Abstract
Tartary buckwheat (Fagopyrum tataricum Gaertn.) originates in mountain regions of Western China, and is cultivated in China, Bhutan, Northern India, Nepal, and Central Europe. The content of flavonoids in Tartary buckwheat grain and groats is much higher than in common buckwheat (Fagopyrum esculentum Moench), and depends on ecological conditions, such as UV-B radiation. Buckwheat intake has preventative effects in chronic diseases, such as cardiovascular diseases, diabetes, and obesity, due to its content of bioactive substances. The main bioactive compounds in Tartary buckwheat groats are flavonoids (rutin and quercetin). There are differences in the bioactivities of buckwheat groats obtained using different husking technologies, based on husking raw or pretreated grain. Husking hydrothermally pretreated grain is among the traditional ways of consuming buckwheat in Europe and some parts of China and Japan. During hydrothermal and other processing of Tartary buckwheat grain, a part of rutin is transformed to quercetin, the degradation product of rutin. By adjusting the humidity of materials and the processing temperature, it is possible to regulate the degree of conversion of rutin to quercetin. Rutin is degraded to quercetin in Tartary buckwheat grain due to the enzyme rutinosidase. The high-temperature treatment of wet Tartary buckwheat grain is able to prevent the transformation of rutin to quercetin.
Keywords:
Fagopyrum tataricum; Fagopyrum esculentum; flavonoids; rutin; quercetin; food; grain; groats; metabolites 1. Introduction
Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.) originates in regions of South-western China, and is cultivated in China, Korea, northern parts of India, Bhutan, and Nepal [1,2]. It is also traditionally cultivated in several European countries (Luxembourg, Germany, Italy, and Slovenia). In Bosnia and Herzegovina, Tartary buckwheat is cultivated as a mixture of Tartary buckwheat and common buckwheat (Fagopyrum esculentum Moench). Recently, Tartary buckwheat has been cultivated in Sweden. Generally, common buckwheat is cultivated more widely than Tartary buckwheat in Asian countries (China, Korea, Japan, India, Nepal, Bhutan, and Pakistan), Central and Eastern Europe (mainly in Russia, Ukraine, Belarus, Kazakhstan, Estonia, Latvia, Lithuania, Poland, Czech Republic, Slovenia, Croatia, Serbia, Italy, and France), Southern and Eastern Africa (mainly Tanzania), Australia (mainly in Tasmania for exporting to Japan as Tasmania soba), Canada, USA, and Brazil [3,4,5].
Buckwheat is used to produce flour dishes, often mixed with wheat flour for bread, pasta, and pancakes. In Central and Eastern Europe, there are traditionally popular dishes made from groats, i.e., husked buckwheat grain. Newly harvested and freshly husked common buckwheat grain is greenish; after time in storage, it becomes light yellow and eventually red. If common buckwheat grain is precooked before husking and/or toasted after husking, it is called kasha. This reddish-brown kasha grain has a nutty flavor. Husked Tartary buckwheat is used in China for making vinegar and beer and in Japan for making tea. Common buckwheat is used in Japan for making liquor. In Korea (the Kangwon area), Tartary buckwheat sprouts are a popular vegetable [6]. Buckwheat husks (after husking the grain to obtain groats) are traditionally used in Japan and China for making cushions. Common buckwheat is a rich source of honey; it is dark, with a unique taste, and is a rich source of antioxidants. Tartary buckwheat is a source of nectar only for small wild insects, but does not attract bees. Thus, fields of Tartary and common buckwheat are important to feed wild insects to maintain their biodiversity.
Zhang et al. [1] discussed how Tartary buckwheat has high nutritional value, including high protein content and quality in regard to balanced amino acid composition [7,8], and very high levels of antioxidants such as rutin [9]. Rutin and quercetin are flavonoids known for their ability to strengthen blood vessels, and they provide many other potential health benefits, such as a reduction in cholesterol levels and blood clots [10,11]. There is a large difference in the concentration of flavonoids between Tartary buckwheat and common buckwheat. It was found that Tartary buckwheat has higher (at least two-fold) contents of 61 flavonoids and 94 nonflavonoid secondary metabolites than common buckwheat. It is reported that Tartary and common buckwheat grain are rich in secondary metabolites beneficial to human health; among them are nonflavonoid metabolites that also contribute to Tartary buckwheat’s higher health-promoting value in comparison to common buckwheat [12,13].
The goal of this review is to focus on the bioactive compounds of Tartary buckwheat groats, including the impact of mechanical, thermal, and hydrothermal treatments during food preparation on the transformation of compounds.
2. Synthesis of Bioactive Substances in Tartary Buckwheat
The ancestors of today’s Tartary buckwheat were plants that were consumed by herbivorous dinosaurs. About 85 million years ago [1], the common ancestors of today’s sugar beet and Tartary buckwheat were among the herbs available during the time of the dinosaurs.
