Abstract
The Tanacetum vulgare L. (Tansy) has several ethnobotanical uses, mostly related to the essential oil and sesquiterpene lactones, whereas information regarding other compounds is scarce. This research is designed to characterize the phenolic compounds (flavonoids, phenolic acids, and tannins) to analyze the thujone (which is toxic in high concentrations) content and to detect the antioxidant activity (DPPH assay) of extracts. The main highlights of our work provide a chemical profile of phenolic compounds of T. vulgare harvested from different regions of Latvia, as well as simultaneously support the ethnomedicinal uses for wild T. vulgare through the integration of phenolic compounds as one of the value constituents of leaves and flowers. The extraction yield was 18 to 20% for leaves and 8 to 16% for flowers. The total phenol content in the extracts of T. vulgare as well as their antioxidant activity was different between collection regions and the aerial parts ranging from 134 to 218 mg GAE/g and 32 to 182 mg L−1, respectively. A remarkable variation in the thujone (α + β) content (0.4% up to 6%) was detected in the extracts. T. vulgare leaf extracts were rich in tannins (up to 19%). According to the parameters detected, the extracts of T. vulgare could be considered promising for the development of new herbal products.
1. Introduction
Tanacetum vulgare L., commonly known as tansy, is an intensely aromatic plant of the Asteraceae family, native to Europe and Asia, where it grows along roadsides, hedges, and wastelands [1]. T. vulgare subspecies vulgare is a common plant in Latvia, and its range of distribution covers the whole country [2]. It has traditionally been used as a spicy additive for food, in cosmetics, and as a herbal remedy due to its biologically active compounds. Besides the scientifically important and closely related species, feverfew (T. parthenium) and dalmatian insect flower (T. cinariifolium), significant applications for common tansy have not yet been found [3].
T. vulgare has been used for centuries as a medicinal plant and flavoring herb, but in order to confirm its therapeutic value, detailed phytochemical and pharmacological analyses are still required [4,5].
Plant extracts and essential oil of T. vulgare are known for their biological activities, mainly antioxidant, antimicrobial, insecticidal, cytotoxic, antivirus, and anti-inflammatory activities [6,7]. The extracts of T. vulgare have been reported to have anticancer properties and an antiproliferative effect on human cervical adenocarcinoma (HeLa) cells in the micrograms range [6,8]. However, pharmaceutical activities are mainly attributable to specific compounds, such as sesquiterpene lactones, volatile oil, flavonoids, and phenolic acids [4,9,10]. The evaluation of the approximate composition of T. vulgare in scientific research focuses primarily on determining the composition and biological properties of essential oil, while information on other compounds is scarce [1,5,11,12,13].
Chemovariation is a well-known fact in Tanacetum species, which is encountered at the species level and at the subspecies level. Essential oil isolated from Tanacetum species has variable chemical constituents and is made of more than 200 compounds [14]. The non-volatile extractives of T. vulgare are mainly characterized as hydrophilic antioxidants [12]. So far, neither the basic composition of the leaves nor the tansy inflorescences have been analyzed. T. vulgare is considered a rich source of sterols (stigmasterol, campesterol, and cholesterol), non-volatile sesquiterpene lactones (tanacetine, parthenolide, and tanachine), flavonoids (including luteolin, quercetin, apigenin, and their glycosides), and phenolic acids (chlorogenic, caffeic, and dicaffeoylquinic acids) [9,15,16]. The high variation of secondary metabolites in T. vulgare still appears to be a problem for further research as well as the optimization and standardization of cultivation conditions.
The use of extracts and essential oil of T. vulgare is limited by the presence of toxic thujones [1,17]. Thujones are bioactive compounds with valuable medicinal properties; however, they are toxic at high concentrations [18,19]. Certain amounts of thujone are allowed in foods and other products in the European Union. Due to the toxic effects of thujones, tansy flower extracts are mainly used externally as antiparasitic agents [1].
The biological and pharmacological assays are reported to strongly depend not only on the morphological part of T. vulgare but also on the extraction solvents used [4,18,20]. Generally, hydroalcoholic extracts and infusions could be considered more active compared to lipophilic n-hexane extracts. In our investigation, we obtained hydroalcoholic extracts directly without any previous separation of lipophilic extractives. In all experiments, the crude extracts were used without any modification. An important advantage of using extracts versus isolated molecules is the presence of other molecules in the extract that can synergistically interact with the bioactive compound, potentiating its beneficial effect [21].
Identifying bioactive compounds is the initial step for drug discovery. Target-based screening is used mainly to identify compounds that modulate the activity. Firstly, the assays can be selected considering that structurally similar compounds have similar biological activity. However, it must be noted that similarity in structure does not always mean similar biological activity, since even a single change in a chiral center can cause significant changes in activity (e.g., the Thalidomide tragedy) [22]. The investigation of antioxidant properties is mandatory in almost every study of biological activities [21]. This is because oxidant stress caused by an excessive amount of oxidants is associated with a number of human ailments, which include but are not limited to cancer and the development of neurological disorders and cardiovascular diseases [23]. Our research is designed to identify the major chemical active groups, focusing on phenolic compounds as one of the most abundant phytochemicals with antioxidant properties.
The aim of this study was to determine and compare the chemical composition of the phenolic compounds and the thujone content, as well as the antioxidant activity of aqueous ethanol (50%, v/v) extracts of T. vulgare wild-growing in different regions of Latvia.
2. Results
2.1. Extraction Yield of T. vulgare
The dryness of the analyzed leaves, stems, and aerial parts of T. vulgare was even and averaged at 5.5%. The exception was tansy flowers with detected moisture up to 9%. Considering that tansy flowers are rich in essential oil and that the gravimetric method was applied, the mass loss could be attributed not only to water content but also to essential oil content.
Depending on the collection place, the leaf extracts had the highest extraction yields of 18 to 20% (w/w) per dry biomass of T. vulgare. The biomass samples of T. vulgare obtained by mixing an equal proportion of leaves or flowers together without sorting them according to their collection place were designated as mix samples. The flower extracts had greater variation in extract yields (8 to 16%, w/w), depending on the collection place (Figure 1), which could be explained by the higher content of volatile substances, mainly essential oil in the samples. This chemical divergence concerns both major and minor compounds.
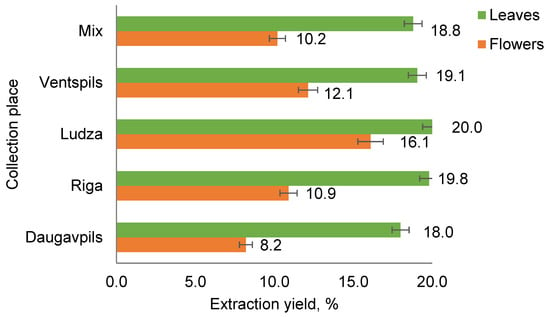
Figure 1.
Extraction yields of T. vulgare flowers and leaves collected from different places.
In order to evaluate the extraction yield of different morphological parts of wild T. vulgare, the sample harvested in Daugavpils was additionally separated into leaf, flower, and stem parts (Table 1).

Table 1.
