Abstract
Java plum is widely recognized as a plant with valuable medicinal properties, originating from Indonesia and India and distributed globally in the tropic and sub-tropic regions of the world. The plant is rich in alkaloids, flavonoids, phenyl propanoids, terpenes, tannins, and lipids. The phytoconstituents of the plant seeds possess various vital pharmacological activities and clinical effects including their antidiabetic potential. The bioactive phytoconstituents of Java plum seeds include jambosine, gallic acid, quercetin, β-sitosterol, ferulic acid, guaiacol, resorcinol, p-coumaric acid, corilagin, ellagic acid, catechin, epicatechin, tannic acid, 4,6 hexahydroxydiphenoyl glucose, 3,6-hexahydroxy diphenoylglucose, 1-galloylglucose, and 3-galloylglucose. Considering all the potential beneficial effects of the major bioactive compounds present in the Jamun seeds, in the current investigation, the specific clinical effects and the mechanism of action for the major bioactive compounds along with the extraction procedures are discussed.
1. Introduction
Syzygium cumini (L.) Skeels., more popularly referred to as black Jamun or Java plum, belongs to the family Myrtaceae, and is a vital indigenous plant with medicinal applications originally from India and Indonesia; it is distributed in the tropics and sub-tropics around the globe [1,2,3]. The plant is fast-growing and can grow 30 m or more in height and its lifespan is more than 100 years [4]. The plant is treated as economically important as all of its parts, starting from the seeds and leaves to the wood, have great medicinal and economical values [2,5]. The plant possesses various phytoconstituents and has high antioxidant potential, which is very much beneficial for our bodies. It possesses phytoconstituents that include glucoside, anthocyanin, steroids, phenols, flavonoids, and terpenoids [6]. The fruit is rich in carbohydrates, vitamins, and minerals; the fruit pulp contains some important minerals including manganese, calcium, potassium, iron, zinc, and sodium [7,8].
Its purple-to-blackish color is the result of the anthocyanins present within the plant [9]. Other than the fruits, leaves and bark also have medicinal properties [2]. They are used in diabetes, ringworm, and diarrhea [1,2]. The bark is used as a digestive anthelmintic and diuretic [4]. In addition, seeds are used in various traditional and oriental systems of medicine such as in Ayurvedic, Unani, and Chinese medicines as a natural substitute for the treatment of hyperglycemia, ulcers, dysentery, asthma, glycosuria, and bronchitis [2,5]. The functional properties of the Jamun seeds as food are shown in Figure 1 [10].
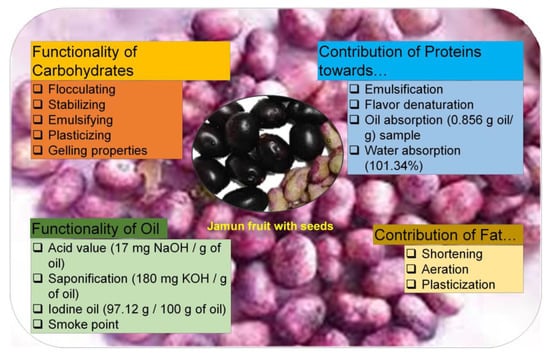
Figure 1.
Functional properties of Jamun seeds. Adopted and reproduced from Kumar et al. [10], under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), 2022, Licensee MDPI, Basel, Switzerland (Originally Figure 1 in the main source).
The seeds have promising high levels of alkaloids, jambosine, and glycoside jamboline or antimellin compounds known for stumbling starch’s diastatic conversion into sugars that makes it a good nutraceutical against type 2 diabetes [11,12,13]. As an overview, pharmacological activities of the plants include chemoprotective, analgesic, hypoglycemic, anti-inflammatory, anti-allergic, anti-oxidant, antihyperlipidemic, hyperglycemic, antiplaque, astringent to bowels, antimicrobial, gastro-protective, antidiarrheal, and antibacterial [2,6]. Moreover, there are reports that the functionally altered seed fibers are used for different applications in the food sector industries such as a thickener, bulking agent, a replacement of fat, and as dietary supplements. [10]. Some of these applications of Jamun seeds are shown in Figure 2 [10].
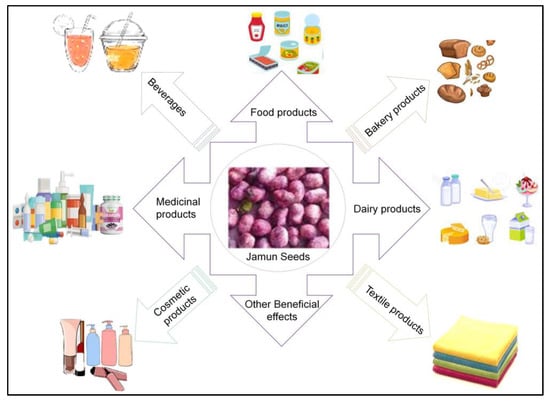
Figure 2.
Multiple uses of Jamun seeds. Adopted and reproduced from Kumar et al. [10], under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/) 2022, Licensee MDPI, Basel, Switzerland (Originally Figure 2 in the main source).
Considering all these potential properties of Jamun seeds, in this review, we exclusively describe the clinical effects and mode of action of the major bioactive constituents present in Jamun seeds.
2. Major Bioactive Constituents
Jamun seed, located in the center of the Jamun fruit, is 1–2 cm long and has a slightly bitter taste [14]. Jamun contains many phytochemicals, and not all the dominant bioactive constituents in different parts are the same (flower, fruit, seed, fruit pulp, leaves, and stem bark) [9]. The nutritional and phytochemical composition of Jamun fruit may differ depending on the maturity level, climatic conditions, growing region, agricultural practices, and post-harvest processing steps [15].
The nutritional composition of Jamun fruit appears to contain significant amounts of carbohydrates (including glucose, sucrose, fructose, and galactose), protein (asparagine, alanine, glutamine, tyrosine, and cysteine; free amino acids), vitamins (ascorbic acid, thiamine and niacin), and minerals (potassium, calcium, sodium, phosphorous, and iron) [16]. Jamun has furthermore been confirmed to possess various bioactive constituents that are shown to be supportive of human health [17]. The color, taste, and aroma of Jamun are closely related to the number of phytochemicals (polyphenols, tannins, and gallic acid) in its composition [9].
The nutritional composition of Jamun seed may differ slightly from its fruit. Raza et al. [18] analyzed the Jamun seeds’ and Jamun fruits’ nutritional composition and found the seeds to have the following approximate composition (%): moisture level of 16.34 ± 0.49, ash level of 2.18 ± 0.06, crude protein level of 1.97 ± 0.59, crude fat level of 0.65 ± 0.01, and crude fiber level of 4.19 ± 0.12; and calculated the composition of Jamun fruit as 82.19 ± 2.46, 2.04 ± 0.06, 2.15 ± 0.06, 0.83 ± 0.02, and 1.76 ± 0.05, respectively [18]. In another study, Jamun seed composition (%), in light of the results obtained from previous studies, has been reported as moisture 3.21–53, ash 1.5–21.72, carbohydrate 6.05–89.68, fiber 1.21–16.9, protein 1.97–8.5, fat 0.65–4.86, ascorbic acid 1.84–35.75, and iron (mg) 1.25–18.62 [19]. Jamun seeds were recently analyzed by collecting the fruits of ripe Jamun grown in Brazil, and the amount of moisture, ash, carbohydrate, protein, and lipid (%) was calculated as 62.25 ± 6.32, 0.36 ± 0.02, 14.95 ± 6.09, 19.96 ± 0.00, and 2.47 ± 0.24, respectively [20]. The amount of oil contained in Jamun seed varies according to its fruit, and although the solvent used is different, the dominant fatty acid is oleic acid [21,22]. The fatty acids present in Jamun seed oil were reported to include oleic acid-32.2%, myristic acid-31.7%, linoleic acid-16.1%, stearic acid-6.5%, palmitic acid-4.7%, vernolic acid-3.0%, lauric acid-2.8%, sterculic acid-1.8%, and malvalic acid-1.2% [23].
More recently, fatty acid extraction of Jamun seed was carried out using different solvents. The fatty acid composition in this analysis using hexane is as follows (%); oleic acid-26.8, linoleic acid-25.2, palmitic acid-19.9, stearic acid-6.4, linoleic acid-2.6, arachidic acid-1.2, eicosapentaenoic acid-0.6, erucic acid-0.5, myristic acid-0.4, docosahexaenoic acid-0.3, and lauric acid-0.3 [21]. This plant species also contains significant amounts of many bioactive constituents including jambosine, quercetin, β-sitosterol, gallic acid, guaiacol, resorcinol, p-coumaric acid, corilagin, ellagic acid, catechin, epicatechin, and tannic acid [24,25]. Jamun’s total flavonoid contents (TFC), total phenolic contents (TPC), and antioxidant capacity differ among the assorted parts of the plant. Ali et al. [25] found that the amount of TPC (mg GAE/g), TFC (mg quercetin/100 g), anthocyanin (mg cyanidin 3-rutinoside equivalent/100 g), and free-radical scavenging capacity (%) in Jamun fruit skin is higher than in the pulp and seed [25]. The amount of the Jamun seed’s bioactive constituents may also differ depending on the type of solvent used [26]. Researchers furthermore found that methanol in the extraction of flavonoids is more effective than methylene chloride [27]. Bajpai et al. [28] determined that the antioxidant activity of the plant’s seeds was 85.49 ± 0.8% and total phenolic contents were 108.79 ± 1.0 mg/g gallic acid equivalent. Moreover, some of the major bioactive constituents of Jamun seed are illustrated in more detail in Figure 3 and Table 1.
