Stimulation of PGP Bacteria on the Development of Seeds, Plants and Cuttings of the Obligate Halophyte Arthrocaulon (Arthrocnemum) macrostachyum (Moric.) Piirainen & G. Kadereit
Abstract
1. Introduction
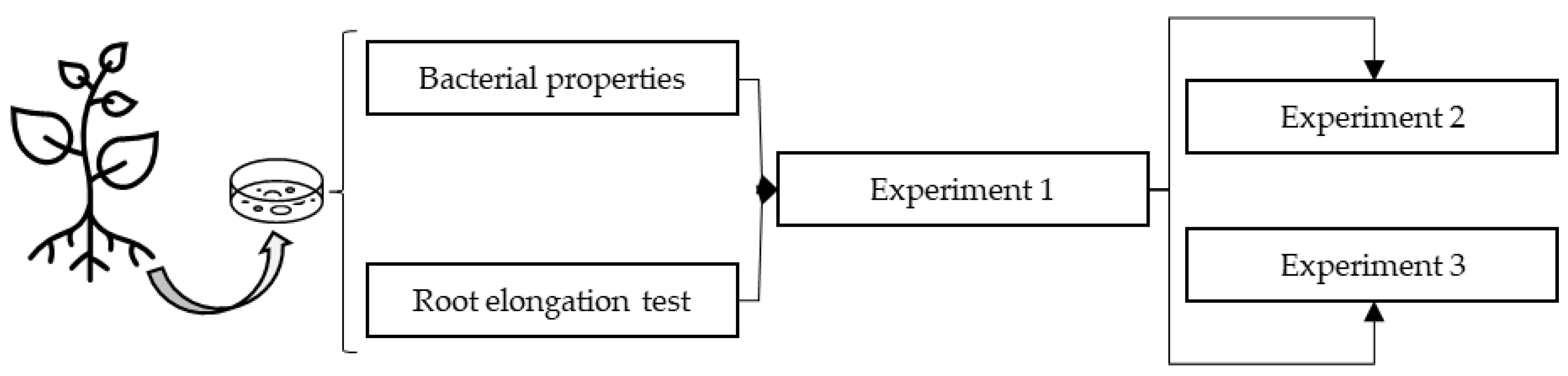
2. Materials and Methods
2.1. Isolation of Bacteria
2.2. Molecular Characterisation of Bacteria: Amplification, Sequencing, and Phylogenetic Analysis of 16S rDNA
2.3. Bacterial Tolerance to High Salinity
2.4. Bacterial Tolerance to High Temperature
2.5. Indole Acetic Acid Production (IAA)
2.6. Acyl-Homoserine Lactone Production (AHL)
2.7. Siderophore Production
2.8. Phosphate Solubilisation
2.9. Vegetal Material
2.10. Inoculation
2.11. Rapeseed Root Elongation Test
2.12. Experiment 1: Influence of Bacterial Strains and Salt on Plant of A. macrostachyum
2.13. Experiment 2: Effects of Bacterial Inoculation and Salt on the Germination of A. macrostachyum
2.14. Experiment 3: Influence of Bacterial Inoculation on Cutting of A. macrostachyum
2.15. Gas Exchange
2.16. Plant Growth
2.17. Statistics
3. Results
3.1. Bacterial Properties
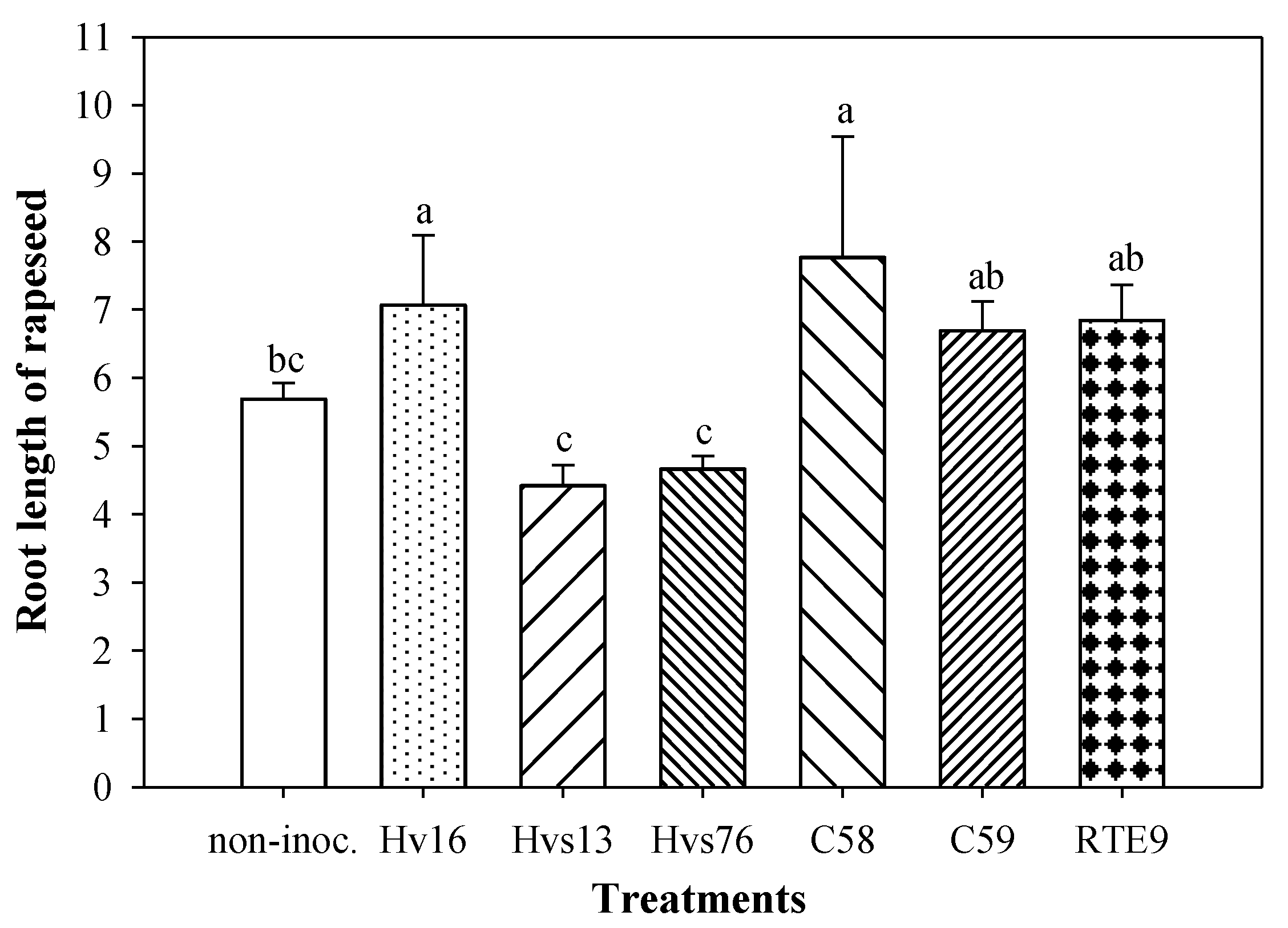
3.2. Rapeseed Root Elongation Test
3.3. Influence of Bacterial Strains and Salt on Plant of A. macrostachyum
3.3.1. Plant Growth
3.3.2. Gas Exchange
3.4. Effects of Bacterial Inoculation and Salt on the Germination of A. macrostachyum
3.5. Influence of Bacterial Inoculation on Cutting of A. macrostachyum
3.5.1. Plant Growth
3.5.2. Gas Exchange
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Oster, J.D. Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Sci. Total Environ. 2004, 323, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rabhi, M.; Atia, A.; Abdelly, C.; Smaoui, A. New parameters for a better evaluation of vegetative bioremediation, leaching, and phytodesalination. J. Theor. Biol. 2015, 383, 7–11. [Google Scholar] [CrossRef]
- Lutts, S.; Lefèvre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 115, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Barroso, F.; Pedreno, A.; Martínez, T.; Robles, A.; González-Rebollar, J. In abstracts book XLV Reunión Científica de la SEEP (Sesión: Sistemas Agrosilvopastorales). Potencialidad de las especies C4 como alimento para el ganado en repoblaciones de zonas semiáridas. Prod. Agroganaderas Gestión Efic. Conserv. Medio Nat. 2005, 1, 347–353. [Google Scholar]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Flowers, T.J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Bazihizina, N.; Barrett-Lennard, E.G.; Colmer, T.D. Plant growth and physiology under heterogeneous salinity. Plant Soil 2012, 354, 1–19. [Google Scholar] [CrossRef]