Extraction yield of different morphological parts of wild T. vulgare harvested in Daugavpils, w/w (%).
The highest 50% aqueous ethanol extraction yield of T. vulgare was found in the leaves (18%), followed by the aerial part (14%), stems (11%), and flowers (8%).
2.2. Phenolic Profile of T. vulgare Extracts
Polyphenols are broad and diverse plant secondary metabolite groups. Their structures range from quite simple molecules, such as phenolic acids, to complex polymerized molecules such as tannins [24]. Furthermore, they appear in conjunction with various sugars in plants. Due to the chemical diversity of phenolic compounds and the complexity of the composition in plant samples, it would be costly and inefficient to separate each phenolic compound and study them individually [25]. However, it is important to identify the main groups and then characterize the types of phenolic compounds [26].
An important group of secondary metabolites presented in T. vulgare is phenolic compounds [9].
Weather conditions can influence the chemical composition and amount of compounds in the plant. The year 2022 was extremely warm, especially in summer when the average temperatures were 0.5 °C above the norm of about 19.8 °C, making it the hottest year since 1924. The temperature of herb harvest sites differed from each other. The average temperature was higher in Ventspils, Daugavpils, Ludza, and Riga by 2.6 °C, 2.9 °C, 3.0 °C, and 3.1 °C, respectively. Even though at the end of August, the average temperature dropped by 11.3 °C, it was still 6.4 °C above the norm. Furthermore, insolation time was longer than average by 15% over the norm. The precipitation was 3% (222.6 mm) over the norm; however, most raindrops were observed in the northern central parts of Latvia (Zoseni), while the least was in the central and southern parts of Latvia. Cases of extreme rainfall were observed throughout all of the study regions during the summer with periods of drought. During summer, August had the least precipitation. In August, out of all the places that were studied, the least amount of precipitation was observed in Riga, and the highest amount of rainfall was observed in Ventspils.
The results in Table 2 indicate that T. vulgare growing at different sites in Latvia is rich in phenolic compounds. The total phenolic content ranges from 127 to 155 mg GAE/g in the flower extracts and from 135 to 219 mg GAE/g in the leaf extracts. Furthermore, depending on the harvest location, all extracts contained at least 15 mg QE/g of flavonoids and 11 mg CAF/g of phenolic acids. The highest total flavonoid content (28 mg QE/g) was observed in the leaf extract of T. vulgare harvested in Ludza. The most abundant in phenolic acids (31 mg CAF/g) was the leaf extract of T. vulgare harvested in Daugavpils.

Table 2.
Total phenolic, flavonoid, and phenolic acid content in leaf and flower extracts of T. vulgare collected in different places.
The data from the literature show that the highest content of total phenols are in the extracts of T. vulgare leaf, followed by extracts of flower and stem [6,27]. The results in Table 3 are in good agreement with these findings. The highest total phenolic content was observed in the leaf extract (156 mg GAE/g) and then in the flower extract (134 mg GAE/g) and in the stem extract (127 mg GAE/g). However, the highest amount of phenolic compounds (240 mg GAE/g), including flavonoids (85 mg QE/g), was obtained from the aerial part of T. vulgare. The highest total phenolic acid content was in the leaf extract (31 mg CAF/g), and the lowest was in the flower extract (15 mg CAF/g).

Table 3.
Total phenolic, flavonoid, and phenolic acid content in extracts obtained from different morphological parts of T. vulgare §.
Among the studied plant tissues (Table 3), leaves were the richest in phenolic compounds with 28.0 mg GAE/g of dry material, most of which corresponds to phenolic acids (6.6 mg CAF/g of dry material) and flavonoids (4.7 mg QE/g).
Considering that ethanol–water is a popular solvent for tannin extraction [28], the colorimetric tannin detection method from European Pharmacopeia was applied. It has to be taken into account that in this method, the tannin content is normalized to the redox potential of pyrogallol, suggesting that there might exist biases due to different redox potentials of the different tannins (e.g., catechines vs. gallotannins) [29]. Tannins are classified as hydrolyzable (gallic acid and ellagic acid) and condensed (proanthocyanidins) tannins [30].
The results in Figure 2 show that the highest content of tannins is found in the extracts of leaves (10 to 19%). Relatively small amounts of tannins (3 to 5%) were observed in the flower extracts of T. vulgare.
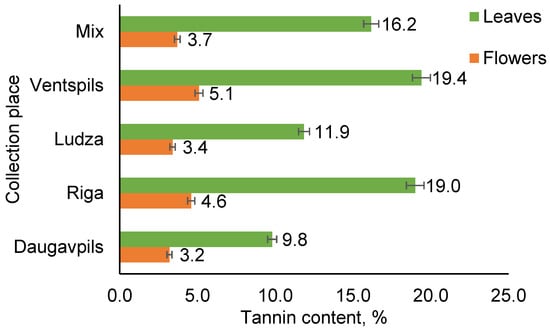
Figure 2.
Tannin content in the leaf and flower extracts of T. vulgare from different collection places.
It has been reported that biologically active properties of tannins not only may be attributed to tannin-rich medicinal plant extracts used in traditional medicine but also could be related to the synergy of tannins with other bioactive polyphenols present in these plants [31]. Considering that tannins affect the biological activities of extracts, it is important to monitor tannin content in T. vulgare.
2.3. Identification of Individual Phenolic Compounds in Extracts of T. vulgare
The chemical composition analysis of the leaf and flower extracts of T. vulgare was performed using liquid chromatography. Since the focus was on phenolic compounds, the liquid chromatography analysis was performed at the absorption maximum of these compounds at 280 nm.
The identification of individual compounds (Figure 3) was carried out using ten different standard substances: quinic acid, gallic acid, chlorogenic acid, caffeic acid, catechin, quercetin, kaempferol, rutin, apingenin, and apingenin glycoside.
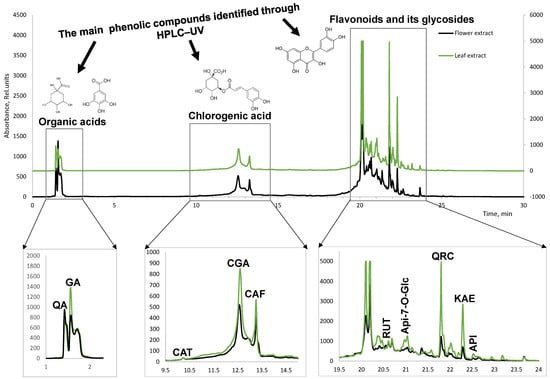
Figure 3.
LC chromatogram of T. vulgare leaf and flower extracts at 280 nm. QA-quinic acid, GA-gallic acid, CAT-catechin, CGA-chlorogenic acid, CAF-caffeic acid, RUT-rutin, Api-7-O-Glc-apingenin-7-O-glucoside, QRC-quercetin, KAE-kaempherol, API-apingenin.
The identification of the abovementioned value compounds was performed by a standard addition method. The results obtained show further perspectives of T. vulgare wild-growing in Latvia as a potential source of these phenolic compounds.