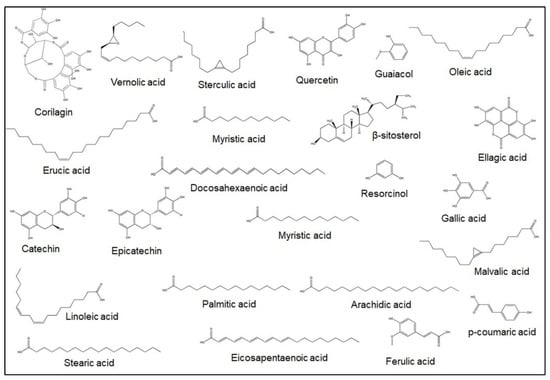
Figure 3.
Primary bioactive constituents in Jamun seed.

Table 1.
The major bioactive constituents of Jamun seed.
3. Clinical Effects Imparted
As mentioned before, the bioactive metabolites found in Jamun and the possible health effects from these metabolites may be different according to the considered parts of the Jamun fruit (pulp, leaf, seed, etc.), botanical type, and/or genotype, extraction techniques, and solvents used to obtain bioactive metabolites [12,13,32]. Although there are predominantly phenolic acids, flavonoids, and tannins, such as anthocyanins, flavonols, flavanols, and flavanonols, in the fruit, the amount of these varies according to the parts of the fruit [33]. Especially in the part of the seed, there are mainly phenolic compounds, including lignans, tannins, coumarins, gallic acid, stilbenoids, ferulic acid, and phloroglucinol derivatives, as well as some flavonoid compounds (e.g., quercetin, rutin, flavones, flavan-3-ols, flavonols, flavanonols, and dihydrochalcones) [1,13,34]. Examination of the evidence-based data in the literature shows that due to these bioactive compounds in its composition, Jamun seeds have anti-inflammatory, cardioprotective, hepatoprotective, anticancer, antimicrobial, and especially antioxidant effects and they can have a regulatory effect on blood glycemia through various mechanisms [33]. However, although it has often been pointed out that there are a limited number of clinical studies on this subject and the possible clinical effects of seeds’ extract forms were evaluated in both cell culture applications and laboratory animal models, the nutraceutical value of this pharmacological agent for many diseases makes it more and more popular day by day [32]. Various pharmacological activities of Jamun seeds are presented in Figure 4. Recently, do Nascimento-Silva et al. [2] summarized a variety of animal experiment studies related to the health-beneficial effect of Jamun seeds, which signifies how beneficial Jamun seeds are (Table 2).
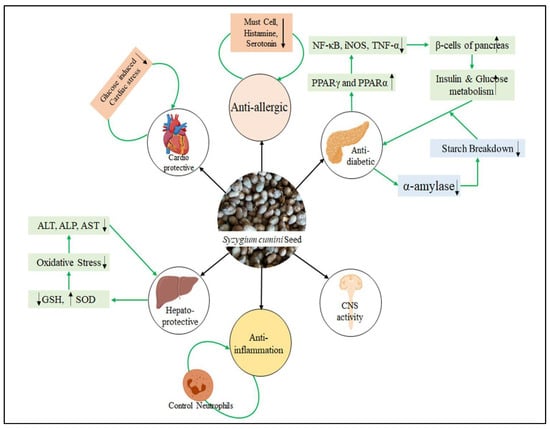
Figure 4.
Pharmacological activity of Jamun seeds.

Table 2.
A summary of various experimental studies on the health-beneficial effects of Jamun seeds in an animal model.
3.1. Phenolic Compounds
Phenolic acids may be best described as non-flavonoid phenolic substances that derive from benzoic acid and cinnamic acid and exist in the free or conjugated (soluble and insoluble) form [40]. They are largely present in plant species and have roles in a promisingly diverse assortment of cellular processes that have been shown to date to include growth and reproduction in plant physiology, and are secondary metabolites generated in the processes of defensive mechanisms in the fight against an array of environmental stressors [41]. Work conducted to date on phenolic acids shows that consuming them regularly has an effective therapeutic potential in protecting humans from various health problems [42]. The underlying mechanism here is that phenolics demonstrate antioxidant activities because they are scavengers of superoxide radical anions, hydroxyl radicals, some organic radicals, singlet oxygens, peroxyl radicals, and peroxynitrite, and, in addition, these compounds function as reducing agents and chain breakers [43]. Moreover, in the case of the phenolic acids, the gallic and the ellagic acids and their various simple and polymeric forms such as gallotannins and ellagitannins are studied enormously [24,34,44,45]. Balyan and Sarkar have found that around 21.9% and 8.65% of gallic acid and ellagic acid are present in the aqueous extract of seeds [24]. With the help of advanced techniques such as liquid chromatography–mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) spectroscopy, various researchers have identified around seventeen ellagitannins and nine gallotannins from the Jamun seeds [6,45,46].
Apart from these, there are other types of phenolic acids such as caffeic acids (1.02–4.73 µg/g seed), chlorogenic acids (0.89–6.80 µg/g seed), ferulic acids (1.50–8.21 µg/g seed), and p-cumaric acids (14.06 µg/g seed), that has been identified from the Jamun seeds [30,47]. Other authors have also identified 5-hydroxyveratric acid, syringic acid, and protocatechuic acid in the Jamun seeds [45,46]. Due to the high phenolic content such as ellagitannins and ellagic acid in Jamun seeds, it has been proven in many studies that it has high levels of antioxidant activities [29,48]. In research supporting this, Aqil et al. [49] performed the antioxidant analysis of the Jamun seeds using different methods (oxygen radical absorbance capacity (ORAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) assay, and ferric reducing antioxidant power (FRAP) assay). Other authors have also proved that hydrolyzable tannins have strong antioxidant and antibacterial properties [50,51]. In another study, the author studied the antioxidant potential of Jamun seeds after the extraction of phenolic compounds [52]. The author concluded the potent antioxidant properties of the Jamun seeds in terms of the DPPH (IC50 value 0.24 μg/mL), ABTS (IC50 value 0.31 μg/mL, hydroxyl, metal chelating (IC50 value 48.39 μg/mL), nitric oxide radical scavenging (IC50 value 37.01 μg/mL), and lipid peroxidation inhibitory activity (IC50 value 5.38 μg/mL) [52]. In light of these studies, it can be said that the antioxidant potential of Jamun seeds brings this fruit to the forefront, especially in terms of its protective effect against oxidative stress-related diseases.
It is determined that the major polyphenols such as ellagic, gallic, cinnamic, ferulic, and syringic acids present in S. cumini seeds can modulate and reduce tertiary butyl hydrogen peroxide (TBHP)-induced oxidative stress–angiotensin converting enzyme (ACE), β-Hydroxy β-methylglutaryl-CoA (HMG-CoA) reductase, and low-density lipoprotein (LDL) oxidation in H9c2 cardiomyoblasts and it may have a cardioprotective effect and this effect has been attributed to Jamun phenolics and flavonoids [53]. Other researchers successfully proved that S. cumini seed extract (0.9 mg/kg × 2 doses) has demonstrated protective effects to combat the systemic toxicity generated with methylmercury in neonatal rats and reduced the increased N-acetyl-β- kidney and urine and the level of lipid peroxidation observed in the kidney and liver, and also modulates adenosine deaminase (ADA) activities occurring within the hippocampus [54]. This protective effect is understood to be the outcome of antioxidant activities caused by phenolic acids including chlorogenic and gallic acids found in Jamun seed extract and rutin compounds [54]. It has been furthermore determined that both ethanolic acidic pulp and seed extract with high polyphenol content obtained from S. cumini have high oxygen radical absorbance (respectively; 1.445 ± 64 μmol Trolox equivalent (TE)/g vs. 3.379 ± 151 µM of TE/g) together with DPPH and ABTS scavenger and ferrous ion chelation abilities [29].