- Ungar, I.A. Halophyte seed germination. Bot. Rev. 1978, 44, 233–264. [Google Scholar] [CrossRef]
- Vicente, M.J.; Conesa, E.; Álvarez-Rogel, J.; Franco, J.A.; Martínez-Sánchez, J.J. Effects of various salts on the germination of three perennial salt marsh species. Aquat. Bot. 2007, 87, 167–170. [Google Scholar] [CrossRef]
- Francis, R.A.; Gurnell, A.M.; Petts, G.E.; Edwards, P.J. Survival and growth responses of Populus nigra, Salix elaeagnos and Alnus incana cuttings to varying levels of hydric stress. For. Ecol. Manag. 2005, 210, 291–301. [Google Scholar] [CrossRef]
- Moggridge, H.L.; Gurnell, A.M. Controls on the sexual and asexual regeneration of Salicaceae along a highly dynamic, braided river system. Aquat. Sci. 2009, 71, 305. [Google Scholar] [CrossRef]
- Van Tassel, D.L.; DeHaan, L.R.; Cox, T.S. Missing domesticated plant forms: Can artificial selection fill the gap? Evol. Appl. 2010, 3, 434–452. [Google Scholar] [CrossRef]
- Piccoli, P.; Travaglia, C.; Cohen, A.; Sosa, L.; Cornejo, P.; Masuelli, R.; Bottini, R. An endophytic bacterium isolated from roots of the halophyte Prosopis strombulifera produces ABA, IAA, gibberellins A1 and A3 and jasmonic acid in chemically-defined culture medium. Plant Growth Regul. 2011, 64, 207–210. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trend Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Hernandez, J.P.; Bashan, Y. The potential contribution of plant growth-promoting bacteria to reduce environmental degradation–A comprehensive evaluation. Appl. Soil Ecol. 2012, 61, 171–189. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Iqbal, S.; Amjad, M.; Naveed, M.; Zahir, Z.A.; Jacobsen, S. Enhancing salt tolerance in quinoa by halotolerant bacterial inoculation. Funct. Plant Biol. 2016, 43, 632–642. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Galyamova, M.R.; Sedykh, S.E. Bacterial Siderophores: Classification, Biosynthesis, Perspectives of Use in Agriculture. Plants 2022, 11, 3065. [Google Scholar] [CrossRef]
- Behera, B.C.; Singdevsachan, S.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Diversity, mechanism and biotechnology of phosphate solubilising microorganism in mangrove—A review. Biocatal. Agric. Biotechnol. 2014, 3, 97–110. [Google Scholar] [CrossRef]
- Hartmann, A.; Klink, S.; Rothballer, M. Plant growth promotion and induction of systemic tolerance to drought and salt stress of plants by quorum sensing auto-inducers of the N-acyl-homoserine lactone type: Recent developments. Front. Plant Sci. 2021, 12, 683546. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Dev, K.; Sourirajan, A.; Choudhary, M. Isolation and characterization of salt-tolerant bacteria with plant growth-promoting activities from saline agricultural fields of Haryana, India. J. Genet. Eng. Biotechnol. 2021, 19, 99. [Google Scholar] [CrossRef]