The HPLC method provides distinctive chemical profiles, also known as fingerprints [32]. The significant difference observed was the quantitative amount of compounds in the fingerprint patterns of the leaf and flower extracts.
2.4. Thujone Content in Extracts of T. vulgare
As previously mentioned, the use of T. vulgare is limited due to the presence of thujones.
The thujone (α + β) content in the T. vulgare samples was less than 1% on average, but two regions, Daugavpils and Ludza, had more than 4% (Table 4). It is worth noting that these regions are geographically closer to each other than the other regions that were studied. The results suggest that in these two regions, another chemotype with different first major constituents could be more common.

Table 4.
Thujone content (%) in leaf and flower extracts of T. vulgare.
The amount of thujone also varied in a wide range of extractives from the T. vulgare plants investigated before. The variation in the different chemical markers (camphor, thujone, and 1,8—cineole) of the essential oil in T. vulgare plants collected in the Baltic States has been reported [33].
2.5. Antioxidant Activity
The antioxidant properties of plants have become one of the most important indicators for evaluating their potential use in medicine [34]. The DPPH radical scavenging assay is the most common method used in the study of the antioxidant activity of plant extracts. The antioxidant activity of all extracts measured by the ability to scavenge (DPPH) free radicals was compared to the standard antioxidant trolox (water-soluble analogue of vitamin E).
All extracts of T. vulgare were found to have concentration-dependent inhibitory activity against the DPPH radical. In this study, the crude extracts showed DPPH radical scavenging effects with IC50 values in the range of 32 to 181 mg/L (Figure 4). The lower the IC50 value, the more powerful the antioxidant capacity.
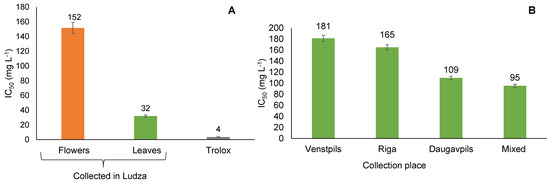
Figure 4.
Radical scavenging activity of leaf and flower extracts (A) and of leaf extracts (B) of T. vulgare harvested from different collection places by DPPH test.
Our results suggest that the aqueous ethanol extracts of T. vulgare have antioxidant activity on non-biological radical DPPH but are less efficient than Trolox (3.6 ± 0.2 mg L−1 (n = 3)). There are four categories of antioxidant activity: very strong (IC50 < 50 mg L−1), strong (IC50 between 50 to 100 mg L−1), moderate (IC50 between 100 to 150 mg L−1), and weak (IC50 between 150 to 200 mg L−1) [35]. In our study, the extracts obtained from T. vulgare leaves harvested in Ludza had very strong antioxidant activity (IC50 = 32.4 mg L−1, respectively). The leaf extracts obtained from T. vulgare harvested in Daugavpils and the mixed leaf sample belong to the active antioxidant category because they have IC50 values of 109.3 mg L−1 and 95.2 mg L−1, respectively. The other aqueous ethanol extracts of T. vulgare showed weak antioxidant activity, with IC50 values between 152 to 181 mg L−1. The data provided in the literature shows that the crude tansy extract could have DPPH radical scavenging effects with an IC50 value of 37 mg L−1 [16]. The findings are in good agreement with this value.
In addition, the total phenolic, tannin, and flavonoid contents of the leaf extract of T. vulgare collected from Ludza, which has high antioxidant activity compared to other extracts, were found to be high. Therefore, these compounds can be considered responsible for the antioxidant activity of the extract. The previously reported correlation between total phenolic compounds and various in vitro tests suggests that phenolic compounds are the main carriers of antioxidant power in the extracts of T. vulgare [36].
3. Discussion
In the present study, different morphological parts (leaves, flowers, stems, and aerial parts) of T. vulgare were analyzed for the chemical profile of phenolic compounds and antioxidant activity. Additionally, flowers and leaves were collected from four different regions of Latvia. Moreover, along with the analysis of phenolic compounds, the potentially toxic co-eluted thujone was qualitatively and quantitatively detected.
The T. vulgare is a common plant in Latvia. It is widely distributed throughout the whole country as the only Tanacetum genus species; however, its practical application has not yet been extensively studied. Our research is the starting point for further investigations aimed at the utilization potential of T. vulgare growing in Latvia.
The investigations of T. vulgare focused mainly on volatile compounds, especially in essential oil [1,13,18,37]. However, the average amount of essential oil found in the aerial parts of T. vulgare plants is from 0.1% up to 1.9% [38]. Some air-dried T. vulgare plants have been reported to contain up to 3% essential oil [39,40]. The phenolic compound-rich extracts of T. vulgare can reach up to 30% [41]. It should be considered that some components of essential oil could be co-extracted with hydrophilic extractives. The essential oil of T. vulgare is essentially composed of terpenoids and phenolic compounds. For the separation of terpenes (the main part of essential oil) from phenolic compounds, several techniques could be applied, e.g., solid phase extraction, the Craig-type apparatus, and circular chromatography. It is known that the phytochemical profiles and the bioactivities of T. vulgare are heavily impacted by the type and ratio of solvents used for extraction. The extraction method depends on the chemistry of the substance to be extracted, and for efficient extraction of phenolic compounds, combinations of aqueous and organic solvents are preferred [9]. Therefore, 50% aqueous ethanol (v/v) was selected as the extraction solvent for phenolic compounds.
By comparing the experimentally obtained extraction yields of hydrophilic extractives to the data provided in the literature, the results obtained in this research show similarity towards classical extraction methods (15 to 20%, w/w [38,41]), and the numbers are relatively comparable to advanced extraction techniques applied for T. vulgare (20 to 30%, w/w [41]). This means that the experimentally obtained extraction yields could be further improved by advanced extraction techniques (ultrasonic-assisted extraction, accelerated solvent extraction, etc.).
Tanacetum populations show high variability with regard to the essential oil composition, and more than 15 distinct chemotypes have been described in Scandinavia and the Baltic countries so far [42]. The variation of secondary metabolites, depending on chemotypes, has also been reported in other natural populations [43]. While there are differences in the essential oil composition, significant variation in the content of phenolic compounds has also been detected [44]. The content of lipophilic extractives depends on the aerial part. It is generally considered that the essential oil content in the inflorescences is higher than in other aerial parts [1]. Our results show that the hydrophilic extractives in T. vulgare have the same tendency, and the leaves have a higher content of hydrophilic extractives than the other parts.
In a study [18], the T. vulgare was demonstrated to be an under-investigated plant, even though comprehensive data on its properties and chemical composition as well as processing technologies may lead to the development of valuable products for various applications. The significant need for experimentally proven scientific investigations of T. vulgare was emphasized by several other researchers [6,11,38]. Our research supports the knowledge-based application potential of T. vulgare by offering additional, alternative resources on T. vulgare plants in the Baltic States.