Similarly, in other research, it was successfully demonstrated that administering S. cumini seed extract for 5 days has a reducing effect on oxidative stress against strand breaks induced by hydroxyl radicals in pBR322 DNA and reduces genomic damages in an in vivo setting [26]. It was determined in a study that dietary intake of Jamun seed extract for 26 weeks delayed tumor development in breast cells of Swiss albino mice, decreased the incidence of tumors, and reduced tumor multiplicity and burden. Jamun has been reported to reduce breast cancer by significantly balancing the estrogen-mediated changes in breast cell proliferations, estrogen receptor-alpha (ER-α), cyclinD1, and candidate miRNAs (overexpression of miR-375 and miR-182 or underexpression of miR-206 and miR-127) [55]. It has been stated that many bioactive substances (including phenolics, oleanolic acid, betulinic acid, and dimethyl cardamonins) found in Syzygium spp. also have anticancer activity and this is often seen in conjunction with inhibited cell proliferation and the induction of apoptotic events [56]. It is reported that Jamun has an antiproliferative effect upon exposure to A549 cells, a human lung cancer line, and the major bioactive compounds that may have this effect originate from the ellagic acid/ellagitannin (0.5%) in seeds and anthocyanin (0.54%) and ellagic acid/ellagitannin (0.17%) in pulp powder [29]. As supported in this study, mainly ellagitanines, phenolic acids, and flavonols found in S. cumini seeds may have a chemoprotective effect [29]. Likewise, ellagitannins, especially the ellagic acid part, are known to exert strong therapeutic effects in many types of cancer, such as prostate, colon, breast, and skin [57]. Ellagic acid can have this effect through an assortment of diverse mechanisms, such as the inducing of pathways of apoptosis in many cell lines, preventing damage to DNA induced by carcinogens, and the inhibition of metastasis, along with various previously described anti-inflammatory and antioxidant mechanisms [57].
In addition, S. cumini was known throughout history to have antidiabetic potential employing its antihyperglycemic and hypoglycemic properties to different degrees, and has been used in alternative and complementary medicine before the discovery of insulin [58,59]. The effects of Jamun seed powder in glycemic control for individuals with type 2 diabetes were also researched in a randomized controlled study which determined fasting plasma glucose to decrease by 30.0%, post-prandial plasma to decrease by 22.0%, and hemoglobin A1c (HbA1c) to decrease from 8.99% to 8.31% in diabetic individuals with poor glycemic control after a 10 g/day seed powder supplementation applied for 90 days [60]. Additionally, the said antidiabetic effect may be the result of a single component or a specific combination of components including phenolic acids and flavonoids which may exert indirect or direct effects on insulin resistance and β-cell function of the seed extract of Syzigium cumini [60]. In another pilot study, 1 g of a combination of raw and cooked bitter gourd, Jamun seeds, and fenugreek seeds, was given to 60 non-insulin-dependent rats for 1.5 months, followed by 2 g, and after 3 months, blood sugar was improved in the diabetic rats and the need for oral hypoglycemic drug intake decreased [61]. There is even evidence suggesting that this effect is dose dependent (500–200 mg/kg body weight (BW) [62].
The dry seeds and their boiled extract have produced hypoglycemic effects and both the aqueous extract (5 g kg−1) and alcoholic extract (100 mg kg−1) of the seeds have shown positive effects against tissue damage in the brain of diabetic rats [63]. The potential mechanism often suggested by clinical studies evaluating the regulation of blood glucose by Jamun seed is that Jamun modulates carbohydrate metabolism and stimulates insulin secretions from the pancreas (β-cells) [64]. In a study that supports this, it was determined that both α-glucosidase and α-amylase were inhibited in vitro after gallic acid and catechin and S. cumini seed kernel extract with high bioactive substance content were combined, and this combination may have antidiabetic potential [65]. The ability to inhibit protein tyrosine phosphatase 1B and aldose reductase has been shown by the ethyl acetate fraction of seeds containing, amongst other valuable compounds, 5-furfural, valoneic acid dilactone, and rubuphenol (IC50 values: 0.77, 0.165, and 0.12 mg/mL, respectively); aldoreductase inhibition was determined. It has been determined that dilactone, rubuphenol, and ellagic acid have protein tyrosine phosphatase 1B activity (IC50: respectively; 9.37, 28.14, and 25.96 mg/mL) and that seeds have an antidiabetic effect because they contain these bioactive compounds [6].
Similarly, in one of the recent studies, a total of 21 phenolics from Jamun seeds that may have antidiabetic potential have been isolated and identified, and it was determined that they may demonstrate antidiabetic potential by reducing the levels of advanced glycation end products (AGEs) that are formed and inhibiting the activities of enzymes such as alpha-glucosidase that hydrolyze carbohydrates [45]. There are also some findings in the literature showing that extracts of different parts of Jamun reduce hepatotoxicity in rats. Especially, pulp aqueous, methanol, and other alcohol extracts are reported to decrease serum levels of liver enzymes, increase antioxidant enzyme levels such as glutathione peroxidase, decrease lipid peroxidation, and thus decrease fibrosis and necrosis [66,67,68]. These effects are thought to be caused by natural antioxidants such as gallic acid and anthocyanin [69]. Considering this information, the phytochemical contents and antioxidant activities of seeds and extracts obtained from Jamun can be promising for hepatoprotective activity, which should be taken into consideration in further clinical trials. In light of these studies, it can be said that the Jamun seed phenolics and Jamun seed extracts as a combination of bioactive compounds have an antioxidant effect and play a promising role in both preventing and treating many ailments that occur in association with oxidative states.
Flavonoids are secondary metabolites found in many fruits and vegetables as aglycones or glycosides. Chemically flavonoids have basic structures of 15-carbon skeletons that comprise one heterocyclic and two phenyl rings. Flavonols, flavones, flavonoids, flavanones, anthocyanidins, and isoflavones are important flavonoids [70]. It has been reported by several authors, that among the type of flavonoids found in the Jamun seeds, catechin, quercetin, kaempferol, and epicatechin are found abundantly [30,47]. Moreover, myricetin and its derivatives such as myricetin glycosides, syringetin, and laricitrin are widely present in the seeds [45,46]. While the main flavonoid found in the pulp of S. cumini is anthocyanins, there are other important phytochemical constituents in the seeds of Jamun such as quercetin and rutin, and myricetin [57]. Among these compounds, quercetin, kaempferol, and rutin are mainly responsible for their effective bioactive potentials [71]. It is to be noted that the anthocyanins which are found in the fruit pulp are absent in the seeds, which might be affected during the ripening stage of the fruit [4,29]. Some other authors have also detected resveratrol, coumarins, phloroglucinol derivatives, lignans, etc. in the Jamun seeds [30,72]. Additionally, resveratrol has been reported to be responsible for the high antioxidant potential of the Jamun seeds [30].
In one study, Gajera et al. [47] measured the levels of quercetin in seed (0.04 ± 0.001 µg/g) and kernel (0.05 ± 0.002 µg/g) parts of only one Jamun species in their analysis with six different Jamun species [47]. Quercetin, a flavonoid, is among the dietary antioxidants of the highest significance [73]. Recently, it has been emphasized that quercetin might have the capability of exerting helpful effects in both prophylaxis and treatment in cases of the novel coronavirus disease (COVID-19) due to its anti-inflammatory, strong scavenger, and thrombin-inhibitory properties [74,75]. Flavonoids such as quercetin are among the promising target compounds that will surely be explored in clinical trials against COVID-19 infections in the future due to their pleiotropic activities and the fact that they do not cause systemic toxicity [76]. Alongside its antiviral effects, studies over the years have shown that quercetin can be considered for applications in treating many disorders or diseases including type 2 diabetes mellitus, cardiovascular disease, osteoporosis, many different cancers, blood pressure, and mental-cognitive and pulmonary disorders [77]. Therefore, as a source of quercetin, the powder forms of the parts of Jamun such as seeds (total flavonoids: 457.2 ± 31.44 mg 100 g−1) and pulp (total flavonoids: 36.00 ± 5.95 100 g−1) are used to increase the antioxidant capacity of foods as well as to increase their clinical benefits [73,78]. It has a special ability to scavenge highly reactive species such as superoxide anions or hydrogen peroxide that may trigger oxidative damage within cellular components such as lipids, proteins, and DNA acids. In this way, it has important protective roles in pathophysiological and degenerative processes caused by oxygen radicals [79].
In particular, its anticancer activity comes to the fore and it can play therapeutic roles against many diseases encompassing malignancies, inflammatory disorders, and oxidative stress [80]. In this context, there are some hypotheses that quercetin can be used as an adjuvant or stand-alone treatment in many metabolic diseases [81]. In one study, 50 Sprague–Dawley rats (all male) with diabetes induced by the administration of streptozotocin were given 30 mg/kg of quercetin for 14 days; their elevated serum blood glucose and insulin decreased, together with their dyslipidemia, and oxidative stress and tissue injury biomarkers, and hyperlactatemia and ketoacidosis markers, which are complications of diabetes, all dramatically improving [81]. Quercetin, given at doses of 15 mg kg/day for four weeks, has also been found to decrease blood sugar and raise the level of plasma insulin in rats with diabetes induced by administering streptozotocin and simultaneously improved bone mineral metabolism and structural bone matrix [82]. There is also some evidence that quercetin may have positive effects on obesity and its comorbidities, which are associated with low-grade inflammation, due to its anti-inflammatory effects [83].
It has been suggested that the performance of quercetin is due to the result of the ability to alleviate intracellular oxidative stresses, reduce low-grade chronic inflammation, inhibit lipogenesis and adipogenesis, and suppress preadipocytes differentiating from matured adipocytes [84]. In one study, it was reported that quercetin has an antiobesity effect via the signaling pathways of adenine monophosphate-activated protein kinase and mitogen-activated protein kinase [85]. In addition, it has also been found in a study conducted on Wistar rats that it has a corrective effect on dysbiosis within the intestinal microbiota caused by diets that are high in fats as a result of administering a mixture containing quercetin at 30 mg/kg body weight and resveratrol at 15 mg/kg body weight daily for 10 weeks [86]. In addition, in a systematic meta-analysis study reviewing the effects of quercetin on levels of blood pressure, it was reported that quercetin supplementation at doses of >500 mg/day may have a significant effect in reducing blood pressure [87]. Similarly, in another study, it was found that quercetin may have an anti-hypertensive effect by activation of Na⁺-K⁺-2Cl− cotransporter 1 (NKCC1) within the epithelial cells of the kidneys [88]. In light of these clinical studies, it is obvious that Jamun seed may have important clinical benefits as a source of quercetin, but more randomized controlled clinical studies are needed in this context.