- Sukhorukov, A.P.; Nilova, M.V. A new species of Arthrocnemum (Salicornioideae: Chenopodiaceae-Amaranthaceae) from West Africa, with a revised characterization of the genus. Bot. Lett. 2016, 163, 237–250. [Google Scholar] [CrossRef]
- Piirainen, M.; Liebisch, O.; Kadereit, G. Phylogeny, biogeography, systematics and taxonomy of Salicornioideae (Amaranthaceae/Chenopodiaceae)—A cosmopolitan, highly specialized hygrohalophyte lineage dating back to the Oligocene. Taxon 2017, 66, 109–132. [Google Scholar] [CrossRef]
- Ramírez, E.; Sánchez-Gavilán, I.; Rufo, L.; Sánchez-Mata, D.; de la Fuente, V. Morphology, anatomy and phylogeny of the two sister halophytic genera Microcnemum and Arthrocnemum (Salicornioideae/Amaranthaceae). Plant Biosyst. 2022, 156, 1422–1437. [Google Scholar] [CrossRef]
- Barcia-Piedras, J.M.; Pérez-Romero, J.A.; Mateos-Naranjo, E.; Camacho, M.; Redondo-Gómez, S. Effect of prior salt experience on desalination capacity of the halophyte Arthrocnemum macrostachyum. Desalination 2019, 463, 50–54. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Barcia-Piedras, J.M.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Camacho, M.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Assessing the role of endophytic bacteria in the halophyte Arthrocnemum macrostachyum salt tolerance. Plant Biol. 2017, 19, 249–256. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Pereira, C.; Rodrigues, M.J.; Fernandes, E.; Neng, N.R.; Bandarra, N.M.; Custódio, L. Short Lecture Biotechnological valorisation of seeds of two halophyte species, Suaeda vera and Arthrocaulon macrostachyum. Planta Med. 2022, 88, 1413–1414. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; Moreira da Silva, M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Comp. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- ElNaker, N.A.; Yousef, A.F.; Yousef, L.F. A review of Arthrocnemum (Arthrocaulon) macrostachyum chemical content and bioactivity. Phytochem. Rev. 2020, 19, 1427–1448. [Google Scholar] [CrossRef]
- Vincent, J.M. A manual for the practical study of the root-nodule bacteria. In A Manual for the Practical Study of the Root-Nodule Bacteria; Blackwell Scientific Publications: Oxford, UK, 1970; p. 164. [Google Scholar]
