Characterization, Antioxidant Potential, and Pharmacokinetics Properties of Phenolic Compounds from Native Australian Herbs and Fruits
Abstract
1. Introduction
2. Results and Discussion
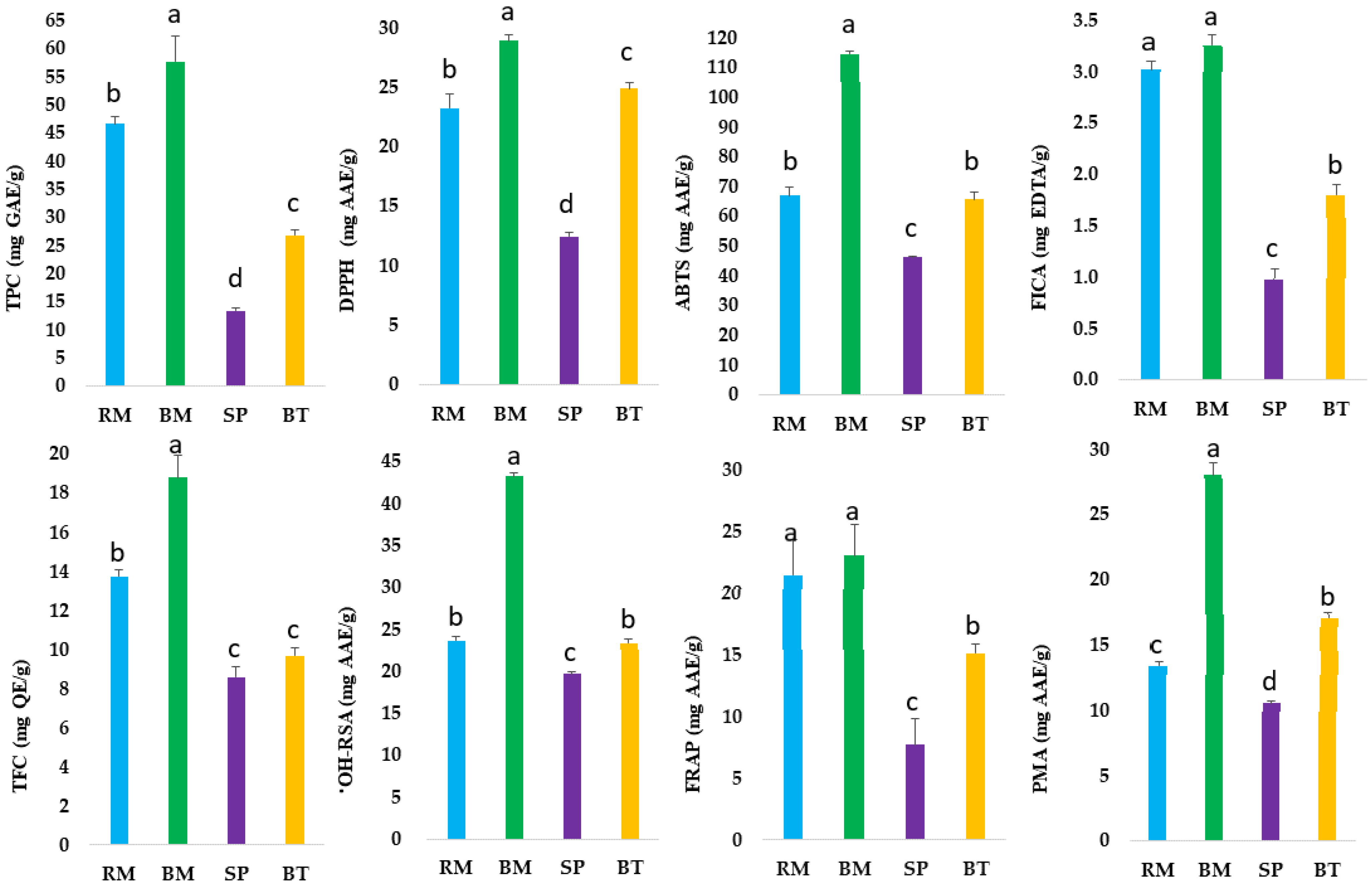
2.1. Estimation of Total Polyphenols and Total Flavonoids
2.2. Antioxidant Potential of Australian Native Herbs and Medicinal Plants
2.3. Correlation of Total Phenolic Content and Antioxidant Activities
2.4. LC-MS/MS Analysis
2.4.1. Phenolic Acids
2.4.2. Flavonoids
2.4.3. Stilbenes and Lignans
2.4.4. Other Compounds
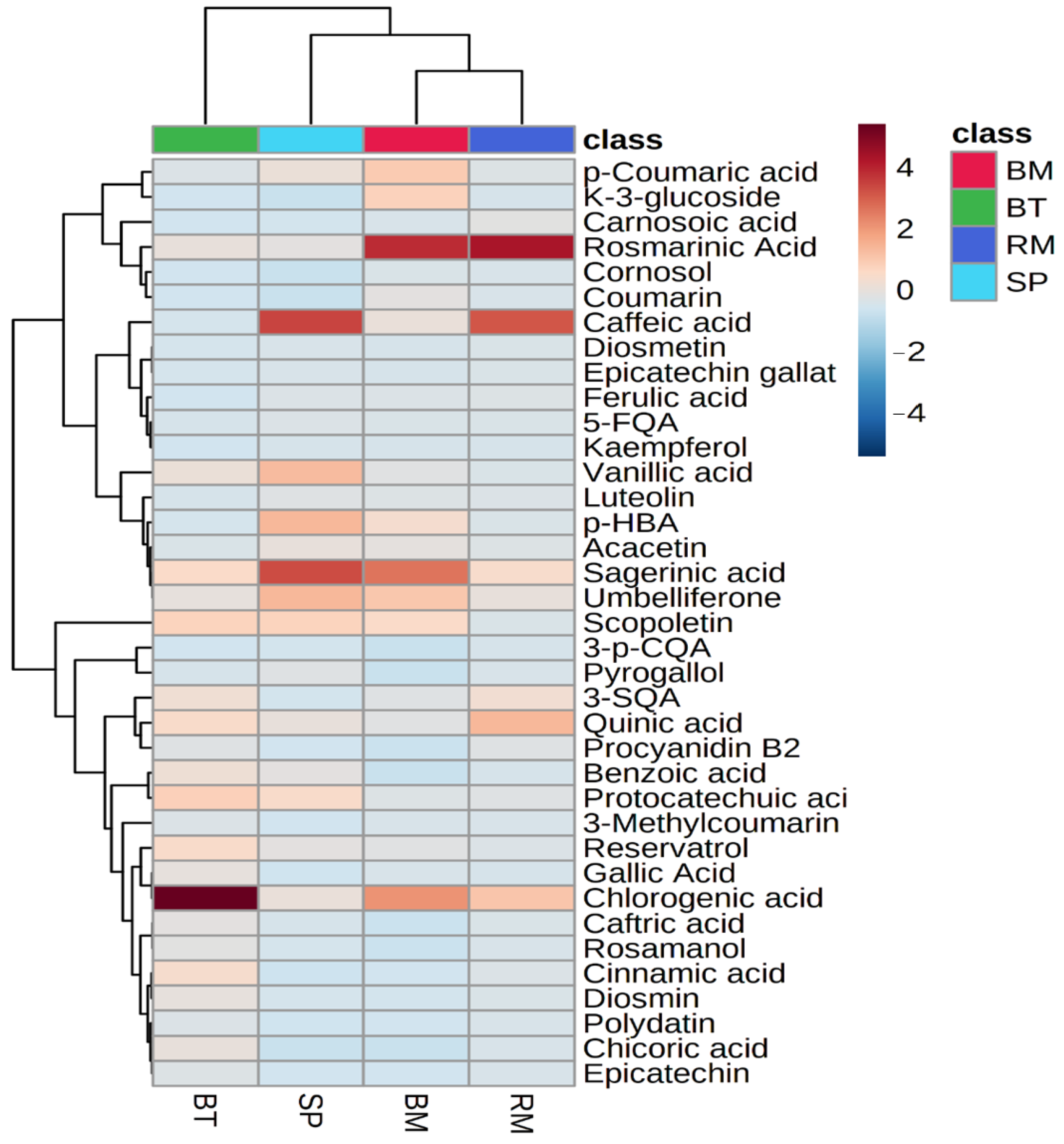
2.5. Distribution of Bioactive Phenolic Metabolites in Selected Native Australian Plants
2.6. LC-MS/MS Quantification/Semi-Quantification of Individual Phenolic Metabolites
2.6.1. Phenolic Acids
2.6.2. Flavonoids
2.6.3. Other Polyphenols
2.6.4. Heatmap and Hierarchical Clustering of Quantified Phenolic Metabolites
2.7. Pharmacokinetics Properties of the Abundant Phenolic Metabolites
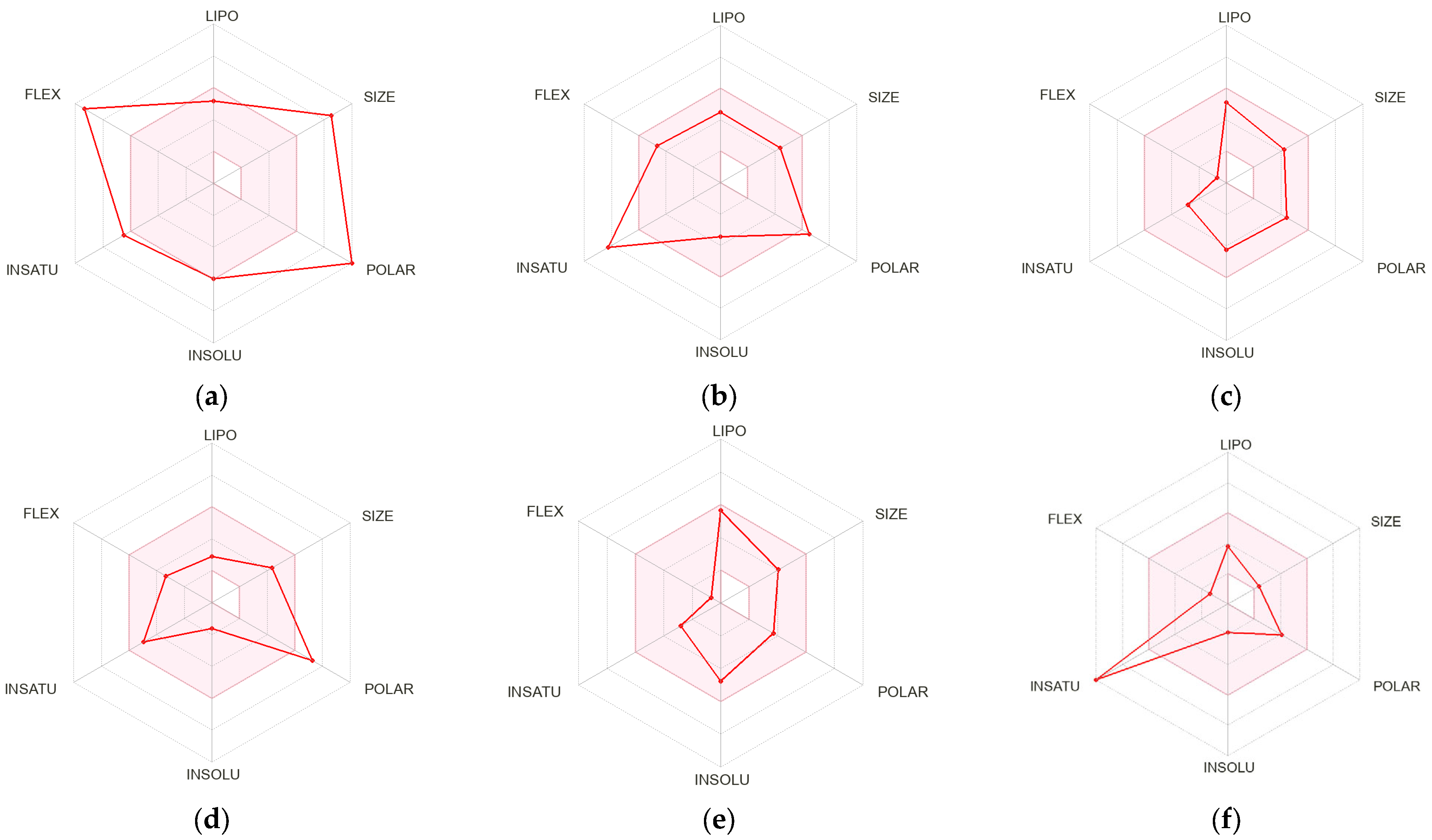
2.7.1. Predicted Absorption and Distribution of Phenolic Compounds
2.7.2. Drug Likeness
2.7.3. Metabolism, Excretion, and Toxicity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Extraction Process of Phenolic Compounds
3.3. Polyphenols Estimation and Their Antioxidant Activities
3.3.1. Quantification of TPC and TFC
3.3.2. Antioxidant Activities
3.4. LC-ESI-QTOF-MS/MS Analysis
3.5. Pharmacokinetics Study of the Most Abundant Phenolic Compounds
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Kiloni, S.M.; Cáceres-Vélez, P.R.; Jusuf, P.R.; Cottrell, J.J.; Dunshea, F.R. Phytochemicals, antioxidant activities, and toxicological screening of native australian fruits using zebrafish embryonic model. Foods 2022, 11, 4038. [Google Scholar] [CrossRef]
- Balentine, D.A.; Albano, M.C.; Nair, M.G. Role of medicinal plants, herbs, and spices in protecting human health. Nutr. Rev. 1999, 57, S41–S45. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Lc-ms/ms-qtof screening and identification of phenolic compounds from australian grown herbs and their antioxidant potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, J.J.; Le, H.H.; Artaiz, O.; Iqbal, Y.; Suleria, H.A.; Ali, A.; Celi, P.; Dunshea, F.R. Recent advances in the use of phytochemicals to manage gastrointestinal oxidative stress in poultry and pigs. Anim. Prod. Sci. 2022, 62, 1140–1146. [Google Scholar] [CrossRef]
- Kiani, H.S.; Ali, A.; Zahra, S.; Hassan, Z.U.; Kubra, K.T.; Azam, M.; Zahid, H.F. Phytochemical composition and pharmacological potential of lemongrass (cymbopogon) and impact on gut microbiota. AppliedChem 2022, 2, 229–246. [Google Scholar] [CrossRef]
- Zhong, L.; Bornman, J.F.; Wu, G.; Hornoff, A.; Dovi, K.A.P.; Al-Ali, H.; Aslam, N.; Johnson, S.K. The nutritional and phytochemical composition of the indigenous australian pindan walnut (terminalia cunninghamii) kernels. Plant Foods Hum. Nutr. 2018, 73, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive profiling of most widely used spices for their phenolic compounds through lc-esi-qtof-ms2 and their antioxidant potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef]
- Kumar, G.; Dey, S.K.; Kundu, S. Herbs and their bioactive ingredients in cardio-protection: Underlying molecular mechanisms and evidences from clinical studies. Phytomedicine 2021, 92, 153753. [Google Scholar] [CrossRef]
- Tapsell, L.C.; Hemphill, I.; Cobiac, L.; Patch, C.S.; Sullivan, D.R.; Fenech, M.; Roodenrys, S.; Keogh, J.B.; Clifton, P.M.; Williams, P.G.; et al. Health benefits of herbs and spices: The past, the present, the future. Med. J. Aust. 2006, 185, S1–S24. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S. A review on health benefits of phenolics derived from dietary spices. Curr. Res. Food Sci. 2022, 5, 1508–1523. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and phenolic compounds in commercially grown native australian herbs and spices. Food Chem. 2010, 122, 260–266. [Google Scholar] [CrossRef]
