticosus leaves and blood–brain barrier-permeated saponins profiling using a pseudotargeted monitoring strategy. Food Funct. 2022, 13, 3603–3620. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Kim, S.H.; Kim, Y.; Kim, J.-J.; Baek, K.-H. Microwave-Assisted Seed Essential Oil of Eleutherococcus senticosus and Its Antioxidant and Free Radical-Scavenging Activities. J. Food Biochem. 2013, 37, 119–127. [Google Scholar] [CrossRef]
- Gromovaya, V.F.; Shapoval, G.S.; Mironyuk, I.E.; Nestyuk, N.V. Antioxidant properties of medicinal plants. Pharm. Chem. J. 2008, 42, 25–28. [Google Scholar] [CrossRef]
- Tohda, C.; Ichimura, M.; Bai, Y.; Tanaka, K.; Zhu, S.; Komatsu, K. Inhibitory Effects of Eleutherococcus senticosus Extracts on Amyloid β(25-35)–Induced Neuritic Atrophy and Synaptic Loss. J. Pharmacol. Sci. 2008, 107, 329–339. [Google Scholar] [CrossRef]
- Ge, Y.-W.; Tohda, C.; Zhu, S.; He, Y.-M.; Yoshimatsu, K.; Komatsu, K. Effects of Oleanane-Type Triterpene Saponins from the Leaves of Eleutherococcus senticosus in an Axonal Outgrowth Assay. J. Nat. Prod. 2016, 79, 1834–1841. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Ge, Y.-W.; Yoshimatsu, K.; Komatsu, K.; Kuboyama, T.; Yang, X.; Tohda, C. Memory Enhancement by Oral Administration of Extract of Eleutherococcus senticosus Leaves and Active Compounds Transferred in the Brain. Nutrients 2019, 11, 1142. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Derosa, G.; Brillante, R.; Bernardi, R.; Nascetti, S.; Gaddi, A. Effects of Siberian ginseng (Eleutherococcus senticosus maxim.) on elderly quality of life: A randomized clinical trial. Arch. Gerontol. Geriatr. 2004, 38, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Tohda, C.; Matsui, M.; Inada, Y.; Yang, X.; Kuboyama, T.; Kimbara, Y.; Watari, H. Combined Treatment with Two Water Extracts of Eleutherococcus senticosus Leaf and Rhizome of Drynaria fortunei Enhances Cognitive Function: A Placebo-Controlled, Randomized, Double-Blind Study in Healthy Adults. Nutrients 2020, 12, 303. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Gyllenhaal, C.; Fong, H.H.; Farnsworth, N.R. Ginsengs: A Review of Safety and Efficacy. Nutr. Clin. Care 2000, 3, 90–101. [Google Scholar] [CrossRef]
- Gerontakos, S.; Taylor, A.; Avdeeva, A.Y.; Shikova, V.A.; Pozharitskaya, O.N.; Casteleijn, D.; Wardle, J.; Shikov, A.N. Findings of Russian literature on the clinical application of Eleutherococcus senticosus (Rupr. & Maxim.): A narrative review. J. Ethnopharmacol. 2021, 278, 114274. [Google Scholar] [CrossRef]
- Bleakney, T.L. Deconstructing an Adaptogen: Eleutherococcus senticosus. Holist. Nurs. Pract. 2008, 22, 220–224. [Google Scholar] [CrossRef]
- Schmidt, M.; Thomsen, M.; Kelber, O.; Kraft, K. Myths and facts in herbal medicines: Eleutherococcus senticosus (Siberian ginseng) and its contraindication in hypertensive patients. Botanics 2014, 4, 27–32. [Google Scholar] [CrossRef]
- Crane, P.R.; Crane, P.; von Knorring, P. Ginkgo: The Tree That Time Forgot; Yale University Press: New Haven, CT, USA, 2013. [Google Scholar]
- Hori, S.; Hori, T. A Cultural History of Ginkgo in Japan and the Generic Name Ginkgo. In Ginkgo biloba a Global Treasure: From Biology to Medicine; Hori, T., Ridge, R.W., Tulecke, W., Del Tredici, P., Trémouillaux-Guiller, J., Tobe, H., Eds.; Springer: Tokyo, Japan, 1997; pp. 385–411. [Google Scholar] [CrossRef]
- Zhao, Y.; Paule, J.; Fu, C.; Koch, M.A. Out of China: Distribution history of Ginkgo biloba L. Taxon 2010, 59, 495–504. [Google Scholar] [CrossRef]
- Jacobs, B.P.; Browner, W.S. Ginkgo biloba: A living fossil. Am. J. Med. 2000, 108, 341–342. [Google Scholar] [CrossRef]
- Huh, H.; Staba, E.J. The Botany and Chemistry of Ginkgo biloba L. J. Herbs Spices Med. Plants 1992, 1, 91–124. [Google Scholar] [CrossRef]
- van Beek, T.A. Chemical analysis of Ginkgo biloba leaves and extracts. J. Chromatogr. A 2002, 967, 21–55. [Google Scholar] [CrossRef] [PubMed]
- Boateng, I.D.; Yang, X.-M. Effect of different drying methods on product quality, bioactive and toxic components of Ginkgo biloba L. seed. J. Sci. Food Agric. 2021, 101, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Masteikova, R.; Muselik, J.; Bernatonienė, J.; Bernatonienė, R. Antioxidative activity of Ginkgo, Echinacea, and Ginseng tinctures. Medicina 2007, 43, 306. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.-S.; Yoon, N.; Oh, B.-S.; Jeong, H.-S.; Yeoun, P.-S.; Kim, D.-J. Variation of Toxin Content in Ginkgo Fruits according to Thermal Treatment. Natl. Acad. Sci. Lett. 2020, 43, 673–676. [Google Scholar] [CrossRef]
- Maitra, I.; Marcocci, L.; Droy-Lefaix, M.T.; Packer, L. Peroxyl radical scavenging activity of Ginkgo biloba extract EGb 761. Biochem. Pharmacol. 1995, 49, 1649–1655. [Google Scholar] [CrossRef]
- Verma, S.; Ranawat, P.; Sharma, N.; Nehru, B. Ginkgo biloba attenuates aluminum lactate-induced neurotoxicity in reproductive senescent female rats: Behavioral, biochemical, and histopathological study. Environ. Sci. Pollut. Res. 2019, 26, 27148–27167. [Google Scholar] [CrossRef]
