Contributions to the Flora of Tropical East Africa
Abstract
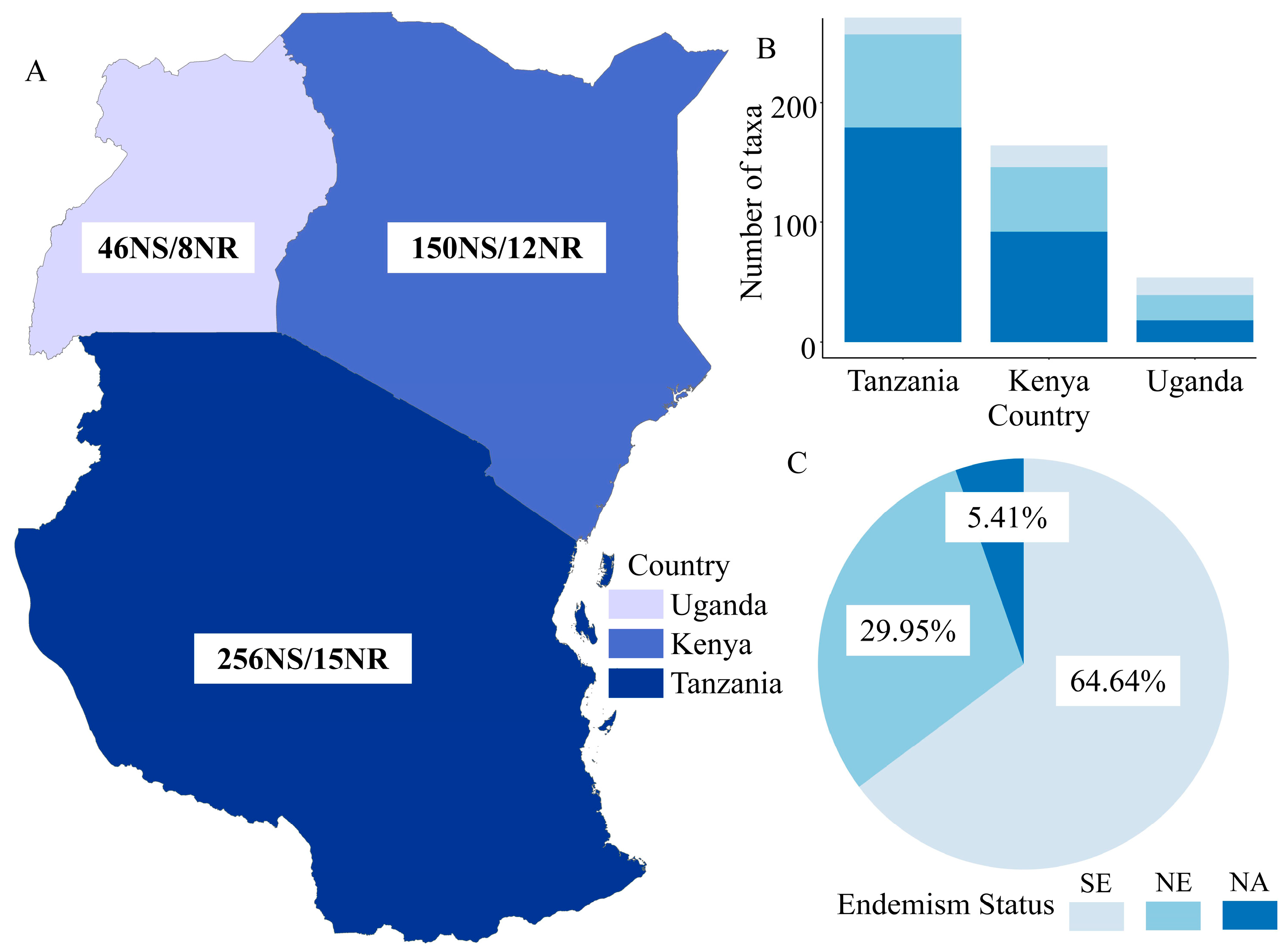
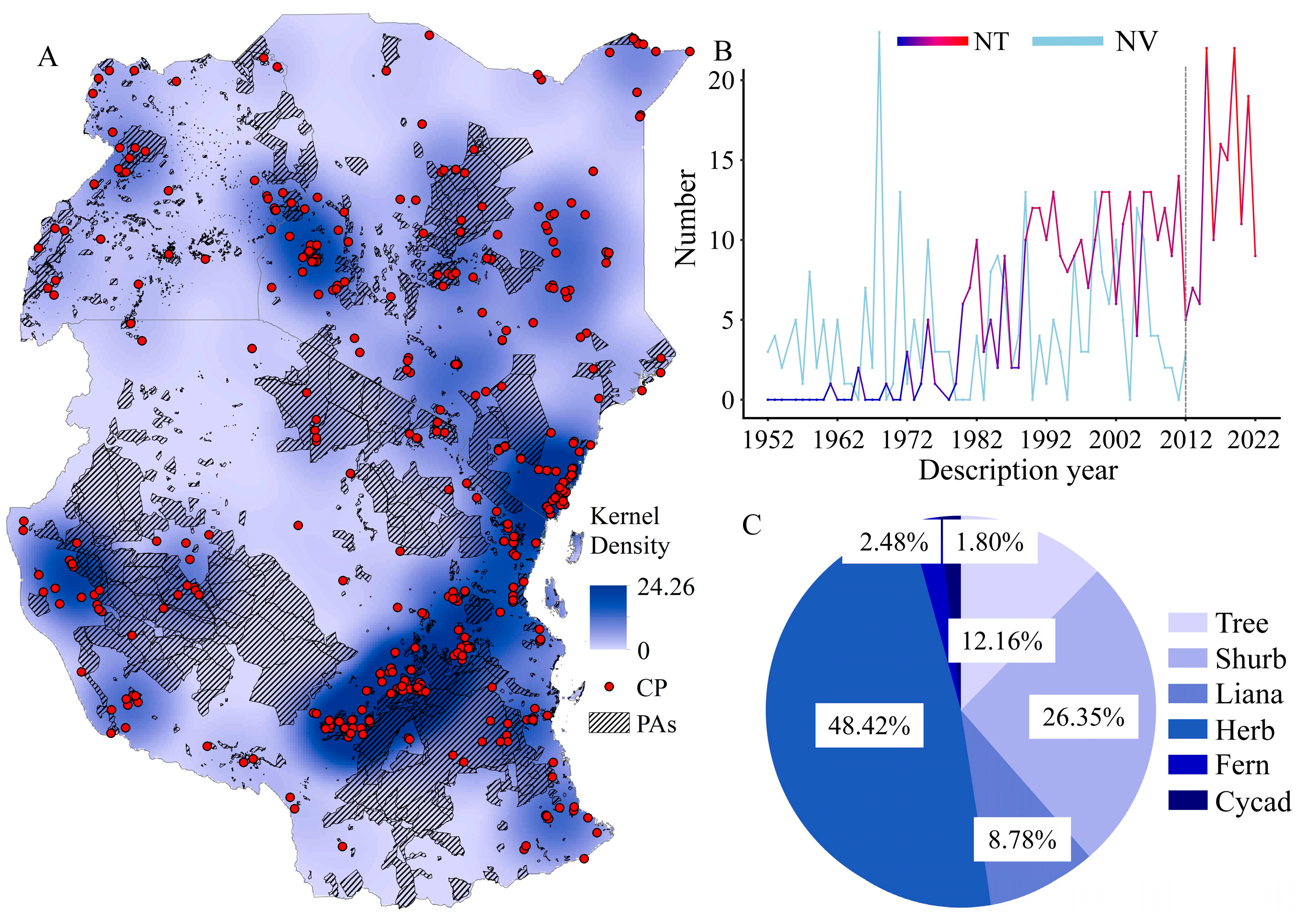
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
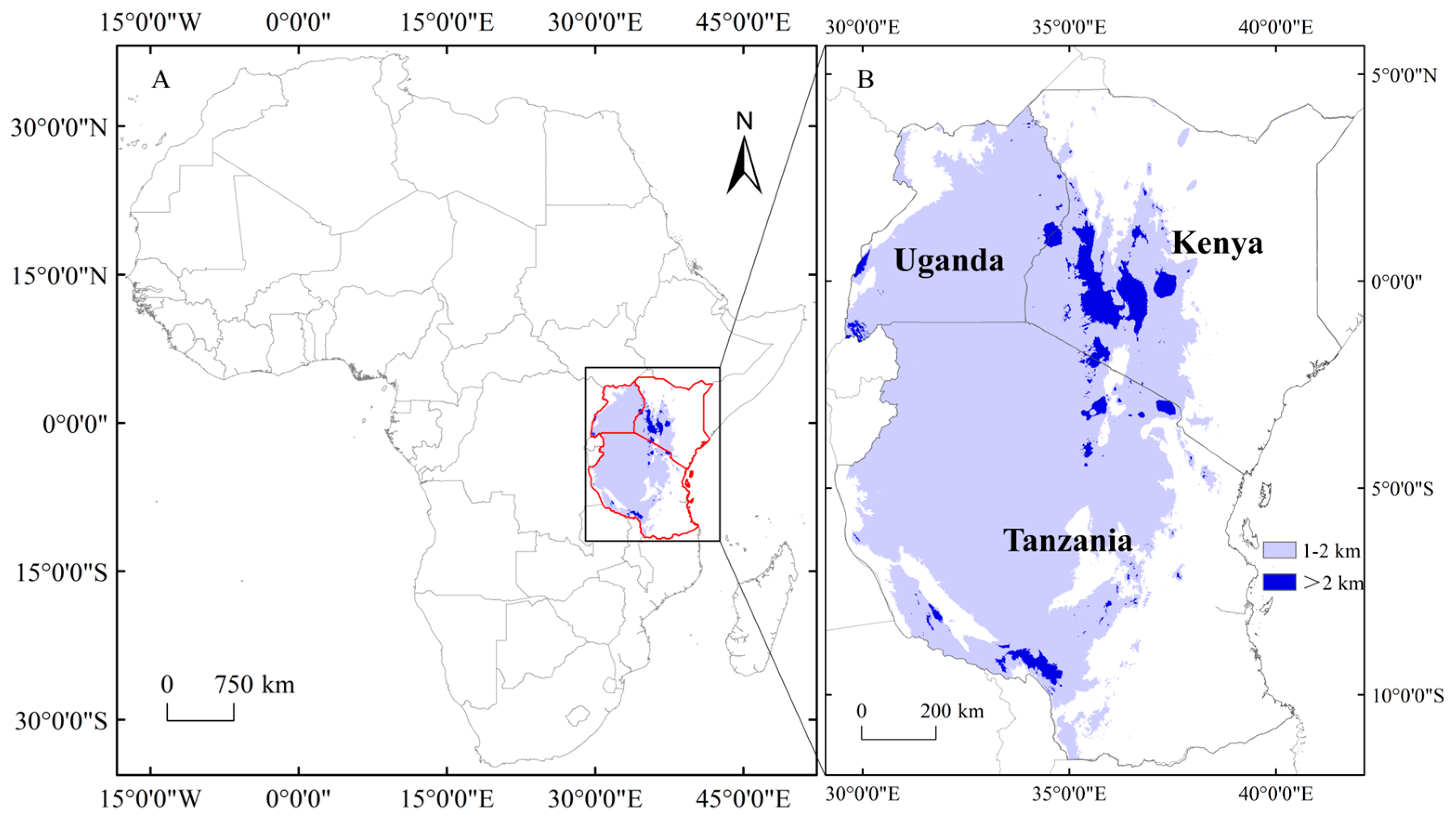
4.1. Study Area
4.2. Data Collection
4.3. Checklist Outline
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klopper, R.R.; Gautier, L.; Chatelain, C.; Smith, G.F.; Spichiger, R. Floristics of the angiosperm flora of Sub-Saharan Africa: An analysis of the African Plant Checklist and Database. Taxon 2007, 56, 201–208. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Mutke, J.; Kier, G.; Braun, G.; Schultz, C.; Barthlott, W. Patterns of African vascular plant diversity: A GIS based analysis. Syst. Geogr. Plants 2001, 71, 1125. [Google Scholar] [CrossRef]
- Mutke, J.; Sommer, J.H.; Kreft, H.; Kier, G.; Barthlott, W. Vascular Plant Diversity in a Changing World: Global Centres and Biome—Specific Patterns. In Biodiversity Hotspots; Zachos, F., Habel, J., Eds.; Springer: Berlin, Germany, 2011; pp. 83–96. [Google Scholar]
- Beentje, H. Science comes from collaboration and communication: The Flora of Tropical East Africa as an example. Webbia 2015, 70, 171–179. [Google Scholar] [CrossRef]
- Turrill, W.B.; Mllne-redhead, E. Flora of Tropical East Africa: Oleaceae; Crown Agents for the Colonies: London, UK, 1952. [Google Scholar]