- Ali, A.; Ponnampalam, E.N.; Pushpakumara, G.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Cinnamon: A natural feed additive for poultry health and production-a review. Animals 2021, 11, 2026. [Google Scholar] [CrossRef] [PubMed]
- Kiselova-Kaneva, Y.; Galunska, B.; Nikolova, M.; Dincheva, I.; Badjakov, I. High resolution lc-ms/ms characterization of polyphenolic composition and evaluation of antioxidant activity of sambucus ebulus fruit tea traditionally used in bulgaria as a functional food. Food Chem. 2022, 367, 130759. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Antioxidant, alpha-glucosidase inhibition activities, in silico molecular docking and pharmacokinetics study of phenolic compounds from native australian fruits and spices. Antioxidants 2023, 12, 254. [Google Scholar] [CrossRef]
- Cáceres-Vélez, P.R.; Ali, A.; Fournier-Level, A.; Dunshea, F.R.; Jusuf, P.R. Phytochemical and safety evaluations of finger lime, mountain pepper, and tamarind in zebrafish embryos. Antioxidants 2022, 11, 1280. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capacity of 68 chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Sommano, S.; Caffin, N.; Kerven, G. Screening for antioxidant activity, phenolic content, and flavonoids from australian native food plants. Int. J. Food Prop. 2013, 16, 1394–1406. [Google Scholar] [CrossRef]
- Tang, K.S.C.; Konczak, I.; Zhao, J. Identification and quantification of phenolics in australian native mint (mentha australis r. Br.). Food Chem. 2016, 192, 698–705. [Google Scholar] [CrossRef]
- Kee, K.T.; Koh, M.; Oong, L.X.; Ng, K. Screening culinary herbs for antioxidant and α-glucosidase inhibitory activities. Int. J. Food Sci. Technol. 2013, 48, 1884–1891. [Google Scholar] [CrossRef]
- Tang, K.S.C.; Konczak, I.; Zhao, J. Phenolic compounds of the australian native herb prostanthera rotundifolia and their biological activities. Food Chem. 2017, 233, 530–539. [Google Scholar] [CrossRef]
- Chanput, W.; Krueyos, N.; Ritthiruangdej, P. Anti-oxidative assays as markers for anti-inflammatory activity of flavonoids. Int. Immunopharmacol. 2016, 40, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-H.; Shin, H.-J. Anti-oxidative and anti-melnogenesis effects of buleberry extract. Kor. J. Aesthet Cosmetol. 2015, 13, 261–266. [Google Scholar]
- Lee, S.-H.; Jang, M.; Kim, G.-H. Antioxidative effects of extracts from different parts of epimedium koreanum nakai. J. Korean Soc. Food Sci. Nutr. 2016, 45, 188–193. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of caffeic acid (3, 4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Tarwadi, K.; Agte, V. Antioxidant and micronutrient quality of fruit and root vegetables from the indian subcontinent and their comparative performance with green leafy vegetables and fruits. J. Sci. Food Agric. 2005, 85, 1469–1476. [Google Scholar] [CrossRef]
- Kim, S.Y. Fluctuations in phenolic content and antioxidant capacity of green vegetable juices during refrigerated storage. Prev. Nutr. Food Sci. 2015, 20, 169. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of abts/dpph assays to measure antioxidant capacity in popular antioxidant-rich us foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Freeman, B.L.; Eggett, D.L.; Parker, T.L. Synergistic and antagonistic interactions of phenolic compounds found in navel oranges. J. Food Sci. 2010, 75, C570–C576. [Google Scholar] [CrossRef]
