Physicochemical Characteristics and Microstructure of Ancient and Common Wheat Grains Cultivated in Romania
Abstract
1. Introduction
2. Results
2.1. Wheat Flour Physicochemical Characteristics
2.2. Wheat Flour Mineral Elements
2.3. Wheat Grain Microstructure
2.4. Principal Component Analysis between Wheat Flours Characteristics
3. Discussion
4. Materials and Methods
4.1. Wheat Flour Samples
4.2. Physicochemical Composition of Wheat Flour Samples
4.2.1. Moisture Analysis
4.2.2. Ash Analysis
4.2.3. Protein Analysis
4.2.4. Analysis of Wet Gluten Content
4.2.5. Analysis of Lipids
4.2.6. Analysis of Total Starch
4.2.7. Analysis of Carbohydrates
4.2.8. Analysis of Test Weight and Thousand-Kernel Weight
4.3. Mineral Elements Analysis of Wheat Flour Samples
4.4. Microstructural Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mitura, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Szablewski, T.; Studnicki, M. Yield and Grain Quality of Common Wheat (Triticum aestivum L.) Depending on the Different Farming Systems (Organic vs. Integrated vs. Conventional). Plants 2023, 12, 1022. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Cini, E. Challenges and Opportunities in Wheat Flour, Pasta, Bread, and Bakery Product Production Chains: A Systematic Review of Innovations and Improvement Strategies to Increase Sustainability, Productivity, and Product Quality. Sustainability 2021, 13, 2608. [Google Scholar] [CrossRef]
- Rasheed, F.; Markgren, J.; Hedenqvist, M.; Johansson, E. Modeling to Understand Plant Protein Structure-Function Relationships—Implications for Seed Storage Proteins. Molecules 2020, 25, 873. [Google Scholar] [CrossRef]
- Baillière, J.; Laureys, D.; Vermeir, P.; Van Opstaele, F.; De Rouck, G.; De Cooman, L.; Vanderputten, D.; De Clippeleer, J. 10 Unmalted Alternative Cereals and Pseudocereals: A Comparative Analysis of Their Characteristics Relevant to the Brewing Process. J. Cereal Sci. 2022, 106, 103482. [Google Scholar] [CrossRef]
- Marzocchi, S.; Caboni, M.F.; Miani, M.G.; Pasini, F. Wheat Germ and Lipid Oxidation: An Open Issue. Foods 2022, 11, 1032. [Google Scholar] [CrossRef] [PubMed]