- Kim, M.-S.; Bang, J.H.; Lee, J.; Han, J.-S.; Baik, T.G.; Jeon, W.K. Ginkgo biloba L. extract protects against chronic cerebral hypoperfusion by modulating neuroinflammation and the cholinergic system. Phytomedicine 2016, 23, 1356–1364. [Google Scholar] [CrossRef]
- Williams, B.; Watanabe, C.M.H.; Schultz, P.G.; Rimbach, G.; Krucker, T. Age-related effects of Ginkgo biloba extract on synaptic plasticity and excitability. Neurobiol. Aging 2004, 25, 955–962. [Google Scholar] [CrossRef]
- Luo, Y.; Smith, J.V.; Paramasivam, V.; Burdick, A.; Curry, K.J.; Buford, J.P.; Khan, I.; Netzer, W.J.; Xu, H.; Butko, P. Inhibition of amyloid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA 2002, 99, 12197–12202. [Google Scholar] [CrossRef]
- Kanowski, S.; Herrmann, W.M.; Stephan, K.; Wierich, W.; Hörr, R. Proof of efficacy of the Ginkgo biloba special extract EGb 761 in outpatients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Phytomedicine 1997, 4, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Stough, C.; Clarke, J.; Lloyd, J.; Nathan, P.J. Neuropsychological changes after 30-day Ginkgo biloba administration in healthy participants. Int. J. Neuropsychopharmacol. 2001, 4, 131–134. [Google Scholar] [CrossRef]
- Bidzan, L.; Biliekiewicz, A.; Turczyński, J. Preliminary assessment of Ginkgo biloba (Ginkofar) in patients with dementia. Psychiatr. Pol. 2005, 39, 559–566. [Google Scholar]
- Canter, P.; Ernst, E. Ginkgo biloba is not a smart drug: An updated systematic review of randomised clinical trials testing the nootropic effects of G. biloba extracts in healthy people. Hum. Psychopharmacol. 2007, 22, 265–278. [Google Scholar] [CrossRef]
- Vellas, B.; Coley, N.; Ousset, P.-J.; Berrut, G.; Dartigues, J.-F.; Dubois, B.; Grandjean, H.; Pasquier, F.; Piette, F.; Robert, P.; et al. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. Lancet Neurol. 2012, 11, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Diamond, B.J.; Shiflett, S.C.; Feiwel, N.; Matheis, R.J.; Noskin, O.; Richards, J.A.; Schoenberger, N.E. Ginkgo biloba extract: Mechanisms and clinical indications. Arch. Phys. Med. Rehabil. 2000, 81, 668–678. [Google Scholar] [CrossRef]
- Le Bars, P.L.; Kastelan, J. Efficacy and safety of a Ginkgo biloba extract. Public Health Nutr. 2000, 3, 495–499. [Google Scholar] [CrossRef] [PubMed]
- R&D. EGb 761. Drugs R D 2003, 4, 188–193. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Mahady, G.B. Ginkgo biloba: A Review of Quality, Safety, and Efficacy. Nutr. Clin. Care 2001, 4, 140–147. [Google Scholar] [CrossRef]
- Stoddard, G.J.; Archer, M.; Shane-McWhorter, L.; Bray, B.E.; Redd, D.F.; Proulx, J.; Zeng-Treitler, Q. Ginkgo and warfarin interaction in a large veterans administration population. In Proceedings of the AMIA Annual Symposium Proceedings, San Francisco, CA, USA, 14–18 November 2015; pp. 1174–1183. [Google Scholar]
- Jang, H.-s.; Roh, S.Y.; Jeong, E.H.; Kim, B.-S.; Sunwoo, M.K. Ginkgotoxin Induced Seizure Caused by Vitamin B6 Deficiency. J. Epilepsy Res. 2015, 5, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Kosaki, Y.; Naito, H.; Nojima, T.; Nakao, A. Epileptic Seizure from Ginkgo Nut Intoxication in an Adult. Case Rep. Emerg. Med. 2020, 2020, 5072954. [Google Scholar] [CrossRef]
- Boateng, I.D. A critical review of current technologies used to reduce ginkgotoxin, ginkgotoxin-5′-glucoside, ginkgolic acid, allergic glycoprotein, and cyanide in Ginkgo biloba L. seed. Food Chem. 2022, 382, 132408. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B. Ginseng: Its History, Dispersion, and Folk Tradition. Am. J. Chin. Med. 1975, 3, 223–234. [Google Scholar] [CrossRef]
- Nair, R.; Sellaturay, S.; Sriprasad, S. The history of ginseng in the management of erectile dysfunction in ancient China (3500–2600 BCE). Indian J. Urol. 2012, 28, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Flagg, A.J. Traditional and current use of ginseng. Nurs. Clin. 2021, 56, 109–121. [Google Scholar] [CrossRef]
- Proctor, J.T.A.; Bailey, W.G. Ginseng: Industry, Botany, and Culture. Hortic. Rev. 1987, 9, 187–236. [Google Scholar] [CrossRef]
- Choi, K.-t. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol. Sin. 2008, 29, 1109–1118. [Google Scholar] [CrossRef]
- Proctor, J.T.; Lee, J.C.; Lee, S.-S. Ginseng production in Korea. HortScience 1990, 25, 746–750. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Ginsenosides analysis of New Zealand-grown forest Panax ginseng by LC-QTOF-MS/MS. J. Ginseng. Res. 2020, 44, 552–562. [Google Scholar] [CrossRef]
- Chen, W.; Balan, P.; Popovich, D.G. Analysis of Ginsenoside Content (Panax ginseng) from Different Regions. Molecules 2019, 24, 3491. [Google Scholar] [CrossRef]
- Lu, J.-M.; Yao, Q.; Chen, C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef]
- Hou, J.P. The chemical constituents of ginseng plants. Am. J. Chin. Med. 1977, 5, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.-C.; Ryu, G.-H. Chemical components of red, white and extruded root ginseng. J. Korean Soc. Food Sci. Nutr. 2005, 34, 247–254. [Google Scholar] [CrossRef]
- Kwon, I.-S.; Kim, H.; Hong, G.-P. Utilization of pulsed infrared for the rapid semidrying of fresh ginseng with advanced qualities and extended shelf life. Food Control 2022, 138, 109043. [Google Scholar] [CrossRef]