- Camacho, M.; Medina, C.; Rodríguez-Navarro, D.N.; Vera, F.T. Biodiversity of rhizobia present in plant nodules of Biserrula pelecinus across Southwest Spain. Syst. Appl. Microbiol. 2019, 42, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J. 16S/23S rRNA Sequencing. In Nucleic Acid Techniques in Bacterial Systematics; John Wiley and Sons: New York, NY, USA, 1991; p. 329. [Google Scholar]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida Indoleacetic Acid in Development of the Host Plant Root System. Appl Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef]
- Pérez-Montaño, F.; Jiménez-Guerrero, I.; Contreras Sánchez-Matamoros, R.; López-Baena, F.J.; Ollero, F.J.; Rodríguez-Carvajal, M.A.; Bellogín, R.A.; Espuny, M.R. Rice and bean AHL-mimic quorum-sensing signals specifically interfere with the capacity to form biofilms by plant-associated bacteria. Res. Microbiol. 2013, 164, 749–760. [Google Scholar] [CrossRef]
- Alexander, D.B.; Zuberer, D.A. Use of Chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fert. Soils 1991, 12, 39–45. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil connection with the vital activity of some microbial species. Microbiology 1948, 17, 362–370. [Google Scholar]
- Montero-Calasanz, M.C.; Santamaría, C.; Albareda, M.; Daza, A.; Duan, J.; Glick, B.R.; Camacho, M. Alternative rooting induction of semi-hardwood olive cuttings by several auxin-producing bacteria for organic agriculture systems. Span. J. Agric. Res. 2013, 11, 146–154. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition; CABI: Farnham Royal, UK, 1952; p. 547. [Google Scholar]
- Ellis, R.H.; Roberts, E.H. Towards a Rational Basis for Testing Seed Quality. In Seed Production; Butterworths: London, UK, 1980; pp. 605–635. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Figueroa, E.; Davy, A. Salt stimulation of growth and photosynthesis in an extreme halophyte, Arthrocnemum macrostachyum. Plant Biol. 2010, 12, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Medrano, H.; Flexas, J. Relaciones Hídricas de las Plantas. In La Ecofisiología Vegetal: Una Ciencia de Síntesis; Reigosa, M., Pedrol, N., Sánchez, A., Eds.; Thomson Editores: Madrid, Spain, 2004; pp. 1141–1174. [Google Scholar]
- Pérez-Romero, J.A.; Barcia-Piedras, J.M.; Redondo-Gómez, S.; Caçador, I.; Duarte, B.; Mateos-Naranjo, E. Salinity modulates Juncus acutus L. tolerance to diesel fuel pollution. Plants 2022, 11, 758. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, X.; Zhang, E.; Wang, C.; Ruan, C.; Xu, Y.; Liu, X.; Luo, X. Study of microbial distribution in the arid desert terrain, Beishan mountains area, Gansu. J. Pure Appl. Microbiol. 2013, 7, 3111–3119. Available online: https://microbiologyjournal.org/archive_mg/jmabsread.php?snoid=1567&month=&year= (accessed on 19 February 2023).