Several genes important for the regulation of quercetin and rutin biosynthesis have been identified [1]. The capability of buckwheat to survive under high levels of abiotic stress is due to the appearance of several gene groups involved in the transfer of signals, the regulation of the activity of genes, and the transfer of molecules. These genetic resources have facilitated the discovery of physiologically and nutritionally important plant genes, and the possibility of genetic improvement in Tartary buckwheat [1,5]. There are 769 gene groups involved in the evolutionary side branching of Tartary buckwheat and sugar beet, establishing the divergence of Caryophyllales before the separation of asterids and rosids [14]. Zhang et al. [1] reported that the ancestors of Tartary buckwheat and sugar beet split from each other about 85 million years ago. The basic biosynthesis pathway for the production of the flavonoids rutin and quercetin and phenolic acids is highlighted in Figure 1. The solar UV-B irradiation of plants may increase the amount of rutin and other UV-absorbing secondary substances in buckwheat grain and the green parts of plants [3,4].
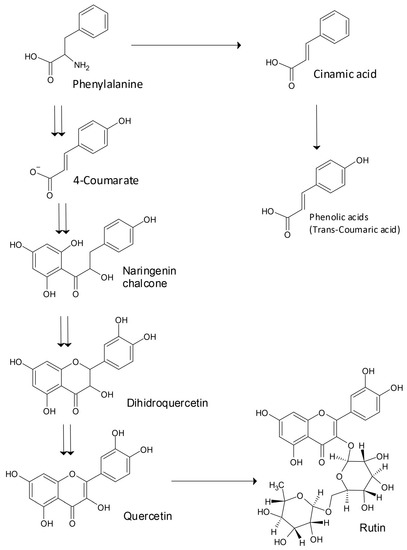
Figure 1.
Basic biosynthesis pathway for the synthesis of flavonoids rutin and quercetin, and phenolic acids.
3. Technologies for Husking Buckwheat Grain and Preparing Groats
Due to their robust husk, dormant Tartary buckwheat seeds may remain alive in the soil for several years and, under favorable conditions, can be activated. Tartary buckwheat may appear as a weed in other crops and is often a weed in a crop of common buckwheat (Fagopyrum esculentum Moench) [4].
One method to husk buckwheat is to use non-precooked grain. The raw husked common buckwheat groats are green when newly harvested buckwheat is husked. After some weeks, chlorophyll fades and phenolic substances oxidize, so the older non-precooked buckwheat groats are yellow, and later become reddish. From the green color it is possible to estimate if raw husked groats are fresh and made from newly harvested common buckwheat. By pressing the flat side of the knife, non-precooked groats are broken into floury particles, and when cooking the groats, they become slimy [3,4].
Buckwheat groats obtained by precooking are harder, and break under pressure into larger particles with a vitreous appearance (Figure 2). Precooked buckwheat groats (husked buckwheat grain) cook nicely, and they also have a special taste. Substances of traditional buckwheat groats, important for this distinctive taste, have been studied [15]. Buckwheat groats are popular in Slovenia, Croatia, Poland, Belarus, Ukraine, Russia, some parts of China (Shanxi and Shaanxi), and Japan (Shikoku island) [3,4]. They were also once known in Carinthia, Austria. In Karelia (Finland), buckwheat groats cooked in milk or cream are added on the top of traditional open pierogi (karjalan piirakka) on a base of rye dough [3,4].

Figure 2.
Buckwheat groats obtained using the traditional precooking method: left, common buckwheat; right, Tartary buckwheat kasha (husked buckwheat grain).
For husking, precooked buckwheat grains are firstly soaked in boiling water, and during this treatment, the starch swells. In doing so, the husk bursts on one of the three corners. When ready, the water is removed and the grain is dried at a moderate temperature. In old times, the grain was traditionally dried on canvas in the shade or on a warm top of a wood-burning stove. The grains were repeatedly mixed to dry evenly. They were dried only so that the husk was dry and brittle, and the inside of the grain was elastic, but solid enough not to squeeze or smear when the process continued. Properly dried grain was placed in a husking device called a stope. A stope consists of a stable hollowed trunk with an opening for at least five liters of grain. Above the opening is a fixed beam with a downward-facing iron tip with horizontal ribs. The beam went up several tens of inches and then fell freely into the prepared grain. This was repeated as needed, even a hundred times if necessary. When the grains were struck, the husk was separated from the grain, and groats (kasha) were formed. When most grains were husked, farmers used ventilation to separate light husks from heavier kasha grains. In areas rich in streams, the stopes were powered by water wheels. Stopes were foot-powered in plain landscapes. In Carinthia, Austria, hydrothermally pretreated buckwheat was husked by treading on grain with heavy wooden shoes [16]. Preparing husked buckwheat grain, kasha, is very challenging. The process has been known among Slovenians for a long time, as was described by Valvasor [17].