- Yu, J.; Eto, M.; Akishita, M.; Kaneko, A.; Ouchi, Y.; Okabe, T. Signaling pathway of nitric oxide production induced by ginsenoside Rb1 in human aortic endothelial cells: A possible involvement of androgen receptor. Biochem. Biophys. Res. Commun. 2007, 353, 764–769. [Google Scholar] [CrossRef]
- Churchill, J.D.; Gerson, J.L.; Hinton, K.A.; Mifek, J.L.; Walter, M.J.; Winslow, C.L.; Deyo, R.A. The nootropic properties of ginseng saponin Rb1 are linked to effects on anxiety. Integr. Psychol. Behav. Sci. 2002, 37, 178–187. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Zhang, Z.; Bi, P.; Qi, Z.; Zhang, C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci. Lett. 2011, 487, 70–72. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, D.W.; Jung, B.H.; Lee, J.H.; Lee, H.; Hwang, G.S.; Kang, K.S.; Lee, J.W. Ginsenoside Rb2 suppresses the glutamate-mediated oxidative stress and neuronal cell death in HT22 cells. J. Ginseng. Res. 2019, 43, 326–334. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, J.; Zheng, K.; Shen, H.; Chen, X. Long-term Ginsenoside Rg1 Supplementation Improves Age-Related Cognitive Decline by Promoting Synaptic Plasticity Associated Protein Expression in C57BL/6J Mice. J. Gerontol. A 2013, 69A, 282–294. [Google Scholar] [CrossRef]
- Shin, S.J.; Park, Y.H.; Jeon, S.G.; Kim, S.; Nam, Y.; Oh, S.-M.; Lee, Y.Y.; Moon, M. Red Ginseng Inhibits Tau Aggregation and Promotes Tau Dissociation In Vitro. Oxid. Med. Cell Longev. 2020, 2020, 7829842. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M. Tau protein and neurodegeneration. Semin. Cell Dev. Biol. 2004, 15, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Petkov, V.D.; Belcheva, S.; Konstantinova, E.; Kehayov, R.; Petkov, V.V.; Hadjiivanova, C. Participation of the serotonergic system in the memory effects of Ginkgo biloba L. and Panax ginseng C. A. Mey. Phytother. Res. 1994, 8, 470–477. [Google Scholar] [CrossRef]
- Kim, H.-G.; Yoo, S.-R.; Park, H.-J.; Lee, N.-H.; Shin, J.-W.; Sathyanath, R.; Cho, J.-H.; Son, C.-G. Antioxidant effects of Panax ginseng C.A. Meyer in healthy subjects: A randomized, placebo-controlled clinical trial. Food Chem. Toxicol. 2011, 49, 2229–2235. [Google Scholar] [CrossRef]
- Park, K.-C.; Jin, H.; Zheng, R.; Kim, S.; Lee, S.-E.; Kim, B.-H.; Yim, S.-V. Cognition enhancing effect of panax ginseng in Korean volunteers with mild cognitive impairment: A randomized, double-blind, placebo-controlled clinical trial. Transl. Clin. Pharmacol. 2019, 27, 92–97. [Google Scholar] [CrossRef]
- Kiefer, D.S.; Pantuso, T. Panax ginseng. Am. Fam. Physician 2003, 68, 1539–1542. [Google Scholar]
- Lee, M.-H.; Kwak, J.H.; Jeon, G.; Lee, J.-W.; Seo, J.-H.; Lee, H.-S.; Lee, J.H. Red ginseng relieves the effects of alcohol consumption and hangover symptoms in healthy men: A randomized crossover study. Food Funct. 2014, 5, 528–534. [Google Scholar] [CrossRef]
- Je, J.; Kim, H.; Park, E.J.; Kim, S.R.; Dusabimana, T.; Jeong, K.; Yun, S.P.; Kim, H.J.; Cho, K.M.; Park, S.W. Fermentation of Sprouted Ginseng (Panax ginseng) Increases Flavonoid and Phenolic Contents to Attenuate Alcoholic Hangover and Acute Liver Injury in Mice. Am. J. Chin. Med. 2020, 49, 131–146. [Google Scholar] [CrossRef]
- Karmazyn, M.; Gan, X.T. Chemical components of ginseng, their biotransformation products and their potential as treatment of hypertension. Mol. Cell Biochem. 2021, 476, 333–347. [Google Scholar] [CrossRef]
- Park, S.H.; Chung, S.; Chung, M.-Y.; Choi, H.-K.; Hwang, J.-T.; Park, J.H. Effects of Panax ginseng on hyperglycemia, hypertension, and hyperlipidemia: A systematic review and meta-analysis. J. Ginseng. Res. 2022, 46, 188–205. [Google Scholar] [CrossRef]
- Kim, K.-M.; Kwon, H.-S.; Jeon, S.-G.; Park, C.-H.; Sohn, S.-W.; Kim, D.-I.; Kim, S.-S.; Chang, Y.-S.; Kim, Y.-K.; Cho, S.-H.; et al. Korean Ginseng-Induced Occupational Asthma and Determination of IgE Binding Components. J. Korean Med. Sci. 2008, 23, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.L.M.; Ferreira, E.D.F.; Paula, M.N.d.; Klein, T.; Mello, J.C.P.d. Paullinia cupana: A multipurpose plant-a review. Rev. Bras. Farmacogn. 2019, 29, 77–110. [Google Scholar] [CrossRef]
- d’Angelo, S.; Ascione, A. Guarana and physical performance: A myth or reality? J. Hum. Sport. Exerc. 2020, 15, S539–S551. [Google Scholar] [CrossRef]
- Hamerski, L.; Somner, G.V.; Tamaio, N. Paullinia cupana Kunth (Sapindaceae): A review of its ethnopharmacology, phytochemistry and pharmacology. J. Med. Plant Res. 2013, 7, 2221–2229. [Google Scholar] [CrossRef]
- Erickson, H.T.; Correa, M.P.F.; Escobar, J.r. Guaraná (Paullinia cupana) as a commercial crop in Brazilian Amazonia. Econ. Bot. 1984, 38, 273–286. [Google Scholar] [CrossRef]
- Ushirobira, T.A.; Yamaguti, E.; Uemura, L.M.; Nakamura, C.V.; Dias Filho, B.P.; Mello, J.P. Chemical and microbiological study of extract from seeds of guaraná (Paullinia cupana var. sorbilis). Lat. Am. J. Pharm. 2007, 26, 5–9. [Google Scholar]