- El-Akad, R.H.; El-Din, M.G.S.; Farag, M.A. How does lagenaria siceraria (bottle gourd) metabolome compare to cucumis sativus (cucumber) f. Cucurbitaceae? A multiplex approach of hr-uplc/ms/ms and gc/ms using molecular networking and chemometrics. Foods 2023, 12, 771. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (rosmarinus officinalis l.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic acid-human pharmacokinetics and health benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.I.; Jungfer, E.; Ritter, C.; Santiago-Schübel, B.; Thiele, B.; Fett, R.; Galensa, R. Characterization of flavan-3-ols in seeds of grape pomace by ce, hplc-dad-msn and lc-esi-fticr-ms. Food Res. Int. 2012, 48, 848–855. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. Uflc-q-tof-ms/ms-based screening and identification of flavonoids and derived metabolites in human urine after oral administration of exocarpium citri grandis extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef]
- Chou, O.; Ali, A.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof-ms/ms characterisation of phenolics in herbal tea infusion and their antioxidant potential. Fermentation 2021, 7, 73. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.; Wang, H.; Fu, Z.; Wen, Q.; Chang, H.; Huang, X. Comparison of different methods for extracting polyphenols from ipomoea batatas leaves, and identification of antioxidant constituents by hplc-qtof-ms2. Food Res. Int. 2015, 70, 101–109. [Google Scholar] [CrossRef]
- Hanhineva, K.; Rogachev, I.; Aura, A.-M.; Aharoni, A.; Poutanen, K.; Mykkänen, H. Identification of novel lignans in the whole grain rye bran by non-targeted lc–ms metabolite profiling. Metabolomics 2012, 8, 399–409. [Google Scholar] [CrossRef]
- Velamuri, R.; Sharma, Y.; Fagan, J.; Schaefer, J. Application of uhplc-esi-qtof-ms in phytochemical profiling of sage (salvia officinalis) and rosemary (rosmarinus officinalis). Planta Med. Int. Open 2020, 7, e133–e144. [Google Scholar] [CrossRef]
- Serrano, C.A.; Villena, G.K.; Rodríguez, E.F. Phytochemical profile and rosmarinic acid purification from two peruvian lepechinia willd. Species (salviinae, mentheae, lamiaceae). Sci. Rep. 2021, 11, 7260. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yeap Foo, L. Antioxidant activities of polyphenols from sage (salvia officinalis). Food Chem. 2001, 75, 197–202. [Google Scholar] [CrossRef]
- Kitts, D.D.; Yuan, Y.V.; Wijewickreme, A.N.; Thompson, L.U. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol. Cell. Biochem. 1999, 202, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fei, Y.J.; Cao, X.H.; Xu, D.; Tang, W.J.; Yang, K.; Xu, W.X.; Tang, J.H. Lignans intake and enterolactone concentration and prognosis of breast cancer: A systematic review and meta-analysis. J. Cancer 2021, 12, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gan, C.; Wang, Z.; Liu, L.; Gao, M.; Li, Q.; Yang, C. Determination and pharmacokinetic study of three diterpenes in rat plasma by uhplc-esi-ms/ms after oral administration of rosmarinus officinalis l. Extract. Molecules 2017, 22, 934. [Google Scholar] [CrossRef]
- Zabot, G.L.; Moraes, M.N.; Rostagno, M.A.; Meireles, M.A.A. Fast analysis of phenolic terpenes by high-performance liquid chromatography using a fused-core column. Anal. Methods 2014, 6, 7457–7468. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by hplc. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Ali, A.; Zahid, H.F.; Cottrell, J.J.; Dunshea, F.R. A comparative study for nutritional and phytochemical profiling of coffea arabica (c. Arabica) from different origins and their antioxidant potential and molecular docking. Molecules 2022, 27, 5126. [Google Scholar] [CrossRef]