The investigation of phenolic compounds in T. vulgare has recently become relevant [6,38], but the available data are limited and controversial in terms of amount (84–142 GAE mg/g in Serbia and Lithuania [6,15]; 40–50 GAE mg/g in Poland and Rumania [18,26]). In addition, the groups of dominant compounds (flavonoids, organic acids, and tannins) differ significantly. The crude aqueous ethanolic extract from aerial parts of T. vulgare contains flavonoids, phenolic acids, coumarins, and tannins [45]. However, the concentration of phenolic compounds in T. vulgare depends on the type of habitat. The distribution of phenolic compounds in plants is difficult to explain by geographical differences in locations [46].
According to the data provided in the literature, depending on the harvesting region, T. vulgare can contain total tannins from 4.3% up to 8.75%. Based on the study about tannin content in the extracts of T. vulgare, it is recommended that the extracts contain no less than 4% tannins [47]. However, only limited information is available on the chemical characterization of T. vulgare tannins. It has been reported that in the inflorescences part and leaves, hydrolyzable tannins are dominant [48]. The tannin content is of high importance because the topicality of tannin application in various industries (pharmaceutical, cosmetic, food, etc.) grows very fast [49]. Plant tannins are more abundant in vulnerable parts of plants, such as new leaves and flowers. The presence of tannins is highly variable among different plants, growth stages, and morphological parts [50].
Quinic acid, caffeic acid, and their esterified derivatives have been reported as characteristic non-volatile compounds in T. vulgare [38]. Gallic acid has been mentioned as one of the biologically active constituents of the liquid extract (ethanol 70%) of the common tansy herb recommended as a hepatoprotective agent [51]. All flavonoids (catechin, quercetin, kaempferol, rutin, and apingenin) identified in our research have been previously reported in T. vulgare as value components with a significant contribution to pharmaceutical activities [6,47,52]. Among the metabolites identified in T. vulgare, phenolic compounds, including caffeoylquinic acids and flavonoids, were considered a particularly important group because they contribute to the major multifunctional biological activity that may be linked to their antioxidant potential [53]. The performed chemical analyses allowed us to highlight the differences in the profiles of the leaves and flowers as well as to evaluate the variability within each biomass of T. vulgare depending on the harvesting location. It was found that the leaves and inflorescences of the T. vulgare plant synthesize the same phenolic compounds but of different compositions. The comparable results of the chemical composition and antioxidant activity detected in our study fit well with already reported findings of T. vulgare plants harvested in other regions, supporting the evidence-based opportunities of practical application.
The significant finding of our study was the co-eluted thujone in ethanol–water extracts. Considering that T. vulgare is one of the plant species that accumulates thujones [19], it is important to detect the amount of these constituents. Thujone is a volatile monoterpene ketone that is usually present in nature in two stereoisomers, α-thujone and β-thujone. For regulatory purposes, the sum of both isomers is generally assessed [54].
More than 15 different chemotypes of T. vulgare from Scandinavia and the Baltic states have been described, and most researchers identify β-thujone, α-thujone, camphor, and chrysanthenyl acetate as the main components of the essential oil [1]. Thujone occurs in different quantities in Tanacetum species [19]. The widespread nature of the thujone-chemotype essential oil has been reported in 15 countries on the European and American continents [33].
Thujones are generally characterized by gas chromatography methods [55]. Considering that the predicted content of thujone in the analyzed hydrophilic extracts must be low, the extract solubility in organic solvents could be lower as well. Therefore, the liquid chromatography method is more suitable for thujones characterization in hydrophilic extracts [56,57].
Although thujones have toxic effects, estimates for the allowable daily intake via herbal preparations and diet for humans are 3 to 7 mg/day [58]. Furthermore, the European Medicines Agency (EMA) also proposed a daily maximum intake of thujone in Absinthii herba, which was established at 3.0 mg thujone/day/person as acceptable for a maximum duration of 2 weeks in the wormwood (A. absinthium) monograph. Low doses of thujone may activate the DNA repair mechanism and act as antigenotoxic agents [19]. There are still important gaps in the knowledge required to assess thujone toxicity, the most important being human dose–concentration–effect relationships, including the elucidation of bioavailability and the actual toxicological consequences of potential pharmacogenetic variations [58]. Thujone-containing species are frequently used as natural remedies in ethnobotanical applications. However, it has been demonstrated that global climate change often alters the chemical composition of plants [59]. The potentially toxic compounds must be constantly monitored in plants.
The extracts obtained from T. vulgare leaves have not been reported to have significant toxicity. In view of the dose of T. vulgare consumed in traditional medicine, there is a wide margin of safety for the therapeutic use of the extracts of T. vulgare leaves [60].
According to the information available in the literature, thujone (α + β) has previously been reported only for the essential oil of T. vulgare.
The antioxidant activity found in the extract is supported by the results reported in other studies of T. vulgare and the traditional medicinal uses of the plant for various conditions such as injury recovery, arthritis, inflammatory processes, etc. [45].
4. Materials and Methods
4.1. Plant Material
The study object was wild-growing tansy plants (Tanacetum vulgare L.) in Latvia. The aerial parts (flowering tops up to lengths of 20 cm, flowers, leaves, and stems) of the wild tansy plants were harvested in the period of mass flowering (August 2022) from four different localities in Latvia: Ventspils (57°14′40″ N 21°39′44″ E), Riga (56°54′18″ N 24°05′57″ E), Daugavpils (55°53′40″ N 26°16′35″ E), and Ludza (56°32′32″ N 27°43′20″ E). The plants were identified by Prof. Dace Bandere, Ph.D. in Pharmacy. The voucher herbariums of the plants are kept in the internal collection of Riga Stradiņš University Department of Pharmaceutical Chemistry and labeled BLM-2022; BLV-2022; BLL-2022; BLR-2022; BLD-2022; BZM-2022; BZV-2022; BZL-2022; BZR-2022; and BZD-2022. The plants were dried at room temperature (20 to 25 °C) in shade. All the samples were dried to equilibrium moisture content and ground in a mill to obtain particles with a size of <2 mm.
4.2. Chemicals and Reagents
Reference standards: caffeic acid (≥98%), quinic acid (≥98%), catechin (≥98%), apigenin (≥95%), and apigenin-7-glucoside were purchased from Sigma-Aldrich (St. Louis, MO, USA). Rutin was purchased from PhytoLab (Vestenbergsgreuth, Germany), and chlorogenic acid was purchased from the HWI group (Rülzheim, Germany). All solvents used were analytical or HPLC grade. The water was distilled and purified using the Stakpure GmpH water system (Niederahr, Germany). Gallic acid and AlCl3 and Trolox were purchased from Acros Organics (Geel, Belgium), and Na2CO3 and NaNO2 were obtained from Honeywell (Charlotte, NC, USA). 2,2-diphenyl-1-picrylhydrazyl (DPPH) was from Alfa Aesar (Kandel, Germany). Folin–Ciocalteu reagent, H2SO4, HCl, and NaOH reagents were purchased from Fisher Scientific (Loughborough, UK). LC-grade acetonitrile, methanol, and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA), and water for LC analysis was purified using a Stakpure GmpH water purification system (Niederahr, Germany).
4.3. Moisture Content
The gravimetric method was applied for the determination of the moisture content. The weight loss on drying was measured by an oven method, dried at 105 °C for 3 h. The 5 g tansy sample was accurately weighed and dried to a constant mass in a vacuum oven at 105 °C for 3 h. The sample was reweighed after 30 min, and the moisture content was calculated and expressed as the percentage moisture content of the fresh weight.