- Cavalcanti, V.; Marques, M.; do Nascimento, W.M.; Rocha, A.W.D.O.; Ferreira, I.D.J.; Leao, D.P.; Félix, P.H.C.; de Oliveira, C.M.C. Bioproducts based on guarana (Paulinia cupana) for practitioners of physical activity. Eur. Acad. Res. 2020, 8, 1746–1759. [Google Scholar]
- Banga, S.; Kumar, V.; Suri, S.; Kaushal, M.; Prasad, R.; Kaur, S. Nutraceutical Potential of Diet Drinks: A Critical Review on Components, Health Effects, and Consumer Safety. J. Am. Coll. Nutr. 2020, 39, 272–286. [Google Scholar] [CrossRef]
- Boasquívis, P.F.; Silva, G.M.M.; Paiva, F.A.; Cavalcanti, R.M.; Nunez, C.V.; de Paula Oliveira, R. Guarana (Paullinia cupana) Extract Protects Caenorhabditis elegans Models for Alzheimer Disease and Huntington Disease through Activation of Antioxidant and Protein Degradation Pathways. Oxid. Med. Cell Longev. 2018, 2018, 9241308. [Google Scholar] [CrossRef]
- Bittencourt, L.d.S.; Zeidán-Chuliá, F.; Yatsu, F.K.J.; Schnorr, C.E.; Moresco, K.S.; Kolling, E.A.; Gelain, D.P.; Bassani, V.L.; Moreira, J.C.F. Guarana (Paullinia cupana Mart.) Prevents β-Amyloid Aggregation, Generation of Advanced Glycation-end Products (AGEs), and Acrolein-Induced Cytotoxicity on Human Neuronal-Like Cells. Phytother. Res. 2014, 28, 1615–1624. [Google Scholar] [CrossRef]
- Rangel, M.P.; de Mello, J.C.P.; Audi, E.A. Evaluation of neurotransmitters involved in the anxiolytic and panicolytic effect of the aqueous fraction of Paullinia cupana (guaraná) in elevated T maze. Rev. Bras. Farmacogn. 2013, 23, 358–365. [Google Scholar] [CrossRef]
- Otobone, F.J.; Sanches, A.C.; Nagae, R.L.; Martins, J.V.C.; Obici, S.; Mello, J.C.P.d.; Audi, E.A. Effect of crude extract and its semi purified constituents from guaraná seeds [Paullinia cupana var. sorbilis (Mart.) lucke] on cognitive performance in Morris water maze in rats. Braz. Arch. Biol. Technol. 2005, 48, 723–728. [Google Scholar] [CrossRef]
- Espinola, E.B.; Dias, R.F.; Mattei, R.; Carlini, E.A. Pharmacological activity of Guarana (Paullinia cupana Mart.) in laboratory animals. J. Ethnopharmacol. 1997, 55, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Veloso, C.F.; Machado, A.K.; Cadoná, F.C.; Azzolin, V.F.; Cruz, I.B.M.; Silveira, A.F. Neuroprotective Effects of Guarana (Paullinia cupana Mart.) against Vincristine in Vitro Exposure. J. Prev. Alzheimers Dis. 2018, 5, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Martins, C.A.; Sampaio, G.R.; Monteiro, M.P.; César, L.A.M.; Mioto, B.M.; Mori, C.S.; Mendes, T.M.N.; Ribeiro, M.L.; Arçari, D.P.; et al. Bioavailability of catechins from guaraná (Paullinia cupana) and its effect on antioxidant enzymes and other oxidative stress markers in healthy human subjects. Food Funct. 2016, 7, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Haskell, C.F.; Wesnes, K.A.; Scholey, A.B. Improved cognitive performance in human volunteers following administration of guarana (Paullinia cupana) extract: Comparison and interaction with Panax ginseng. Pharmacol. Biochem. Behav. 2004, 79, 401–411. [Google Scholar] [CrossRef]
- Patrick, M.; Kim, H.A.; Oketch-Rabah, H.; Marles, R.J.; Roe, A.L.; Calderón, A.I. Safety of Guarana Seed as a Dietary Ingredient: A Review. J. Agric. Food. Chem. 2019, 67, 11281–11287. [Google Scholar] [CrossRef]
- Marques, L.L.M.; Klein, T.; de Mello, J.C.P. Chapter 3.24—Guarana. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 283–288. [Google Scholar] [CrossRef]
- Turnbull, D.; Rodricks, J.V.; Mariano, G.F.; Chowdhury, F. Caffeine and cardiovascular health. Regul. Toxicol. Pharmacol. 2017, 89, 165–185. [Google Scholar] [CrossRef]
- Torres, E.A.F.S.; Pinaffi-Langley, A.C.d.C.; Figueira, M.d.S.; Cordeiro, K.S.; Negrão, L.D.; Soares, M.J.; da Silva, C.P.; Alfino, M.C.Z.; Sampaio, G.R.; de Camargo, A.C. Effects of the consumption of guarana on human health: A narrative review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 272–295. [Google Scholar] [CrossRef]
- Silva, C.P.; Sampaio, G.R.; Freitas, R.A.M.S.; Torres, E.A.F.S. Polyphenols from guaraná after in vitro digestion: Evaluation of bioacessibility and inhibition of activity of carbohydrate-hydrolyzing enzymes. Food Chem. 2018, 267, 405–409. [Google Scholar] [CrossRef]
- Pinaffi, A.C.d.C.; Sampaio, G.R.; Soares, M.J.; Shahidi, F.; de Camargo, A.C.; Torres, E.A.F.S. Insoluble-Bound Polyphenols Released from Guarana Powder: Inhibition of Alpha-Glucosidase and Proanthocyanidin Profile. Molecules 2020, 25, 679. [Google Scholar] [CrossRef] [PubMed]
- Rienks, J.; Barbaresko, J.; Oluwagbemigun, K.; Schmid, M.; Nöthlings, U. Polyphenol exposure and risk of type 2 diabetes: Dose-response meta-analyses and systematic review of prospective cohort studies. Am. J. Clin. Nutr. 2018, 108, 49–61. [Google Scholar] [CrossRef]
- León, J. The “Maca” (Lepidium meyenii), a little known food plant of peru. Econ. Bot. 1964, 18, 122–127. [Google Scholar] [CrossRef]
- Muhammad, I.; Zhao, J.; Khan, I.A. Maca (Lepidium meyenii). In Encyclopedia of Dietary Supplement; Coates, P., Blackman, M.R., Cragg, G., Levine, M., Moss, J., White, J., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 522–531. [Google Scholar] [CrossRef]