The modern husking of buckwheat grain is essentially the same as it used to be—cooking, drying, husking, drying again, and blowing off the husks. The details of technology are the intellectual property of each producer, especially in husking technology for Tartary buckwheat, which is because of the significant issue of thick husks. To separate the remaining unhusked grain from the husked grain, producers use photocell-supported equipment. Buckwheat kasha obtained the traditional way by precooking before husking has a special taste and properties, including the composition of bioactive substances (Table 1). Tartary buckwheat grains have a much higher content of rutin and total flavonoids than common buckwheat grains (Table 1). In addition, the husked grains of Tartary buckwheat have a much higher content of rutin than the husked grains of common buckwheat. As a result of hydrothermal treatment, husked Tartary buckwheat grains contain much less rutin in comparison to intact grains, but a much higher content of quercetin. This is the result of the enzymatic conversion of rutin to quercetin during hydrothermal treatment.

Table 1.
Content of major bioactive compounds isolated from buckwheat grain and groats.
Phenolic acids and other phenolic metabolites are present in Tartary buckwheat grain at low concentrations in comparison to rutin and quercetin (Table 1) [40,41]. Their content is more substantial in the green parts (leaves) and flowers of buckwheat plants [43]. For example, the concentration of neochlorogenic acid is 0.3% in buckwheat leaves, much higher than its concentration in grain [40,44].
4. Interaction among Substances in Buckwheat Groats and Impact on Bioactivity
4.1. Impact of Buckwheat Phenolic Substances on Proteins and Cholesterol
During husking and the hydrothermal pretreatment of buckwheat grain, phenolic substances have an impact on protein digestibility [45]. Substantial interactions have been reported between proteins and phenolic substances, and as such, the digestibility of buckwheat proteins is reduced. In any way, microbial processes in the large intestine enhance the digestion of proteins [45]. It was established that phenolic substances in buckwheat grain lower the digestibility of proteins. Ikeda et al. [46] reported that tannic acid and catechin significantly inhibit the in vitro peptic and pancreatic digestion of buckwheat globulin. Ikeda et al. [46] and Ikeda and Kishida [47] studied the impact of secondary buckwheat metabolites on the in vitro digestibility of buckwheat grain proteins. It was established that undigested buckwheat proteins can reduce cholesterol levels in serum by increasing the fecal excretion of steroids, which is induced by the binding of steroids to proteins. Ma and Xiong [48] reported that digestion-resistant peptides are largely responsible for the elimination of bile acids and lowering the risk of the appearance of a high level of cholesterol in human serum. In this way, buckwheat proteins, in their interaction with phenolic substances, protect the human cardiovascular system.
4.2. Transformation of Phenolic Substances
The treatment of Tartary buckwheat grain with water impacts the transformation of rutin to quercetin by the rutin-degrading enzyme (Figure 3) [49,50,51,52]. The activity of the rutin-degrading enzyme is prevented when buckwheat grains are exposed to temperatures at about 80 °C or higher during husking treatment [52].

Figure 3.
Transformation of rutin to quercetin and sugar rutinose.
Ingested quercetin can cross the blood–brain barrier and accumulate in the brain tissue [53]. Indeed, important bioactivities have been established for quercetin and its derivatives, not just in blood vessels, muscle, and the gastrointestinal system, but also in the brain. Quercetin and other phenolic metabolites have been isolated from the stool samples of people who had eaten food rich in phenolic substances [53].
Phenolic compounds are often transformed in the gut before their absorption. The gut microbiota are essential in this process [54]. Large-sized dietary phenolic substances are poorly absorbed, while small-sized products of microbial conversion are more easily absorbed in the colon. It is interesting to note the suitability of buckwheat groats for feeding dogs. There are nutritional differences between buckwheat groats that are husked raw and those that are husked after the precooking of the grain. The first are toxic to dogs and cause them liver damage, and the second can be safely consumed [55]. Carnivores are susceptible to low-molecular-weight tannins, but not to high-molecular-weight tannins. Conversion occurs in the case of precooked buckwheat groats, but not in groats obtained without precooking.
4.3. Tartary Buckwheat Diet in the Prevention of Diabetes
The complexation of quercetin in Tartary buckwheat materials with molecules of starch has an influence on the in vitro digestibility and physicochemical properties of starch [56]. The effects of quercetin–starch complexation indicate that food products based on Tartary buckwheat have lower starch digestibility. Indeed, quercetin in Tartary buckwheat can reduce body weight, serum triacylglycerols, and low-density lipoprotein. In rats, a diet with 0.1% quercetin was shown to have the significant impact of lowering low-density lipoprotein concentrations in serum, with no such effects on high-density lipoprotein. Tartary buckwheat has also been shown to prevent increases in body weight and fat deposition during high-fat intake in rats, although on the other hand, this was reported to protect against hepatic stenosis [10,11,57]. A buckwheat diet can also reduce insulin and ameliorate glucose intolerance in humans [58].