- Shin, Y.; Lee, B.H.; Lee, K.E.; Park, W. Pseudarthrobacter psychrotolerans sp. nov., a cold-adapted bacterium isolated from Antarctic soil. Int. J. Syst. Evol. Microbiol. 2020, 70, 6106–6114. [Google Scholar] [CrossRef]
- Djedidi, S.; Yokoyama, T.; Ohkama-Ohtsu, N.; Risal, C.P.; Abdelly, C.; Sekimoto, H. Stress tolerance and symbiotic and phylogenic features of root nodule bacteria associated with Medicago species in different bioclimatic regions of Tunisia. Microbes Environ. 2011, 16, 36–45. [Google Scholar] [CrossRef]
- Yahaghi, Z.; Shirvani, M.; Nourbakhsh, F.; De La Pena, T.C.; Pueyo, J.J.; Talebi, M. Isolation and characterisation of Pb-solubilising bacteria and their effects on Pb uptake by Brassica juncea: Implications for microbe-assisted phytoremediation. J. Microbiol. Biotechnol. 2018, 28, 1156–1167. [Google Scholar] [CrossRef]
- Mohammad, S.; Sen, D.N. Germination behaviour of some halophytes in Indian desert. Indian J. Exp. Biol. 1990, 28, 545–549. [Google Scholar]
- Qu, X.; Baskin, J.M.; Wang, L.; Huang, Z. Effects of cold stratification, temperature, light and salinity on seed germination and radicle growth of the desert halophyte shrub, Kalidium caspicum (Chenopodiaceae). Plant Growth Regul. 2008, 54, 241–248. [Google Scholar] [CrossRef]
- Nada, R.M.; Abogadallah, G.M. Developmental acquisition of salt tolerance in the halophyte Atriplex halimus L. is related to differential regulation of salt inducible genes. Plant Growth Regul. 2015, 75, 165–178. [Google Scholar] [CrossRef]
- Pujol, J.A.; Calvo, J.F.; Ramirez-Diaz, L. Recovery of germination from different osmotic conditions by four halophytes from south-eastern Spain. Ann. Bot. 2000, 85, 279–286. [Google Scholar] [CrossRef]
- Vicente, M.J.; Conesa, E.; Alvarez-Rogel, J.; Franco, J.A.; Martínez-Sánchez, J.J. Relationships between salt type and seed germination in three plant species growing in salt marsh soils of semi-arid Mediterranean environments. Arid. Land Res. Manag. 2009, 23, 103–114. [Google Scholar] [CrossRef]
- Rubio-Casal, A.E.; Castillo, J.M.; Luque, C.J.; Figueroa, M.E. Influence of salinity on germination and seeds viability of two primary colonizers of Mediterranean salt pans. J. Arid. Environ. 2003, 53, 145–154. [Google Scholar] [CrossRef]
- Nisar, F.; Gul, B.; Khan, M.A.; Hameed, A. Germination and recovery responses of heteromorphic seeds of two co-occurring Arthrocnemum species to salinity, temperature and light. S. Afr. J. Bot. 2019, 121, 143–151. [Google Scholar] [CrossRef]
- Ramírez, E.; Chaâbene, Z.; Hernández-Apaolaza, L.; Rekik, M.; Elleuch, A.; de la Fuente, V. Seed priming to optimize germination in Arthrocnemum Moq. BMC Plant Biol. 2022, 22, 527. [Google Scholar] [CrossRef] [PubMed]
- Vyas, P.; Joshi, R.; Sharma, K.C.; Rahi, P.; Gulati, A.; Gulati, A. Cold-adapted and rhizosphere-competent strain of Rahnella sp. with broad-spectrum plant growth-promotion potential. J. Microbiol. Biotechnol. 2010, 20, 1724–1734. [Google Scholar] [CrossRef]