- Edmonds, J.M. Flora of Tropical East Africa: Solanaceae; Royal Botanic Gardens, Kew: London, UK, 2012. [Google Scholar]
- Wang, S.; Zhou, Y.; Musili, P.M.; Mwachala, G.; Hu, G.; Wang, Q. Inventory incompleteness and collecting priority on the plant diversity in tropical East Africa. Biol. Conserv. 2020, 241, 108313. [Google Scholar] [CrossRef]
- Robinson, H.; Keeley, S.C.; Skvarla, J.J.; Chan, R. Two new genera, Hoffmannanthus and Jeffreycia, mostly from East Africa (Erlangeinae, Vernonieae, Asteraceae). PhytoKeys 2014, 39, 49–64. [Google Scholar] [CrossRef]
- Couvreur, T.L.P.; van der Ham, R.W.J.M.; Mbele, Y.M.; Mbago, F.M.; Johnson, D.M. Molecular and morphological characterization of a new monotypic genus of Annonaceae, Mwasumbia from Tanzania. Syst. Bot. 2009, 34, 266–276. [Google Scholar] [CrossRef]
- Descourvières, P.; Farminhão, J.N.M.; Droissart, V.; Dubuisson, J.-Y.; Simo-Droissart, M.; Stévart, T. A new genus of angraecoid orchids (Orchidaceae: Angraecinae) with highly distinctive pollinaria morphology, including three new species from tropical West and Central Africa. Phytotaxa 2018, 373, 99–120. [Google Scholar] [CrossRef]
- Cheek, M.; Luke, W.Q.; Gosline, G. Lukea gen. nov. (Monodoreae-Annonaceae) with two new species of shrub from the forests of the Udzungwas, Tanzania & Kaya Ribe, Kenya. Kew Bull. 2022, 77, 647–664. [Google Scholar]