- Kaldas, M.I.; Walle, U.K.; Walle, T. Resveratrol transport and metabolism by human intestinal caco-2 cells. J. Pharm. Pharmacol. 2003, 55, 307–312. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Noumi, E.; Belaabed, S.; Aouadi, K.; Lamjed, B.; Adnan, M.; Defant, A.; Kadri, A.; Snoussi, M.; Khan, M.A.; et al. Lc-esi/ms-phytochemical profiling with antioxidant, antibacterial, antifungal, antiviral and in silico pharmacological properties of algerian asphodelus tenuifolius (cav.) organic extracts. Antioxidants 2021, 10, 628. [Google Scholar] [CrossRef]
- Hase, T.; Shishido, S.; Yamamoto, S.; Yamashita, R.; Nukima, H.; Taira, S.; Toyoda, T.; Abe, K.; Hamaguchi, T.; Ono, K. Rosmarinic acid suppresses alzheimer’s disease development by reducing amyloid β aggregation by increasing monoamine secretion. Sci. Rep. 2019, 9, 8711. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of rosmarinic acid in intestinal caco-2 cell monolayers. Biosci. Biotechnol. Biochem. 2005, 69, 583–591. [Google Scholar] [CrossRef]
- Konishi, Y.; Hitomi, Y.; Yoshida, M.; Yoshioka, E. Pharmacokinetic study of caffeic and rosmarinic acids in rats after oral administration. J. Agric. Food Chem. 2005, 53, 4740–4746. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary flavonoids: Cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of phenolics profile in freeze-dried apple peel and their bioactivities during in vitro digestion and colonic fermentation. Int. J. Mol. Sci. 2023, 24, 1514. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. Swissadme: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Osakabe, N.; Sanbongi, C.; Yanagisawa, R.; Inoue, K.; Yasuda, A.; Natsume, M.; Baba, S.; Ichiishi, E.; Yoshikawa, T. Extract of perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp. Biol. Med. (Maywood) 2004, 229, 247–254. [Google Scholar] [CrossRef]
- Herrlinger, K.A.; Nieman, K.M.; Sanoshy, K.D.; Fonseca, B.A.; Lasrado, J.A.; Schild, A.L.; Maki, K.C.; Wesnes, K.A.; Ceddia, M.A. Spearmint extract improves working memory in men and women with age-associated memory impairment. J. Altern. Complement. Med. 2018, 24, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and characterization of anthocyanins and non-anthocyanin phenolics from australian native fruits and their antioxidant, antidiabetic, and anti-alzheimer potential. Food Res. Int. 2022, 162, 111951. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Lc-ms/ms characterization of phenolic metabolites and their antioxidant activities from australian native plants. Metabolites 2022, 12, 1016. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Dunshea, F.R.; Ajlouni, S. In vitro bioaccessibility of phenolic compounds and alpha-glucosidase inhibition activity in yoghurts enriched with mango peel powder. Food Biosci. 2022, 50, 102011. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Song, S.; Ali, A.; Subbiah, V.; Taheri, Y.; Suleria, H.A.R. Lc-esi-qtof-ms/ms characterization of phenolic compounds from pyracantha coccinea m. Roem. And their antioxidant capacity. Cell. Mol. Biol. 2021, 67, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Bashmil, Y.M.; Ali, A.; BK, A.; Dunshea, F.R.; Suleria, H.A.R. Screening and characterization of phenolic compounds from australian grown bananas and their antioxidant capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]


| Variables | TPC | TFC | DPPH | ABTS | FRAP | PMA | •OH-RSA |
|---|---|---|---|---|---|---|---|
| TFC | 0.96 ** | ||||||
| DPPH | 0.84 | 0.76 | |||||
| ABTS | 0.45 | 0.46 | 0.80 | ||||
| FRAP | 0.96 ** | 0.85 | 0.91* | 0.50 | |||
| PMA | 0.76 | 0.84 | 0.82 | 0.84 | 0.68 | ||
| •OH-RSA | 0.81 | 0.92 * | 0.74 | 0.68 | 0.69 | 0.97 ** | |
| FICA | 0.99 ** | 0.91 * | 0.83 | 0.38 | 0.98 ** | 0.67 | 0.72 |
| No. | RT (min) | Mode of Ionization | Theoretical (m/z) | Observed (m/z) | Mass Error (ppm) | MS/MS Productions | Molecular Formula | Proposed Compounds | Herbs and Fruits |