- Arzani, A.; Ashraf, M. Cultivated Ancient Wheats (Triticum spp.): A Potential Source of Health-Beneficial Food Products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, L.H.; Morales, D.A.; Rodríguez, E.R.; Romero, C.D. Minerals and Trace Elements in a Collection of Wheat Landraces from the Canary Islands. J. Food Compos. Anal. 2011, 24, 1081–1090. [Google Scholar] [CrossRef]
- de Sousa, T.; Ribeiro, M.; Sabença, C.; Igrejas, G. The 10,000-Year Success Story of Wheat! Foods 2021, 10, 2124. [Google Scholar] [CrossRef]
- Mefleh, M.; Boukid, F.; Fadda, C. Suitability of Improved and Ancient Italian Wheat for Bread-Making: A Holistic Approach. Life 2022, 12, 1613. [Google Scholar] [CrossRef]
- Pagnotta, M.A.; Mondini, L.; Atallah, M.F. Morphological and Molecular Characterization of Italian Emmer Wheat Accessions. Euphytica 2005, 146, 29–37. [Google Scholar] [CrossRef]
- Valli, V.; Taccari, A.; Di Nunzio, M.; Danesi, F.; Bordoni, A. Health Benefits of Ancient Grains. Comparison among Bread Made with Ancient, Heritage and Modern Grain Flours in Human Cultured Cells. Food Res. Int. 2018, 107, 206–215. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S. Do “Ancient” Wheat Species Differ from Modern Bread Wheat in Their Contents of Bioactive Components? J. Cereal Sci. 2015, 65, 236–243. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A.; Stankowski, S.; Sobolewska, M.; Kępińska-Pacelik, J. Comparison of Yield, Chemical Composition and Farinograph Properties of Common and Ancient Wheat Grains. Eur. Food Res. Technol. 2021, 247, 1525–1538. [Google Scholar] [CrossRef]
- Whitton, C.; Nicholson, S.K.; Roberts, C.; Prynne, C.J.; Pot, G.; Olson, A.; Fitt, E.; Cole, D.; Teucher, B.; Bates, B.; et al. The National Diet and Nutrition Survey: Adults Aged 19 to 64 Years: Types and Quantities of Foods Consumed. Br. J. Nutr. 2011, 106, 1899–1914. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Whittaker, A.; Pagliai, G.; Benedettelli, S.; Sofi, F. Ancient Wheat Species and Human Health: Biochemical and Clinical Implications. J. Nutr. Biochem. 