Another important flavonoid found in Jamun seeds with clinical implications is rutin. Rutin, a type of flavonol, is found in many plants. In clinical terms, it has been reported that it exerts various biological effects, increasing the strength of blood capillaries and performing antihypertensive, antioxidant, and alpha-glucosidase inhibitory activities [89]. A study by Khan et al. [90] showed that the ethyl acetate fraction of S. cumini seeds displayed encouraging antimutagenic and DNA-protective activity, and its flavonoid constituents, including rutin, contributed ominously to the experiential activity.
Jamun, which has a complex matrix of both flavonoid content and other phytochemicals, can scavenge free radicals, increasing the antioxidant capacity of cells, and reducing lipid peroxidation as a result of the increased levels of major antioxidant enzymes. Furthermore, it can suppress transcription of nuclear factor kappa B, inducible nitric oxide synthase, peroxisome proliferator-activated receptor, tumor necrosis factor-alpha, and many other pro-inflammatory cytokines, simultaneously modulating the upregulating of the transcription of nuclear factor erythroid 2-related factor 2, and it has important physiological roles in the antioxidant system and the associated clinical problems [91].
In addition to its antioxidant effects, compounds such as 3,5,7,4′-tetrahydroxy flavanone found in the seeds of this plant were also identified as amylase inhibitors [92]. Similarly, in another study, it has been determined that the flavonoids and other functional groups in the seed can inhibit the alpha-amylase enzyme by up to 96%, depending on the dose, and thus have an antidiabetic effect [93]. Chemically, rutin is a compound with low water solubility (0.125 g/L), which makes it difficult to integrate into functional foods and nutritional supplements [94]. This solubility-related disadvantage highlights Jamun as a potential functional nutrient because of its potential clinical benefits and as a source of rutin.
Tannins are another important secondary plant metabolite. Although they have different classifications according to their chemical structures and stability, they are generally classified as hydrolyzable, complex, and/or proanthocyanidins [95]. In Syzygium cumini fruit, there are some hydrolyzed and condensed tannins. Hydrolyzed tannins, defined as ellagitannins, consist of glucose in the center and gallic and ellagic acid units surrounding it. As condensed tannins, there are B-type oligomers of epiafzelechin (propelargonidin) with different degrees of polymerization [96]. However, in one study conducted, it was determined in an HPLC-DAD-ESI-MS/MS analyses that non-tannin phenolics are mostly predominant in the skin part of the Jambolan fruit in S. cumini and the existing tannin varieties are hydrolyzable tannins (equal amounts of gallotannins and ellagitanins) [97]. In addition, it has been reported that extraction using acetone can extract tannins better than ethanolic extraction [98].
In the literature, it has been stated that the tannins extracted from the fruit and seeds of this plant have strong DPPH radical scavenging and ferric-reducing/antioxidant activities, and therefore, this fruit is a natural antioxidant source [96]. Some tannins come to the forefront with antiviral, antibacterial, and antitumor activities [99]. Studies with S. cumini seed tannins are mostly related to their gastroprotective and antidiabetic effects. One study determined that S. cumini tannins (20.0 g tannins/kg) reduce gastric damage by reducing free-radical formation in the stomach in Sprague–Dawley rats with induced gastric mucosal damage and thus have an antiulcerogenic effect [100]. In another interesting study, it was reported that ellagic acid and urolithin A (3,8-dihydroxy-6H-dibenzopyran-6-one), which are colonic metabolites of ellagitannins (total phenolic substance: 20.5% GAE), which are hydrolyzable tannins found in Jamun, reduce colon carcinogenesis in human 293T cell line by modulating Wnt pathway-mediated transcriptional activation, and especially, urolithin A is a bioactive metabolite that is effective in modulating gut microbiota [101].
In a preclinical assessment of diabetes, 250, 500, and 1000 mg/kg doses of powdered seeds (identified bioactive components: tannins, gallic acid, oxalic acid, and triterpenoids) were supplemented to STZ-induced diabetic rats for 15 days, and especially, the seed powder at 500 and 1000 mg/kg doses caused antidiabetic effects on fasting blood sugar, peak blood sugar, and liver glycogen stores. In the same study, a subacute toxicity phase 2 study was also conducted and it was demonstrated that the 2.5 and 5.0 g/kg seed powder did not cause deaths or any morbidity [102]. In another study, three novel hydrolyzable tannins, namely iso-oenothein C and jamutannins A and B, were identified in Jamun seeds, in addition to ellagitannin, which has an antidiabetic effect similar to acarbose, which is one of the oral antidiabetic drugs, and it was reported that they have α-glucosidase inhibitory effects [103].
3.2. Terpenes and Terpenoids
In S. cumini, there are monoterpenoids (e.g., linalool oxide, 1,8-cineole, nerol, terpinolene, citronellol) and triterpenoids (e.g., acetyl oleanolic acid, oleanolic acid), and sesquiterpenes at a lower level [104]. It has demonstrated that these compounds, also known as the essential oil fraction of S. cumini seeds, have anti-inflammatory, antimicrobial, antihyperlipidemic, and chemopreventive effects [105,106]. Researchers assessed the anti-inflammatory effects (total leukocyte, neutrophil, and eosinophil migration) of S. cumini seed essential oil (100 mg/kg) in male Swiss mice by a lipopolysaccharide-induced pleurisy model and it was determined that eosinophil migration decreased by 67%. It was determined that this inhibitory effect was correlated primarily with β-caryophyllene and β-pinene found in the oil, and α-pinene increased this effect synergistically [105].
Betulinic acid, which has recently come to the fore among terpenes, is a bioactive compound also known as pentacyclic lupine-type triterpenoid and is isolated from different parts of Jamun [107,108]. In the studies on betulinic acid and its derivatives, it has been often reported that it has therapeutic and anti-HIV and antimalarial activities against anti-inflammatory, antidiabetic, antihyperlipidemic, and nonalcoholic fatty liver disease and it has even been reported that it has an antiviral effect against Dengue virus (DENV) due to its antioxidant effect [108,109,110,111]. It has been even stated that it is an important antineoplastic/chemopreventive agent, which has been discovered recently [112]. A study determined that betulunic acid causes apoptosis of differentiated PC12 cells caused by ROS via the mitochondrial apoptotic pathway [106]. In an experimental study that supports this, it was reported that betulinic acid (50 μM) can induce apoptotic processes in ovarian A2780 cells via mitochondria-dependent and -independent pathways and may be considered a novel chemopreventive agent [113]. It has been determined by other researchers that betulinic acid inhibits pancreatic cancer formation through mTOR-caspases/Bcl2/Bax apoptotic signaling modulation and may have a chemopreventive effect [114]. It has even been reported that it is safe in mice up to 500 mg/kg body weight [109]. Considering the results of these studies, it is predicted that Jamun, which is a valuable provider of both betulinic acid and assorted bioactive substances, will become more popular day by day and the number of clinical studies to be conducted in this context will increase.
3.3. Alkaloids
Alkaloids are nitrogenous organic molecules and naturally occurring [115]. Several researchers have reported the presence of alkaloids and their beneficial effects in the Jamun seeds [116,117]. Hasanuzzaman et al. reported on the presence of alkaloids in the acetone and chloroform seed extracts of the S. cumini fruit [118]. They also stated that alkaloids are used as medicines in serious illnesses including cancer, heart failure, and blood pressure, besides their applications as euphoric and addicting drugs and pesticides or insect repellents [118,119,120]. Older reports have shown the anti-inflammatory effects and antioxidant properties of the seed extract on rats [119,121,122]. Moreover, some studies have also proved the beneficial effects of seeds on diabetic humans [119,120,123]. Other older research on the seeds has shown that the seed extracts have a high-hypoglycemic effect on diabetic rabbits [124]. Ayurveda’s traditional system of medicine has suggested that the average dose of 1–3 g of seed powder per day is ideal [125]. Moreover, there are no side effects of seed and bark extracts in the traditional reports, but high-tannin bark extracts might source slight gastrointestinal distress in some people if taken with food [120]. Some authors have written that the Jamun seeds contain an alkaloid, jambosine, which helps in the halting of the diastatic conversion of starch into sugar [116,126]. The seed extract has lowered blood pressure by 34.6% and this act is attributed to the ellagic acid content in the seeds [126]. Rajkumar et al. reported finding alkaloid contents of 81.07 mg/g in the seed extract [115]. Alkaloids are highly useful in the management of diabetes and they are responsible for the reverse conversion of starch into sugar in human blood levels [115]. Despite the useful and medicinal properties of Jamun, some adverse effects of Jamun have also been reported in humans, such as lowering blood sugar [91]. Hence, it was suggested to avoid its consumption after immediate surgery or by pregnant women, etc. [91].