4.4. Extraction
The 5.00 g of air-dried fine tansy powder was extracted with 50 mL of 50% aqueous ethanol by maceration with shaking using an orbital shaker (PSU-10i Biosan, Riga, Latvia) for 24 h at room temperature. Subsequently, the mixture was filtered through a 9 mm diameter filter paper (Sartorius, FT-3-303-110, Goettingen, Germany). The ethanol was evaporated in a rotary vacuum evaporator (Heidolph Laborota 4002 control, Schwabach, Germany), and extracts in water were freeze-dried by lyophilization at −80 °C, 0.05 mBar.
4.5. Total Phenolic Content
The total phenolic content was determined using the Folin–Ciocalteu method with minor modifications using gallic acid as the standard [27]. A total of 5 to 10 mg of the lyophilized extracts were dissolved in 25 mL of 50% ethanol. Briefly, 400 µL of extracts were mixed with 4 mL of 7.5% sodium carbonate and 5 mL of 10% Folin–Ciocalteu phenol reagent, and then the mixture was vortexed briefly. After incubation for 30 min at room temperature in the dark, absorbance was read at 750 nm using an ultraviolet (UV)-visible spectrophotometer (Mettler Toledo, LabX™, Greifensee, Switzerland). Total phenolic content was calculated based on a calibration curve of gallic acid. The results were expressed as milligram gallic acid equivalents per gram of lyophilized extract and tansy plant (milligram GAE per gram dry weight (DW)).
4.6. Total Flavonoid Content
The total flavonoid content was determined according to Sun et al. [61] with slight modifications as follows. A total of 5 to 10 mg of the lyophilized extracts were dissolved in 25 mL of 96% ethanol. Then, 0.4 mL of the extract solutions was added to a 10 mL test tube containing 2 mL of water. After that, 0.12 mL of 5% sodium nitrite (NaNO2) solution was added and incubated for 5 min at room temperature, and then 0.24 mL of 10% aluminum nitrate solution was added to the mixture. After 6 min, 0.8 mL of 1 mol/L sodium hydroxide was added. The absorbance was measured at 420 nm. The equation obtained for the calibration curve of the standard quercetin solution was y = 1.0369x − 0.0228 (R2 = 0.9954), and the result was expressed as mg QE/g (quercetin equivalents) per gram of dry weight (DW) extract.
4.7. Total Phenolic Acids
The method determined the total content of phenolic acids based on the proportional increase in the color intensity of the solution relating to the content of phenolic acids in the test [1]. A total of 10 to 15 mg of the lyophilized extracts were dissolved in 10 mL of water. The test solution was prepared by measuring 1 mL of extract solution, 1 mL of distilled water, 1 mL of 0.5 M hydrochloric acid, and 1 mL of Arnov’s reagent (10 g of sodium molybdate and 10 g of sodium nitrite dissolved in water and supplemented to 100 mL) into measuring tubes with a capacity of 10 mL. After 6 min, 1 mL of 0.1 M sodium hydroxide was added, supplemented with 5 mL of distilled water. The absorbance was measured at 490 nm. The total contents of phenolic acids in terms of caffeic acid were calculated according to the following formula, y = 0.7505x − 0.0505 (R2 = 0.9993), and the result was expressed as mg CAF/g (caffeic acid equivalents) per gram of dry weight (DW) extract.
4.8. Total Tannin Content
The analyses of the extracts were carried out in accordance with the monograph 2.8.14 available in the European Pharmacopoeia. In short, according to Neumann 2022 [29], the drugs were pulverized, and approximately 500 mg was extracted with boiling water (150 mL) for 30 min. The water extract was filtered and split into two parts of equal volume. Half of the filtered water extract was mixed with hide powder CRS and filtered once again. Phosphomolybdotungstic reagent R was added to both filtered water extracts, and their pH was adjusted using a sodium carbonate solution. The solutions were allowed to equilibrate for approximately 30 min. The total polyphenol contents were then obtained by measuring the absorbance of the solutions at a wavelength of 760 nm. Pyrogallol mixed with phosphomolybdotungstic reagent R was used as the standard. Since the hide powder CRS adsorbs the tannin polyphenols, it was possible to quantify the total tannin content by calculating the concentration difference between the two results obtained from the absorbance measurements. The hide powder CRS is a reference standard of the European Pharmacopoeia for the detection of phenolic compounds and tannins. The tannin content was calculated in the dried extracts.
4.9. Identification of Individual Phenolic Compounds
Standard stock solutions of ten different substances—quinic acid, gallic acid, chlorogenic acid, caffeic acid, catechin, quercetin, kaempferol, rutin, apingenin, and apingenin glycoside—were prepared in 50% acetonitrile (2 mg/mL). All solutions were filtered prior to analysis through a 0.45 μm syringe filter and injected three times into the HPLC. The phenolic compounds were identified by analyzing the same extracts with no added standard compounds and with added standard compounds. LC analysis was performed on a DIONEX UltiMate 3000 UHPLC+ focused system (Thermo Fisher Scientific, Waltham, MA, USA), which consists of an UltiMate 3000 pump, UltiMate 3000 autosampler, UltiMate 3000 column compartment, UltiMate 3000 variable wavelength detector, and Chromeleon software. As a stationary phase, an Ascentis Express 90 Å AQ-C18 column (15 cm × 3.0 mm, 2.7 μm, Supelco, Darmstadt, Germany) was used. The mobile phase was composed of 0.1% aqueous trichloroacetic acid (v/v) (A) and acetonitrile (B) with the following gradient elution: 0 min—95% A, 10 min—75% A, 25 min—20% A, and 30 min—95% A. Before each sample analysis, a 4 min equilibration with 5% B was performed. The flow rate was set at 0.425 mL/min, the column temperature was 40 °C, the injection volume was 1 μL, and the time of analysis was 30 min.
4.10. Determination of Thujone Content
The purified standard of α, β-thujone (80% purity; 70% α-thujone and 10% β-thujone) was from Sigma-Aldrich (Steinheim, Germany). The reference standard solution contained 0.724 g/mL of α- and β-thujone (taking into consideration a purity of 80.0% and density of 0.925 g/mL). An aliquot of 25 μL of the standard solution was diluted in 25 mL of methanol to a final concentration of 0.724 mg/mL. The extracts were dissolved in methanol with an approximate of concentration 2 to 6 mg/mL, filtered (Nylon filter, 0.45 μm pore size), and then used for reversed phase liquid chromatography (DIONEX UltiMate 3000 UHPLC, Thermo Fisher Scientific, Waltham, MA, USA) with fluorescence detection experiments. The peak areas were recorded, and the amount of thujone was calculated using the calibration plot on LC.
The LC analysis was carried out using a Zorbax Eclipse XDB-C18 (Agilent, 5 µm, 15 cm × 0.46 cm i.d.) column heated at 35 °C and an acetonitrile to water ratio of 55 to 45 as the mobile phase; the flow rate was 1 mL/min, and the injection volume was 20 µL. The fluorescence detector was set at 220/290 nm (λex/λem). The thujones were detected as only one peak as the sum of the two isomers. The quantitative analysis was performed both by using linear regression analysis, obtained by injecting various concentrations of thujone solution in methanol, and by the standard addition method.