2018, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Karayigit, B.; Colak, N.; Ozogul, F.; Gundogdu, A.; Inceer, H.; Bilgiçli, N.; Ayaz, F.A. The Biogenic Amine and Mineral Contents of Different Milling Fractions of Bread and Durum Wheat (Triticum L.) Cultivars. Food Biosci. 2020, 37, 100676. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Rizzi, R.; Pannacciulli, E.; Della Gatta, C. Mineral Composition in Hulled Wheat Grains: A Comparison between Emmer (Triticum Dicoccon Schrank) and Spelt (T. spelta L.) Accessions. Int. J. Food Sci. Nutr. 1997, 48, 381–386. [Google Scholar] [CrossRef]
- Erba, D.; Hidalgo, A.; Bresciani, J.; Brandolini, A. Environmental and Genotypic Influences on Trace Element and Mineral Concentrations in Whole Meal Flour of Einkorn (Triticum monococcum L. subsp. monococcum). J. Cereal Sci. 2011, 54, 250–254. [Google Scholar] [CrossRef]
- Golea, M.C.; Şandru, M.D.; Codină, G.G. Mineral composition of flours produced from modern and ancient wheat varieties cultivated in Romania. Ukr. Food J. 2022, 11, 78–89. [Google Scholar] [CrossRef]
- Golea, C.M.; Codină, G.G.; Oroian, M. Prediction of Wheat Flours Composition Using Fourier Transform Infrared Spectrometry (FT-IR). Food Control 2023, 143, 109318. [Google Scholar] [CrossRef]
- Athinaiou, A.; Aja, S.; Haros, C.M. Nutritional Characterization of Ancestral Organic Wheats: Emmer, Khorasan and Spelt. Biol. Life Sci. Forum 2022, 17, 6. [Google Scholar] [CrossRef]
- Boukid, F.; Folloni, S.; Sforza, S.; Vittadini, E.; Prandi, B. Current Trends in Ancient Grains-Based Foodstuffs: Insights into Nutritional Aspects and Technological Applications. Compr. Rev. Food Sci. Food Saf. 2018, 17, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Mondini, L.; Grausgruber, H.; Pagnotta, M.A. Evaluation of European Emmer Wheat Germplasm for Agro-Morphological, Grain Quality Traits and Molecular Traits. Genet. Resour. Crop Evol. 2014, 61, 69–87. [Google Scholar] [CrossRef]
- Longin, C.F.H.; Ziegler, J.; Schweiggert, R.; Koehler, P.; Carle, R.; Würschum, T. Comparative Study of Hulled (Einkorn, Emmer, and Spelt) and Naked Wheats (Durum and Bread Wheat): Agronomic Performance and Quality Traits. Crop Sci. 2016, 56, 302–311. [Google Scholar] [CrossRef]