- Winter, P.J.; Tilney, P.M.; Van Wyk, B.-E. Clarification of the concept of Aframmi (Heteromorpheae, Apiaceae) and a new monotypic genus, Normantha. Phytotaxa 2017, 298, 71. [Google Scholar] [CrossRef]
- Darbyshire, I.; Kiel, C.A.; Daniel, T.F.; McDade, L.A.; Luke, W.R.Q. Two new genera of Acanthaceae from tropical Africa. Kew Bull. 2019, 74, 39. [Google Scholar] [CrossRef]
- Muasya, A.M.; Goetghebeur, P.; Larridon, I. Zulustylis (Abildgaardieae, Cyperaceae)—A new genus from sub-Saharan Africa. S. Afr. J. Bot. 2020, 128, 326–332. [Google Scholar] [CrossRef]
- Shen, J.-H.; Hu, G.-W.; Gao, T.-G. Calyptocarpus (Asteraceae: Heliantheae: Ecliptinae), a newly naturalized genus in Tropical East Africa. Phytotaxa 2020, 441, 69–77. [Google Scholar] [CrossRef]
- Veranso-Libalah, M.C.; Stone, R.D.; Kadereit, G. Argyrella richardsiae, a new species of Melastomataceae from the wet miombo woodlands of south-central Africa. PhytoKeys 2017, 82, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.-W.; Lorence, D.; Wood, K.R.; Liao, W.-B.; Zhang, L.-B. A revision of the Hymenasplenium unilaterale subclade (Aspleniaceae; Pteridophyta) with the description of nine new species. Phytotaxa 2019, 419, 1–27. [Google Scholar] [CrossRef]