4.11. Antioxidant Activity Using DPPH Method
The antioxidant capacity was determined with the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay [62]. The 10 µg/mL DPPH solution was prepared in methanol. The antioxidant activities were expressed as the half maximal inhibitory concentration (IC50), defined as the antioxidant concentration needed to reduce the absorbance of the control by 50%. The lower the IC50 value, the higher the antioxidant activity. A sample solution in DMSO (0.03 mL) was mixed for 15 min with 3.0 mL of a DPPH in methanol solution, and then the absorbance at 517 nm of the mixture was immediately measured using a Metter Toledo UV7 spectrometer.
4.12. Statistical Analysis
The measured values were shown as an average with a confidence interval (at a level of significance α = 0.05). Each measurement was performed at least in triplicate. Statistical calculations were carried out using Microsoft Office Professional Plus 2019 Excel.
5. Conclusions
In terms of the extraction efficiency of hydrophilic extractives (up to 20% w/w for leaves and up to 16% w/w for flowers), the flowers and leaves of T. vulgare have been proven to be promising for the development of new herbal products.
Phytochemical screening of the ethanol–water extracts of T. vulgare showed phenolic compounds, mainly tannins, flavonoids, and phenolic acids. The total tannin content in tansy leaf extracts was up to 19%, and considering its potential biological activities and synergic effect with other compounds, tannins should be characterized in more detail. The value constituents were identified as chlorogenic acid, apigenin and its glycoside, quinic acid, rutin, and kaempherol.
The chemical profile of T. vulgare leaves and flowers was dependent on the sampling location. Thujone variation was significantly high not only between aerial parts (leaves, flowers) but also between collection sites. The toxic thujone content should be controlled in all extracts of T. vulgare regardless of the plant part or extraction solvent used. The aqueous ethanol extracts of T. vulgare have antioxidant activity (IC50 between 181 to 32 mg L−1) on non-biological radical DPPH but are less efficient than Trolox, a hydrophilic analogue of vitamin E (IC50 3.6 ± 0.2 mg L−1).
Further studies may obtain data on important relationships between the chemical composition and biological activities of T. vulgare extracts.
The data obtained from this study are indicative and may be helpful for future cultivation and the practical application of T. vulgare plants.
Author Contributions
Conceptualization, R.Š., L.L., A.B. (Agnese Brangule), L.K. and D.B.; methodology, R.Š., K.L. and L.L.; investigation, R.Š., Z.M.H., A.B. (Ance Bārdziņa) and L.L.; resources, D.B.; data curation, Z.M.H., R.Š. and A.B. (Ance Bārdziņa); writing—original draft preparation, L.L., R.Š., K.L., A.B. (Agnese Brangule), L.K. and D.B.; writing—review and editing, L.L., D.B., R.Š., A.B. (Agnese Brangule), L.K. and K.L.; visualization, Z.M.H., A.B. (Ance Bārdziņa) and L.L.; supervision, D.B. and L.K.; project administration, D.B. and L.K.; funding acquisition, L.K. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 857287 and European Agricultural Fund for Rural Development—development of the medication form from the extract of the leaves of the Latvian traditional medicinal plant tansy and its impact on the sheep digestive tract microbiome and antiparasitic control (22-00-A01612-000007). The project is supported by the Ministry of Agriculture and Rural Support Service Republic of Latvia, project no: 22-00-A01612-000007.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
The authors would like to thank Samanta Petrēvica for helping carry out some extraction and spectrophotometric experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nurzynska-Wierdak, R.; Salata, A.; Kniaziewicz, M. Tansy (Tanacetum vulgare L.)-A Wild-Growing Aromatic Medicinal Plant with a Variable Essential Oil Composition. Agronomy 2022, 12, 277. [Google Scholar] [CrossRef]
- Prūse, B.; Kalle, R.; Buffa, G.; Simanova, A.; Mežaka, I.; Sõukand, R. We need to appreciate common synanthropic plants before they become rare: Case study in Latgale (Latvia). Ethnobiol. Conserv. 2020, 10. [Google Scholar] [CrossRef]
- Rohloff, J.; Mordal, R.; Dragland, S. Chemotypical variation of tansy (Tanacetum vulgare L.) from 40 different locations in Norway. J. Agric. Food Chem. 2004, 52, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Ak, G.; Gevrenova, R.; Sinan, K.I.; Zengin, G.; Zheleva, D.; Mahomoodally, M.F.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Tanacetum vulgare L. (Tansy) as an effective bioresource with promising pharmacological effects from natural arsenal. Food Chem. Toxicol. 2021, 153, 112268. [Google Scholar] [CrossRef]
- Ivanescu, B.; Tuchilus, C.; Corciova, A.; Lungu, C.; Mihai, C.T.; Gheldiu, A.M.; Vlase, L. Antioxidant, Antimicrobial and Cytotoxic Activity of Tanacetum Vulgare, Tanacetum Corymbosum and Tanacetum Macrophyllum Extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Devrnja, N.; Krstic-Milosevic, D.; Janosevic, D.; Tesevic, V.; Vinterhalter, B.; Savic, J.; Calic, D. In vitro cultivation of tansy (Tanacetum vulgare L.): A tool for the production of potent pharmaceutical agents. Protoplasma 2021, 258, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Cote, H.; Boucher, M.A.; Pichette, A.; Legault, J. Anti-Inflammatory, Antioxidant, Antibiotic, and Cytotoxic Activities of Tanacetum vulgare L. Essential Oil and Its Constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Rosselli, S.; Bruno, M.; Raimondo, F.M.; Spadaro, V.; Varol, M.; Koparal, A.T.; Maggio, A. Cytotoxic Effect of Eudesmanolides Isolated from Flowers of Tanacetum vulgare ssp. siculum. Molecules 2012, 17, 8186–8195. [Google Scholar] [CrossRef] [PubMed]
- Baczek, K.B.; Kosakowska, O.; Przybyl, J.L.; Pioro-Jabrucka, E.; Costa, R.; Mondello, L.; Gniewosz, M.; Synowiec, A.; Weglarz, Z. Antibacterial and antioxidant activity of essential oils and extracts from costmary (Tanacetum balsamita L.) and tansy (Tanacetum vulgare L.). Ind. Crops Prod. 2017, 102, 154–163. [Google Scholar] [CrossRef]