- Tekin, M.; Cengiz, M.F.; Abbasov, M.; Aksoy, A.; Canci, H.; Akar, T. Comparison of Some Mineral Nutrients and Vitamins in Advanced Hulled Wheat Lines. Cereal Chem. 2018, 95, 436–444. [Google Scholar] [CrossRef]
- Zhao, F.J.; Su, Y.H.; Dunham, S.J.; Rakszegi, M.; Bedo, Z.; McGrath, S.P.; Shewry, P.R. Variation in Mineral Micronutrient Concentrations in Grain of Wheat Lines of Diverse Origin. J. Cereal Sci. 2009, 49, 290–295. [Google Scholar] [CrossRef]
- Antonini, E.; Zara, C.; Valentini, L.; Gobbi, P.; Ninfali, P.; Menotta, M. Novel Insights into Pericarp, Protein Body Globoids of Aleurone Layer, Starchy Granules of Three Cereals Gained Using Atomic Force Microscopy and Environmental Scanning Electronic Microscopy. Eur. J. Histochem. 2018, 62, 20–27. [Google Scholar] [CrossRef]
- Panato, A.; Antonini, E.; Bortolotti, F.; Ninfali, P. The Histology of Grain Caryopses for Nutrient Location: A Comparative Study of Six Cereals. Int. J. Food Sci. Technol. 2017, 52, 1238–1245. [Google Scholar] [CrossRef]
- Golea, C.M.; Galan, P.-M.; Leti, L.-I.; Codină, G.G. Genetic Diversity and Physicochemical Characteristics of Different Wheat Species (Triticum aestivum L., Triticum monococcum L., Triticum spelta L.) Cultivated in Romania. Appl. Sci. 2023, 13, 4992. [Google Scholar] [CrossRef]
- Tamba-Berehoiu, R.-M.; Mioara, V.; Codina, G.G. Predictive Model of the Alveografic Parameters in Flours Obtained from Romanian Grains. Rom. Biotechnol. Lett. 2009, 14, 4234–4242. [Google Scholar]
- Codina, G.G.; Mironeasa, S.; Bordei, D.; Leahu, A. Mixolab versus Alveograph and Falling Number. Czech J. Food Sci. 2010, 28, 185–191. [Google Scholar] [CrossRef]
- Sobczyk, A.; Pycia, K.; Jaworska, G. Charakterystyka porównawcza warto´sci technologicznej ziarna starych odmian i nowych rodów orkiszu (Triticum spelta L.) oraz ziarna pszenicy zwyczajnej (Triticum vulgare). Zesz. Probl. Post. Nauk Roln. 2017, 589, 81–91. [Google Scholar]
- Subira, J.; Peña, R.J.; Álvaro, F.; Ammar, K.; Ramdani, A.; Royo, C. Breeding Progress in the Pasta-Making Quality of Durum Wheat Cultivars Released in Italy and Spain during the 20th Century. Crop Pasture Sci. 2014, 65, 16–26. [Google Scholar] [CrossRef]