|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||||
| Hydroxybenzoic acids | |||||||||
| 1 | 6.624 | [M−H]− | 331.0671 | 331.0682 | 3.3 | 169, 151, 125 | C13H16O10 | Gallic acid 4-O-glucoside | RM |
| 2 | 7.054 | ** [M−H]− | 169.0142 | 169.0134 | −4.7 | 125 | C7H6O5 | * Gallic acid | BT, BM, SP |
| 3 | 8.447 | [M−H]− | 315.0721 | 315.0715 | −1.9 | 153, 109 | C13H16O9 | Protocatechuic acid 4-O-glucoside | SP, BM, BT |
| 4 | 10.323 | [M−H]− | 167.0350 | 167.0350 | 0.0 | 152, 123, 108 | C8H8O4 | * Vanillic acid | BM, SP, BT |
| 5 | 12.718 | [M−H]− | 153.0193 | 153.0193 | 0.0 | 109 | C7H6O4 | Protocatechuic acid | BM, SP, BT, RM |
| 6 | 13.279 | [M−H]− | 299.0772 | 299.0788 | 5.3 | 255, 137 | C13H16O8 | 4-Hydroxybenzoic acid 4-O-glucoside | BM, BT |
| 7 | 16.152 | [M−H]− | 137.0244 | 137.0248 | 2.9 | 93, 65 | C7H6O3 | p-Hydroxybenzoic acid | SP, BM, BT |
| 8 | 17.753 | [M−H]− | 121.0295 | 121.0295 | 0.0 | 103, 77 | C7H6O2 | * Benzoic acid | BT, RM, SP, BM |
| Hydroxycinnamic acids | |||||||||
| 9 | 4.122 | [M−H]− | 191.0561 | 191.0567 | 3.1 | 171, 127, 85 | C7H12O6 | Quinic acid | BT, RM, SP, BM |
| 10 | 6.152 | [M−H]− | 311.0408 | 311.0411 | 0.9 | 267, 179, 135 | C13H12O9 | Caftaric acid | BT |
| 11 | 7.342 | ** [M−H]− | 355.0671 | 355.0673 | 0.6 | 179, 135 | C15H16O10 | Caffeic acid 3-O-glucuronide | BT, BM, SP, RM |
| 12 | 12.816 | [M−H]− | 723.2142 | 723.2167 | 3.5 | 529, 499 | C33H40O18 | 1-Sinapoyl-2-feruloylgentiobiose | BT, SP |
| 13 | 13.212 | [M−H]− | 369.0827 | 369.0832 | 1.4 | 193, 178, 134 | C16H18O10 | Ferulic acid 4-O-glucuronide | BT, BM |
| 14 | 14.722 | ** [M−H]− | 325.0565 | 325.0571 | 1.8 | 193, 149 | C14H14O9 | Feruloyl tartaric acid | RM, BM |
| 15 | 15.595 | ** [M−H]− | 325.0929 | 325.0933 | 1.2 | 163, 119 | C15H18O8 | p-Coumaric acid 4-O-glucoside | BM, BT, RM, SP |
| 16 | 16.282 | [M−H]− | 355.1034 | 355.1037 | 0.8 | 193, 176, 161, 134 | C16H20O9 | Ferulic acid 4-glucoside | BM, RM, BT |
| 17 | 16.611 | [M−H]− | 223.0612 | 223.0608 | −1.8 | 193, 179, 149, 134 | C11H12O5 | * Sinapic acid | BT |
| 18 | 16.733 | ** [M−H]− | 341.0878 | 341.0872 | −1.8 | 179 | C15H18O9 | Caffeic acid 4-O-glucoside | BM, SP, BT |
| 19 | 17.234 | ** [M−H]− | 337.0929 | 337.0921 | −2.4 | 191, 119 | C16H18O8 | 3-p-Coumaroylquinic acid | BT, BM, SP |
| 20 | 17.431 | [M−H]− | 385.1140 | 385.1144 | 1.0 | 223, 193 | C17H22O10 | 1-O-Sinapoyl-ꞵ-d-glucose | RM, SP, BM, BT |
| 21 | 17.558 | ** [M−H]− | 295.0459 | 295.0473 | 4.7 | 115 | C13H12O8 | p-Coumaroyl tartaric acid | RM, SP, BM, BT |
| 22 | 17.608 | [M−H]− | 163.0400 | 163.0395 | −3.1 | 119 | C9H8O3 | * p-Coumaric acid | RM, BM, SP, BT |
| 23 | 17.619 | [M−H]− | 193.0506 | 193.0504 | −1.0 | 178, 149, 134 | C10H10O4 | * Ferulic acid | RM, SP |
| 24 | 19.625 | [M−H]− | 367.1034 | 367.1036 | 0.5 | 191 | C17H20O9 | 3-Feruloylquinic acid | BT, RM |
| 25 | 19.701 | [M−H]− | 197.0450 | 197.0439 | −5.7 | 182, 153, 138, 121 | C9H10O5 | * Syringic acid | BM, BT |
| 26 | 21.648 | [M−H]− | 147.0451 | 147.0451 | 0.0 | 129, 103 | C9H8O2 | * Cinnamic acid | BM, RM, BT |
| 27 | 23.958 | [M−H]− | 397.1140 | 397.1152 | 3.0 | 223, 191 | C18H22O10 | 3-Sinapoylquinic acid | BT, RM |
| 28 | 27.531 | ** [M−H]− | 353.0878 | 353.0873 | −1.4 | 191, 179, 161, 135 | C16H18O9 | * 3-Caffeoylquinic acid | SP, BT, BM, RM |
| 29 | 27.531 | ** [M−H]− | 515.1195 | 515.1196 | 0.2 | 191, 179, 135 | C25H24O12 | 1,5-Dicaffeoylquinic acid | SP, BM |
| 30 | 29.235 | [M−H]− | 179.0350 | 179.0353 | 1.7 | 135 | C9H8O4 | * Caffeic acid | BT, BM, SP, RM |
| 31 | 29.423 | [M−H]− | 473.0725 | 473.0748 | 4.9 | 293, 311 | C22H18O12 | Chicoric acid | RM, BT |
