Comparative Analysis of Qualitative and Bioactive Compounds of Whole and Refined Flours in Durum Wheat Grains with Different Year of Release and Yield Potential
Abstract
1. Introduction
- characterize different durum wheat genotypes, including four Italian varieties selected in the South of Italy, and an elite USA variety, in terms of quality traits and bioactive compounds, considering both wholemeal and semolina products;
- provide novel insights about the correlation between such features and their relation with the genotypes’ yield potential and year of release.
2. Results and Discussion
2.1. Whole Seed Morphological and Yield-Related Traits in Durum Wheat Genotypes
2.2. Quality Traits of Wholemeal and Semolina Samples
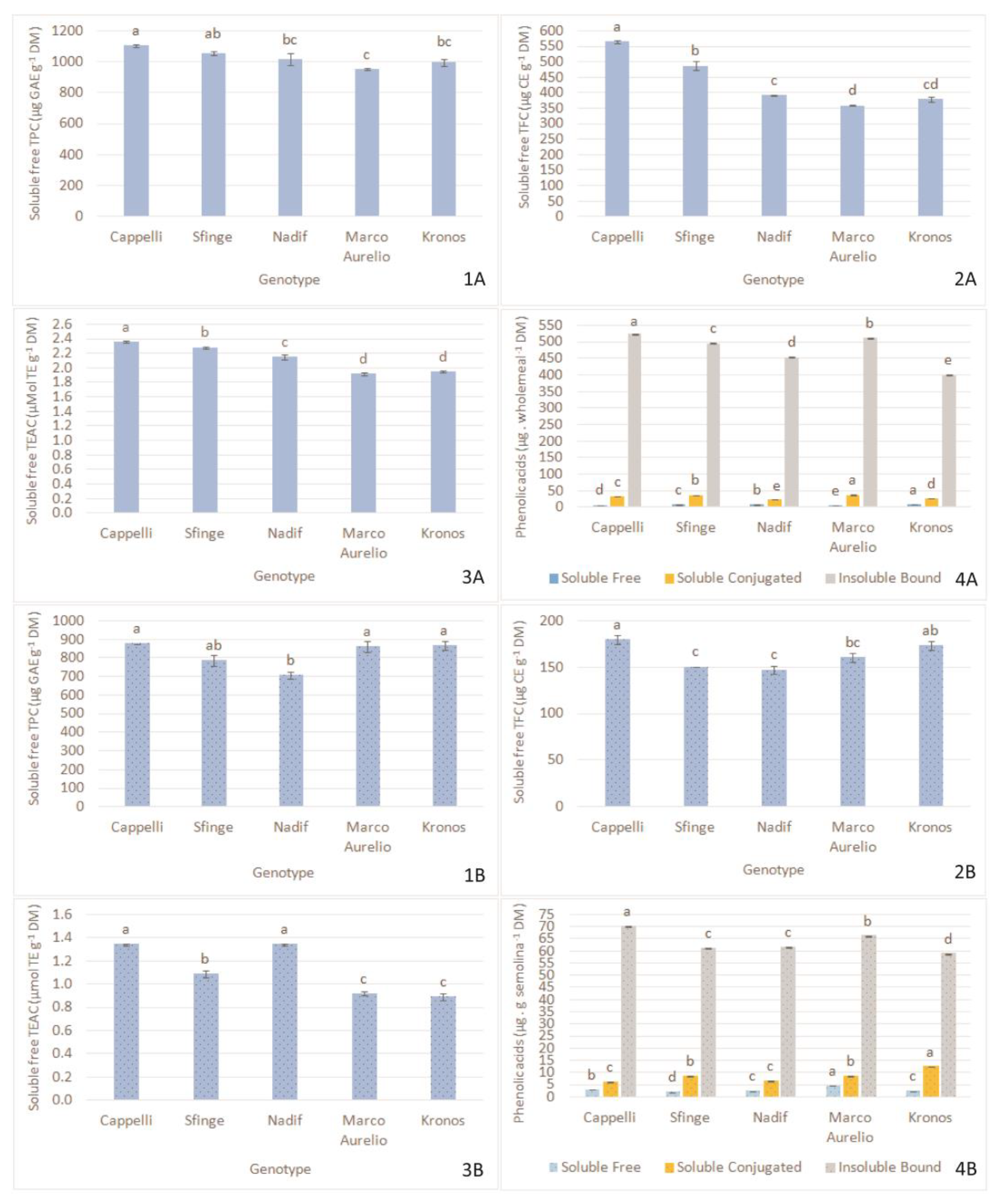
2.3. Variability of Antioxidants and Phenolic Acids in Wholemeal and Semolina Samples
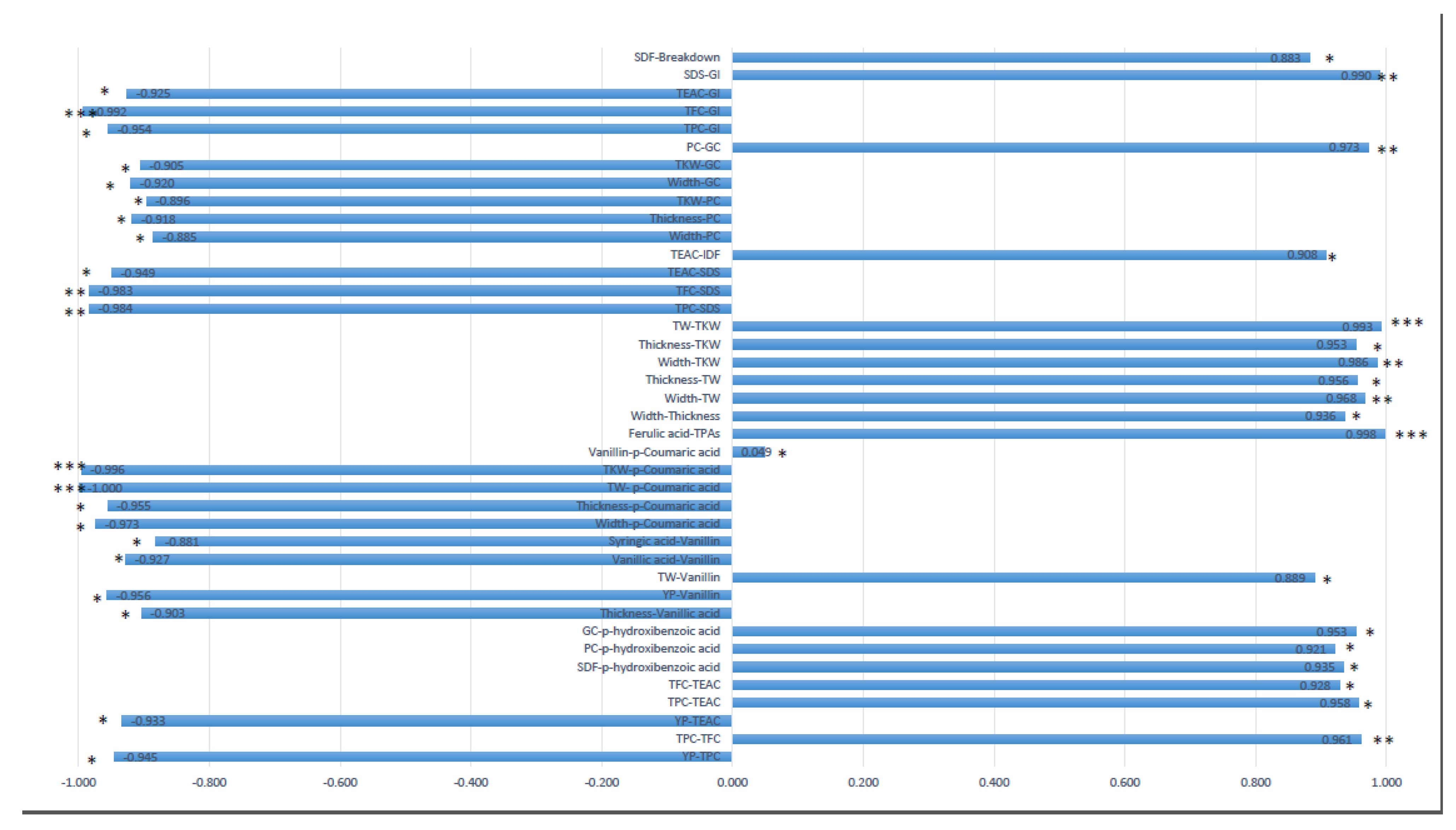
2.4. Correlation and Multivariate Analysis for Yield-Related Components, Quality and Phenolics
- the first one, with some phenolic acids such as YP, sinapic acid and SDS being part of the same cluster, and the related genotypes Marco Aurelio and Kronos.