Rat experiments with common buckwheat have further suggested the complexity of the impact of the gut microbiota. Indeed, Peng et al. [57] suggested that the link between weight gain and the gut microbiota is very complex, with a need for further studies. Luo et al. [59] studied the slow digestion properties of Tartary buckwheat starch treated with ethanol extract. The slow digestibility of this starch appeared to be due to the impact of phenolic substances on starch. In the in vivo experiments, mice showed reduced postprandial glycemic responses. The data of Luo et al. [59] for Tartary buckwheat grain and glycemic responses were similar to those obtained earlier in common buckwheat [58].
4.4. Buckwheat Groats in a Health-Preserving Diet
In Japan, buckwheat groats are less well known than in Europe [3]. However, soup with buckwheat groats (soba-gome) is served in Tokushima on Shikoku island [60]. In China, Tartary buckwheat groats are known in some regions, for example, in Shanxi and Shaanxi [3].
Many dishes can be made with common or Tartary buckwheat kasha [61,62]. Some attractive dishes can be made simply by replacing white rice or husked barley with common or Tartary buckwheat kasha (Figure 4). It is possible to cook buckwheat kasha one day, preserve it overnight in the refrigerator, and mix it into oil-fried vegetables (sliced pumpkins, tomatoes, etc.) the next day, or mix it with cottage cheese, cream, and/or walnuts, and/or sliced apples for baking in the oven [61]. In addition to rutin, buckwheat dishes, especially Tartary buckwheat dishes, contain metabolites such as tannins and quercetin, which also inhibit starch degradation [4,63,64]. As a food rich in quercetin, Tartary buckwheat groats may hold nutraceutic potential against SARS-CoV-2 due to their ability to inhibit the virus at various stages of its life cycle [65,66].

Figure 4.
One of the simplest dishes with Tartary buckwheat kasha is Tartary buckwheat soup. About 10 percent (volume) Tartary buckwheat kasha is added to very lightly salted water, boiled for few minutes, and a few spoons of spicy olive oil are added.
4.5. Bioactivity Impact of Metabolites
During the treatment of Tartary buckwheat groat, the concentrations of bioactive substances with strong impacts on human health are altered. This includes the concentration of rutin and quercetin in buckwheat groats (Table 1).
The supplementation of rutin-rich diets with vitamin C is able to reduce oxidative stress, have an impact on glycemic stress, and reduce fasting blood glucose in patients with type 2 diabetes [10]. A rutin-rich Tartary buckwheat diet may be effective in reducing body weight through its antioxidant effects [11,13]. The fermentation of dietary fiber from Tartary buckwheat helps to improve its solubility, in addition to the impact of flavonoids on countering obesity [13].
Phenolic substances in buckwheat have significant bioactivities in addition to their antioxidative and anti-inflammatory effects [43]. Phenolic substances incorporated in the hydrophilic erythrocyte membranes are barriers against free radicals. This is possible because of the double nature of phenolic substances: a lipophilic phenolic aglycone and a hydrophilic sugar part. Buckwheat flavonoid extracts have an impact on the antioxidant system in the liver and brain [44].
Ingested quercetin and its glycosides are metabolized during human digestion, and are absorbed and transported as conjugates with the blood. A major metabolite of quercetin is quercetin-glucuronide, which is transported to the target tissues. Following the separation of the sugar part of hydrophilic quercetin-glucuronide, the hydrophobic aglycone remains at injured sites to perform the improvement of pathological conditions [53].
High levels of rutin and quercetin in Tartary buckwheat, and high levels of antioxidative impacts, have further effects on the cytotoxic and antigenotoxic impacts [64,67]. A study of the antigenotoxic effects of Tartary buckwheat in human hepatoma cell lines has shown that flavonoid-rich Tartary buckwheat products are more effective for maintaining health in their complexed form than as the single active substances, rutin or quercetin [67]. It has been suggested that the Tartary buckwheat metabolites rutin and quercetin may be effective against cancers, based on experiments in cell lines and animal models for mammary, colon, skin, and other cancers [68,69,70,71,72,73].
5. Conclusions and Future Perspectives
The grain and groat of common buckwheat contain a low concentration of rutin, while its concentration in the grain of Tartary buckwheat could be up to 1%. The solar UV irradiation of plants may increase the amount of rutin and other UV-absorbing secondary metabolites in buckwheat grain.
During the processing of buckwheat grain using hydrothermal treatment to obtain husked product (groats or kasha), there are various possible transformations and interactions among the constituents, including the formation of quercetin, as a degradation product of rutin by rutin-degrading enzyme. The activity of rutin-degrading enzyme is prevented by hydrothermal treatment of buckwheat grain products at about 80 °C or more.