| 32 | 30.095 | [M−H]− | 959.2826 | 959.2826 | 0.0 | 887, 223, 207, 163 | C45H52O23 | 1,2,2′-Trisinapoylgentiobiose | BT, BM |
| 33 | 30.671 | [M−H]− | 359.0772 | 359.0770 | −0.6 | 197, 179, 161, 135 | C18H16O8 | * Rosmarinic acid | BM, BT, SP, RM |
| 34 | 32.125 | [M−H]− | 543.1508 | 543.1502 | −1.1 | 193, 191, 134 | C27H28O12 | 3,5-Diferuloylquinic acid | SP, BM, BT, RM |
| 35 | 34.042 | [M−H]− | 693.2036 | 693.2037 | 0.1 | 193, 134 | C32H38O17 | 1,2-Diferuloylgentiobiose | BM, SP, BT |
| Flavonoids | |||||||||
| Flavanols | |||||||||
| 36 | 17.181 | [M−H]− | 577.1351 | 577.1353 | 0.3 | 451, 425, 407, 289 | C30H26O12 | * Procyanidin dimer B2 | BT, RM |
| 37 | 17.465 | ** [M−H]− | 289.0717 | 289.0704 | −4.5 | 245, 205, 179 | C15H14O6 | * Epicatechin | BT, BM |
| 38 | 19.595 | [M−H]− | 865.1985 | 865.2004 | 2.2 | 739, 713, 695, 577, 451 | C45H38O18 | Procyanidin trimer C1 | BM |
| 39 | 19.625 | ** [M−H]− | 481.0987 | 481.0999 | 2.5 | 305 | C21H22O13 | (-)-Epigallocatechin 3′-O-glucuronide | BT, SP, BM |
| 40 | 21.869 | [M−H]− | 451.1246 | 451.1250 | 0.9 | 289, 245 | C21H24O11 | Catechin 3′-glucoside | BM, BT, SP |
| 41 | 22.699 | [M−H]− | 1153.2619 | 1153.2599 | −1.7 | 1135, 577, 289, 125 | C60H50O24 | Cinnamtannin A2 | BM |
| 42 | 25.918 | [M−H]− | 609.1250 | 609.1262 | 2.0 | 591, 539 | C30H26O14 | Prodelphinidin dimer B3 | SP, RM, BT, BM |
| Flavanones | |||||||||
| 43 | 18.43 | ** [M−H]− | 477.1038 | 477.1040 | 0.4 | 301 | C22H22O12 | Hesperetin 3′-O-glucuronide | SP, BM, BT |
| 44 | 19.782 | [M−H]− | 595.1668 | 595.1665 | −0.5 | 459, 287, 151 | C27H32O15 | Neoeriocitrin | BT, BM, RM |
| 45 | 20.855 | [M−H]− | 433.1140 | 433.1139 | −0.2 | 271 | C21H22O10 | Naringenin 7-O-glucoside | BT |
| 46 | 22.011 | [M−H]− | 407.1864 | 407.1881 | 4.2 | 287, 243, 159, 119 | C25H28O5 | 6-Geranylnaringenin | BT |
| 47 | 24.293 | [M−H]− | 579.1719 | 579.1716 | −0.5 | 459, 313, 271 | C27H32O14 | Naringin | RM, BT, SP, BM |
| 48 | 39.991 | [M−H]− | 741.2247 | 741.2249 | 0.3 | 579 | C33H42O19 | Narirutin 4′-O-glucoside | BM, RM, BT, SP |
| 49 | 52.783 | [M−H]− | 285.0768 | 285.0765 | −1.0 | 243, 164, 151, 136 | C16H14O5 | Isosakuranetin | RM, BT |
| 50 | 65.819 | ** [M+H]+ | 611.1971 | 611.1974 | 0.5 | 303 | C28H34O15 | Hesperidin | BM, BT, RM |
| Flavones | |||||||||
| 51 | 4.268 | [M−H]− | 637.1774 | 637.1754 | −3.1 | 329 | C29H34O16 | Tricin 7-neohesperidoside | SP, BT |
| 52 | 4.911 | ** [M−H]− | 505.0987 | 505.1003 | 3.2 | 329 | C23H22O13 | Tricin 7-O-glucuronide | BM, RM |
| 53 | 18.274 | ** [M−H]− | 637.1046 | 637.1044 | −0.3 | 285 | C27H26O18 | Luteolin 7-O-diglucuronide | BM, RM, BT |
| 54 | 18.905 | [M−H]− | 285.0404 | 285.0418 | 4.8 | 151 | C15H10O6 | Kaempferol | BM, BT |
| 55 | 19.447 | ** [M−H]− | 343.0823 | 343.0814 | −2.6 | 327, 255, 241 | C18H16O7 | Cirsilineol | BT, RM, BM |
| 56 | 21.165 | [M−H]− | 577.1563 | 577.1555 | −1.4 | 431, 269 | C27H30O14 | Rhoifolin | BT, BM, RM |
| 57 | 25.466 | [M−H]− | 445.1140 | 445.1134 | −1.3 | 325, 297, 282 | C22H22O10 | * Swertisin | BT, RM |
| 58 | 25.83 | ** [M−H]− | 285.0404 | 285.0403 | −0.4 | 177, 151, 119 | C15H10O6 | 3,4′,7-Tetrahydroxyflavone | BT, BM, SP, RM |
| 59 | 27.21 | ** [M−H]− | 593.1512 | 593.1513 | 0.2 | 449, 287 | C27H30O15 | Apigenin 6,8-di-C-glucoside | BT, BM, SP |
| 60 | 27.29 | ** [M−H]− | 607.1668 | 607.1668 | 0 | 300, 299 | C28H32O15 | * Diosmin | BT, RM, SP, BM |
| 61 | 29.297 | ** [M−H]− | 461.1089 | 461.1097 | 1.7 | 299 | C22H22O11 | Chrysoeriol 7-O-glucoside | BT, SP, RM |
| 62 | 32.909 | [M−H]− | 431.0983 | 431.0993 | 2.3 | 269 | C21H20O10 | Apigenin 6-C-glucoside | BT, RM |
| 63 | 41.946 | [M−H]− | 299.0561 | 299.0601 | 13.3 | 284 | C16H12O6 | * Diosmetin | RM |
| 64 | 50.821 | [M−H]− | 343.0823 | 343.0809 | −4.1 | 328, 313 | C18H16O7 | Santin | BM |