- The second one, with the morphological and yield-related traits such as length, thickness, width, TW and TKW being associated with Sfinge and Nadif.
- The third one, with antioxidants such as TFC, TPC, TEAC and the breakdown quality trait related to Cappelli.
3. Materials and Methods
3.1. Plant Materials
3.2. Wholegrain Analysis
3.3. Milling
3.4. Quality Traits Assessment
3.5. Phenolics
3.5.1. Colourimetric Assay
3.5.2. Extraction and Determination of Phenolic Acids
3.6. Statistical Analysis
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Graziano, S.; Marando, S.; Prandi, B.; Boukid, F.; Marmiroli, N.; Francia, E.; Pecchioni, N.; Sforza, S.; Visioli, G.; Gullì, M. Technological Quality and Nutritional Value of Two Durum Wheat Varieties Depend on Both Genetic and Environmental Factors. J. Agric. Food Chem. 2019, 67, 2384–2395. [Google Scholar] [CrossRef] [PubMed]
- Troccoli, A.; Borrelli, G.M.; De Vita, P.; Fares, C.; Di Fonzo, N. Durum Wheat Quality: A Multidisciplinary Concept (mini review). J. Cereal Sci. 2000, 32, 99–113. [Google Scholar] [CrossRef]
- Pojić, M.; Hadnađev, M.; Hadnađev, T.D. Gelatinization properties of wheat flour as determined by empirical and fundamental rheometric method. Eur. Food Res. Technol. 2013, 237, 299–307. [Google Scholar] [CrossRef]
- Shamloo, M.; Babawale, E.A.; Furtado, A.; Henry, R.J.; Eck, P.K.; Jones, P.J.H. Effects of genotype and temperature on accumulation of plant secondary metabolites in Canadian and Australian wheat grown under controlled environments. Sci. Rep. 2017, 7, 9133. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Pérez-Jiménez, J.; Touriño, S.; Serrano, J.; Fuguet, E.; Torres, J.L.; Goñi, I. Proanthocyanidin metabolites associated with dietary fibre from in vitro colonic fermentation and proanthocyanidin metabolites in human plasma. Mol. Nutr. Food Res. 2010, 54, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.A.; Dimberg, L.; Åman, P.; Landberg, R. Recent findings on certain bioactive components in whole grain wheat and rye. J. Cereal Sci. 2014, 59, 294–311. [Google Scholar] [CrossRef]
- Van Hung, P.; Hatcher, D.W.; Barker, W. Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities. Food Chem. 2011, 126, 1896–1901. [Google Scholar] [CrossRef]
- Laddomada, B.; Blanco, A.; Mita, G.; D’Amico, L.; Singh, R.P.; Ammar, K.; Crossa, J.; Guzmán, C. Drought and Heat Stress Impacts on Phenolic Acids Accumulation in Durum Wheat Cultivars. Foods 2021, 10, 2142. [Google Scholar] [CrossRef]
- Sall, A.T.; Chiari, T.; Legesse, W.; Seid-Ahmed, K.; Ortiz, R.; van Ginkel, M.; Bassi, F.M. Durum Wheat (Triticum durum Desf.): Origin, Cultivation and Potential Expansion in Sub-Saharan Africa. Agronomy 2019, 9, 263. [Google Scholar] [CrossRef]
- Ficco, D.B.; Beleggia, R.; Pecorella, I.; Giovanniello, V.; Frenda, A.S.; De Vita, P. Relationship between Seed Morphological Traits and Ash and Mineral Distribution along the Kernel Using Debranning in Durum Wheats from Different Geographic Sites. Foods 2020, 9, 1523. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Borrelli, G.M.; Miedico, O.; Giovanniello, V.; Tarallo, M.; Pompa, C.; De Vita, P.; Chiaravalle, A.E. Effects of grain debranning on bioactive compounds, antioxidant capacity and essential and toxic trace elements in purple durum wheats. LWT 2020, 118, 108734. [Google Scholar] [CrossRef]