In Tartary buckwheat groats, quercetin complexation with starch molecules has an impact on the in vitro digestibility of the starch and the appearance of resistant starch, thus altering the physicochemical properties of Tartary buckwheat products. The effects of such flavonoid–starch or flavonoid–protein complexation indicate that food products based on Tartary buckwheat will show lower starch or protein digestibility. Quercetin in Tartary buckwheat can reduce body weight, serum triacylglycerols, and low-density lipoprotein. During digestion in the large intestine, due to protein–phenol interactions of the buckwheat grain, protein digestion is slowed down. However, again, the microbial processes in the colon partly enhance the digestibility of the proteins. Slowly digestible buckwheat groat protein can reduce cholesterol levels in serum by increasing the fecal excretion of steroids. Tartary buckwheat has also been shown to prevent increases in body weight and fat deposition during high fat intake in rats. A buckwheat diet can also reduce insulin and ameliorate glucose intolerance in humans. Important bioactivities have been established for ingested quercetin and its derivatives, not just in blood vessels, muscle, and the gastrointestinal system, but also in the brain.
Further understanding of the metabolism of rutin and quercetin in buckwheat plants and food products, and how different forms of flavonoids impact the health of consumers, should prove useful in achieving better human health. Hopefully, continuous progress on the wider use of Tartary buckwheat and its products, including groats, over time can fulfill the yield gap needed to feed our global community with a health-preserving diet.
Author Contributions
Conceptualization, I.K., A.G., B.V. and M.G.; data curation, A.G. and B.V.; validation, writing original draft preparation, review and editing, all authors equally responsible; visualization, A.G.; supervision, I.K.; project administration and funding acquisition, I.K. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was the result of a study financed by the Slovenian Research Agency, through the programs P1-0212 “Biology of Plants” and P3-0395 “Nutrition and Public Health”, projects J1-3014, J4-3091, and the applied project L4-9305, cofinanced by the Ministry of Agriculture, Forestry and Food, Republic of Slovenia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary Buckwheat Genome Provides Insights into Rutin Biosynthesis and Abiotic Stress Tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-F.; Song, X.-Y.; Xie, G.; Tang, Y.-N.; Wortley, A.H.; Qin, F.; Blackmore, S.; Li, C.-S.; Wang, Y.-F. New Insights into the Origin of Buckwheat Cultivation in Southwestern China from Pollen Data. New Phytol. 2022, 237, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I. Bitter Seed Tartary Buckwheat; Kreft, I., Ed.; Slovenian Academy of Sciences and Arts; Fagopyrum–Slovenian Association for Buckwheat Promotion: Ljubljana, Slovenia, 2022. [Google Scholar]
- Kreft, I. Grenko Seme Tatarske Ajde; Slovenska Akademija Znanosti in Umetnosti: Ljubljana, Slovenia, 2020. [Google Scholar]
- Kreft, I.; Zhou, M.; Golob, A.; Germ, M.; Likar, M.; Dziedzic, K.; Luthar, Z. Breeding Buckwheat for Nutritional Quality. Breed. Sci. 2020, 70, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Park, M.O.; Kim, H.J.; Choi, I.Y.; Park, C.H. Development and Utilization of Buckwheat Sprouts in Korea. Fagopyrum 2022, 39, 19–26. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Gambelli, L.; Fabjan, N.; Kreft, I. Trace Elements in Flour and Bran from Common and Tartary Buckwheat. Food Chem. 2003, 83, 1–5. [Google Scholar] [CrossRef]
- Bonafaccia, G.; Marocchini, M.; Kreft, I. Composition and Technological Properties of the Flour and Bran from Common and Tartary Buckwheat. Food Chem. 2003, 80, 9–15. [Google Scholar] [CrossRef]
- Fabjan, N.; Rode, J.; Kosǐr, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary Buckwheat (Fagopyrum tataricum Gaertn.) as a Source of Dietary Rutin and Quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef]