| 65 | 52.842 | [M−H]− | 255.0658 | 255.0697 | 15.5 | 213, 171 | C15H12O4 | 5,7-Dihydroxyflavanone | BM |
| 66 | 53.416 | [M−H]− | 253.0506 | 253.0507 | 0.4 | 235, 151 | C15H10O4 | * Chrysin | BM |
| 67 | 54.173 | [M−H]− | 283.0612 | 283.0642 | 10.7 | 268 | C16H12O5 | Wogonin | RM |
| 68 | 68.447 | [M+H]+ | 255.0652 | 255.0656 | 1.6 | 213, 137, 119 | C15H10O4 | 7,4′-Dihydroxyflavone | SP |
| 69 | 69.137 | [M+H]+ | 303.0499 | 303.0493 | −2.0 | 285, 169 | C15H10O7 | 6-Hydroxyluteolin | RM |
| 70 | 21.687 | [M−H]− | 283.0611 | 283.0639 | 9.9 | 268 | C16H12O5 | Acacetin | BT, BM |
| 71 | 69.147 | [M+H]+ | 317.0656 | 317.0652 | −1.3 | 302 | C16H12O7 | 8-Methoxyluteolin | BM |
| 72 | 69.48 | [M+H]+ | 549.1239 | 549.1258 | 3.5 | 531, 401, 301 | C25H24O14 | Chrysoeriol 7-O-(6″-malonyl-glucoside) | SP, BM, BT |
| Flavonols | |||||||||
| 73 | 6.656 | [M−H]− | 401.1242 | 401.1250 | 2.0 | 327, 209 | C21H22O8 | 3-Methoxysinensetin | BT, RM |
| 74 | 13.212 | [M−H]− | 623.1617 | 623.1627 | 1.6 | 315 | C28H32O16 | Isorhamnetin 3-O-rutinoside | BT, SP, BM, RM |
| 75 | 13.358 | [M−H]− | 446.0854 | 446.0891 | 8.3 | 285 | C21H19O11 | Kaempferol 7-O-glucoside | RM |
| 76 | 13.844 | ** [M−H]− | 609.1097 | 609.1075 | −3.6 | 301 | C26H26O17 | Quercetin 3-O-xylosyl-glucuronide | RM, BT, BM |
| 77 | 14.873 | ** [M−H]− | 609.1461 | 609.1459 | −0.3 | 447, 285 | C27H30O16 | Kaempferol 3,7-O-diglucoside | BM, RM, SP, BT |
| 78 | 19.099 | ** [M−H]− | 625.1410 | 625.1425 | 2.4 | 317 | C27H30O17 | Myricetin 3-O-rutinoside | BT, SP |
| 79 | 24.49 | ** [M−H]− | 461.0725 | 461.0715 | −2.2 | 285, 113, 85 | C21H18O12 | Kaempferol 3-O-glucuronide | BM, BT |
| 80 | 25.494 | [M−H]− | 491.0831 | 491.0818 | −2.6 | 315 | C22H20O13 | Isorhamnetin 3-O-glucuronide | BM |
| 81 | 27.848 | ** [M−H]− | 535.1093 | 535.1099 | 1.1 | 359 | C24H24O14 | Jaceidin 4′-O-glucuronide | BT, RM, BM |
| 82 | 28.879 | ** [M−H]− | 477.0674 | 477.0686 | 2.5 | 301 | C21H18O13 | Quercetin 4′-O-glucuronide | BT, RM, BM, SP |
| 83 | 31.203 | ** [M−H]− | 449.0725 | 449.0706 | −4.2 | 317 | C20H18O12 | Myricetin 3-O-arabinoside | BM, SP, BT |
| 84 | 31.977 | ** [M−H]− | 269.0455 | 269.0453 | −0.7 | 227, 151, 117 | C15H10O5 | Apigenin | BT, RM, BM |
| 85 | 37.395 | ** [M−H]− | 463.0882 | 463.0870 | −2.6 | 317 | C21H20O12 | Myricetin 3-O-rhamnoside | BT, RM |
| 86 | 44.404 | ** [M−H]− | 329.0667 | 329.0679 | 3.6 | 314, 299, 271 | C17H14O7 | 3,7-Dimethylquercetin | RM, BT, BM |
| Isoflavonoids | |||||||||
| 87 | 8.849 | ** [M−H]− | 315.0874 | 315.0866 | −2.5 | 300, 285, 135 | C17H16O6 | Violanone | RM, BM, SP, BT |
| 88 | 12.208 | [M−H]− | 329.1030 | 329.1033 | 0.9 | 285, 163 | C18H18O6 | 3′-O-Methylviolanone | SP, BT, BM |
| 89 | 15.877 | [M−H]− | 457.1140 | 457.1131 | −2.0 | 253 | C23H22O10 | 6″-O-Acetyldaidzin | BM, RM |
| 90 | 16.8 | ** [M−H]− | 517.0987 | 517.1011 | 4.6 | 271 | C24H22O13 | 6″-O-Malonylgenistin | BM, SP, RM, BT |
| 91 | 20.242 | [M−H]− | 269.0819 | 269.0830 | 4.1 | 253, 239, 223 | C16H14O4 | Dihydroformononetin | BM, RM |
| 92 | 21.949 | [M−H]− | 271.0976 | 271.0989 | 4.8 | 255, 149, 121 | C16H16O4 | 3′-O-Methylequol | BM, BT |
| 93 | 23.941 | [M−H]− | 591.1355 | 591.1357 | 0.3 | 415, 253 | C27H28O15 | Daidzin 4′-O-glucuronide | BM |
| 94 | 28.073 | ** [M−H]− | 531.1144 | 531.1175 | 5.8 | 283, 267 | C25H24O13 | 6″-O-Malonylglycitin | BT |
| 95 | 28.221 | ** [M−H]− | 459.0933 | 459.0933 | 0.0 | 441, 283, 267 | C22H20O11 | Glycitein 4′-O-glucuronide | BT |
| 96 | 28.286 | [M−H]− | 257.0819 | 257.0824 | 1.9 | 239, 135, 121 | C15H14O4 | 3′,4′,7-Trihydroxyisoflavan | BT, BM |
| 97 | 28.885 | [M−H]− | 487.1246 | 487.1262 | 3.3 | 283, 267, 59 | C24H24O11 | 6″-O-Acetylglycitin | RM, BT |
| 98 | 32.314 | [M−H]− | 299.0561 | 299.0558 | −1.0 | 284 | C16H12O6 | 3′-Hydroxymelanettin | BT, BM |