- Williams, C.A.; Harborne, J.B.; Geiger, H.; Hoult, J.R.S. The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflammatory properties (vol 51, pg 417, 1999). Phytochemistry 1999, 52, 1181–1182. [Google Scholar] [CrossRef]
- Kowalonek, J.; Stachowiak, N.; Bolczak, K.; Richert, A. Physicochemical and Antibacterial Properties of Alginate Films Containing Tansy (Tanacetum vulgare L.) Essential Oil. Polymers 2023, 15, 260. [Google Scholar] [CrossRef]
- Piątkowska, E.; Biel, W.; Witkowicz, R.; Kępińska-Pacelik, J. Chemical Composition and Antioxidant Activity of Asteraceae Family Plants. Appl. Sci. 2022, 12, 12293. [Google Scholar] [CrossRef]
- Formisano, C.; Senatore, F.; Bruno, M.; Rosselli, S.; Bellone, G.; Spadaro, V. Essential Oil Composition of Tanacetum vulgare subsp siculum (Guss.) Raimondo et Spadaro (Asteraceae) from Sicily. Nat. Prod. Commun. 2009, 4, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Vinesh Kumar, D.T. hemical Composition and Biological Activities of Essential Oils of Genus Tanacetum—A review. J. Pharmacogn. Phytochem. 2013, 2, 159–163. [Google Scholar]
- Sowa, P.; Marcinčáková, D.; Miłek, M.; Sidor, E.; Legáth, J.; Dżugan, M. Analysis of Cytotoxicity of Selected Asteraceae Plant Extracts in Real Time, Their Antioxidant Properties and Polyphenolic Profile. Molecules 2020, 25, 5517. [Google Scholar] [CrossRef]
- Juan-Badaturuge, M.; Habtemariam, S.; Jackson, C.; Thomas, M.J. Antioxidant principles of Tanacetum vulgare L. aerial parts. Nat. Prod. Commun. 2009, 4, 1561–1564. [Google Scholar] [CrossRef] [PubMed]
- Raal, A.; Orav, A.; Gretchushnikova, T. Essential Oil Content and Composition in Tanacetum vulgare L. Herbs Growing Wild in Estonia. J. Essent. Oil Bear. Plants 2014, 17, 670–675. [Google Scholar] [CrossRef]
- Baranauskiene, R.; Kazernaviciute, R.; Pukalskiene, M.; Mazdzieriene, R.; Venskutonis, P.R. Agrorefinery of Tanacetum vulgare L. into valuable products and evaluation of their antioxidant properties and phytochemical composition. Ind. Crops Prod. 2014, 60, 113–122. [Google Scholar] [CrossRef]
- Nemeth, E.Z.; Nguyen, H.T. Thujone, a widely debated volatile compound: What do we know about it? Phytochem. Rev. 2020, 19, 405–423. [Google Scholar] [CrossRef]
- Šukele, R.; Bārzdiņa, A.; Koka, R.; Skadins, I.; Lauberte, L.; Brangule, A.; Kovalcuka, L.; Bandere, D. Antibacterial Activity of Tanacetum vulgare L. Extracts against Clinical Isolates of Bovine Mastitis. Appl. Sci. 2023, 13, 3369. [Google Scholar] [CrossRef]
- Barba-Ostria, C.; Carrera-Pacheco, S.E.; Gonzalez-Pastor, R.; Heredia-Moya, J.; Mayorga-Ramos, A.; Rodríguez-Pólit, C.; Zúñiga-Miranda, J.; Arias-Almeida, B.; Guamán, L.P. Evaluation of Biological Activity of Natural Compounds: Current Trends and Methods. Molecules 2022, 27, 4490. [Google Scholar] [CrossRef]
- Tokunaga, E.; Yamamoto, T.; Ito, E.; Shibata, N. Understanding the Thalidomide Chirality in Biological Processes by the Self-disproportionation of Enantiomers. Sci. Rep. 2018, 8, 17131. [Google Scholar] [CrossRef] [PubMed]
- Mapfumari, S.; Nogbou, N.-D.; Musyoki, A.; Gololo, S.; Mothibe, M.; Bassey, K. Phytochemical Screening, Antioxidant and Antibacterial Properties of Extracts of Viscum continuum E. Mey. Ex Sprague, a South African Mistletoe. Plants 2022, 11, 2094. [Google Scholar] [CrossRef]
- Tijjani, H.; Zangoma, M.; Mohammed, Z.; Obidola, S.M.; Egbuna, C.; Abdulai, S. Polyphenols: Classifications, Biosynthesis and Bioactivities; Springer: Berlin/Heidelberg, Germany, 2020; pp. 389–414. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Muresan, M.; Benedec, D.; Vlase, L.; Oprean, R.; Toiu, A.; Oniga, I. Screening of Polyphenolic Compounds, Antioxidant and Antimicrobial Properties of Tanacetum Vulgare from Transylvania. Stud. Univ. Babes-Bolyai. Chem. 2015, 60, 127–138. [Google Scholar]
- Devrnja, N.; Andelkovic, B.; Arandelovic, S.; Radulovic, S.; Sokovic, M.; Krstic-Milosevic, D.; Ristic, M.; Calic, D. Comparative studies on the antimicrobial and cytotoxic activities of Tanacetum vulgare L. essential oil and methanol extracts. S. Afr. J. Bot. 2017, 111, 212–221. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Neumann, N.; Honke, M.; Povydysh, M.; Guenther, S.; Schulze, C. Evaluating Tannins and Flavonoids from Traditionally Used Medicinal Plants with Biofilm Inhibitory Effects against MRGN E. coli. Molecules 2022, 27, 2284. [Google Scholar] [CrossRef]
- Okuda, T.; Ito, H. Tannins of Constant Structure in Medicinal and Food Plants-Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef]
- Bārzdiņa, A.; Paulausks, A.; Bandere, D.; Brangule, A. The Potential Use of Herbal Fingerprints by Means of HPLC and TLC for Characterization and Identification of Herbal Extracts and the Distinction of Latvian Native Medicinal Plants. Molecules 2022, 27, 2555. [Google Scholar] [CrossRef]
- Mockute, D.; Judzentiene, A. Composition of the Essential Oils of Tanacetum vulgare L. Growing Wild in Vilnius District (Lithuania). J. Essent. Oil Res. 2004, 16, 550–553. [Google Scholar] [CrossRef]
- Peng, X.W.; He, X.H.; Tang, J.R.; Xiang, J.Y.; Deng, J.; Kan, H.; Zhang, Y.J.; Zhang, G.L.; Zhao, P.; Liu, Y. Evaluation of the in vitro antioxidant and antitumor activity of extracts from Camellia fascicularis leaves. Front. Chem. 2022, 10, 1035949. [Google Scholar] [CrossRef] [PubMed]
- Marjoni, M.R.; Zulfisa, A. Antioxidant Activity of Methanol Extract/Fractions of Senggani Leaves (Melastoma candidum D. Don). Pharm. Anal. Acta 2017, 2017, 1–6. [Google Scholar]
- Stojković, M.B.; Mitić, S.S.; Pavlović, J.L.; Stojanović, B.T.; Paunović, D.Đ. Antioxidant Potential of Tanacetum vulgare L. Extracts; Biologica Nyssana: Niš, Serbia, 2017. [Google Scholar]
- Magierowicz, K.; Górska-Drabik, E.; Sempruch, C. The effect of Tanacetum vulgare essential oil and its main components on some ecological and physiological parameters of Acrobasis advenella (Zinck.) (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2020, 162, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Larina, V.; Krol, O.; Ulrikh, E.; Sukhikh, S.; Gureev, M.A.; Prosekov, A.; Ivanova, S. In Vitro Study of Biological Activity of Tanacetum vulgare Extracts. Pharmaceutics 2023, 15, 616. [Google Scholar] [CrossRef] [PubMed]