- Hubbard, C.E. A New Species of Hypseochloa (Gramineae) from Tanzania. Kew Bull. 1981, 36, 62. [Google Scholar] [CrossRef]
- Gosline, G.; Marshall, A.R.; Larridon, I. Revision and new species of the African genus Mischogyne (Annonaceae). Kew Bull. 2019, 74, 28. [Google Scholar] [CrossRef]
- Bouman, R.W.; Kebler, P.J.; Telford, I.R.; Bruhl, J.J.; Strijk, J.S.; Saunders, R.M.; Esser, H.-J.; Falcón-Hidalgo, B.; Van Welzen, P.C. A revised phylogenetic classification of tribe Phyllantheae (Phyllanthaceae). Phytotaxa 2022, 540, 1–100. [Google Scholar] [CrossRef]
- Leeuwenberg, A.J.M. The Loganiaceae of Africa: A revision of Mostuea Didr. Veenman 1961, 61, 1–31. [Google Scholar]
- Escudero, M.; Luceño, M. Taxonomic revision of the tropical African group of Carex subsect. Elatae (sect. Spirostachyae, Cyperaceae). An. Jardín Botánico Madr. 2011, 68, 225–247. [Google Scholar] [CrossRef]
- Johnson, D.M.; Murray, N.A. A revision of Xylopia L. (Annonaceae): The species of Tropical Africa. PhytoKeys 2018, 97, 1–252. [Google Scholar] [CrossRef]
- Farminhão, J.N.M.; Cribb, P.J. Two new species of Rhipidoglossum (Orchidaceae: Angraecinae) from Tanzania and Zimbabwe. Kew Bull. 2020, 75, 30. [Google Scholar] [CrossRef]
- International Plant Names Index. Available online: http://www.ipni.org (accessed on 15 March 2022).