- Ragheb, S.R.; El Wakeel, L.M.; Nasr, M.S.; Sabri, N.A. Impact of Rutin and Vitamin C Combination on Oxidative Stress and Glycemic Control in Patients with Type 2 Diabetes. Clin. Nutr. ESPEN 2020, 35, 128–135. [Google Scholar] [CrossRef]
- Nishimura, M.; Ohkawara, T.; Sato, Y.; Satoh, H.; Suzuki, T.; Ishiguro, K.; Noda, T.; Morishita, T.; Nishihira, J. Effectiveness of Rutin-Rich Tartary Buckwheat (Fagopyrum tataricum Gaertn.) ‘Manten-Kirari’ in Body Weight Reduction Related to Its Antioxidant Properties: A Randomised, Double-Blind, Placebo-Controlled Study. J. Funct. Foods 2016, 26, 460–469. [Google Scholar] [CrossRef]
- Li, H.; Lv, Q.; Liu, A.; Wang, J.; Sun, X.; Deng, J.; Chen, Q.; Wu, Q. Comparative Metabolomics Study of Tartary (Fagopyrum tataricum (L.) Gaertn) and Common (Fagopyrum esculentum Moench) Buckwheat Seeds. Food Chem. 2022, 371, 131125. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, K.; Guo, W.; Zhang, C.; Chen, H.; Xu, T.; Lu, Y.; Wu, Q.; Li, Y.; Chen, Y. Aspergillus Niger Fermented Tartary Buckwheat Ameliorates Obesity and Gut Microbiota Dysbiosis through the NLRP3/Caspase-1 Signaling Pathway in High-Fat Diet Mice. J. Funct. Foods 2022, 95, 105171. [Google Scholar] [CrossRef]
- Dohm, J.C.; Minoche, A.E.; Holtgräwe, D.; Capella-Gutiérrez, S.; Zakrzewski, F.; Tafer, H.; Rupp, O.; Sörensen, T.R.; Stracke, R.; Reinhardt, R.; et al. The Genome of the Recently Domesticated Crop Plant Sugar Beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Janeš, D.; Prosen, H.; Kreft, I.; Kreft, S. Aroma Compounds in Buckwheat (Fagopyrum esculentum Moench) Groats, Flour, Bran, and Husk. Cereal Chem. 2010, 87, 141–143. [Google Scholar] [CrossRef]
- Scheucher, S. Buchweizen in Österreich: Geschichtliches und Kulinarisches. In Das Buchweizenbuch; Islek ohne Grenzen: Islek, Luxemburg, 1999. [Google Scholar]
- Valvasor, J.V. Die Ehre Dess Hertzogthums Crain, Das Ist, Wahre, Gründliche, und Recht Eigendliche Belegen- und Beschaffenheit Dieses; Römisch-Keyserlichen herrlichen Erblandes: Ljubljana, Slovenia, 1689. [Google Scholar]
- Niu, Q.; Li, J.; Messia, M.C.; Li, X.; Zou, L.; Hu, X. Selenium and Flavonoids in Selenium-Enriched Tartary Buckwheat Roasted Grain Tea: Their Distribution and Correlation to Antioxidant Activity. LWT 2022, 170, 114047. [Google Scholar] [CrossRef]
- Li, H.; Lv, Q.; Ma, C.; Qu, J.; Cai, F.; Deng, J.; Huang, J.; Ran, P.; Shi, T.; Chen, Q. Metabolite Profiling and Transcriptome Analyses Provide Insights into the Flavonoid Biosynthesis in the Developing Seed of Tartary Buckwheat (Fagopyrum tataricum). J. Agric. Food Chem. 2019, 67, 11262–11276. [Google Scholar] [CrossRef] [PubMed]
- Dietrych-Szostak, D.; Oleszek, W. Effect of Processing on the Flavonoid Content in Buckwheat (Fagopyrum Esculentum Möench) Grain. J. Agric. Food Chem. 1999, 47, 4384–4387. [Google Scholar] [CrossRef]
- Jiang, P.; Burczynski, F.; Campbell, C.; Pierce, G.; Austria, J.A.; Briggs, C.J. Rutin and Flavonoid Contents in Three Buckwheat Species Fagopyrum esculentum, F. tataricum, and F. homotropicum and Their Protective Effects against Lipid Peroxidation. Food Res. Int. 2007, 40, 356–364. [Google Scholar] [CrossRef]
- Ghimeray, A.K.; Sharma, P.; Briatia, X. Phenolic Content and Free Radical Scavenging Activity of Seed, Seedling and Sprout of Buckwheat. In Proceedings of the International Symposium of Buckwheat Sprouts—Developement and Utilization of Buckwheat Sprouts as Medicinal Natural Products, Bongpyoung, Republic of Korea, 7–9 September 2009; Park, C.H., Kreft, I., Eds.; pp. 41–45. [Google Scholar]
- Ölschläger, C.; Treutter, D.; Zeller, F.J. Breeding Buckwheat (Fagopyrum esculentum Moench) for Flavonoids. In Proceedings of the 9th International Symposium on Buckwhe, Advances in Buckwheat Research, Prague, Czech Republic, 18–22 August 2004; pp. 674–678. [Google Scholar]