- Giunta, F.; Motzo, R.; Pruneddu, G. Trends since 1900 in the yield potential of Italian-bred durum wheat cultivars. Eur. J. Agron. 2007, 27, 12–24. [Google Scholar] [CrossRef]
- Lovegrove, A.; Pellny, T.K.; Hassall, K.L.; Plummer, A.; Wood, A.; Bellisai, A.; Przewieslik-Allen, A.; Burridge, A.J.; Ward, J.L.; Shewry, P.R. Historical changes in the contents and compositions of fibre components and polar metabolites in white wheat flour. Sci. Rep. 2020, 10, 5920. [Google Scholar] [CrossRef] [PubMed]
- Carucci, F.; Gatta, G.; Gagliardi, A.; De Vita, P.; Bregaglio, S.; Giuliani, M.M. Agronomic Strategies to Improve N Efficiency Indices in Organic Durum Wheat Grown in Mediterranean Area. Plants 2021, 10, 2444. [Google Scholar] [CrossRef]
- Fares, C.; Menga, V.; Codianni, P.; Russo, M.; Perrone, D.; Suriano, S.; Savino, M.; Rascio, A. Phenolic acids variability and grain quality of organically and conventionally fertilised old wheats under a warm climate. J. Sci. Food Agric. 2019, 99, 4615–4623. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blanco, A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef]
- Giunta, F.; Bassu, S.; Mefleh, M.; Motzo, R. Is the Technological Quality of Old Durum Wheat Cultivars Superior to That of Modern Ones When Exposed to Moderately High Temperatures during Grain Filling? Foods 2020, 9, 778. [Google Scholar] [CrossRef]
- Digesù, A.; Platani, C.; Cattivelli, L.; Mangini, G.; Blanco, A. Genetic variability in yellow pigment components in cultivated and wild tetraploid wheats. J. Cereal Sci. 2009, 50, 210–218. [Google Scholar] [CrossRef]
- Andersson, R.; Fransson, G.; Tietjen, M.; Åman, P. Content and Molecular-Weight Distribution of Dietary Fiber Components in Whole-Grain Rye Flour and Bread. J. Agric. Food Chem. 2009, 57, 2004–2008. [Google Scholar] [CrossRef]
- Ianiro, G.; Rizzatti, G.; Napoli, M.; Matteo, M.V.; Rinninella, E.; Mora, V.; Fanali, C.; Leonetti, A.; Benedettelli, S.; Mele, M.C.; et al. A Durum Wheat Variety-Based Product Is Effective in Reducing Symptoms in Patients with Non-Celiac Gluten Sensitivity: A Double-Blind Randomized Cross-Over Trial. Nutrients 2019, 11, 712. [Google Scholar] [CrossRef]
- Khan, J.; Khan, M.Z.; Ma, Y.; Meng, Y.; Mushtaq, A.; Shen, Q.; Xue, Y. Overview of the Composition of Whole Grains’ Phenolic Acids and Dietary Fibre and Their Effect on Chronic Non-Communicable Diseases. Int. J. Environ. Res. Public Health 2022, 19, 3042. [Google Scholar] [CrossRef] [PubMed]
- Montevecchi, G.; Setti, L.; Olmi, L.; Buti, M.; Laviano, L.; Antonelli, A.; Sgarbi, E. Determination of Free Soluble Phenolic Compounds in Grains of Ancient Wheat Varieties (Triticum sp. pl.) by Liquid Chromatography–Tandem Mass Spectrometry. J. Agric. Food Chem. 2019, 67, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Sestili, F.; Janni, M.M.; Doherty, A.; Botticella, E.; D’Ovidio, R.; Masci, S.; Jones, H.D.; Lafiandra, D. Increasing the amylose content of durum wheat through silencing of the SBEIIa genes. BMC Plant Biol. 2010, 10, 144. Available online: http://www.biomedcentral.com/1471-2229/10/144 (accessed on 1 February 2023). [CrossRef]
- Crosbie, G.B.; Ross, A.S.; Moro, T.; Chiu, P.C. Starch and Protein Quality Requirements of Japanese Alkaline Noodles (Ramen). Cereal Chem. 1999, 76, 328–334. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications—A review. Int. J. Food Sci. Technol. 2016, 52, 38–58. [Google Scholar] [CrossRef]