- Haghayegh, G.; Schoenlechner, R. Comparison of Functional Properties of Isolated Emmer and Einkorn Wheat Starches. J. Food Agric. Environ. 2010, 8, 239–243. [Google Scholar]
- Rachoń, L.; Bobryk-Mamczarz, A.; Kiełtyka-Dadasiewicz, A. Hulled Wheat Productivity and Quality in Modern Agriculture against Conventional Wheat Species. Agriculture 2020, 10, 275. [Google Scholar] [CrossRef]
- Packa, D.D.L.W.; Hoscik, M. Reaction of Diploid, Tetraploid and Hexaploid Wheats to Inoculations of Fusarium Culmorum (WG Smith) Sacc. Half. J. Agron. 2013, 12, 38–48. [Google Scholar]
- Krochmal-Marczak, B.; Sawicka, B. Nutritional value of spelled wheat (Triticum spelta L.) grown in Podkarpacie. Herbalism 2016, 1, 146. [Google Scholar] [CrossRef]
- Simsek, S.; Budak, B.; Schwebach, C.S.; Ovando-Martínez, M. Historical vs. Modern Hard Red Spring Wheat: Analysis of the Chemical Composition. Cereal Chem. 2019, 96, 937–949. [Google Scholar] [CrossRef]
- Morgounov, A.I.; Belan, I.; Zelenskiy, Y.; Roseeva, L.; Tömösközi, S.; Békés, F.; Abugalieva, A.; Cakmak, I.; Vargas, M.; Crossa, J. Historical Changes in Grain Yield and Quality of Spring Wheat Varieties Cultivated in Siberia from 1900 to 2010. Can. J. Plant Sci. 2013, 93, 425–433. [Google Scholar] [CrossRef]
- Fan, M.S.; Zhao, F.J.; Fairweather-Tait, S.J.; Poulton, P.R.; Dunham, S.J.; McGrath, S.P. Evidence of Decreasing Mineral Density in Wheat Grain over the Last 160 Years. J. Trace Elem. Med. Biol. 2008, 22, 315–324. [Google Scholar] [CrossRef]
- Gomez-Becerra, H.F.; Erdem, H.; Yazici, A.; Tutus, Y.; Torun, B.; Ozturk, L.; Cakmak, I. Grain Concentrations of Protein and Mineral Nutrients in a Large Collection of Spelt Wheat Grown under Different Environments. J Cereal Sci. 2010, 52, 342–349. [Google Scholar] [CrossRef]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.P.; Liu, D.T.; Yu, X.R.; Xiong, F.; Li, D.L.; Zheng, Y.K.; Hao, Y.F.; Gu, Y.J.; Wang, Z. Development of Endosperm Cells and Starch Granules in Common Wheat. Cereal Res. Commun. 2014, 42, 514–524. [Google Scholar] [CrossRef]