- Briggs, C.J.; Campbell, C.; Pierce, G.; Jiang, P. Bioflavonoid Analysis and Antioxidant Properties of Tartary Buckwheat Accessions. In Proceedings of the 9th International Symposium on Buckwheat, Advances in Buckwheat Research, Prague, Czech Republic, 18–22 August 2004; pp. 593–597. [Google Scholar]
- Morishita, T.; Yamaguchi, H.; Degi, K. Plant Production Science The Contribution of Polyphenols To Antioxidative Activity In Common Buckwheat and Tartary Buckwheat Grain. Plant Prod. Sci. 2007, 10, 99–104. [Google Scholar] [CrossRef]
- Fabjan, N. Zel in Zrnje Tatarske Ajde Kot Vir Flavonoidov; Biotehniška fakulteta Univerze v Ljubljani: Ljubljana, Slovenia, 2007. [Google Scholar]
- Kreft, I.; Fabjan, N.; Yasumoto, K. Rutin Content in Buckwheat (Fagopyrum esculentum Moench) Food Materials and Products. Food Chem. 2006, 98, 508–512. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X. Determination of Rutin Content on Chinese Buckwheat Cultivars. In Proceedings of the 10th International Symposium on Buckwheat, Yangling, China, 1 January 2007; pp. 465–468. [Google Scholar]
- Lee, M.H.; Lee, J.S.; Lee, T.H. Germination of Buckwheat Grain: Effects on Minerals, Rutin, Tannins and Colour. In Proceedings of the 9th International Symposium on Buckwheat, Advances in Buckwheat Research, Prague, Czech Republic, 18–22 August 2004; pp. 50–54. [Google Scholar]
- Chai, Y.; Feng, B.; Hu, Y.G.; Gao, J.; Gao, X. Analysis on the Variation of Rutin Content in Different Buckwheat Genotypes. In Proceedings of the 9th International Symposium on Buckwheat, Advances in Buckwheat Research, Prague, Czech Republic, 18–22 August 2004; pp. 688–691. [Google Scholar]
- Park, B.J.; Park, J.I.; Chang, K.J.; Park, C.H. Comparison in Rutin Content in Seed and Plant of Tartary Buckwheat (Fagopyrum tataricum). In Proceedings of the 9th International Symposium on Buckwheat, Advances in Buckwheat Research, Prague, Czech Republic, 18–22 August 2004; pp. 626–629. [Google Scholar]
- Suzuki, T.; Kim, S.-J.; Takigawa, S.; Mukasa, Y.; Hashimoto, N.; Saito, K.; Noda, T.; Matsuura-Endo, C.; Zaidul, I.S.M.; Yamauchi, H. Changes in Rutin Concentration and Flavonol-3-Glucosidase Activity during Seedling Growth in Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Can. J. Plant Sci. 2007, 87, 83–87. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Flavonoids and Antioxidative Activities in Buckwheat. J. Agric. Food Chem. 1996, 44, 1746–1750. [Google Scholar] [CrossRef]
- Steadman, K.J.; Burgoon, M.S.; Lewis, B.A.; Edwardson, S.E.; Obendorf, R.L. Minerals, Phytic Acid, Tannin and Rutin in Buckwheat Seed Milling Fractions. J. Sci. Food Agric. 2001, 81, 1094–1100. [Google Scholar] [CrossRef]
- Soon Mi, K.; Park, J.I.; Park, B.J.; Chang, K.J.; Park, C.H. Flavonoid Content and Antioxidant Activity of Tartary Buckwheat. In Proceedings of the International Forum on Tartary Industrial Economy, Beijing, China, 1 January 2006; pp. 149–153. [Google Scholar]
- Park, C.H.; Kim, Y.B.; Choi, Y.S.; Heo, K.; Kim, S.L.; Lee, K.C.; Chang, K.J.; Lee, H.B. Rutin Content in Food Products Processed from Groats, Leaves, and Flowers of Buckwheat. Fagopyrum 2000, 17, 63–66. [Google Scholar]
- Liu, Y.; Cai, C.; Yao, Y.; Xu, B. Alteration of Phenolic Profiles and Antioxidant Capacities of Common Buckwheat and Tartary Buckwheat Produced in China upon Thermal Processing. J. Sci. Food Agric. 2019, 99, 5565–5576. [Google Scholar] [CrossRef]
- Kočevar Glavač, N.; Stojilkovski, K.; Kreft, S.; Park, C.H.; Kreft, I. Determination of Fagopyrins, Rutin, and Quercetin in Tartary Buckwheat Products. LWT-Food Sci. Technol. 2017, 79, 423–427. [Google Scholar] [CrossRef]
- Tian, Q.; Li, D.; Patil, B.S. Identification and Determination of Flavonoids in Buckwheat (Fagopyrum esculentum Moench, Polygonaceae) by High-Performance Liquid Chromatography with Electrospray Ionisation Mass Spectrometry and Photodiode Array Ultraviolet Detection. Phytochem. Anal. 2002, 13, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Vollmannová, A.; Musilová, J.; Lidiková, J.; Árvay, J.; Šnirc, M.; Tóth, T.; Bojňanská, T.; Čičová, I.; Kreft, I.; Germ, M. Concentrations of Phenolic Acids Are Differently Genetically Determined in Leaves, Flowers, and Grain of Common Buckwheat (Fagopyrum esculentum Moench). Plants 2021, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.D.; Ma, Y.J.; Parry, J.; Gao, J.M.; Yu, L.L.; Wang, M. Phenolics Content and Antioxidant Activity of Tartary Buckwheat from Different Locations. Molecules 2011, 16, 9850–9867. [Google Scholar] [CrossRef]
