Contrasting Pollination Strategies and Breeding Systems in Two Native Useful Cacti from Southern Brazil
Abstract
1. Introduction
2. Results
2.1. Overall Flower Features and Nectar Concentration
2.2. Breeding Systems
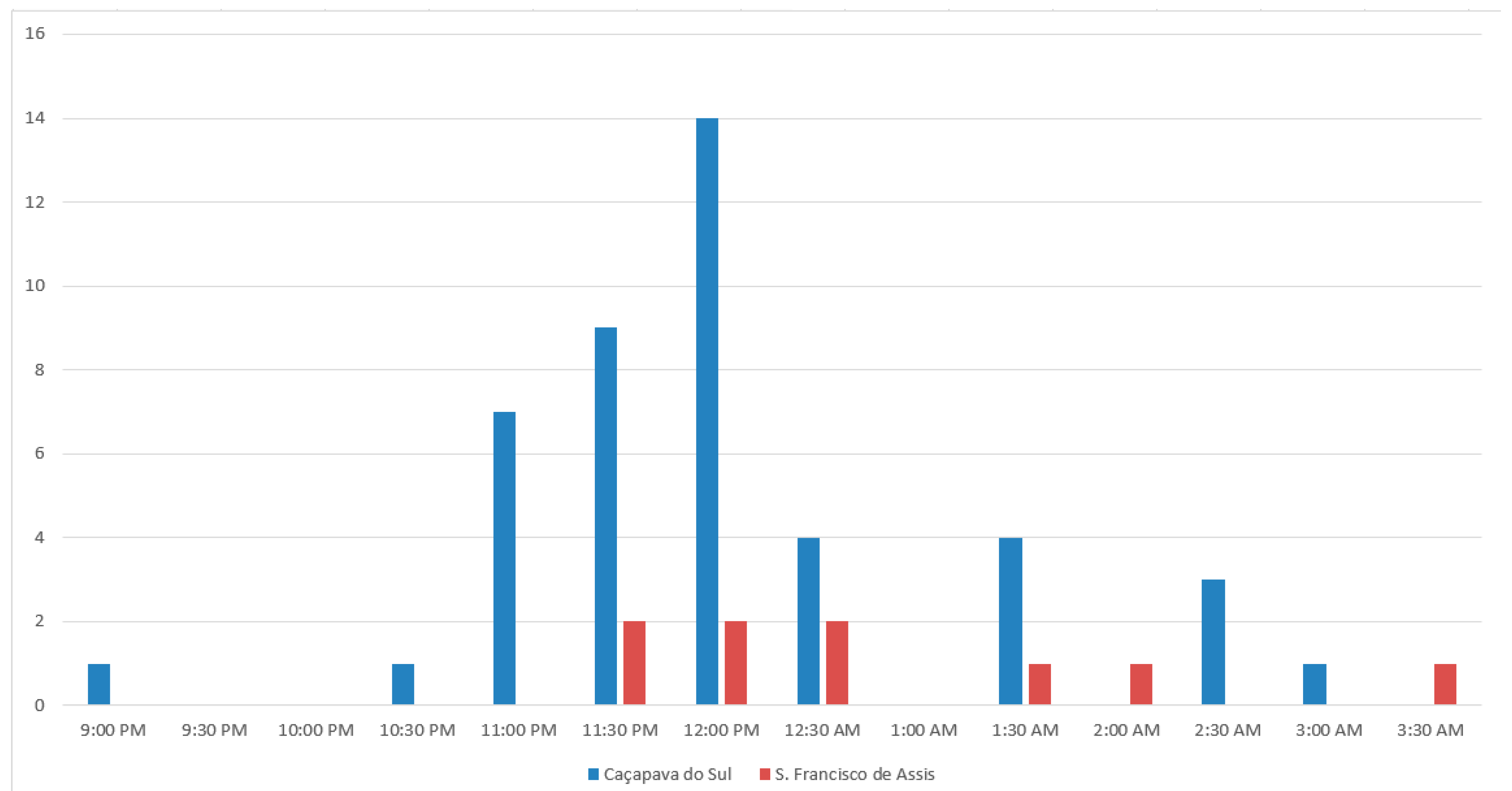
2.3. Pollinators, Pollinator Behavior, and Pollinator Frequency
3. Discussion
3.1. Overall Flower Features
3.2. Breeding Systems
3.3. Pollinators, Pollinator Behavior, and Pollinator Frequency
4. Materials and Methods
4.1. Study Species
4.2. Study Sites
4.3. Nectar Concentration
4.4. Reproductive Biology
4.5. Pollination Observations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zappi, D.; Taylor, N.; Santos, M.R. Conservação das Cactaceae do Brasil. In Plano Nacional para Conservação das Cactáceas; Série espécies ameaçadas, n. 24; Instituto Chico Mendes de Conservação da Biodiversidade, ICMBio: Brasília, Brazil, 2011. [Google Scholar]
- Tremlett, C.J.; Peh, K.S.H.; Zamora-Gutierrez, V.; Schaafsma, M. Value and benefit distribution of pollination services provided by bats in the production of cactus fruits in central Mexico. Ecosyst. Serv. 2021, 47, 101197. [Google Scholar] [CrossRef]
- Carneiro, A.M.; Farias-Singer, R.; Ramos, R.A.; Nilson, A.D. Cactos do Rio Grande do Sul; Fundação Zoobotânica do Rio Grande do Sul: Porto Alegre, Brazil, 2016. [Google Scholar]
- Lucena, C.M.; de Lucena, R.F.P.; Costa, G.M.; Carvalho, T.K.N.; Costa, G.G.D.S.; Alves, R.R.D.N.; Nunes, E.N. Use and knowledge of Cactaceae in Northeastern Brazil. J. Ethnobiol. Ethnomed. 2013, 9, 1–11. [Google Scholar] [CrossRef]
- Silva Menezes, L.; Vogel, C.E.; Lucas, D.B.; Minervini, G.H.; Boldrini, I.I.; Overbeck, G.E. Plant species richness record in Brazilian Pampa grasslands and implications. Braz. J. Bot. 2018, 41, 817–823. [Google Scholar] [CrossRef]
- Castro, J.B.; Perdomo, O.; Singer, R.B. Pollination biology and reproductive success in four Brazilian species of Gomesa (Orchidaceae: Oncidiinae): Specific pollinators, but high pollen loss and low fruit set. Plant Species Biol. 2022, 37, 132–147. [Google Scholar] [CrossRef]
- Cerceau, I.; Siriani-Oliveira, S.; Dutra, A.L.; Oliveira, R.; Schlindwein, C. The cost of fidelity: Foraging oligolectic bees gather huge amounts of pollen in a highly specialized cactus–pollinator association. Biol. J. Linn. Soc. 2019, 128, 30–43. [Google Scholar] [CrossRef]
- Schlindwein, C.; Wittmann, D. Stamen movements in flowers of Opuntia (Cactaceae) favour oligolectic pollinators. Plant Syst. Evol. 1997, 204, 179–193. [Google Scholar] [CrossRef]