- Din, B.U.; Sarfraz, S.; Xia, Y.; Kamran, M.A.; Javed, M.T.; Sultan, T.; Munis MF, H.; Chaudhary, H.J. Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing Bacillus strains under induced salinity stress. Ecotoxicol. Environ. Saf. 2019, 183, 109466. [Google Scholar] [CrossRef]
- Michaud, M.; Martinez, C.; Simao-Beaunoir, A.M.; Bélanger, R.R.; Tweddell, R.J. Selection of antagonist microorganisms against Helminthosporium solani, causal agent of potato silver scurf. Plant Dis. 2002, 86, 717–720. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showalter, A.M. Salt stimulation and tolerance in an intertidal stem-succulent halophyte. J. Plant Nutr. 2005, 28, 1365–1374. [Google Scholar] [CrossRef]
- Bashan, Y.; Moreno, M.; Troyo, E. Growth promotion of the seawater-irrigated oilseed halophyte Salicornia bigelovii inoculated with mangrove rhizosphere bacteria and halotolerant Azospirillum spp. Biol. Fertil. Soils 2000, 32, 265–272. [Google Scholar] [CrossRef]
- Ozawa, T.; Wu, J.; Fujii, S. Effect of inoculation with a strain of Pseudomonas pseudoalcaligenes isolated from the endorhizosphere of Salicornia europea on salt tolerance of the glasswort. Soil Sci. Plant Nutr. 2007, 53, 12–16. [Google Scholar] [CrossRef]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Springer: Dordrecht, The Netherland, 2007; pp. 329–339. [Google Scholar] [CrossRef]
- Peng, J.; Wu, D.; Liang, Y.; Li, L.; Guo, Y. Disruption of acdS gene reduces plant growth promotion activity and maize saline stress resistance by Rahnella aquatilis HX2. J. Basic Microbiol. 2019, 59, 402–411. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Davranov, K.; Wirth, S.; Hashem, A.; Fathi; Abdallah, E. Impact of soil salinity on the plant-growth-promoting and biological control abilities of root associated bacteria. Saudi J. Biol. Sci. 2017, 24, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, R.; Alikhani, H.A.; Towfighi, H.; Khavazi, K.; Pourbabaee, A.A. Isolated bacteria from saline–sodic soils alter the response of wheat under high adsorbed sodium and salt stress. Int. J. Environ. Sci. Technol. 2017, 14, 143–150. [Google Scholar] [CrossRef]
- Lu, C.; Qiu, N.; Lu, Q.; Wang, B.; Kuang, T. Does salt stress lead to increased susceptibility of photosystem II to photoinhibition and changes in photosynthetic pigment composition in halophyte Suaeda salsa grown outdoors? Plant Sci. 2002, 163, 1063–1068. [Google Scholar] [CrossRef]
- Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops. Plants 2023, 12, 549. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Mitsios, I.; Fragkakis, I.; Nektarios, P.; Ntoulas, N.; Londra, P.; Papafotiou, M. The growth of Arthrocnemum macrostachyum and Halimione portulacoides in an extensive green roof system under two watering regimes. Agri. Agric. Sci. Procedia 2015, 4, 242–249. [Google Scholar] [CrossRef]
- Zenginbal, H.; Demir, T. Effects of some rhizobacteria and indole-3-butyric acid on rooting of black and white mulberry hardwood cuttings. J. Anim. Plant Sci. 2018, 28, 1426–1431. Available online: https://www.thejaps.org.pk/docs/v-28-05/24.pdf (accessed on 13 March 2023).

| Strain | Environment | Related Species | Identity (%) | Accession Number |