- Flores, H.E.; Walker, T.S.; Guimarães, R.L.; Bais, H.P.; Vivanco, J.M. Andean Root and Tuber Crops: Underground Rainbows. HortSci 2003, 38, 161–167. [Google Scholar] [CrossRef]
- Zaytseva, O.; Terrel Gutierrez, M.; Graeff-Hönninger, S. Effect of Day Length on Growth and Root Morphology of Yellow Maca (Lepidium meyenii) Seedlings. Int. J. Plant Biol. 2022, 13, 71–81. [Google Scholar] [CrossRef]
- Tafuri, S.; Cocchia, N.; Vassetti, A.; Carotenuto, D.; Esposito, L.; Maruccio, L.; Avallone, L.; Ciani, F. Lepidium meyenii (Maca) in male reproduction. Nat. Prod. Res. 2021, 35, 4550–4559. [Google Scholar] [CrossRef] [PubMed]
- Huarancca Reyes, T.; Esparza, E.; Crestani, G.; Limonchi, F.; Cruz, R.; Salinas, N.; Scartazza, A.; Guglielminetti, L.; Cosio, E. Physiological responses of maca (Lepidium meyenii Walp.) plants to UV radiation in its high-altitude mountain ecosystem. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Dini, A.; Migliuolo, G.; Rastrelli, L.; Saturnino, P.; Schettino, O. Chemical composition of Lepidium meyenii. Food Chem. 1994, 49, 347–349. [Google Scholar] [CrossRef]
- Muhammad, I.; Zhao, J.; Dunbar, D.C.; Khan, I.A. Constituents of Lepidium meyenii ‘maca’. Phytochemistry 2002, 59, 105–110. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Peng, X.-R.; Qiu, M.-H. Progress on the Chemical Constituents Derived from Glucosinolates in Maca (Lepidium meyenii). Nat. Prod. Bioprospect. 2018, 8, 405–412. [Google Scholar] [CrossRef]
- Brinckmann, J.; Smith, E. Maca Culture of the Junín Plateau. J. Altern. Complement. Med. 2004, 10, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Smith, E. MACA ROOT: Modern Rediscovery of an Ancient Andean Fertility Food. J. Am. Herbal. Guild 2003, 4, 15–21. [Google Scholar]
- Jin, W.; Chen, X.; Huo, Q.; Cui, Y.; Yu, Z.; Yu, L. Aerial parts of maca (Lepidium meyenii Walp.) as functional vegetables with gastrointestinal prokinetic efficacy in vivo. Food Funct. 2018, 9, 3456–3465. [Google Scholar] [CrossRef] [PubMed]
- Caicai, K.; Limin, H.; Liming, Z.; Zhiqiang, Z.; Yongwu, Y. Isolation, purification and antioxidant activity of polysaccharides from the leaves of maca (Lepidium meyenii). Int. J. Biol. Macromol. 2018, 107, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hao, L.; Kang, Q.; Cui, Y.; Jiang, H.; Liu, X.; Lu, J. Purification, characterization and biological activities of a polysaccharide from Lepidium meyenii leaves. Int. J. Biol. Macromol. 2017, 103, 1302–1310. [Google Scholar] [CrossRef]
- Pino-Figueroa, A.; Nguyen, D.; Maher, T.J. Neuroprotective effects of Lepidium meyenii (Maca). Ann. N. Y. Acad. Sci. 2010, 1199, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, P.; Brantner, A.; Wang, H.; Shu, X.; Yang, J.; Si, N.; Han, L.; Zhao, H.; Bian, B. Chemical profiling analysis of Maca using UHPLC-ESI-Orbitrap MS coupled with UHPLC-ESI-QqQ MS and the neuroprotective study on its active ingredients. Sci. Rep. 2017, 7, 44660. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.; Caldas, M.; Dávila, S.; Gasco, M.; Gonzales, G.F. Effect of three different cultivars of Lepidium meyenii (Maca) on learning and depression in ovariectomized mice. BMC Complement. Altern. Med. 2006, 6, 23. [Google Scholar] [CrossRef]
- Guo, S.-S.; Gao, X.-F.; Gu, Y.-R.; Wan, Z.-X.; Lu, A.M.; Qin, Z.-H.; Luo, L. Preservation of Cognitive Function by Lepidium meyenii (Maca) Is Associated with Improvement of Mitochondrial Activity and Upregulation of Autophagy-Related Proteins in Middle-Aged Mouse Cortex. J. Evid.-Based. Complement. Altern. Med. 2016, 2016, 4394261. [Google Scholar] [CrossRef]
- Yu, Z.; Li, D.; Zhai, S.; Xu, H.; Liu, H.; Ao, M.; Zhao, C.; Jin, W.; Yu, L. Neuroprotective effects of macamide from maca (Lepidium meyenii Walp.) on corticosterone-induced hippocampal impairments through its anti-inflammatory, neurotrophic, and synaptic protection properties. Food Funct. 2021, 12, 9211–9228. [Google Scholar] [CrossRef]
- Honma, A.; Fujiwara, Y.; Takei, S.; Kino, T. The improvement of daily fatigue in women following the intake of maca (Lepidium meyenii) extract containing benzyl glucosinolate. Funct. Food Health Dis. 2022, 12, 175–187. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Córdova, A.; Vega, K.; Chung, A.; Villena, A.; Góñez, C.; Castillo, S. Effect of Lepidium meyenii (MACA) on sexual desire and its absent relationship with serum testosterone levels in adult healthy men. Andrologia 2002, 34, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Arimborgo, C.; Yupanqui, I.; Montero, E.; Alarcón-Yaquetto, D.E.; Zevallos-Concha, A.; Caballero, L.; Gasco, M.; Zhao, J.; Khan, I.A.; Gonzales, G.F. Acceptability, Safety, and Efficacy of Oral Administration of Extracts of Black or Red Maca (Lepidium meyenii) in Adult Human Subjects: A Randomized, Double-Blind, Placebo-Controlled Study. Pharmaceuticals 2016, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; He, H.-Y.; Chen, Q.; Ma, S.-W.; Chen, X. Drug-induced Liver Injury Due to Lepidium meyenii (Maca) Medicinal Liquor. Chin. Med. J. 2017, 130, 3005–3006. [Google Scholar] [CrossRef] [PubMed]