- Giacosa, A.; Peroni, G.; Rondanelli, M. Phytochemical Components and Human Health Effects of Old versus Modern Italian Wheat Varieties: The Case of Durum Wheat Senatore Cappelli. Nutrients 2022, 14, 2779. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Fernandez-Orozco, R.; Li, L.; Harflett, C.; Shewry, P.R.; Ward, J.L. Effects of Environment and Genotype on Phenolic Acids in Wheat in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2010, 58, 9341–9352. [Google Scholar] [CrossRef]
- Fares, C.; Platani, C.; Baiano, A.; Menga, V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010, 119, 1023–1029. [Google Scholar] [CrossRef]
- Dinelli, G.; Segura Carretero, A.; Di Silvestro, R.; Marotti, I.; Fu, S.; Benedettelli, S.; Ghiselli, L.; Fernandez Gutierrez, A. Determination of phenolic compounds in modern and old varieties of durum wheat using liquid chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 7229–7240. [Google Scholar] [CrossRef]
- Suchowilska, E.; Bieńkowska, T.; Stuper-Szablewska, K.; Wiwart, M. Concentrations of Phenolic Acids, Flavonoids and Carotenoids and the Antioxidant Activity of the Grain, Flour and Bran of Triticum polonicum as Compared with Three Cultivated Wheat Species. Agriculture 2020, 10, 591. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Hassan, N.D.; Mamat, S.N.H.; Nawi, N.M.; Rashid, W.A.; Tan, N.A. Extraction technologies and solvents of phytocompounds from plant materials: Physicochemical characterization and identification of ingredients and bioactive compounds from plant extract using various instrumentations. In Handbook of Food Bioengineering, Ingredients Extraction by Physicochemical Methods in Food; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 523–560. ISBN 9780128115213. [Google Scholar] [CrossRef]
- Șerban, L.R.; Păucean, A.; Man, S.M.; Chiş, M.S.; Mureşan, V. Ancient Wheat Species: Biochemical Profile and Impact on Sourdough Bread Characteristics—A Review. Processes 2021, 9, 2008. [Google Scholar] [CrossRef]
- Aalami, M.; Rao, U.P.; Leelavathi, K. Physicochemical and biochemical characteristics of Indian durum wheat varieties: Relationship to semolina milling and spaghetti making quality. Food Chem. 2007, 102, 993–1005. [Google Scholar] [CrossRef]
- Suriano, S.; Iannucci, A.; Codianni, P.; Fares, C.; Russo, M.; Pecchioni, N.; Marciello, U.; Savino, M. Phenolic acids profile, nutritional and phytochemical compounds, antioxidant properties in colored barley grown in southern Italy. Food Res. Int. 2018, 113, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]

| Genotype | Length (mm) | Width (mm) | Thickness (mm) | TW (kg hL−1) | TKW (g) |
|---|---|---|---|---|---|
| Cappelli | 7.33 ± 0.43 b | 3.26 ± 0.36 c | 2.99 ± 0.25 b | 83.52 ± 0.1 c | 47.71 ± 0.2 c |
| Sfinge | 7.21 ± 0.58 b | 3.49 ± 0.35 b | 3.20 ± 0.25 a | 85.84 ± 0.3 b | 57.25 ± 0.3 b |
| Marco Aurelio | 6.94 ± 0.44 c | 3.09 ± 0.4 e | 2.86 ± 0.26 c | 80.05 ± 0.2 d | 38.68 ± 0.1 d |
| Nadif | 7.59 ± 0.52 a | 3.61 ± 0.32 a | 3.17 ± 0.25 a | 87.36 ± 0.2 a | 63.27 ± 0.2 a |
| Kronos | 6.74 ± 0.56 c | 3.17 ± 0.39 d | 3 ± 0.29 b | 82.95 ± 0.3 c | 46.54 ± 0.4 c |
| Genotype | PC (g kg−1) | SDS (mL g−1) | YP (µg g−1 DM) | IDF (g 100 g−1) | SDF (g 100 g−1) |
|---|---|---|---|---|---|
| Cappelli | 183 ± 0.1 a | 23.9 ± 0.1 e | 5.51 ± 0.05 e | 10.86 ± 0.3 ab | 3.34 ± 0.1 a |