- Grundas, S.; Wrigley, C.W. Ultrastructure of wheat grain, flour and dough. In Encyclopedia of Food Grains; Elsevier: Amsterdam, The Netherlands, 2016; Volume 3, pp. 384–395. [Google Scholar] [CrossRef]
- Barrera, G.N.; Calderón-Domínguez, G.; Chanona-Pérez, J.; Gutiérrez-López, G.F.; León, A.E.; Ribotta, P.D. Evaluation of the Mechanical Damage on Wheat Starch Granules by SEM, ESEM, AFM and Texture Image Analysis. Carbohydr. Polym. 2013, 98, 1449–1457. [Google Scholar] [CrossRef]
- Kłosok, K.; Welc, R.; Fornal, E.; Nawrocka, A. Effects of Physical and Chemical Factors on the Structure of Gluten, Gliadins and Glutenins as Studied with Spectroscopic Methods. Molecules 2021, 26, 508. [Google Scholar] [CrossRef] [PubMed]
- Tremmel-Bede, K.; Láng, L.; Török, K.; Tömösközi, S.; Vida, G.; Shewry, R.; Bedő, Z.; Rakszegi, M. Development and Characterization of Wheat Lines with Increased Levels of Arabinoxylan. Euphytica 2017, 213, 291. [Google Scholar] [CrossRef]
- Zhou, W.; Therdthai, N.; Hui, Y.H. Introduction to Baking and Bakery Products. In Bakery Products Science and Technology, 2nd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–16. [Google Scholar] [CrossRef]
- International Association for Cereal Science and Technology. ICC Standard Methods (Methods No. 110/1); ICC: Vienna, Austria, 2005. [Google Scholar]
- International Association for Cereal Science and Technology. ICC Standard Methods (Methods No. 104/1); ICC: Vienna, Austria, 2005. [Google Scholar]
- International Association for Cereal Science and Technology. ICC Standard Methods (Methods No. 105/2); ICC: Vienna, Austria, 2005. [Google Scholar]
- International Association for Cereal Science and Technology. ICC Standard Methods (Methods No. 137/1); ICC: Vienna, Austria, 2005. [Google Scholar]
- International Association for Cereal Science and Technology. ICC Standard Methods (Methods No. 136); ICC: Vienna, Austria, 2005. [Google Scholar]
- AACC International. Approved Methods of Analysis (Methods No. 76-13.01), 11th ed.; AACC International, Inc.: St. Paul, MN, USA, 2011. [Google Scholar]