- Valerio, L.G.; Gonzales, G.F. Toxicological Aspects of the South American Herbs Cat’s Claw (Uncaria tomentosa) and Maca (Lepidium meyenii). Toxicol. Rev. 2005, 24, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Buckiová, D.; Křen, V.; Pěknicová, J.; Ulrichová, J.; Šimánek, V. The in vitro biological activity of Lepidium meyenii extracts. Cell Biol. Toxicol. 2006, 22, 91–99. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, G.; Benavides, V.; Pino, J. Preliminary Evaluation Effect of Lepidium meyenii Walp on the embryonic development of mouse. Rev. Peru Biol. 2004, 11, 103–106. [Google Scholar] [CrossRef]
- Brown, R.P.; Gerbarg, P.L.; Ramazanov, Z. Rhodiola rosea A phytomedicinal overview. HerbalGram 2002, 56, 40–52. [Google Scholar]
- Galambosi, B. Cultivation of Rhodiola rosea in Europe. In Rhodiola rosea; Cuerrier, A., Ampong-Nyarko, K., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 87–124. [Google Scholar]
- Tasheva, K.; Kosturkova, G. The Role of Biotechnology for Conservation and Biologically Active Substances Production of Rhodiola rosea: Endangered Medicinal Species. Sci. World J. 2012, 2012, 274942. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Liu, C.; Song, Z.; Li, Q.; Zha, Q.; Lu, C.; Wang, C.; Ning, Z.; Zhang, Y.; et al. The chemotaxonomic classification of Rhodiola plants and its correlation with morphological characteristics and genetic taxonomy. Chem. Cent. J. 2013, 7, 118. [Google Scholar] [CrossRef]
- Bejar, E.; Upton, R.; John, H. Adulteration of Rhodiola (Rhodiola rosea) rhizome and root and extracts. Bot. Adulterants Bull. 2017, Fall 2017, 1–8. [Google Scholar]
- Panossian, A.; Wikman, G.; Sarris, J. Rosenroot (Rhodiola rosea): Traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine 2010, 17, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Kołodziej, B.; Sugier, D. Influence of plants age on the chemical composition of roseroot (Rhodiola rosea L.). Acta Sci. Pol. 2013, 12, 147–160. [Google Scholar]
- Mardones, V.; Cuerrier, A.; Hermanutz, L. Developing a community-based enterprise: Nunatsiavut Inuit knowledge and perspectives on the use of medicinal plant Rhodiola rosea. Ethnobot. Res. Appl. 2021, 22, 1–13. [Google Scholar] [CrossRef]
- Jang, S.I.; Pae, H.O.; Choi, B.M.; Oh, G.S.; Jeong, S.; Lee, H.J.; Kim, H.Y.; Kang, K.J.; Yun, Y.G.; Kim, Y.C.; et al. Salidroside from Rhodiola sachalinensis Protects Neuronal PC12 Cells Against Cytotoxicity Induced by Amyloid-β. Immunopharmacol. Immunotoxicol. 2003, 25, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, M.; Gu, X.; Ding, F. Neuroprotective Effects of Salidroside in the PC12 Cell Model Exposed to Hypoglycemia and Serum Limitation. Cell Mol. Neurobiol. 2008, 28, 1067. [Google Scholar] [CrossRef]
- Zhou, L.; Yao, P.; Jiang, L.; Wang, Z.; Ma, X.; Wen, G.; Yang, J.; Zhou, B.; Yu, Q. Salidroside-pretreated mesenchymal stem cells contribute to neuroprotection in cerebral ischemic injury in vitro and in vivo. J. Mol. Histol. 2021, 52, 1145–1154. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Z.; Ren, Y.; Wu, X.; Liu, Y.; Wang, T.; Li, Y.; Cong, Y.; Guo, Y. Neuroprotective effects of salidroside on ageing hippocampal neurons and naturally ageing mice via the PI3K/Akt/TERT pathway. Phytother. Res. 2021, 35, 5767–5780. [Google Scholar] [CrossRef]
- Perfumi, M.; Mattioli, L. Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice. Phytother. Res. 2007, 21, 37–43. [Google Scholar] [CrossRef]
- Spasov, A.A.; Wikman, G.K.; Mandrikov, V.B.; Mironova, I.A.; Neumoin, V.V. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine 2000, 7, 85–89. [Google Scholar] [CrossRef]
- Cropley, M.; Banks, A.P.; Boyle, J. The Effects of Rhodiola rosea L. Extract on Anxiety, Stress, Cognition and Other Mood Symptoms. Phytother. Res. 2015, 29, 1934–1939. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.S. Rhodiola rosea: A Possible Plant Adaptogen. Altern. Med. Rev. 2001, 6, 293–302. [Google Scholar] [PubMed]
- Jagtap, P.N.; Mhetre, O.S.; Malavdkar, P.R. A Review Article on Rhodiola rosea: An Adaptogen Having Multiple Benefits. Int. J. Pharmacogn. 2020, 7, 62–69. [Google Scholar] [CrossRef]
- Kucinskaite, A.; Briedis, V.; Savickas, A. Experimental analysis of therapeutic properties of Rhodiola rosea L. and its possible application in medicine. Medicina 2004, 40, 614–619. [Google Scholar] [PubMed]
- Razgonova, M.; Zakharenko, A.; Pikula, K.; Kim, E.; Chernyshev, V.; Ercisli, S.; Cravotto, G.; Golokhvast, K. Rapid mass spectrometric study of a supercritical CO2-extract from woody liana Schisandra chinensis by HPLC-SPD-ESI-MS/MS. Molecules 2020, 25, 2689. [Google Scholar] [CrossRef]
- Saunders, R.M.K. Monograph of Schisandra (Schisandraceae). Syst. Bot. Monogr. 2000, 58, 1–146. [Google Scholar] [CrossRef]
- Hancke, J.L.; Burgos, R.A.; Ahumada, F. Schisandra chinensis (Turcz.) Baill. Fitoterapia 1999, 70, 451–471. [Google Scholar] [CrossRef]
- Szopa, A.; Ekiert, R.; Ekiert, H. Current knowledge of Schisandra chinensis (Turcz.) Baill. (Chinese magnolia vine) as a medicinal plant species: A review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochem. Rev. 2017, 16, 195–218. [Google Scholar] [CrossRef]