4. Major Extraction Procedures
Many factors such as the extraction methods of Jamun seeds, solvents used in extraction, and duration of extraction cause significant changes in extracted bioactive constituents and reaction kinetics [24]. This naturally leads to the potential health benefits, which are medicinal properties, attributed to Jamun seeds [29,127]. The most common and important methods used to extract the major bioactive components of Jamun seed and the results of these methods are given in Table 3. While Jamun seed contains about 3–10% oil, the main fatty acids are reported as myristic acid and oleic acid [128]. Soxhlet extraction (SJE) and gas chromatography–mass spectrometry (GC-MS) are the main methods in Jamun seed oil analysis. While the Soxhlet method is a traditional technique used to extract fat from foods, GC-MS is a prominent method in the analysis of fatty acids. Although the Soxhlet method has some disadvantages, it is one of the most popularly applied techniques due to its unattended and simple use [129]. While the oil content of Jamun seed was reported as 10% in one of the two different studies using the Soxhlet method, it was calculated as 2.47% in the other study [20,128]. Another reliable and frequently used method for the quantitative extraction of lipids and the extraction of fatty acids is the Folch method [130]. Although it is frequently used in the oil analysis of some plant seeds, no study has been found in the literature in which Jamun seed has been analyzed by this method.

Table 3.
Some research results on the extraction of Jamun seeds’ major constituents.
The extraction of phenolic compounds in fruits such as Jamun is recommended from fresh samples. However, due to the high perishability of these fruits, methods such as freeze-drying are generally used. The phenolic contents may be seriously affected by the particular approaches used to prepare the extract [49]. While certain methods used in the preparation of seed extracts from this plant provide high efficiency for phenolics, on the other hand, some methods in the literature may have problems with reproducibility [33].
Different methods are used in the analysis of the bioactive components of Jamun seed. While the most common traditional methods are the conventional solvent extraction method and SJE, modern methods include UJE or MJE, ultrafiltration, solid-phase microextraction, and supercritical fluid extraction [117]. do Carmo Brito et al. [136] emphasized that ethanol 95% with 1% of HCl (v/v) is the most efficient extraction method to extract anthocyanins from Jamun fruits [136]. In another study, as a result of analysis using different extraction methods, the total phenolic contents (mg GAE/100 g), flavonoid content (mg quercetin equivalents (QE)/100 g), and total anthocyanin contents (mg cyanidin-3-glucoside equivalent (CYE)/g) were obtained with the highest amount in the ethanol extracts of both Jamun fruit and seeds [131]. This was followed by methanol and water extracts [131]. In addition to the extraction technique and the characteristics of the solvent used, pH is also one of the important factors in the analysis of bioactive components; especially anthocyanins [136]. Although different results are obtained in different analysis methods, an ideal method for the extraction of the bioactive components of Jamun seed has not been reported yet.
5. Conclusions and Future Perspectives
Jamun is easily available, and highly nutritious, with multiple medicinal properties. Along with its taste, the fruit is loaded with flavonoids, phenyl propanoids, alkaloids, tannins, terpenes, and lipids. The phytoconstituents of Jamun seeds have pharmacological activities such as antidiabetic, hepatoprotective, antiallergic, and cardioprotective properties which support its facts, i.e., Jamun is a highly health-beneficial fruit. It also discloses numerous medicinally imperative bioactive compounds existing in Jamun seeds and validates their use as a conventional medication for the management of different diseases. Among all the pharmacological aspects, Jamun seed is widely popular for its antidiabetic activity. As diabetes is a disease of concern globally, Jamun might assume a crucial future role in controlling hyperglycemia by ingesting it in the raw form. Moreover, it is highly significant to study the phytoconstituents of Jamun seeds and explore their possibilities in finding a cure for diabetes or other related diseases.
Author Contributions
J.K.P. and G.D. conceptualized the whole concept. J.K.P., G.D., A.D.T., D.A. and R.C. wrote, review, and edited the manuscript. R.N., B.Y. and H.-S.S. helped in the collection of literature, review, and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to this manuscript are available in the form of figures and tables in the manuscript.
Acknowledgments
All authors are grateful to their respective institutions for their support. JK Patra, G Das, and H-S Shin are grateful to Dongguk University, Republic of Korea for their support.
Conflicts of Interest
The authors declare no conflict of interest with the manuscript.
References
- Gajera, H.P.; Gevariya, S.N.; Patel, S.V.; Golakiya, B.A. Nutritional profile and molecular fingerprints of indigenous black jamun (Syzygium cumini L.) landraces. J. Food Sci. Technol. 2018, 55, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento-Silva, N.R.R.; Bastos, R.P.; da Silva, F.A. Jambolan (Syzygium cumini (L.) Skeels)): A review on its nutrients, bioactive compounds and health benefits. J. Food Compos. Anal. 2022, 109, 104491. [Google Scholar] [CrossRef]
- Qamar, M.; Akhtar, S.; Ismail, T.; Wahid, M.; Abbas, M.W.; Mubarak, M.S.; Yuan, Y.; Barnard, R.T.; Ziora, Z.M.; Esatbeyoglu, T. Phytochemical profile, biological properties, and food applications of the medicinal plant Syzygium cumini. Foods 2022, 11, 378. [Google Scholar] [CrossRef]
- Benherlal, P.S.; Arumughan, C. Chemical composition and in vitro antioxidant studies on Syzygium cumini fruit. J. Sci. Food Agric. 2007, 87, 2560–2569. [Google Scholar] [CrossRef]
- Chaudhary, B.; Mukhopadhyay, K. Syzygium cumini (L.) Skeels: A potential source of nutraceuticals. Int. J. Pharm. Biol. Sci. 2012, 2, 46–53. [Google Scholar]
- Sawant, L.; Singh, V.K.; Dethe, S.; Bhaskar, A.; Balachandran, J.; Mundkinajeddu, D.; Agarwal, A. Aldose reductase and protein tyrosine phosphatase 1B inhibitory active compounds from Syzygium cumini seeds. Pharm. Biol. 2015, 53, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Roy, G.; Malla, S.; Chakravarty, S. Integrated processing of jamun (Syzygium cumini (L.) Skeels) fruit for value addition and assessment of its impact on health and nutrition. Clin. Diagn. Lab. Immunol. 2013, 21, 65–69. [Google Scholar]
- Jenke, D.R. Chromatographic Method Validation: A Review of current practices and procedures. II. Guidelines for Primary Validation Parameters. J. Liq. Chromatogr. Relat. Technol. 1996, 19, 737–757. [Google Scholar] [CrossRef]
- Chhikara, N.; Kaur, R.; Jaglan, S.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds and pharmacological and food applications of Syzygium cumini—A review. Food Funct. 2018, 9, 6096–6115. [Google Scholar] [CrossRef]
- Kumar, M.; Zhang, B.; Nishad, J.; Verma, A.; Sheri, V.; Dhumal, S.; Sharma, N.; Chandran, D.; Senapathy, M.; Dey, A. Jamun (Syzygium cumini (L.) Skeels) Seed: A Review on Nutritional Profile, Functional food properties, health-promoting applications, and safety aspects. Processes 2022, 10, 2169. [Google Scholar] [CrossRef]
- Gowri, S.S.; Vasantha, K. Phytochemical screening and antibacterial activity of Syzygium cumini (L.)(Myrtaceae) leaves extracts. Int. J. PharmTech Res. 2010, 2, 1569–1573. [Google Scholar]
- Ayyanar, M.; Subash-Babu, P. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop. Biomed. 2012, 2, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Chandra, D. Pharmacological potentials of Syzygium cumini: A review. J. Sci. Food Agric. 2013, 93, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Ramya, S.; Neethirajan, K.; Jayakumararaj, R. Profile of bioactive compounds in Syzygium cumini—A review. J. Pharm. Res. 2012, 5, 4548–4553. [Google Scholar]