- Yang, B.; Deng, M.; Zhang, M.-X.; Moe, A.Z.; Ding, H.-B.; Maw, M.B.; Win, P.P.; Corlett, R.T.; Tan, Y.-H. Contributions to the flora of Myanmar from 2000 to 2019. Plant Divers. 2020, 42, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Costello, M.J.; May, R.M.; Stork, N.E. Can we name Earth’s species before they go extinct? Science 2013, 339, 413–416. [Google Scholar] [CrossRef]
- Camberlin, P. Climate of Eastern Africa; Oxford Research Encyclopedia of Climate Science: Oxford, UK, 2018. [Google Scholar]
- Weber, O.; von Blittersdorff, R.; Beentje, H. Eriospermum adpressifolium (Asparagaceae) and Emilia blittersdorffii (Asteraceae)—Two new species from Tanzania with nearly identical leaves. Kew Bull. 2015, 70, 28. [Google Scholar] [CrossRef]
- Goldblatt, P.; Manning, J.; Demissew, S.S. Two new species of Zygotritonia Mildbr. (Iridaceae: Crocoideae) from eastern tropical Africa with notes on the morphology of the genus. S. Afr. J. Bot. 2015, 96, 37–41. [Google Scholar] [CrossRef]
- Funk, V.A.; Richardson, K.S. Systematic Data in Biodiversity Studies: Use It or Lose It. Syst. Biol. 2002, 51, 303–316. [Google Scholar] [CrossRef]
- Romo, H.; García-Barros, E.; Lobo, J.M. Identifying recorder-induced geographic bias in an Iberian butterfly database. Ecography 2006, 29, 873–885. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Cagnetta, M.; Padoa-Schioppa, E.; Quas, A.; Razzetti, E.; Sindaco, R.; Bonardi, A. Sampling bias inverts ecogeographical relationships in island reptiles. Glob. Ecol. Biogeogr. 2014, 23, 1303–1313. [Google Scholar] [CrossRef]
- Forrest, T.G. Sansevieria marachiensis (Asparagaceae) a New Species in North-West Uganda. Cactus Succul. J. 2021, 93, 207–210. [Google Scholar] [CrossRef]
- Cole, T.C. Aloe lukeana: A New, Caulescent Aloe from Uganda. Cactus Succul. J. 2015, 87, 152–159. [Google Scholar] [CrossRef]
- Muasya, A.M.; Musili, P.M.; Vrijdaghs, A. Kyllinga mbitheana (Cyperaceae)—Description, floral ontogeny and pollen micromorphology of a new species from Kenya. J. East Afr. Nat. Hist. 2010, 99, 65–75. [Google Scholar] [CrossRef]
- Muasya, A.M.; Vollesen, K. Cyperus volkielloides (Cyperaceae), a new ephemeral species from Tanzania. Kew Bull. 2015, 70, 53. [Google Scholar] [CrossRef]
- Kustanto, A. Does Trade Openness Cause Deforestation? A Case Study from Indonesia. J. Ekon. Pembang. 2022, 19, 165–182. [Google Scholar] [CrossRef]