- Raj, S.P.; Solomon, P.R.; Thangaraj, B. Schisandraceae. In Biodiesel from Flowering Plants; Springer: Singapore, 2022; pp. 529–532. [Google Scholar] [CrossRef]
- Qiu, F.; Liu, H.; Duan, H.; Chen, P.; Lu, S.-J.; Yang, G.-Z.; Lei, X.-X. Isolation, Structural Elucidation of Three New Triterpenoids from the Stems and Leaves of Schisandra chinensis (Turcz) Baill. Molecules 2018, 23, 1624. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Q.-L.; Tadmor, Y.; Simon, J.E.; Sang, S.; Ho, C.-T. Schisandra chinensis: Chemistry and Analysis. In Oriental Foods and Herbs; American Chemical Society: Washington, DC, USA, 2003; Volume 859, pp. 234–246. [Google Scholar]
- Lu, Y.; Chen, D.-F. Analysis of Schisandra chinensis and Schisandra sphenanthera. J. Chromatogr. A 2009, 1216, 1980–1990. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, Y.; Cai, G.; Gong, J.; Wang, Y.; Liu, S. Chemical composition analysis of Schisandra chinensis fructus and its three processed products using UHPLC-Q-Orbitrap/MS-based metabolomics approach. Nat. Prod. Res. 2022, 36, 3464–3468. [Google Scholar] [CrossRef]
- Song, Y.; Shan, B.; Zeng, S.; Zhang, J.; Jin, C.; Liao, Z.; Wang, T.; Zeng, Q.; He, H.; Wei, F.; et al. Raw and wine processed Schisandra chinensis attenuate anxiety like behavior via modulating gut microbiota and lipid metabolism pathway. J. Ethnopharmacol. 2021, 266, 113426. [Google Scholar] [CrossRef]
- Chen, X.; Tang, R.; Liu, T.; Dai, W.; Liu, Q.; Gong, G.; Song, S.; Hu, M.; Huang, L.; Wang, Z. Physicochemical properties, antioxidant activity and immunological effects in vitro of polysaccharides from Schisandra sphenanthera and Schisandra chinensis. Int. J. Biol. Macromol. 2019, 131, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Shang, L.; Wang, M.; Zhang, C.; Zhao, X.; Bi, K.; Jia, Y. Lignans from Schisandra chinensis ameliorate cognition deficits and attenuate brain oxidative damage induced by D-galactose in rats. Metab. Brain Dis. 2016, 31, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Giridharan, V.V.; Thandavarayan, R.A.; Sato, S.; Ko, K.M.; Konishi, T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free Radic. Res. 2011, 45, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Sa, F.; Zhang, L.Q.; Chong, C.M.; Guo, B.J.; Li, S.; Zhang, Z.J.; Zheng, Y.; Hoi, P.M.; Lee, S.M.Y. Discovery of novel anti-parkinsonian effect of schisantherin A in in vitro and in vivo. Neurosci. Lett. 2015, 593, 7–12. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Sa, F.; Chong, C.M.; Wang, Y.; Zhou, Z.Y.; Chang, R.C.C.; Chan, S.W.; Hoi, P.M.; Yuen Lee, S.M. Schisantherin A protects against 6-OHDA-induced dopaminergic neuron damage in zebrafish and cytotoxicity in SH-SY5Y cells through the ROS/NO and AKT/GSK3β pathways. J. Ethnopharmacol. 2015, 170, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, C.; Jing, S.; Wang, M.; Wang, H.; Sun, J.; Wang, C.; Chen, J.; Li, H. Compound Schisandra-Ginseng-Notoginseng-Lycium Extract Ameliorates Scopolamine-Induced Learning and Memory Disorders in Mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 8632016. [Google Scholar] [CrossRef] [PubMed]
- Koncic, M.Z.; Tomczyk, M. New insights into dietary supplements used in sport: Active substances, pharmacological and side effects. Curr. Drug Targets 2013, 14, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Abascal, K.; Yarnell, E. Bacopa for the Brain: A Smart Addition to Western Medicine. Altern. Complement. Ther. 2011, 17, 21–25. [Google Scholar] [CrossRef]
- Kean, J.; Stough, C. Role of the Ayurvedic Medicinal Herb Bacopa monnieri in Child and Adolescent Populations. In Natural Medicines; CRC Press: Boca Raton, FL, USA, 2019; pp. 333–348. [Google Scholar]
- Devendra, P.; Patel, S.S.; Birwal, P.; Basu, S.; Deshmukh, G.; Datir, R. Brahmi (Bacopa monnieri) as functional food ingredient in food processing industry. J. Pharmacogn. Phytochem. 2018, 7, 189–194. [Google Scholar]
- Srivastava, A.; Srivastava, P.; Pandey, A.; Khanna, V.K.; Pant, A.B. Chapter 24—Phytomedicine: A Potential Alternative Medicine in Controlling Neurological Disorders. In New Look to Phytomedicine; Ahmad Khan, M.S., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 625–655. [Google Scholar] [CrossRef]
- Binita, B.C.; Ashok, M.D.; Yogesh, T.J. Bacopa monnieri (L.) Pennell: A rapid, efficient and cost effective micropropagation. Plant Tissue Cult Biotechnol. 2005, 15, 167–175. [Google Scholar]
- Akbar, S. Bacopa monnieri (L.) Wettst. (Plantaginaceae). In Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; Springer International Publishing: Cham, Switzerland, 2020; pp. 401–412. [Google Scholar] [CrossRef]
- Deolankar, S.C.; Najar, M.A.; Ramesh, P.; Kanichery, A.; Kudva, A.K.; Raghu, S.V.; Prasad, T.S.K. Discovery of Molecular Networks of Neuroprotection Conferred by Brahmi Extract in Aβ42-Induced Toxicity Model of Drosophila melanogaster Using a Quantitative Proteomic Approach. Mol. Neurobiol. 2023, 60, 303–316. [Google Scholar] [CrossRef]
- Bhandari, P.; Kumar, N.; Singh, B.; Kaur, I. Dammarane triterpenoid saponins from Bacopa monnieri. Can. J. Chem. 2009, 87, 1230–1234. [Google Scholar] [CrossRef]