- Schlindwein, C.; Wittmann, D. Specialized solitary bees as effective pollinators of South Brazilian species of Notocactus and Gymnocalycium (Cactaceae). Bradleya 1995, 13, 25–34. [Google Scholar] [CrossRef]
- Becker, R.; Dal Ri, L.; Farias-Singer, R.; Singer, R.B. Unveiling the germination requirements for Cereus hildmannianus (Cactaceae), a potential new crop from southern and southeastern Brazil. Acta Bot. Bras. 2021, 34, 765–771. [Google Scholar] [CrossRef]
- Mizrahi, Y. Cereus peruvianus (Koubo) new cactus fruit for the world. Rev. Bras. Frutic. 2014, 36, 68–78. [Google Scholar] [CrossRef]
- Silva Santos, E.; Oliveira, A.J.; Silva, M.D.; Mangolin, C.A.; Gonçalves, R.A. Cereus hildmannianus (K.) Schum. (Cactaceae): Ethnomedical uses, phytochemistry and biological activities. J. Ethnopharmacol. 2021, 264, 113339. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.D.; Cambraia, J. Estudo do valor nutritivo do “ora-pro-nobis” (Pereskia aculeata Mill.). Rev. Ceres 1974, 21, 105–111. [Google Scholar]
- Kinupp, V.F.; Barros, I. Riqueza de plantas alimentícias não-convencionais na região metropolitana de Porto Alegre, Rio Grande do Sul. Rev. Bras. Biociências 2007, 5, 63–65. [Google Scholar]
- Carvalho, E.G.; Soares, C.P.; Blau, L.; Menegon, R.F.; Joaquim, W.M. Wound healing properties and mucilage content of Pereskia aculeata from different substrates. Rev. Bras. Farmacogn. 2014, 24, 677–682. [Google Scholar] [CrossRef]
- Souza, M.R.; Correa, E.J.; Guimarães, G.; Pereira, P.R. O potencial do ora-pro-nobis na diversificação da produção agrícola familiar. Rev. Bras. Agroecol. 2009, 4, 2. [Google Scholar]
- Mercê, A.L. Complexes of arabinogalactan of Pereskia aculeata and Co2+, Cu2+, Mn2+, and Ni2+. Bioresour. Technol. 2001, 76, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.F.; Gasparetto, B.F.; Lopes, R.R.; Barros, I.B. Temperature requirements for seed germination of Pereskia aculeata and Pereskia grandifolia. J. Therm. Biol. 2016, 57, 6–10. [Google Scholar] [CrossRef]
- Silva, W.R.; Sazima, M. Hawk moth pollination in Cereus peruvianus, a columnar cactus from southeastern Brazil. Flora 1995, 190, 339–343. [Google Scholar] [CrossRef]
- Locatelli, E.; Machado, I.C. Floral biology of Cereus fernambucensis: A sphingophilous cactus of the restinga. Bradleya 1999, 17, 86–94. [Google Scholar] [CrossRef]
- Gorostiague, P.; Ollerton, J.; Ortega-Baes, P. Latitudinal gradients in biotic interactions: Are cacti pollination systems more specialized in the tropics? Plant Biol. 2022, 25, 187–197. [Google Scholar] [CrossRef]