|---|---|---|---|---|
| Hv16 | Rhizosphere of A. macrostachyum from Guadalquivir River saltmarsh. | Kocuria polaris | 98.6 | LN794848 |
| Hvs13 | Rhizosphere of A. macrostachyum from Guadalquivir River saltmarsh (isolated at 350 mM NaCl). | Brevibacterium frigoritolerans | 100 | OU596762 |
| Hvs76 | Rhizosphere of A. macrostachyum from Guadalquivir River saltmarsh (isolated at 350 mM NaCl). | Pseudarthrobacter siccitolerans | 99.4 | OU596760 |
| C58 | Rhizosphere of A. macrostachyum from an irrigation canal close to Guadalquivir River saltmarsh. | Pseudarthrobacter psychrotolerans | 99.3 | OU596763 |
| C59 | Rhizosphere of A. macrostachyum from an irrigation canal close to Guadalquivir River saltmarsh. | Ensifer meliloti | 99.9 | OU596759 |
| RTE9 | Interior of an indeterminate plant of the Tinto River saltmarsh. | Rahnella aceris | 99.3 | OU596761 |
| Strain | SAL (%) | TEM | IAA (μg g−1) | AHL (%) | SID (mm) | PHO (mm) |
|---|---|---|---|---|---|---|
| Hv16 | 0–9 | − | 200 | 50 | 20 | − |
| Hvs13 | 0–6 | + | 50 | 75 | 10 | − |
| Hvs76 | 0–4 | − | 150 | 100 | 10 | − |
| C58 | 0–4 | − | 150 | 25 | 10 | − |
| C59 | 0–1 | − | 100 | 50 | - | − |
| RTE9 | 0–4 | − | 150 | 0 | - | 30 |
| Treatment | Branch Length (cm) | Branch Diameter (mm) | Branch Surface Area (cm2) | Secondary Branches |
|---|---|---|---|---|
| Non-inoculated | 2.2 ± 0.2 b | 1.7 ± 0.1 ab | 1.2 ± 0.1 c | 0 ± 0 c |
| Hv16 | 2.9 ± 0.2 a | 1.9 ± 0.1 a | 1.7 ± 0.1 a | 2 ± 0 a |
| C58 | 2.1 ± 0.2 bc | 1.9 ± 0.1 a | 1.4 ± 0.1 ab | 1 ± 0 bc |
| C59 | 2.0 ± 0.2 c | 1.6 ± 0.1 bc | 1.1 ± 0.1 c | 1 ± 0 bc |
| RTE9 | 2.7 ± 0.2 a | 1.8 ± 0.1 a | 1.7 ± 0.2 a | 1 ± 0 bc |
| 0 mM NaCl | |||
|---|---|---|---|
| Treatment | Aerial Dried Weight (g) | Root Dried Weight (g) | Water Content (%) |
| Non-inoculated | 0.27 ± 0.10 c | 0.19 ± 0.06 c | 84.1 ± 1.8 ab |
| Hv16 | 0.59 ± 0.12 a | 0.73 ± 0.18 a | 85.3 ± 0. 8 a |
| C58 | 0.45 ± 0.09 ab | 0.81 ± 0.23 a | 78.9 ± 1.2 c |
| C59 | 0.34 ± 0.07 abc | 0.40 ± 0.11 bc | 80.4 ± 0.7 bc |
| RTE9 | 0.57 ± 0.13 ab | 0.67 ± 0.19 ab | 81.4 ± 0.5 abc |
| 171 mM NaCl | |||
| Non-inoculated | 1.25 ± 0.27 ab | 0.75 ± 0.18 c | 87.6 ± 0.4 ab |
| Hv16 | 1.66 ± 0.30 a | 1.14 ± 0.15 ab | 88.0 ± 0.2 ab |
| C58 | 0.89 ± 0.15 b | 0.77 ± 0.13 bc | 88.6 ± 0.9 ab |
| C59 | 1.84 ± 0.32 a | 1.21 ± 0.19 ab | 87.4 ± 0.6 b |
| RTE9 | 1.93 ± 0.23 a | 1.28 ± 0.13 a | 87.7 ± 0.5 ab |
| Treatment | 0 mM NaCl | |||
|---|---|---|---|---|
| A (μmol m−2s−1) | gs (mmol m−2s−1) | Ci (μmol mol−1) | iWUE (mmol mol−1) | |
| No-inoculated | 7.5 ± 1.4 c | 90 ± 20 c | 215 ± 20 bc | 85 ± 12 |
| Hv16 | 14.1 ± 1.0 a | 240 ± 30 a | 250 ± 6 a | 70 ± 14 |
| C58 | 8.6 ± 1.7 bc | 120 ± 40 b | 215 ± 17 bc | 82 ± 12 |
| C59 | 5.8 ± 1.7 c | 90 ± 20 c | 224 ± 19 ab | 78 ± 12 |
| RTE9 | 10.9 ± 1.6 ab | 150 ± 30 b | 250 ± 10 a | 76 ± 16 |
| 171 mM NaCl | ||||
| No-inoculated | 7.3 ± 0.7 | 140 ± 20 | 275 ± 7 a | 53 ± 4 b |
| Hv16 | 5.8 ± 1.1 | 100 ± 30 | 252 ± 9 ab | 61 ± 7 ab |
| C58 | 6.8 ± 1.2 | 90 ± 20 | 232 ± 19 ab | 86 ± 15 ab |
| C59 | 5.5 ± 0.6 | 90 ± 10 | 246 ± 19 ab | 69 ± 13 ab |
| RTE9 | 5.6 ± 0.9 | 90 ± 40 | 215 ± 13 b | 86 ± 1 a |