| 99 | 53.600 | [M−H]− | 283.0612 | 283.0601 | −3.8 | 268 | C16H12O5 | Biochanin A | BM |
| 100 | 54.173 | ** [M−H]− | 283.0612 | 283.0621 | 3.2 | 255 | C16H12O5 | 2′-Hydroxyformononetin | RM, BM, SP |
| 101 | 54.401 | [M−H]− | 285.0768 | 285.0767 | −0.4 | 269, 203, 175 | C16H14O5 | Dihydrobiochanin A | BT, RM, BM, SP |
| 102 | 56.229 | [M−H]− | 417.1191 | 417.1188 | −0.7 | 241 | C21H22O9 | Equol 7-O-glucuronide | RM, BT, BM |
| Stilbenes | |||||||||
| 103 | 4.630 | [M−H]− | 243.0663 | 243.0643 | −8.2 | 225, 201, 174, 159 | C14H12O4 | Piceatannol | SP, RM, BT |
| 104 | 26.569 | [M−H]− | 419.1347 | 419.1348 | 0.2 | 257, 241 | C21H24O9 | Rhaponticin | BT |
| 105 | 28.073 | [M−H]− | 227.0713 | 227.0709 | −1.8 | 211, 167, 127 | C14H12O3 | * Resveratrol | BT |
| Lignans | |||||||||
| 106 | 26.010 | [M−H]− | 719.1612 | 719.1610 | −0.3 | 360, 359, 197, 179, 161 | C36H32O16 | Sagerinic acid | BM |
| 107 | 29.042 | [M−H]− | 357.1343 | 357.1348 | 1.4 | 341, 327, 191, 151 | C20H22O6 | Pinoresinol | RM, BT, SP |
| 108 | 39.577 | [M−H]− | 557.2392 | 557.2392 | 0.0 | 539, 521, 509, 361 | C30H38O10 | Secoisolariciresinol-sesquilignan | SP, RM |
| 109 | 47.844 | [M−H]− | 361.1656 | 361.1661 | 1.4 | 346, 177, 165 | C20H26O6 | Secoisolariciresinol | RM |
| 110 | 49.423 | [M−H]− | 313.1081 | 313.1088 | 2.2 | 255 | C18H18O5 | 2-Hydroxyenterolactone | BT, RM |
| 111 | 56.027 | [M−H]− | 265.1234 | 265.1244 | 3.9 | 97 | C12H26O4S | Magnolol | BM |
| 112 | 69.400 | ** [M+H]+ | 299.1278 | 299.1279 | 0.3 | 281, 187, 165 | C18H18O4 | Enterolactone | RM, BM, SP |
| Other compounds | |||||||||
| 113 | 4.333 | [M−H]− | 191.0350 | 191.0355 | 2.6 | 175, 147 | C10H8O4 | Scopoletin | BM, SP, BT, RM |
| 114 | 12.917 | [M−H]− | 339.0721 | 339.0731 | 2.9 | 177 | C15H16O9 | Aesculin | BT, BM, RM |
| 115 | 18.146 | [M−H]− | 177.0193 | 177.0192 | −0.6 | 133, 105 | C9H6O4 | Aesculetin | BT, BM, RM |
| 116 | 19.445 | [M−H]− | 159.0451 | 159.0450 | −0.6 | 115 | C10H8O2 | 3-Methylcoumarin | BM |
| 117 | 30.753 | [M−H]− | 161.0244 | 161.0242 | −1.2 | 133 | C9H6O3 | Umbelliferone | BT, BM, SP, RM |
| 118 | 37.435 | [M−H]− | 177.0557 | 177.0557 | 0.0 | 133 | C10H10O3 | Mellein | BT |
| 119 | 39.116 | ** [M+H]+ | 147.0441 | 147.0441 | 0.0 | 103, 91 | C9H6O2 | Coumarin | RM, BM, BT |
| 120 | 50.542 | [M−H]− | 345.1707 | 345.1714 | 2.0 | 301 | C20H26O5 | Rosmanol | BT |
| 121 | 57.603 | [M−H]− | 329.1758 | 329.1770 | 3.6 | 285 | C20H26O4 | Carnosol | BM, RM |
| 122 | 61.237 | [M−H]− | 331.1915 | 331.1910 | −1.5 | 287 | C20H28O4 | Carnosic acid | RM, BM |
| 123 | 6.624 | [M−H]− | 125.0244 | 125.0251 | 5.6 | 107, 97, 79 | C6H6O3 | * Pyrogallol | RM, BT, SP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Cottrell, J.J.; Dunshea, F.R. Characterization, Antioxidant Potential, and Pharmacokinetics Properties of Phenolic Compounds from Native Australian Herbs and Fruits. Plants 2023, 12, 993. https://doi.org/10.3390/plants12050993
Ali A, Cottrell JJ, Dunshea FR. Characterization, Antioxidant Potential, and Pharmacokinetics Properties of Phenolic Compounds from Native Australian Herbs and Fruits. Plants. 2023; 12(5):993. https://doi.org/10.3390/plants12050993
Chicago/Turabian StyleAli, Akhtar, Jeremy J. Cottrell, and Frank R. Dunshea. 2023. "Characterization, Antioxidant Potential, and Pharmacokinetics Properties of Phenolic Compounds from Native Australian Herbs and Fruits" Plants 12, no. 5: 993. https://doi.org/10.3390/plants12050993
APA StyleAli, A., Cottrell, J. J., & Dunshea, F. R. (2023). Characterization, Antioxidant Potential, and Pharmacokinetics Properties of Phenolic Compounds from Native Australian Herbs and Fruits. Plants, 12(5), 993. https://doi.org/10.3390/plants12050993