- Korpinen, R.I.; Välimaa, A.-L.; Liimatainen, J.; Kunnas, S. Essential Oils and Supercritical CO2 Extracts of Arctic Angelica (Angelica archangelica L.), Marsh Labrador Tea (Rhododendron tomentosum) and Common Tansy (Tanacetum vulgare) & Chemical Compositions and Antimicrobial Activities. Molecules 2021, 26, 7121. [Google Scholar] [PubMed]
- Piras, A.; Falconieri, D.; Bagdonaite, E.; Maxia, A.; Goncalves, M.J.; Cavaleiro, C.; Salgueiro, L.; Porcedda, S. Chemical composition and antifungal activity of supercritical extract and essential oil of Tanacetum vulgare growing wild in Lithuania. Nat. Prod. Res. 2014, 28, 1906–1909. [Google Scholar] [CrossRef]
- Herbina, N.; Ruban, O.; Andryushayev, O.; Hohlova, L. Intensification of the Extraction Process of Flavonoids and Hydroxycinnamic Acids from Tanacetum vulgare L. Flowers. J. Rep. Pharm. Sci. 2022, 11, 125–131. [Google Scholar] [CrossRef]
- Dragland, S.; Rohloff, J.; Mordal, R.; Iversen, T.H. Harvest regimen optimization and essential oil production in five tansy (Tanacetum vulgare L.) genotypes under a northern climate. J. Agric. Food Chem. 2005, 53, 4946–4953. [Google Scholar] [CrossRef]
- Li, Y.; Zidorn, C. Seasonal variations of natural products in European herbs. Phytochem. Rev. 2022, 21, 1549–1575. [Google Scholar] [CrossRef]
- Guedri Mkaddem, M.; Zrig, A.; Ben Abdallah, M.; Romdhane, M.; Okla, M.K.; Al-Hashimi, A.; Alwase, Y.A.; Hegab, M.Y.; Madany, M.M.Y.; Hassan, A.H.A.; et al. Variation of the Chemical Composition of Essential Oils and Total Phenols Content in Natural Populations of Marrubium vulgare L. Plants 2022, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Vilhelmova, N.; Simeonova, L.; Nikolova, N.; Pavlova, E.; Gospodinova, Z.; Antov, G.; Galabov, A.; Nikolova, I. Antiviral, Cytotoxic and Antioxidant Effects of Tanacetum Vulgare L. Crude Extract In Vitro. Folia Med. 2020, 62, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Radušienė, J.; Karpavičienė, B.; Raudone, L.; Vilkickyte, G.; Çırak, C.; Seyis, F.; Yayla, F.; Marksa, M.; Rimkienė, L.; Ivanauskas, L. Trends in Phenolic Profiles of Achillea millefolium from Different Geographical Gradients. Plants 2023, 12, 746. [Google Scholar] [CrossRef]
- Ivancheva, S.; Kurteva, M.; Stancheva, B. Dynamic and content of tannins and flavonoids in Achillea millefolium L. and Tanacetum vulgare L. Pharmacia 2000, 47, 17–20. [Google Scholar]
- Khramova, E.; Kukushkina, T.; Shaldaeva, T.; Pshenichkina, Y. Biologically Active Compounds and Antioxidant Activity of the Plants from CSBG SB RAS Collection of the Asteraceae Family. BIO Web Conf. 2021, 38, 00055. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and Actual Industrial Applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef]
- Tong, Z.K.; He, W.F.; Fan, X.; Guo, A.W. Biological Function of Plant Tannin and Its Application in Animal Health. Front. Vet. Sci. 2022, 8, 1597. [Google Scholar] [CrossRef]
- Kalko, K.O.; Mishchenko, O.Y.; Derymedvid, L.V.; Zolotaikina, M.Y.; Gontova, T.M.; Mashtaler, V.V.; Kutsenko, S.A. A Screening Study of Hepatoprotective Activity of Liquid Extract from Common Tansy Tanacetum vulgare L. Herb in the setting of Subchronic Hepatitis in Rats. Res. J. Pharm. Technol. 2018, 11, 4393–4396. [Google Scholar] [CrossRef]
- Arıtuluk, Z.; Çankaya, I.I.T.; Özkan, A.M.G. Antioxidant activity, total phenolic and flavonoid contents of some Tanacetum L. (Asteraceae) taxa growing in Turkey. Fabad J. Pharm. Sci. 2016, 41, 17–25. [Google Scholar]
- Raudone, L.; Radušiene, J.; Seyis, F.; Yayla, F.; Vilkickyte, G.; Marksa, M.; Ivanauskas, L.; Cırak, C. Distribution of Phenolic Compounds and Antioxidant Activity in Plant Parts and Populations of Seven Underutilized Wild Achillea Species. Plants 2022, 11, 447. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Uebelacker, M. Risk assessment of thujone in foods and medicines containing sage and wormwood—Evidence for a need of regulatory changes? Regul. Toxicol. Pharmacol. 2010, 58, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Alshishani, A.A.; Saad, B.; Semail, N.F.; Salhimi, S.M.; Talib, M.K.M. Salting-out assisted liquid-liquid extraction method coupled to gas chromatography for the simultaneous determination of thujones and pulegone in beverages. Int. J. Food. Prop. 2018, 20, S2776–S2785. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Ferretti, F.; Moro, E.; Ceschi, A.; Colombo, F.; Frigerio, G.; Lude, S.; Restani, P. Identification and Quantification of Thujone in a Case of Poisoning Due to Repeated Ingestion of an Infusion of Artemisia Vulgaris L. J. Food Sci. 2018, 83, 2257–2264. [Google Scholar] [CrossRef]
- Micali, G.; Lanuzza, F. HPLC determination of β- and β-thujone, potentially toxic components of natural flavourings, in alcoholic beverages. Flavour Fragr. J. 1995, 10, 329–333. [Google Scholar] [CrossRef]
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and thujone-containing herbal medicinal and botanical products: Toxicological assessment. Regul. Toxicol. Pharmacol. 2013, 65, 100–107. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Khan, N. Climate Change and Salinity Effects on Crops and Chemical Communication Between Plants and Plant Growth-Promoting Microorganisms Under Stress. Front. Sustain. Food Syst. 2021, 5, 618092. [Google Scholar] [CrossRef]
- Lahlou, S.; Israili, Z.H.; Lyoussi, B. Acute and chronic toxicity of a lyophilised aqueous extract of Tanacetum vulgare leaves in rodents. J. Ethnopharmacol. 2008, 117, 221–227. [Google Scholar] [CrossRef]
- Sun, Y.T.; Li, M.; Mitra, S.; Muhammad, R.H.; Debnath, B.; Lu, X.C.; Jian, H.X.; Qiu, D.L. Comparative Phytochemical Profiles and Antioxidant Enzyme Activity Analyses of the Southern Highbush Blueberry (Vaccinium corymbosum) at Different Developmental Stages. Molecules 2018, 23, 2209. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).