- Saeed, A.; Kauser, S.; Iqbal, M. Nutrient, mineral, antioxidant, and anthocyanin profiles of different cultivars of Syzygium cumini (jamun) at different stages of fruit maturation. Pak. J. Bot. 2018, 50, 1791–1804. [Google Scholar]
- Longvah, T.; Ananthan, R.; Bhaskarachary, K.; Venkaiah, K. Indian Food Composition Tables; National Institute of Nutrition (Indian Council of Medical Research): Hyderabad, Telangana, India, 2017. [Google Scholar]
- Baliga, M.S.; Bhat, H.P.; Baliga, B.R.V.; Wilson, R.; Palatty, P.L. Phytochemistry, traditional uses and pharmacology of Eugenia jambolana Lam. (black plum): A review. Food Res. Int. 2011, 44, 1776–1789. [Google Scholar] [CrossRef]
- Raza, A.; Ali, M.U.; Nisar, T.; Qasrani, S.A.; Hussain, R.; Sharif, M.N. Proximate Composition of Jamun (Syzygium cumini) Fruit and Seed. Am.-Eurasian J. Agric. Environ. Sci. 2015, 15, 1221–1223. [Google Scholar]
- Sharma, R.; Oberoi, K.; Sharma, K.; Nagraik, R.; Kumar, D.; Sharma, A.; Sharma, S. Bioactive compounds and ethnomedicinal uses of Syzygium cumini (L.) Skeels—A comprehensive review. Ann. Univ. Dunarea Jos Galati. Fascicle VI Food Technol. 2020, 44, 230–254. [Google Scholar] [CrossRef]
- Santos, C.A.; Almeida, F.A.; Quecan, B.X.; Pereira, P.A.; Gandra, K.; Cunha, L.R.; Pinto, U.M. Bioactive properties of Syzygium cumini (L.) skeels pulp and seed phenolic extracts. Front. Microbiol. 2020, 11, 990. [Google Scholar] [CrossRef]
- Kumar, A.; Padmanabhan, N.; Krishnan, M. Extraction of Fatty Acids from Syzygium cumini Seeds with Different Solvents. Asian J. Chem. 2007, 19, 2779–2782. [Google Scholar]
- Hameed, F.; Gupta, N.; Rahman, R.; Anjum, N.; Nayik, G.A. Jamun. In Antioxidants in Fruits: Properties and Health Benefits; Ahmad, N.G., Amir, G., Eds.; Springer: Singapore, 2020; pp. 615–637. [Google Scholar]
- Daulatabad, C.M.J.D.; Mirajkar, A.M.; Hosamani, K.M.; Mulla, G.M.M. Epoxy and cyclopropenoid fatty acids in Syzygium cuminii seed oil. J. Sci. Food Agric. 1988, 43, 91–94. [Google Scholar] [CrossRef]
- Balyan, U.; Sarkar, B. Aqueous extraction kinetics of phenolic compounds from jamun (Syzygium cumini L.) seeds. Int. J. Food Prop. 2017, 20, 372–389. [Google Scholar] [CrossRef]
- Ali, S.M.; Masud, T.; Abbasi, K.S.; Ali, A.; Hussain, A. Some compositional and biochemical attributes of jaman fruit (Syzygium cumini L.) from Potowar region of Pakistan. Res. Pharm. 2013, 3, 1–9. [Google Scholar]
- Arun, R.; Prakash, M.V.; Abraham, S.K.; Premkumar, K. Role of Syzygium cumini seed extract in the chemoprevention of in vivo genomic damage and oxidative stress. J. Ethnopharmacol. 2011, 134, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Ali, S.I.; El-Baz, F.K. Antioxidant and antibacterial activities of crude extracts and essential oils of Syzygium cumini leaves. PLoS ONE 2013, 8, e60269. [Google Scholar] [CrossRef]
- Bajpai, M.; Pande, A.; Tewari, S.; Prakash, D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int. J. Food Sci. Nutr. 2005, 56, 287–291. [Google Scholar] [CrossRef]
- Aqil, F.; Gupta, A.; Munagala, R.; Jeyabalan, J.; Kausar, H.; Sharma, R.J.; Singh, I.P.; Gupta, R.C. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr. Cancer 2012, 64, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef]
- Ahmed, S.; Jahan, I.A.; Hossain, M.H.; Ahmed, K.S.; Rahman, M.; Zzaman, W.; Hoque, M.M. Bioactive compounds, antioxidant properties and phenolic profile of pulp and seed of Syzygium cumini. J. Food Meas. Charact. 2021, 15, 1991–1999. [Google Scholar] [CrossRef]
- Chagas, V.T.; França, L.M.; Malik, S.; Paes, A.M.d.A. Syzygium cumini (L.) skeels: A prominent source of bioactive molecules against cardiometabolic diseases. Front. Pharmacol. 2015, 6, 259. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Insights into the phenolic compounds present in jambolan (Syzygium cumini) along with their health-promoting effects. Int. J. Food Sci. Technol. 2018, 53, 2431–2447. [Google Scholar] [CrossRef]
- Tak, Y.; Kaur, M.; Jain, M.C.; Samota, M.K.; Meena, N.K.; Kaur, G.; Kumar, R.; Sharma, D.; Lorenzo, J.M.; Amarowicz, R. Jamun seed: A review on bioactive constituents, nutritional value and health benefits. Pol. J. Food Nutr. Sci. 2022, 72, 211–228. [Google Scholar] [CrossRef]
- Raza, A.; Butt, M.S.; Suleria, H.A.R. Jamun (Syzygium cumini) seed and fruit extract attenuate hyperglycemia in diabetic rats. Asian Pac. J. Trop. Biomed. 2017, 7, 750–754. [Google Scholar] [CrossRef]
- Nahid, S.; Mazumder, K.; Rahman, Z.; Islam, S.; Rashid, M.H.; Kerr, P.G. Cardio-and hepato-protective potential of methanolic extract of Syzygium cumini (L.) Skeels seeds: A diabetic rat model study. Asian Pac. J. Trop. Biomed. 2017, 7, 126–133. [Google Scholar] [CrossRef]
- Sharma, S.; Pathak, S.; Gupta, G.; Sharma, S.K.; Singh, L.; Sharma, R.K.; Mishra, A.; Dua, K. Pharmacological evaluation of aqueous extract of Syzigium cumini for its antihyperglycemic and antidyslipidemic properties in diabetic rats fed a high cholesterol diet—Role of PPARγ and PPARα. Biomed. Pharmacother. 2017, 89, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Sharafeldin, K.; Rizvi, M.R. Effect of traditional plant medicines (Cinnamomum zeylanicum and Syzygium cumini) on oxidative stress and insulin resistance in streptozotocin-induced diabetic rats. J. Basic Appl. Zool. 2015, 72, 126–134. [Google Scholar] [CrossRef]
- Alikatte, K.L.; Akondi, B.R.; Yerragunta, V.G.; Veerareddy, P.R.; Palle, S. Antiamnesic activity of Syzygium cumini against scopolamine induced spatial memory impairments in rats. Brain Dev. 2012, 34, 844–851. [Google Scholar] [CrossRef]
- Vincente, A.R.; Manganaris, G.A.; Ortiz, C.M.; Sozzi, G.O.; Crisosto, C.H. Nutritional quality of fruits and vegetables. In Postharvest Handling; Elsevier: Amsterdam, The Netherlands, 2014; pp. 69–122. [Google Scholar]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Saibabu, V.; Fatima, Z.; Khan, L.A.; Hameed, S. Therapeutic potential of dietary phenolic acids. Adv. Pharmacol. Sci. 2015, 2015, 823539. [Google Scholar] [CrossRef]
- Chandrasekara, A. Phenolic acids. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 535–545. [Google Scholar]
- Mashkor, I. Phenolic content and antioxidant activity of fenugreek seeds extract. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 841–844. [Google Scholar]
- Liu, F.; Ma, H.; Wang, G.; Liu, W.; Seeram, N.P.; Mu, Y.; Xu, Y.; Huang, X.; Li, L. Phenolics from Eugenia jambolana seeds with advanced glycation endproduct formation and alpha-glucosidase inhibitory activities. Food Funct. 2018, 9, 4246–4254. [Google Scholar] [CrossRef] [PubMed]
- Eldin Elhawary, S.S.; Elmotayam, A.k.E.; Alsayed, D.k.; Zahran, E.M.; Fouad, M.A.; Sleem, A.A.; Elimam, H.; Rashed, M.H.; Hayallah, A.M.; Mohammed, A.F. Cytotoxic and anti-diabetic potential, metabolic profiling and in silico studies of Syzygium cumini (L.) Skeels belonging to family Myrtaceae. Nat. Prod. Res. 2022, 36, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Gajera, H.; Gevariya, S.N.; Hirpara, D.G.; Patel, S.; Golakiya, B. Antidiabetic and antioxidant functionality associated with phenolic constituents from fruit parts of indigenous black jamun (Syzygium cumini L.) landraces. J. Food Sci. Technol. 2017, 54, 3180–3191. [Google Scholar] [CrossRef]
- Margaret, E.; Shailaja, A.; Rao, V.V. Evaluation of antioxidant activity in different parts of Syzygium cumini (Linn.). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 372–379. [Google Scholar]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Joshi, T.; Gupta, R.C.; Singh, I.P. Chapter 10—The Indian blackberry (Jamun), antioxidant capacity, and cancer protection. In Cancer; Preedy, V., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 101–113. [Google Scholar]
- Karamać, M. In-vitro study on the efficacy of tannin fractions of edible nuts as antioxidants. Eur. J. Lipid Sci. Technol. 2009, 111, 1063–1071. [Google Scholar] [CrossRef]
- Puljula, E.; Walton, G.; Woodward, M.J.; Karonen, M. Antimicrobial activities of ellagitannins against Clostridiales perfringens, Escherichia coli, Lactobacillus plantarum and Staphylococcus aureus. Molecules 2020, 25, 3714. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Pal, A.; Sihag, S.; Nagesh, C. Antioxidant activity profiling of acetonic extract of jamun (Syzygium cumini L.) seeds in different models. Open Food Sci. J. 2020, 12, 3–8. [Google Scholar] [CrossRef]