- Alonso-Miravalles, L.; O’Mahony, J.A. Composition, protein profile and rheological properties of pseudocereal-based protein-rich ingredients. Foods 2018, 7, 73. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO 2009). Determination of Bulk Density, Called Mass per Hectolitre-Part 1: Reference Method; Method 7971-1:2009; ISO: Geneva, Switzerland, 2009; p. 8. [Google Scholar]
- International Organization for Standardization (ISO 2010). Cereals and Pulses-Determination of the Mass of 1000 Grains; Method 520:2010; ISO: Geneva, Switzerland, 2010; p. 10. [Google Scholar]
- European Union. Foodstuffs—Determination of Trace Elements—Determination of Lead, Cadmium, Zinc, Copper, Iron and Chromium by Atomic Absorption Spectrometry (AAS) after Dry Ashing; European Union: Brussels, Belgium, 2003; Volume EN 14082:2003. [Google Scholar]
- Atudorei, D.; Stroe, S.G.; Codină, G.G. Impact of Germination on the Microstructural and Physicochemical Properties of Different Legume Types. Plants 2021, 10, 592. [Google Scholar] [CrossRef]


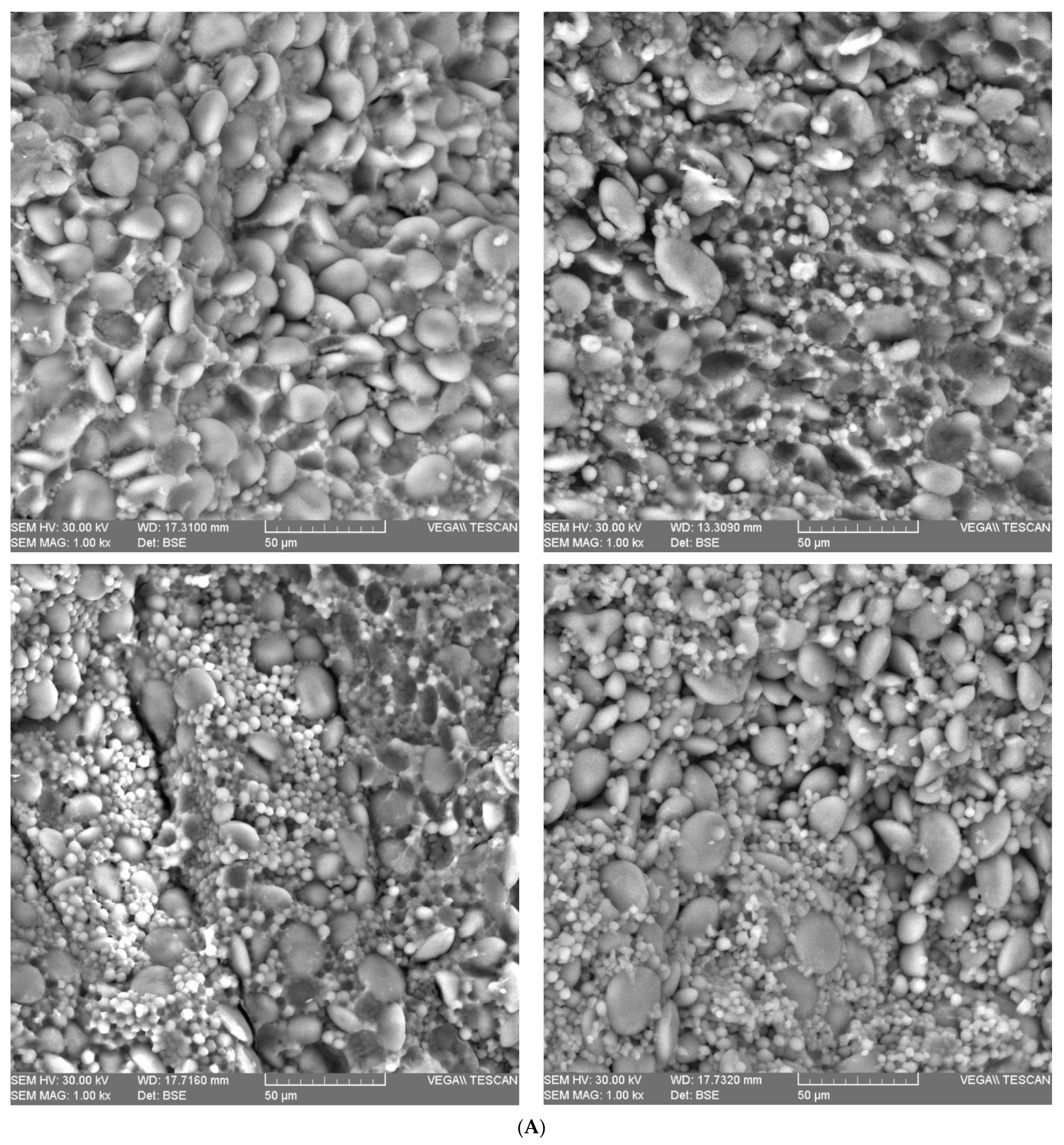
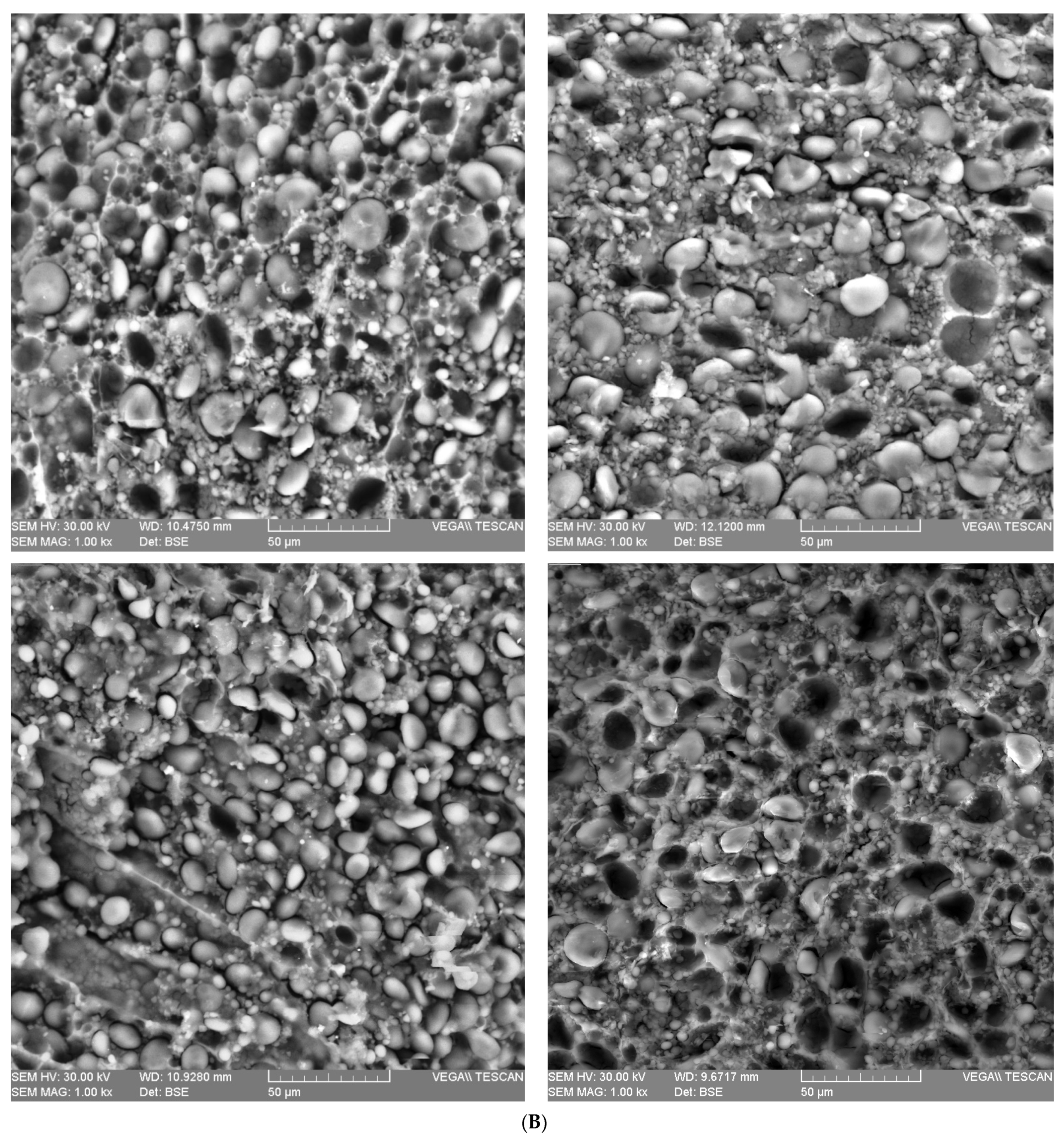

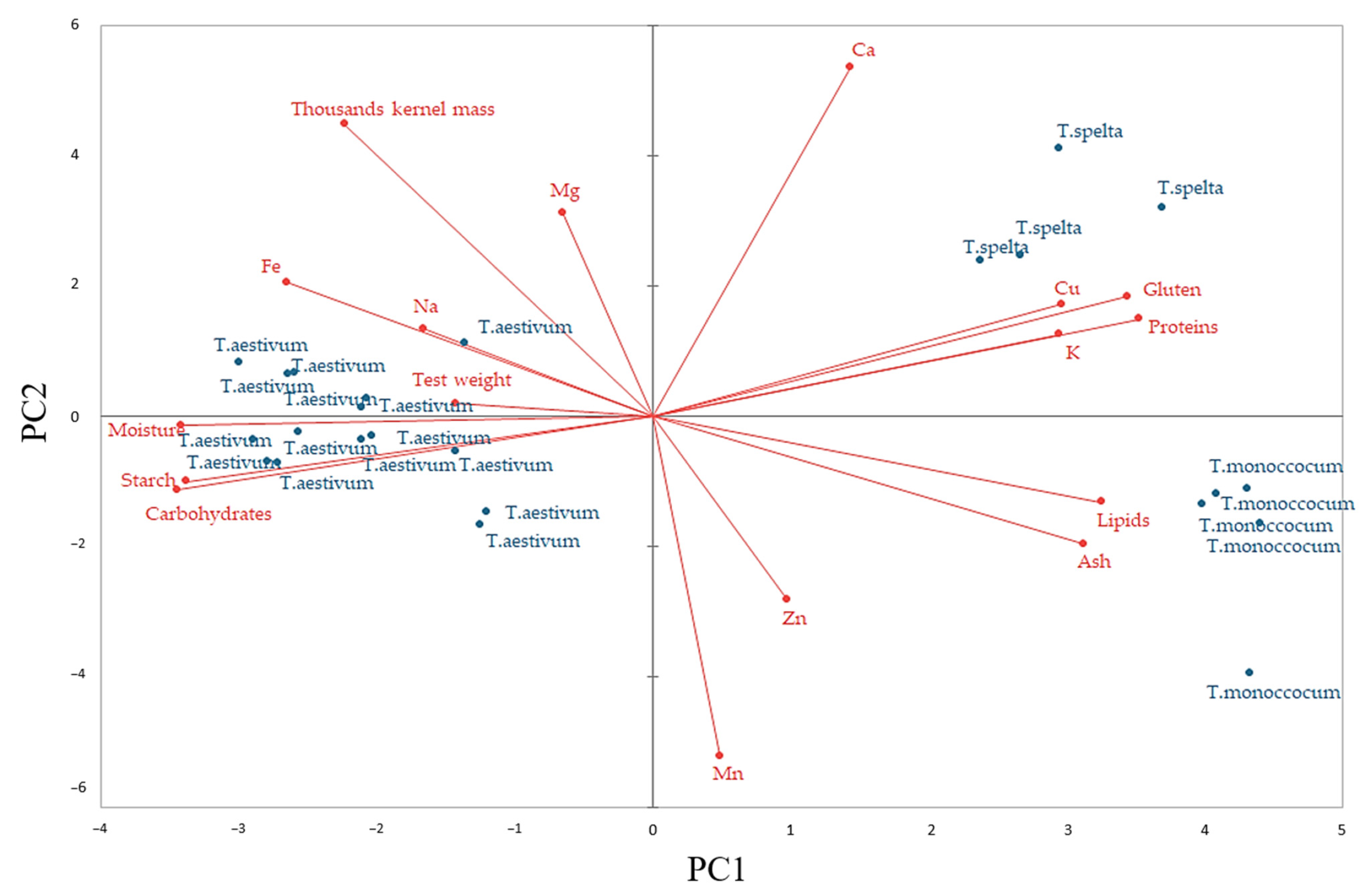
| Parameter | Wheat Species | F Value | ||
|---|---|---|---|---|
| Triticum aestivum | Triticum monococcum | Triticum spelta | ||
| Moisture (%) | 13.24 b (12.48–13.76) | 11.52 a (11.40–11.69) | 11.78 a (11.68–11.85) | 67.37 *** |
| Ash (%) | 1.77 a (1.63–2.25) | 2.47 b (2.34–2.61) | 2.03 a (1.82–2.54) | 26.07 *** |
| Protein (%) | 12.83 a (10.52–14.43) | 18.15 b (17.29–18.77) | 18.95 b (18.24–19.27) | 87.47 *** |
| Wet gluten (%) | 27.8 a (21.00–33.00) | 43.00 b (40.00–45.00) | 47.50 b (45.00–49.00) | 78.91 *** |
| Lipid (%) | 1.77 a (1.42–2.27) | 2.35 c (2.28–2.42) | 2.07 b (1.91–2.31) | 24.48 *** |
| Starch (%) | 63.77 b (60.90–67.7) | 56.18 a (55.90–56.4) | 56.90 a (53.10–59.80) | 34.48 *** |
| Carbohydrates (%) | 70.44 b (67.96–73.31) | 66.48 a (65.07–65.99) | 65.15 a (64.03–65.91) | 43.55 *** |
| Test weight (kg hl−1) | 79.06 a (59–86) | 73.68 a (71.2–75.7) | 73.55 a (67.80–77.40) | 2.42 ns |
| Thousand-kernel mass (g) | 38.31 b (33.1–42.9) | 24.92 a (19.60–28.1) | 40.42 b (38.30–41.50) | 45.90 *** |