| Sfinge | 127 ± 0.2 c | 31.2 ± 0.3 d | 6.08 ± 0.01 d | 11.22 ± 0.1 a | 2.35 ± 0.1 bc |
| Marco Aurelio | 179 ± 0.1 a | 40.3 ± 0.3 a | 11.28 ± 0.19 a | 9.88 ± 0.1 c | 2.55 ± 0.1 bc |
| Nadif | 124 ± 0.2 c | 35.2 ± 0.2 c | 7.16 ± 0.10 c | 10.21 ± 0.1 bc | 2.14 ± 0.2 c |
| Kronos | 160 ± 0.1 b | 38.2 ± 0.3 b | 8.71 ± 0.10 b | 9.90 ± 0.3 c | 2.90 ± 0.3 ab |
| Genotype | GC (g kg−1) | GI | YP (µg g−1 DM) | YI | RI | BI | Peak Viscosity (BU) | Breakdown (BU) | Setback (BU) |
|---|---|---|---|---|---|---|---|---|---|
| Cappelli | 163.6 ± 0.05 a | 13.77 ± 0.24 d | 3.92 ± 0.01 e | 18.83 ± 0.11 d | −0.95 ± 0.06 a | 14.60 ± 0.60 a | 874 ± 2.83 c | 86 ± 2.83 a | 583 ± 1.41 c |
| Sfinge | 107.3 ± 0.28 b | 50.20 ± 0.79 c | 4.80 ± 0.02 d | 19.58 ± 0.30 d | −1.92 ± 0.08 b | 13.28 ± 0.67 a | 793 ± 1.41 d | 33 ± 1.41 c | 621 ± 2.83 b |
| Marco Aurelio | 154.6 ± 0.24 a | 88.98 ± 0.87 a | 9.32 ± 0.28 a | 28.40 ± 0.46 a | −2.50 ± 0.06 c | 14.91 ± 0.52 a | 1054 ± 0.71 a | 5 ± 0.35 d | 370 ± 2.83 e |
| Nadif | 90.3 ± 0.81 c | 75.83 ± 0.82 b | 6.55 ± 0.02 c | 22.64 ± 0.19 c | −3.16 ± 0.11 d | 13.70 ± 0.37 a | 1018 ± 1.41 b | 6 ± 0.71 d | 542 ± 2.83 d |
| Kronos | 148.6 ± 0.19 a | 87.18 ± 0.32 a | 8.44 ± 0.01 b | 26.21 ± 0.10 b | −2.43 ± 0.13 c | 14.70 ± 0.06 a | 787 ± 2.83 d | 76 ± 2.83 b | 632 ± 1.41 a |
| Groups | Genotype | Taxonomic Classification | Year of Release | Pedigree/Country of Origin | Yield Potential |
|---|---|---|---|---|---|
| Old | Cappelli | T. turgidum ssp. durum | 1915 | Selection from the Tunisian population ‘Jean Retifah’-Italy | Low |
| Modern | Sfinge | T. turgidum ssp. durum | 2003 | Ofanto/Tavoliere//Doro–Italy | High |
| Modern | Marco Aurelio | T. turgidum ssp. durum | 2010 | Orobel//Arcobaleno/Svevo–Italy | Intermediate |
| Modern | Nadif | T. turgidum ssp. durum | 2016 | Claudio/Orobel–Italy | High |
| Modern | Kronos | T. turgidum ssp. durum | 1992 | APB MSFRS Pop selection D03–21–USA | Very high |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menga, V.; Giovanniello, V.; Savino, M.; Gallo, A.; Colecchia, S.A.; De Simone, V.; Zingale, S.; Ficco, D.B.M. Comparative Analysis of Qualitative and Bioactive Compounds of Whole and Refined Flours in Durum Wheat Grains with Different Year of Release and Yield Potential. Plants 2023, 12, 1350. https://doi.org/10.3390/plants12061350
Menga V, Giovanniello V, Savino M, Gallo A, Colecchia SA, De Simone V, Zingale S, Ficco DBM. Comparative Analysis of Qualitative and Bioactive Compounds of Whole and Refined Flours in Durum Wheat Grains with Different Year of Release and Yield Potential. Plants. 2023; 12(6):1350. https://doi.org/10.3390/plants12061350
Chicago/Turabian StyleMenga, Valeria, Valentina Giovanniello, Michele Savino, Antonio Gallo, Salvatore Antonio Colecchia, Vanessa De Simone, Silvia Zingale, and Donatella Bianca Maria Ficco. 2023. "Comparative Analysis of Qualitative and Bioactive Compounds of Whole and Refined Flours in Durum Wheat Grains with Different Year of Release and Yield Potential" Plants 12, no. 6: 1350. https://doi.org/10.3390/plants12061350
APA StyleMenga, V., Giovanniello, V., Savino, M., Gallo, A., Colecchia, S. A., De Simone, V., Zingale, S., & Ficco, D. B. M. (2023). Comparative Analysis of Qualitative and Bioactive Compounds of Whole and Refined Flours in Durum Wheat Grains with Different Year of Release and Yield Potential. Plants, 12(6), 1350. https://doi.org/10.3390/plants12061350