- Syama, H.; Arya, A.; Dhanya, R.; Nisha, P.; Sundaresan, A.; Jacob, E.; Jayamurthy, P. Quantification of phenolics in Syzygium cumini seed and their modulatory role on tertiary butyl-hydrogen peroxide-induced oxidative stress in H9c2 cell lines and key enzymes in cardioprotection. J. Food Sci. Technol. 2017, 54, 2115–2125. [Google Scholar] [CrossRef]
- Abdalla, F.; Bellé, L.; Bitencourt, P.; de Bona, K.; Zanette, R.; Boligon, A.; Athayde, M.; Pigatto, A.; Moretto, M. Protective effects of Syzygium cumini seed extract against methylmercury-induced sistemic toxicity in neonatal rats. Biometals 2011, 24, 349–356. [Google Scholar] [CrossRef]
- Aqil, F.; Jeyabalan, J.; Munagala, R.; Singh, I.P.; Gupta, R.C. Prevention of hormonal breast cancer by dietary jamun. Mol. Nutr. Food Res. 2016, 60, 1470–1481. [Google Scholar] [CrossRef]
- Chua, L.K.; Lim, C.L.; Ling, A.P.K.; Chye, S.M.; Koh, R.Y. Anticancer potential of Syzygium species: A review. Plant Foods Hum. Nutr. 2019, 74, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Qais, F.A.; Ahmad, I. Indian berries and their active compounds: Therapeutic potential in cancer prevention. In New Look to Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 179–201. [Google Scholar]
- Grover, J.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Fernandes, S.; Thilakchand, K.R.; D’souza, P.; Rao, S. Scientific validation of the antidiabetic effects of Syzygium jambolanum DC (black plum), a traditional medicinal plant of India. J. Altern. Complement. Med. 2013, 19, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Sidana, S.; Singh, V.; Meena, B.; Beniwal, S.; Singh, K.; Kumar, D.; Singla, R. Effect of Syzygium cumini (jamun) seed powder on glycemic control: A double-blind randomized controlled trial. J. Med. Soc. 2017, 31, 185. [Google Scholar] [CrossRef]
- Kochhar, A.; Nagi, M. Effect of supplementation of traditional medicinal plants on blood glucose in non–insulin-dependent diabetics: A pilot study. J. Med. Food 2005, 8, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Lavania, A.; Tomar, R.; Prasad, G.; Jain, S.; Yadav, H. Complementary and comparative study on hypoglycemic and antihyperglycemic activity of various extracts of Eugenia jambolana seed, Momordica charantia fruits, Gymnema sylvestre, and Trigonella foenum graecum seeds in rats. Appl. Biochem. Biotechnol. 2010, 160, 2388–2400. [Google Scholar] [CrossRef]
- Prince, P.S.M.; Kamalakkannan, N.; Menon, V.P. Antidiabetic and antihyperlipidaemic effect of alcoholic Syzigium cumini seeds in alloxan induced diabetic albino rats. J. Ethnopharmacol. 2004, 91, 209–213. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, M. Effects of ethanolic extract of Syzygium cumini (Linn) seed powder on pancreatic islets of alloxan diabetic rats. Indian J. Exp. Biol. 2007, 45, 861–867. [Google Scholar]
- Mahindrakar, K.V.; Rathod, V.K. Antidiabetic potential evaluation of aqueous extract of waste Syzygium cumini seed kernel’s by in vitro α-amylase and α-glucosidase inhibition. Prep. Biochem. Biotechnol. 2021, 51, 589–598. [Google Scholar] [CrossRef]
- Das, S.; Sarma, G. Study of the hepatoprotective activity of the ethanolic extract of the pulp of Eugenia jambolana (jamun) in albino rats. J. Clin. Diagn. Res. 2009, 3, 1466–1474. [Google Scholar]
- Veigas, J.M.; Shrivasthava, R.; Neelwarne, B. Efficient amelioration of carbon tetrachloride induced toxicity in isolated rat hepatocytes by Syzygium cumini Skeels extract. Toxicol. Vitr. 2008, 22, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Moresco, R.N.; Sperotto, R.L.; Bernardi, A.S.; Cardoso, R.F.; Gomes, P. Effect of the aqueous extract of Syzygium cumini on carbon tetrachloride-induced hepatotoxicity in rats. Phytother. Res. 2007, 21, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Evans, W. Trease and Evans Pharmacognosy, 15th ed.; Sanders Co., Ltd.: Singapore, 2002. [Google Scholar]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Chapter 14—Flavonoids: Potential therapeutic agents by their antioxidant capacity. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 265–288. [Google Scholar]
- Jadeja, R.N.; Thouaojam, M.C.; Sankhari, J.M.; Jain, M.; Devkar, R.V.; Ramachandran, A. Standardized flavonoid-rich Eugenia jambolana seed extract retards in vitro and in vivo LDL oxidation and expression of VCAM-1 and P-selectin in atherogenic rats. Cardiovasc. Toxicol. 2012, 12, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yuan, T.; Liu, W.; Ma, H.; Seeram, N.P.; Li, Y.; Xu, L.; Mu, Y.; Huang, X.; Li, L. Phloroglucinol derivatives with protein tyrosine phosphatase 1B inhibitory activities from Eugenia jambolana seeds. J. Nat. Prod. 2017, 80, 544–550. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Chanu, N.R.; Bhattacharjee, A.; Bordoloi, R.; Sahariah, B.J.; Talukdar, A.; Kalita, R. Quercetin for the experimental treatment of COVID-19. In Handbook of Research on Knowledge and Organization Systems in Library and Information Science; IGI Global: Hershey, PA, USA, 2021; pp. 69–87. [Google Scholar]
- Training, K.S.S. Effect of Quercetin on Prophylaxis and Treatment of COVID-19; Clinical Trials: Washington, DC, USA, 2020. [Google Scholar]
- Russo, M.; Moccia, S.; Spagnuolo, C.; Tedesco, I.; Russo, G.L. Roles of flavonoids against coronavirus infection. Chem.-Biol. Interact. 2020, 328, 109211. [Google Scholar] [CrossRef]
- Babaei, F.; Mirzababaei, M.; Nassiri-Asl, M. Quercetin in food: Possible mechanisms of its effect on memory. J. Food Sci. 2018, 83, 2280–2287. [Google Scholar] [CrossRef]
- Wasswa, M.; Tumuhimbise, G.A.; Acham, H. Chemical characterisation of pulp, seed powder and a ready-to-drink juice produced from Syzygium cumini fruit. Mak. Univ. J. Agric. Environ. Sci. 2019, 8, 44–57. [Google Scholar]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular targets underlying the anticancer effects of quercetin: An update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Tuli, H.S.; Yerer, M.B.; Sak, K.; Sharma, A.K.; Kumar, M.; Aggarwal, V.; Sandhu, S.S. Fisetin and quercetin: Promising flavonoids with chemopreventive potential. Biomolecules 2019, 9, 174. [Google Scholar] [CrossRef]
- Yang, D.K.; Kang, H.-S. Anti-diabetic effect of cotreatment with quercetin and resveratrol in streptozotocin-induced diabetic rats. Biomol. Ther. 2018, 26, 130. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Altan, M.F.; Donmez, S.; Ocakci, A.; Kartal, M.E. The effects of quercetin on bone minerals, biomechanical behavior, and structure in streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2007, 25, 747–752. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, B.; Shen, J.; Wan, L.; Zhu, Y.; Yi, T.; Xiao, Z. The beneficial effects of quercetin, curcumin, and resveratrol in obesity. Oxidative Med. Cell. Longev. 2017, 2017, 1459497. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Russo, G.L.; Daglia, M.; Nabavi, S.M. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015, 179, 305–310. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P. Effects of quercetin on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef] [PubMed]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of quercetin, a polyphenol, on blood pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T. Bitterness generation, rutin hydrolysis, and development of trace rutinosidase variety in tartary buckwheat. In Molecular Breeding and Nutritional Aspects of Buckwheat; Elsevier: Amsterdam, The Netherlands, 2016; pp. 345–353. [Google Scholar]
- Khan, M.S.; Abul Qais, F.; Ahmad, I.; Hussain, A.; Alajmi, M.F. Genotoxicity inhibition by Syzygium cumini (L.) seed fraction and rutin: Understanding the underlying mechanism of DNA protection. Toxicol. Res. 2018, 7, 156–171. [Google Scholar] [CrossRef]
- Jagetia, G.C. Phytochemical Composition and pleotropic pharmacological properties of jamun, Syzygium cumini skeels. J. Explor. Res. Pharmacol. 2017, 2, 54–66. [Google Scholar] [CrossRef]
- Karthic, K.; Kirthiram, K.S.; Sadasivam, S.; Thayumanavan, B. Identification of alpha amylase inhibitors from Syzygium cumini Linn seeds. Indian J. Exp. Biol. 2008, 46, 677–680. [Google Scholar] [PubMed]