- Hulme, P.E. Unwelcome exchange: International trade as a direct and indirect driver of biological invasions worldwide. One Earth 2021, 4, 666–679. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Kumar Rai, P.; Singh, J. Invasive alien plant species: Their impact on environment, ecosystem services and human health. Ecol. Indic. 2020, 111, 106020. [Google Scholar] [CrossRef]
- Darbyshire, I.; Fischer, E. A new species of Craterostigma (Linderniaceae) from Kenya. Phytotaxa 2017, 306, 91. [Google Scholar] [CrossRef]
- Carter, S. Taxonomic changes in Monadenium and Synadenium (Euphorbiaceae) for Flora Zambesiaca. Kew Bull. 2000, 55, 435. [Google Scholar] [CrossRef]
- Knox, E.B.; Luke, Q.; Thulin, M. A new giant Lobelia from the Eastern Arc Mts, Tanzania. Kew Bull. 2004, 59, 189. [Google Scholar] [CrossRef]
- Kennedy, J.D.; Fjeldså, J. Completing Wallace’s journey. Science 2020, 367, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Mutie, F.M.; Mwachala, G.; Grace, O.M.; Hu, G.-W.; Wang, Q.-F. Euphorbia mbuinzauensis, a new succulent species in Kenya from the Synadenium group in Euphorbia sect. Monadenium (Euphorbiaceae). PhytoKeys 2021, 183, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Matheka, E.; Malombe, I.; Mwadime, T.; Wabuyele, E.; Newton, L.N. Aloe ngutwaensis (Asphodelaceae), a new species in Makueni County, south-eastern Kenya. CactusWorld 2019, 38, 211–215. [Google Scholar]
- Malombe, I.; Matheka, K.W.; Mwadime, T.; Mwachala, G. Dorstenia arachniformis (Moraceae), a new species from Combretum wooded grasslands in Makueni County, Kenya. Phytotaxa 2020, 468, 226–230. [Google Scholar] [CrossRef]
- Ngumbau, V.M.; Musili, P.M.; Hu, G.W. Premna mwadimei (Lamiaceae), a new species from Cha Simba, a remnant of coastal forests of Kenya, East Africa. Phytotaxa 2021, 510, 155–162. [Google Scholar] [CrossRef]
- Owino, A.O.; Ryan, P.G. Recent papyrus swamp habitat loss and conservation implications in western Kenya. Wetl. Ecol. Manag. 2007, 15, 1–12. [Google Scholar] [CrossRef]
- St-Laurent, M.-H.; Dussault, C.; Ferron, J.; Gagnon, R. Dissecting habitat loss and fragmentation effects following logging in boreal forest: Conservation perspectives from landscape simulations. Biol. Conserv. 2009, 142, 2240–2249. [Google Scholar] [CrossRef]