- Leuenberger, B.E. Pereskia (Cactaceae) Memoirs of the New York Botanical Garden; New York Botanical Garden: New York, NY, USA, 1986; p. 141. [Google Scholar]
- Mandujano, M.; Carrillo-Ángeles, I.; Martínez-Peralta, C.; Golubov, J. Reproductive Biology of Cactaceae. In Desert Plants; Springer: Berlin/Heidelberg, Germany, 2010; pp. 197–230. [Google Scholar]
- Faegri, K.; Van der Pijl, L. The Principles of Pollination Ecology; Pergamon: New York, NY, USA, 1979. [Google Scholar]
- Eggli, U.; Giorgetta, M. Flowering phenology and observations on the pollination biology of South American cacti. Haseltonia 2015, 20, 3–12. [Google Scholar] [CrossRef]
- Viana, M.L.; Ortega-Baes, P.; Saravia, M.; Badano, E.I.; Schlumpberger, B. Biología floral y polinizadores de Trichocereus pasacana (Cactaceae) en el Parque Nacional Los Cardones, Argentina. Rev. Biol. Tropical. 2001, 49, 279–285. [Google Scholar]
- Rego, J.O.; Franceschinelli, E.V.; Zappi, D.C. Reproductive biology of a highly endemic species: Cipocereus laniflorus NP Taylor & Zappi (Cactaceae). Acta Bot. Bras. 2012, 26, 243–250. [Google Scholar]
- Walter, H.E. Floral biology of Echinopsis chiloensis ssp. chiloensis (Cactaceae): Evidence for a mixed pollination syndrome. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 757–763. [Google Scholar] [CrossRef]
- Schlumpberger, B.O.; Cocucci, A.A.; Moré, M.; Sérsic, A.N.; Raguso, R.A. Extreme variation in floral characters and its consequences for pollinator attraction among populations of an Andean cactus. Ann. Bot. 2009, 103, 1489–1500. [Google Scholar] [CrossRef]
- Ortega-Baes, P.; Saravia, M.; Sühring, S.; Godínez-Alvarez, H.; Zamar, M. Reproductive biology of Echinopsis terscheckii (Cactaceae): The role of nocturnal and diurnal pollinators. Plant Biol. 2011, 13, 33–40. [Google Scholar] [CrossRef]
- Rosas-Guerrero, V.; Aguilar, R.; Martén-Rodríguez, S.; Ashworth, L.; Lopezaraiza-Mikel, M.; Bastida, J.M.; Quesada, M. A quantitative review of pollination syndromes: Do floral traits predict effective pollinators? Ecol. Lett. 2014, 17, 388–400. [Google Scholar] [CrossRef]
- Nassar, J.M.; Ramirez, N.; Linares, O. Comparative pollination biology of Venezuelan columnar cacti and the role of nectar-feeding bats in their sexual reproduction. Am. J. Bot. 1997, 84, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Nassar, J.M.; Hamrick, J.L.; Fleming, T.H. Allozyme diversity and genetic structure of the leafy cactus (Pereskia guamacho [Cactaceae]). J. Hered. 2002, 93, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, W.S. The specialization continuum in pollination systems: Diversity of concepts and implications for ecology, evolution and conservation. Funct. Ecol. 2017, 31, 88–100. [Google Scholar] [CrossRef]