| Parameter | Wheat Species | F Value | ||
|---|---|---|---|---|
| Triticum aestivum | Triticum monococcum | Triticum spelta | ||
| Calcium (Ca) (mg kg−1) | 232.87 a (189.03–277.78) | 244.01 a (168.93–266.04) | 428.46 b (380.69–469.12) | 61.14 *** |
| Magnesium (Mg) (mg kg−1) | 962.21 a (794.82–1147.09) | 851.57 a (627.11–1142.51) | 1016.37 a (771.24–1316.15) | 1.38 ns |
| Potassium (K) (mg kg−1) | 3504.28 a (2757.19–4347.18) | 4782.30 a (3934.51–5404.34) | 5029.32 a (4694.62–5609.11) | 19.88 *** |
| Sodium (Na) (mg kg−1) | 80.62 a (18.98–250.05) | 25.35 a (19.82–33.21) | 38.29 a (27.05–50.47) | 2.19 ns |
| Zinc (Zn) (mg kg−1) | 25.83 a (21.96–34.54) | 30.36 a (17.07–34.13) | 23.22 a (21.18–26.31) | 3.55 * |
| Iron (Fe) (mg kg−1) | 37.34 a (31.11–43.31) | 21.90 a (19.93–23.13) | 31.25 ab (23.63–41.58) | 18.65 *** |
| Manganese (Mn) (mg kg−1) | 58.54 ab (43.89–72.5) | 74.45 b (52.78–93.36) | 37.8 a (29.37–47.97) | 13.46 *** |
| Copper (Cu) (mg kg−1) | 2.30 a (0.82–3.36) | 4.52 b (3.21–5.9) | 4.25 b (3.07–5.42) | 20.36 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golea, C.M.; Stroe, S.-G.; Gâtlan, A.-M.; Codină, G.G. Physicochemical Characteristics and Microstructure of Ancient and Common Wheat Grains Cultivated in Romania. Plants 2023, 12, 2138. https://doi.org/10.3390/plants12112138
Golea CM, Stroe S-G, Gâtlan A-M, Codină GG. Physicochemical Characteristics and Microstructure of Ancient and Common Wheat Grains Cultivated in Romania. Plants. 2023; 12(11):2138. https://doi.org/10.3390/plants12112138
Chicago/Turabian StyleGolea, Camelia Maria, Silviu-Gabriel Stroe, Anca-Mihaela Gâtlan, and Georgiana Gabriela Codină. 2023. "Physicochemical Characteristics and Microstructure of Ancient and Common Wheat Grains Cultivated in Romania" Plants 12, no. 11: 2138. https://doi.org/10.3390/plants12112138
APA StyleGolea, C. M., Stroe, S.-G., Gâtlan, A.-M., & Codină, G. G. (2023). Physicochemical Characteristics and Microstructure of Ancient and Common Wheat Grains Cultivated in Romania. Plants, 12(11), 2138. https://doi.org/10.3390/plants12112138