- Scanes, C.G. Chapter 19—Human Activity and Habitat Loss: Destruction, Fragmentation, and Degradation. In Animals and Human Society; Scanes, C.G., Toukhsati, S.R., Eds.; Academic Press: Amsterdam, The Netherlands, 2018; pp. 451–482. [Google Scholar]
- Runge, C.A.; Plantinga, A.J.; Larsen, A.E.; Naugle, D.E.; Helmstedt, K.J.; Polasky, S.; Donnelly, J.P.; Smith, J.T.; Lark, T.J.; Lawler, J.J.; et al. Unintended habitat loss on private land from grazing restrictions on public rangelands. J. Appl. Ecol. 2019, 56, 52–62. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, S.; Hu, G.; Mwachala, G.; Yan, X.; Wang, Q. Species richness and phylogenetic diversity of seed plants across vegetation zones of Mount Kenya, East Africa. Ecol. Evol. 2018, 8, 8930–8939. [Google Scholar] [CrossRef]
- Mbuni, Y.M.; Zhou, Y.; Wang, S.; Ngumbau, V.M.; Musili, P.M.; Mutie, F.M.; Njoroge, B.; Kirika, P.M.; Mwachala, G.; Vivian, K.; et al. An annotated checklist of vascular plants of Cherangani hills, Western Kenya. PhytoKeys 2019, 120, 1–90. [Google Scholar] [CrossRef]
- Kipkoech, S.; Melly, D.K.; Muema, B.W.; Wei, N.; Kamau, P.; Kirika, P.M.; Wang, Q.; Hu, G. An annotated checklist of the vascular plants of Aberdare Ranges Forest, a part of Eastern Afromontane Biodiversity Hotspot. PhytoKeys 2020, 149, 1–88. [Google Scholar] [CrossRef]
- Ngumbau, V.M.; Luke, Q.; Nyange, M.; Wanga, V.O.; Watuma, B.M.; Mbuni, Y.M.; Munyao, J.N.; Oulo, M.A.; Mkala, E.M.; Kipkoech, S.; et al. An annotated checklist of the coastal forests of Kenya, East Africa. PhytoKeys 2020, 147, 1–191. [Google Scholar] [CrossRef] [PubMed]
- Watuma, B.M.; Kipkoech, S.; Melly, D.K.; Ngumbau, V.M.; Rono, P.C.; Mutie, F.M.; Mkala, E.M.; Nzei, J.M.; Mwachala, G.; Gituru, R.W.; et al. An annotated checklist of the vascular plants of Taita Hills, Eastern Arc Mountain. PhytoKeys 2022, 191, 1–158. [Google Scholar] [CrossRef]
- de Lima, R.A.; Sánchez-Tapia, A.; Mortara, S.R.; ter Steege, H.; de Siqueira, M.F. plantR: An R package and workflow for managing species records from biological collections. Methods Ecol. Evol. 2021, 14, 332–339. [Google Scholar] [CrossRef]
- Group, A.P. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- PPG, I. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 2016, 54, 563–603. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Reveal, J.L.; Farjon, A.; Gardner, M.F.; Mill, R.R.; Chase, M.W. A new classification and linear sequence of extant gymnosperms. Phytotaxa 2011, 19, 55–70. [Google Scholar] [CrossRef]