- Benavides, M.L. Aspectos da Biologia Reprodutiva de Bombus morio e Bombus atratus (Hymenoptera, Apidae). Ph.D. Thesis, Federal University of Viçosa, Viçosa, Brazil, 2008. [Google Scholar]
- Caballero-Villalobos, L.; Silva-Arias, G.A.; Buzatto, C.R.; Nervo, M.H.; Singer, R.B. Generalized food-deceptive pollination in four Cattleya (Orchidaceae: Laeliinae) species from Southern Brazil. Flora 2017, 234, 195–206. [Google Scholar] [CrossRef]




| Treatment | Cereus hildmannianus (n = 16) | Pereskia aculeata (n = 4) |
|---|---|---|
| Control | 0% (0/30) | 0% (0/30) |
| Emasculation | 0% (0/30) | 0% (0/30) |
| Self-pollination | 0% (0/30) | 78% (24/30) |
| Cross-pollination | 83.3% (25/30) | 86.25% (69/80) |
| Natural pollination | 70% (21/30) | 100% (30/30) |
| Species | Foraged Resource | V | S |
|---|---|---|---|
| Scaptotrigona bipunctata (Apidae; Meliponini) | P, N | 73 | 35 ± 21.1 |
| Trigona spinipes (Apidae; Meliponini) | P, N | 58 | 32 ± 17.1 |
| Plebeia droryana (Apidae; Meliponini) | P, N | 35 | 9 ± 3.4 |
| Apis mellifera (Apidae; Apini) | P, N | 33 | 7 ± 3.2 |
| Bombus pauloensis (Apidae; Bombini) | P, N | 13 | 4 ± 2.7 |
| Bombus morio (Apidae; Bombini) | P, N | 13 | 5 ± 2.4 |
| Xylocopa frontalis (Apidae; Xylocopini) | P, N | 12 | 8 ± 4.9 |
| Caryedes sp. (Chrysomelidae; Bruchinae) | P | 9 | 104 ± 28.5 |
| Polybia sp. (Vespidae; Epiponini) | P, N | 7 | 44 ± 15.6 |
| Augochlora sp. (Apidae; Halictinae) | P, N | 6 | 57 ± 29.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, R.; Báez, O.P.; Singer, R.F.; Singer, R.B. Contrasting Pollination Strategies and Breeding Systems in Two Native Useful Cacti from Southern Brazil. Plants 2023, 12, 1298. https://doi.org/10.3390/plants12061298
Becker R, Báez OP, Singer RF, Singer RB. Contrasting Pollination Strategies and Breeding Systems in Two Native Useful Cacti from Southern Brazil. Plants. 2023; 12(6):1298. https://doi.org/10.3390/plants12061298
Chicago/Turabian StyleBecker, Rafael, Oscar Perdomo Báez, Rosana Farias Singer, and Rodrigo Bustos Singer. 2023. "Contrasting Pollination Strategies and Breeding Systems in Two Native Useful Cacti from Southern Brazil" Plants 12, no. 6: 1298. https://doi.org/10.3390/plants12061298
APA StyleBecker, R., Báez, O. P., Singer, R. F., & Singer, R. B. (2023). Contrasting Pollination Strategies and Breeding Systems in Two Native Useful Cacti from Southern Brazil. Plants, 12(6), 1298. https://doi.org/10.3390/plants12061298