| 0 mM NaCl | |||||
|---|---|---|---|---|---|
| Treatment | Total Germination (%) | First Germination (d) | Last Germination (d) | MTG (d) | CFU |
| Non-inoculated | 86 ± 5 | 5 ± 1 | 11 ± 1 | 7.2 ± 0.6 | 2 ± 0 |
| Hv16 | 86 ± 3 | 4 ± 1 | 12 ± 1 | 7.2 ± 0.3 | 2 ± 0 |
| C58 | 83 ± 6 | 4 ± 0 | 11 ± 2 | 6.6 ± 0.3 | 3 ± 1 |
| RTE9 | 88 ± 3 | 4 ± 0 | 12 ± 3 | 6.9 ± 0.2 | 2 ± 1 |
| 171 mM NaCl | |||||
| Non-inoculated | 64 ± 5 a | 6 ± 1 | 14 ± 0 | 9.6 ± 0.4 | 4 ± 1 a |
| Hv16 | 85 ± 6 b | 6 ± 1 | 14 ± 1 | 9.3 ± 0.2 | 2 ± 1 b |
| C58 | 71 ± 2 ab | 6 ± 1 | 13 ± 1 | 9.4 ± 0.2 | 3 ± 0 a |
| RTE9 | 91 ± 3 b | 5 ± 1 | 14 ± 2 | 8.8 ± 0.4 | 3 ± 1 a |
| Branch | Root | |||||
|---|---|---|---|---|---|---|
| Treatment | Length (cm) | Dry Weight (mg) | RWC (%) | RGR (mg g−1 day−1) | Length (cm) | Dry Weight (mg) |
| Non-inoculated | 82 ± 6 | 43 ± 3 | 89 ± 2 a | 25 ± 3 | 44 ± 4 b | 8.6 ± 0.6 b |
| Hv16 | 88 ± 5 | 45 ± 3 | 79 ± 2 b | 25 ± 3 | 52 ± 4 a | 10.4 ± 0.6 a |
| C58 | 84 ± 6 | 38 ± 3 | 87 ± 2 a | 21 ± 3 | 48 ± 5 ab | 10.4 ± 0.6 a |
| RTE9 | 80 ± 5 | 39 ± 3 | 72 ± 2 c | 27 ± 2 | 47 ± 5 ab | 8.8 ± 0.7 ab |
| Treatment | A (μmol m−2s−1) | gs (mmol m−2s−1) | Ci (μmol mol−1) | iWUE (μmol mol−1) |
|---|---|---|---|---|
| Non-inoculated | 6.9 ± 0.2 c | 150 ± 20 | 306 ± 5 | 45 ± 4 c |
| Hv16 | 7.3 ± 0.1 bc | 130 ± 20 | 289 ± 12 | 61 ± 5 ab |
| C58 | 7.9 ± 0.4 ab | 150 ± 10 | 296 ± 6 | 53 ± 3 bc |
| RTE9 | 8.8 ± 0.4 a | 123 ± 17 | 281 ± 11 | 70 ± 10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcia-Piedras, J.-M.; Pérez-Romero, J.-A.; Mateos-Naranjo, E.; Parra, R.; Rodríguez-Llorente, I.-D.; Camacho, M.; Redondo-Gómez, S. Stimulation of PGP Bacteria on the Development of Seeds, Plants and Cuttings of the Obligate Halophyte Arthrocaulon (Arthrocnemum) macrostachyum (Moric.) Piirainen & G. Kadereit. Plants 2023, 12, 1436. https://doi.org/10.3390/plants12071436
Barcia-Piedras J-M, Pérez-Romero J-A, Mateos-Naranjo E, Parra R, Rodríguez-Llorente I-D, Camacho M, Redondo-Gómez S. Stimulation of PGP Bacteria on the Development of Seeds, Plants and Cuttings of the Obligate Halophyte Arthrocaulon (Arthrocnemum) macrostachyum (Moric.) Piirainen & G. Kadereit. Plants. 2023; 12(7):1436. https://doi.org/10.3390/plants12071436
Chicago/Turabian StyleBarcia-Piedras, José-María, Jesús-Alberto Pérez-Romero, Enrique Mateos-Naranjo, Raquel Parra, Ignacio-David Rodríguez-Llorente, María Camacho, and Susana Redondo-Gómez. 2023. "Stimulation of PGP Bacteria on the Development of Seeds, Plants and Cuttings of the Obligate Halophyte Arthrocaulon (Arthrocnemum) macrostachyum (Moric.) Piirainen & G. Kadereit" Plants 12, no. 7: 1436. https://doi.org/10.3390/plants12071436
APA StyleBarcia-Piedras, J.-M., Pérez-Romero, J.-A., Mateos-Naranjo, E., Parra, R., Rodríguez-Llorente, I.-D., Camacho, M., & Redondo-Gómez, S. (2023). Stimulation of PGP Bacteria on the Development of Seeds, Plants and Cuttings of the Obligate Halophyte Arthrocaulon (Arthrocnemum) macrostachyum (Moric.) Piirainen & G. Kadereit. Plants, 12(7), 1436. https://doi.org/10.3390/plants12071436