- Darbyshire, I.; Timberlake, J.; Osborne, J.; Rokni, S.; Matimele, H.; Langa, C.; Datizua, C.; De Sousa, C.; Alves, T.; Massingue, A.; et al. The endemic plants of Mozambique: Diversity and conservation status. PhytoKeys 2019, 136, 45–96. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria; Available online: https://www.R–project.org/ (accessed on 30 October 2022).
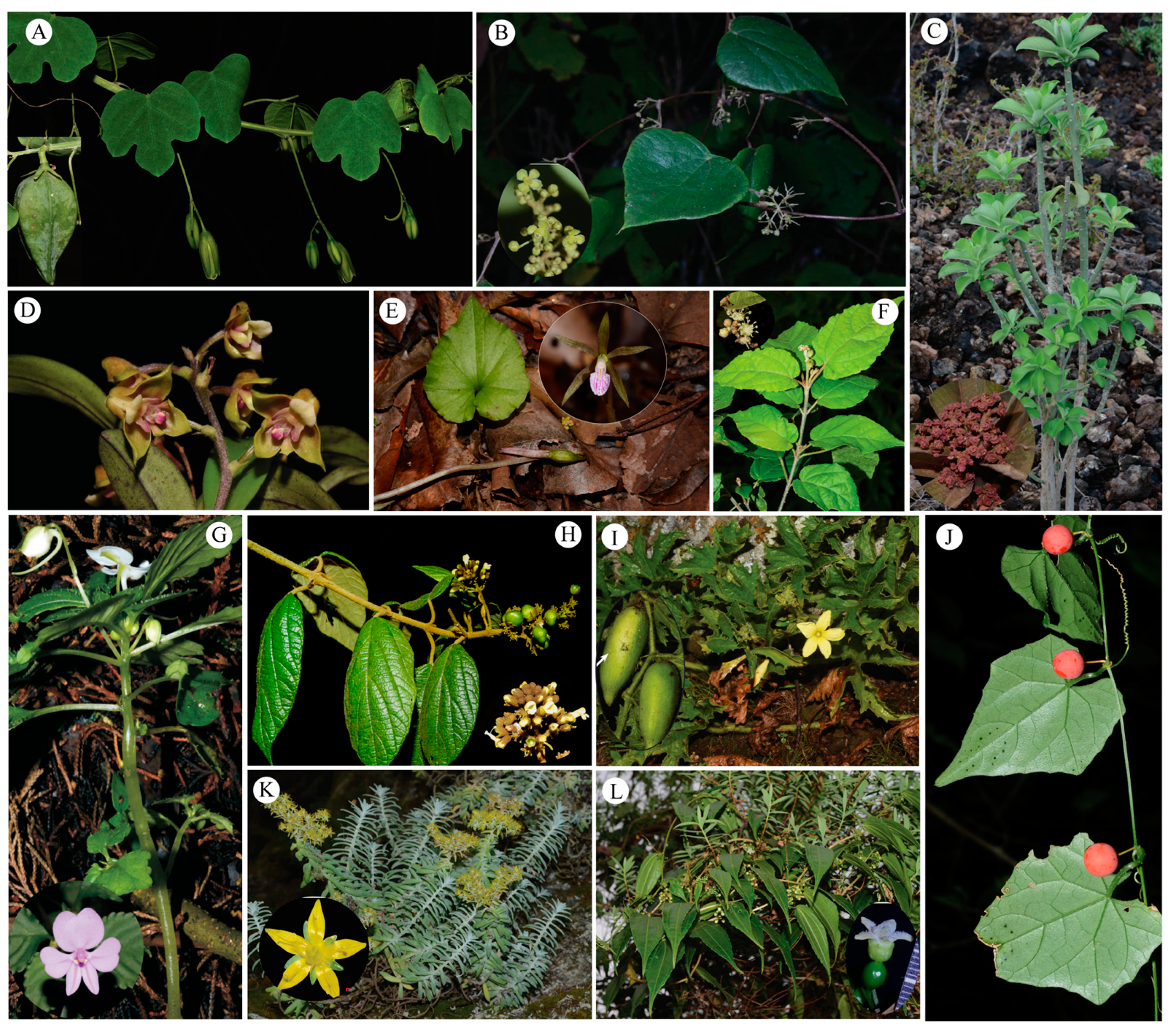
| Family | Genus | Category |
|---|---|---|
| Acanthaceae | Kenyacanthus I.Darbysh. & Kiel | New genus |
| Annonaceae | Mwasumbia Couvreur & D. M. Johnson | New genus |
| Annonaceae | Lukea Gosline & Cheek | New genus |
| Annonaceae | Mischogyne Exell | New recorded genus |
| Apiaceae | Normantha P.J.D.Winter & B-E.van Wyk | New genus |
| Aspleniaceae | Hymenasplenium Hayata | New recorded genus |
| Asteraceae | Calyptocarpus Less. | New recorded genus |
| Asteraceae | Hoffmannanthus H. Rob., S.C. Keeley & Skvarla | New genus |
| Asteraceae | Jeffreycia H. Rob., S.C. Keeley & Skvarla | New genus |
| Cyperaceae | Zulustylis Muasya | New genus |
| Fabaceae | Hypseochloa C.E.Hubb. | New recorded genus |
| Melastomataceae | Argyrella Naudin | New recorded genus |
| Orchidaceae | Kylicanthe Descourvières, Stévart & Droissart | New genus |
| Orchidaceae | Gastrodia R.Br. | New recorded genus |
| Phyllanthaceae | Moeroris Raf. | New recorded genus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Wang, M.; Wei, N.; Mwachala, G.; Hu, G.; Wu, L.; Wang, S.; Wang, Q. Contributions to the Flora of Tropical East Africa. Plants 2023, 12, 1336. https://doi.org/10.3390/plants12061336
Du S, Wang M, Wei N, Mwachala G, Hu G, Wu L, Wang S, Wang Q. Contributions to the Flora of Tropical East Africa. Plants. 2023; 12(6):1336. https://doi.org/10.3390/plants12061336
Chicago/Turabian StyleDu, Shenglan, Miaoxuan Wang, Neng Wei, Geoffrey Mwachala, Guangwan Hu, Lin Wu, Shengwei Wang, and Qingfeng Wang. 2023. "Contributions to the Flora of Tropical East Africa" Plants 12, no. 6: 1336. https://doi.org/10.3390/plants12061336
APA StyleDu, S., Wang, M., Wei, N., Mwachala, G., Hu, G., Wu, L., Wang, S., & Wang, Q. (2023). Contributions to the Flora of Tropical East Africa. Plants, 12(6), 1336. https://doi.org/10.3390/plants12061336