- Phrompittayarat, W.; Wittaya-Areekul, S.; Jetiyanon, K.; Putalun, W.; Tanaka, H.; Ingkaninan, K. Determination of saponin glycosides in Bacopa monnieri by reversed phase high performance liquid chromatography. Thai Pharmaceut. Health Sci. J. 2007, 2, 26–32. [Google Scholar]
- Chadha, M.L. Indigenous vegetables of India with potentials for improving livelihood. Acta Hortic. 2009, 806, 579–586. [Google Scholar] [CrossRef]
- Amaravathi, T.; Geetha, P.S.; Murugan, M.; Selvam, S.; Kanchana, S. Traditional value added products from Indian penny wort (Centella asiatica) and water hyssop (Bacopa monnieri) to alleviate ADHD. J. Pharm. Innov. 2020, 9, 432–441. [Google Scholar]
- Russo, A.; Borrelli, F.; Campisi, A.; Acquaviva, R.; Raciti, G.; Vanella, A. Nitric oxide-related toxicity in cultured astrocytes: Effect of Bacopa monniera. Life Sci. 2003, 73, 1517–1526. [Google Scholar] [CrossRef]
- Brimson, J.M.; Prasanth, M.I.; Plaingam, W.; Tencomnao, T. Bacopa monnieri (L.) wettst. Extract protects against glutamate toxicity and increases the longevity of Caenorhabditis elegans. J. Tradit. Complement. Med. 2020, 10, 460–470. [Google Scholar] [CrossRef]
- Dhanasekaran, M.; Tharakan, B.; Holcomb, L.A.; Hitt, A.R.; Young, K.A.; Manyam, B.V. Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera. Phytother. Res. 2007, 21, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Murthy, V.; Ramassamy, C. Modulation of Hydrogen Peroxide and Acrolein-Induced Oxidative Stress, Mitochondrial Dysfunctions and Redox Regulated Pathways by the Bacopa monniera Extract: Potential Implication in Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 21, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, L.A.; Dhanasekaran, M.; Hitt, A.R.; Young, K.A.; Riggs, M.; Manyam, B.V. Bacopa monniera extract reduces amyloid levels in PSAPP mice. J. Alzheimer’s Dis. 2006, 9, 243–251. [Google Scholar] [CrossRef]
- Le, X.T.; Pham, H.T.N.; Do, P.T.; Fujiwara, H.; Tanaka, K.; Li, F.; Van Nguyen, T.; Nguyen, K.M.; Matsumoto, K. Bacopa monnieri Ameliorates Memory Deficits in Olfactory Bulbectomized Mice: Possible Involvement of Glutamatergic and Cholinergic Systems. Neurochem. Res. 2013, 38, 2201–2215. [Google Scholar] [CrossRef] [PubMed]
- Uabundit, N.; Wattanathorn, J.; Mucimapura, S.; Ingkaninan, K. Cognitive enhancement and neuroprotective effects of Bacopa monnieri in Alzheimer’s disease model. J. Ethnopharmacol. 2010, 127, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Dhawan, B. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn. (Brahmi). Indian J. Pharmacol. 1997, 29, 359. [Google Scholar]
- Kamkaew, N.; Norman Scholfield, C.; Ingkaninan, K.; Taepavarapruk, N.; Chootip, K. Bacopa monnieri Increases Cerebral Blood Flow in Rat Independent of Blood Pressure. Phytother. Res. 2013, 27, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Roodenrys, S.; Booth, D.; Bulzomi, S.; Phipps, A.; Micallef, C.; Smoker, J. Chronic Effects of Brahmi (Bacopa monnieri) on Human Memory. Neuropsychopharmacology 2002, 27, 279–281. [Google Scholar] [CrossRef]
- Dimpfel, W.; Schombert, L.; Biller, A. Psychophysiological Effects of Sideritis and Bacopa Extract and Three Combinations Thereof—A Quantitative EEG Study in Subjects Suffering from Mild Cognitive Impairment (MCI). Adv. Alzheimer’s Dis. 2016, 5, 1–22. [Google Scholar] [CrossRef]
- McPhee, G.M.; Downey, L.A.; Wesnes, K.A.; Stough, C. The Neurocognitive Effects of Bacopa monnieri and Cognitive Training on Markers of Brain Microstructure in Healthy Older Adults. Front. Aging Neurosci. 2021, 13, 638109. [Google Scholar] [CrossRef]
- Gohil, K.J.; Patel, J.A. A review on Bacopa monniera: Current research and future prospects. Int. J. Green Pharm. 2010, 4, 1–9. [Google Scholar] [CrossRef]
- Chaudhari, K.S.; Tiwari, N.R.; Tiwari, R.R.; Sharma, R.S. Neurocognitive Effect of Nootropic Drug Brahmi (Bacopa monnieri) in Alzheimer’s Disease. Ann. Neurosci. 2017, 24, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Kean, J.D.; Downey, L.A.; Stough, C. A systematic review of the Ayurvedic medicinal herb Bacopa monnieri in child and adolescent populations. Complement. Ther. Med. 2016, 29, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Joshua Allan, J.; Damodaran, A.; Deshmukhda, N.S.; Goudar, K.S.; Amit, A. Safety evaluation of a standardized phytochemical composition extracted from Bacopa monnieri in Sprague–Dawley rats. Food Chem. Toxicol. 2007, 45, 1928–1937. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malík, M.; Tlustoš, P. Nootropic Herbs, Shrubs, and Trees as Potential Cognitive Enhancers. Plants 2023, 12, 1364. https://doi.org/10.3390/plants12061364
Malík M, Tlustoš P. Nootropic Herbs, Shrubs, and Trees as Potential Cognitive Enhancers. Plants. 2023; 12(6):1364. https://doi.org/10.3390/plants12061364
Chicago/Turabian StyleMalík, Matěj, and Pavel Tlustoš. 2023. "Nootropic Herbs, Shrubs, and Trees as Potential Cognitive Enhancers" Plants 12, no. 6: 1364. https://doi.org/10.3390/plants12061364
APA StyleMalík, M., & Tlustoš, P. (2023). Nootropic Herbs, Shrubs, and Trees as Potential Cognitive Enhancers. Plants, 12(6), 1364. https://doi.org/10.3390/plants12061364