- Prabakaran, K.; Shanmugavel, G. Antidiabetic activity and phytochemical constituents of Syzygium cumini Seeds in Puducherry Region, South India. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 985–989. [Google Scholar] [CrossRef]
- Frutos, M.J.; Rincón-Frutos, L.; Valero-Cases, E. Rutin. In Nonvitamin and Nonmineral Nutritional Supplements; Elsevier: Amsterdam, The Netherlands, 2019; pp. 111–117. [Google Scholar]
- Sieniawska, E.; Baj, T. Chapter 10—Tannins. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 199–232. [Google Scholar]
- Zhang, L.L.; Lin, Y.M. Antioxidant tannins from Syzygium cumini fruit. Afr. J. Biotechnol. 2009, 8, 2301–2309. [Google Scholar]
- De Carvalho Tavares, I.M.; Lago-Vanzela, E.S.; Rebello, L.P.G.; Ramos, A.M.; Gomez-Alonso, S.; Garcia-Romero, E.; Da-Silva, R.; Hermosin-Gutierrez, I. Comprehensive study of the phenolic composition of the edible parts of jambolan fruit (Syzygium cumini (L.) Skeels). Food Res. Int. 2016, 82, 1–13. [Google Scholar] [CrossRef]
- Bhatia, I.; Bajaj, K. Tannins in black-plum (Syzygium cumini L.) seeds. Biochem. J. 1972, 128, 56. [Google Scholar] [CrossRef]
- Kamal, A. Phytochemical screening of Syzygium cumini seeds. Indian J. Plant Sci. 2014, 3, 1–4. [Google Scholar]
- Ramirez, R.O.; Roa, C.C., Jr. The gastroprotective effect of tannins extracted from duhat (Syzygium cumini Skeels) bark on HCl/ethanol induced gastric mucosal injury in Sprague-Dawley rats. Clin. Hemorheol. Microcirc. 2003, 29, 253–261. [Google Scholar]
- Sharma, M.; Li, L.; Celver, J.; Killian, C.; Kovoor, A.; Seeram, N.P. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J. Agric. Food Chem. 2010, 58, 3965–3969. [Google Scholar] [CrossRef]
- Sridhar, S.; Sheetal, U.; Pai, M.; Shastri, M. Preclinical evaluation of the antidiabetic effect of Eugenia jambolana seed powder in streptozotocin-diabetic rats. Braz. J. Med. Biol. Res. 2005, 38, 463–468. [Google Scholar] [CrossRef]
- Omar, R.; Li, L.; Yuan, T.; Seeram, N.P. α-Glucosidase inhibitory hydrolyzable tannins from Eugenia jambolana seeds. J. Nat. Prod. 2012, 75, 1505–1509. [Google Scholar] [CrossRef]
- Jäger, W.; Höferl, M. Metabolism of terpenoids in animal models and humans. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2020; pp. 275–301. [Google Scholar]
- Siani, A.C.; Souza, M.C.; Henriques, M.G.; Ramos, M.F. Anti-inflammatory activity of essential oils from Syzygium cumini and Psidium guajava. Pharm. Biol. 2013, 51, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, X.; Zhu, R.; Zhang, K.; Li, S.; Chen, Z.; Li, L. Betulinic acid induces apoptosis in differentiated PC12 cells via ROS-mediated mitochondrial pathway. Neurochem. Res. 2017, 42, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Sah, A.K.; Verma, V.K. Syzygium cumini: An overview. J. Chem. Pharm. Res. 2011, 3, 108–113. [Google Scholar]
- Ríos, J.L.; Máñez, S. New pharmacological opportunities for betulinic acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Alakurtti, S.; Mäkelä, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef]
- Huang, Q.X.; Chen, H.F.; Luo, X.R.; Zhang, Y.X.; Yao, X.; Zheng, X. Structure and Anti-HIV Activity of Betulinic Acid Analogues. Curr. Med. Sci. 2018, 38, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Wang, H.; Tong, L.; Fang, Q.; Xiang, M.; Han, L.; Jin, L.; Yang, J.; Qian, Z.; Ning, G. Betulinic acid improves nonalcoholic fatty liver disease through YY1/FAS signaling pathway. FASEB J. 2020, 34, 13033–13048. [Google Scholar] [CrossRef]
- Eiznhamer, D.A.; Xu, Z.-Q. Betulinic acid: A promising anticancer candidate. IDrugs Investig. Drugs J. 2004, 7, 359–373. [Google Scholar]
- Lee, D.; Lee, S.R.; Kang, K.S.; Ko, Y.; Pang, C.; Yamabe, N.; Kim, K.H. Betulinic acid suppresses ovarian cancer cell proliferation through induction of apoptosis. Biomolecules 2019, 9, 257. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, H.; Weng, M.; Wang, C.; Sun, L. Chemopreventive effect of Betulinic acid via mTOR-Caspases/Bcl2/Bax apoptotic signaling in pancreatic cancer. BMC Complement. Med. Ther. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Rajkumar, G.; Jayasinghe, M.R.; Vinotha, S. Comparative analytical study of phytochemicals in selected antidiabetic medicinal plant seeds in Sri Lanka. Pharm. Sci. Res. 2021, 8, 145–155. [Google Scholar]
- Manohar, M. Ayurveda for All; V&S Publishers: Delhi, India, 2012. [Google Scholar]
- Pandhi, S.; Poonia, A. Phytochemical screening of Jamun seeds using different extraction methods. Pharma Innov. 2019, 8, 226–231. [Google Scholar]
- Hasanuzzaman, M.; Islam, W.; Islam, M. Phytochemical screening of Syzygium cumini (L.) extracts in different solvents. J. Bio-Sci. 2016, 24, 11–18. [Google Scholar] [CrossRef]
- Nobori, T.; Miura, K.; Wu, D.J.; Lois, A.; Takabayashi, K.; Carson, D.A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 1994, 368, 753–756. [Google Scholar] [CrossRef]
- Yarnell, E.; Abascal, K.; Rountree, R. Clinical Botanical Medicine; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2009. [Google Scholar]
- Chaudhuri, A.N.; Pal, S.; Gomes, A.; Bhattacharya, S. Anti-inflammatory and related actions of Syzygium cuminii seed extract. Phytother. Res. 1990, 4, 5–10. [Google Scholar] [CrossRef]
- Prince, P.S.M.; Menon, V.P. Effect of Syzigium cumini in plasma antioxidants on alloxan-induced diabetes in rats. J. Clin. Biochem. Nutr. 1998, 25, 81–86. [Google Scholar] [CrossRef]
- Srivastava, Y.; Bhatt, H.; Gupta, O.; Gupta, P. Hypoglycemia induced by Syzygium cumini Linn. seeds in diabetes mellitus. Asian Med. J. 1983, 26, 489–492. [Google Scholar]
- Ratsimamanga, A.; Loiseau, A.; Ratsimamanga-Urverg, S.; Bibal-Prot, P. Action of a hypoglycemic agent found in the young bark of Eugenia jambolania (Myrtacea) on induced hyperglycemia of the rabbit and continuation of its purification. Comptes Rendus Hebd. Seances L’academie Sci. 1973, 277, 2219–2222. [Google Scholar]
- Kapoor, L. CRC Handbook of Ayurvedic Plants; CRC Press: Boca Raton, FL, USA, 1990; p. 183. [Google Scholar]
- Morton, J.F. Fruits of Warm Climates; Echo Point Books & Media: Brattleboro, VT, USA, 1987. [Google Scholar]
- Arya, S.; Pegu, K.; Sadawarte, P. Bioactive compounds and health benefits of jamun (Syzygium cumini). In Bioactive Molecules in Food. Reference Series in Phytochemistry; Springer Publications: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Mathur, A. A comparative physicochemical analysis of crude and refined jamun (Syzygium cumini) seed oil. Iconic Res. Eng. J. 2017, 1, 1–5. [Google Scholar]
- Hewavitharana, G.G.; Perera, D.N.; Navaratne, S.B.; Wickramasinghe, I. Extraction methods of fat from food samples and preparation of fatty acid methyl esters for gas chromatography: A review. Arab. J. Chem. 2020, 13, 6865–6875. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Raza, A.; Saif-ul-Malook; Shahzad, N.; Qasrani, S.A.; Sharif, M.N.; Akram, M.N.; Ali, M.U. Extraction of Bioactive Components from the Fruit and Seed of Jamun (Syzygium cumini) Through Conventional Solvent Extraction Method. Am.-Eurasian J. Agric. Environ. Sci. 2015, 15, 991–996. [Google Scholar]
- Mathur, A. Extraction and characterization of Syzygium cumini (Jamun) seed oil. Int. J. Adv. Res. Eng. Sci. Technol. 2015, 2, 2394–2444. [Google Scholar]
- Abdul Jaleel, A.H.; Mahdi, J.F.; Farooqui, M. Gas chromatography-mass spectroscopic analysis of black plum seed (Syzygium cumini) extract in hexane. Asian J. Pharm. Clin. Res. 2019, 12, 219–222. [Google Scholar] [CrossRef]
- Balyan, U.; Sarkar, B. Ultrafiltration of Syzygium cumini (L.) seeds extract: Analysis of flux decline and extract stability. Asia-Pac. J. Chem. Eng. 2018, 13, e2166. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K. Microwave assisted extraction and characterization of polysaccharide from waste jamun fruit seeds. Int. J. Biol. Macromol. 2020, 152, 1157–1163. [Google Scholar] [CrossRef]
- Do Carmo Brito, B.N.; da Silva Pena, R.; Santos Lopes, A.; Campos Chisté, R. Anthocyanins of Jambolao (Syzygium cumini): Extraction and pH-Dependent Color Changes. J. Food Sci. 2017, 82, 2286–2290. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).