- Germ, M.; Árvay, J.; Vollmannová, A.; Tóth, T.; Kreft, I.; Golob, A. Hydrothermal Treatments Affecting the Concentration of Neochlorogenic Acid in Dough of Tartary Buckwheat. Agriculture 2020, 10, 601. [Google Scholar] [CrossRef]
- Włoch, A.; Strugała, P.; Pruchnik, H.; Żyłka, R.; Oszmiański, J.; Kleszczyńska, H. Physical Effects of Buckwheat Extract on Biological Membrane In Vitro and Its Protective Properties. J. Membr. Biol. 2016, 249, 155–170. [Google Scholar] [CrossRef]
- Sadauskiene, I.; Liekis, A.; Bernotiene, R.; Sulinskiene, J.; Kasauskas, A.; Zekonis, G. The Effects of Buckwheat Leaf and Flower Extracts on Antioxidant Status in Mouse Organs. Oxid. Med. Cell. Longev. 2018, 2018, 6712407. [Google Scholar] [CrossRef]
- Škrabanja, V.; Lærke, H.N.; Kreft, I. Protein-Polyphenol Interactions and in Vivo Digestibility of Buckwheat Groat Proteins. Pflugers Arch. Eur. J. Physiol. 2000, 440, R129–R131. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Oku, M.; Kusano, T.; Yasumoto, K. Inhibitory Potency of Plant Antinutrients towards the In Vitro Digestibility of Buckwheat Protein. J. Food Sci. 1986, 51, 1527–1530. [Google Scholar] [CrossRef]
- Ikeda, K.; Kishida., M. Digestibility of Proteins in Buckwheat Seed. Fagopyrum 1993, 13, 21–24. [Google Scholar]
- Ma, Y.; Xiong, Y.L. Antioxidant and Bile Acid Binding Activity of Buckwheat Protein in Vitro Digests. J. Agric. Food Chem. 2009, 57, 4372–4380. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Morishita, T.; Takigawa, S.; Noda, T.; Ishiguro, K. Characterization of Rutin-Rich Bread Made with ‘Manten-Kirari’, a Trace-Rutinosidase Variety of Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Food Sci. Technol. Res. 2015, 21, 733–738. [Google Scholar] [CrossRef][Green Version]
- Suzuki, T.; Morishita, T.; Kim, S.J.; Park, S.U.; Woo, S.H.; Noda, T.; Takigawa, S. Physiological Roles of Rutin in the Buckwheat Plant. Japan Agric. Res. Q. JARQ 2015, 49, 37–43. [Google Scholar] [CrossRef]
- Suzuki, T.; Morishita, T.; Noda, T.; Ishiguro, K. Acute and Subacute Toxicity Studies on Rutin-Rich Tartary Buckwheat Dough in Experimental Animals. J. Nutr. Sci. Vitaminol. 2015, 61, 175–181. [Google Scholar] [CrossRef][Green Version]
- Germ, M.; Árvay, J.; Vollmannová, A.; Tóth, T.; Golob, A.; Luthar, Z.; Kreft, I. The Temperature Threshold for the Transformation of Rutin to Quercetin in Tartary Buckwheat Dough. Food Chem. 2019, 283, 28–31. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and Related Polyphenols: New Insights and Implications for Their Bioactivity and Bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Lineva, A.; Benković, E.T.; Kreft, S.; Kienzle, E. Remarkable Frequency of a History of Liver Disease in Dogs Fed Homemade Diets with Buckwheat. Tierarztl. Prax. Ausg. K Kleintiere. Heimtiere 2019, 47, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, S.; Ji, X.; Liu, H.; Liu, N.; Yang, J.; Lu, M.; Han, L.; Wang, M. Evaluation Studies on Effects of Quercetin with Different Concentrations on the Physicochemical Properties and in Vitro Digestibility of Tartary Buckwheat Starch. Int. J. Biol. Macromol. 2020, 163, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of Tartary Buckwheat, Rutin, and Quercetin on Lipid Metabolism in Rats during High Dietary Fat Intake. Food Sci. Nutr. 2020, 8, 199–213. [Google Scholar] [CrossRef]
- Škrabanja, V.; Liljeberg Elmståhl, H.G.M.; Kreft, I.; Björck, I.M.E. Nutritional Properties of Starch in Buckwheat Products: Studies in Vitro and in Vivo. J. Agric. Food Chem. 2000, 49, 490–496. [Google Scholar] [CrossRef]
- Luo, K.; Zhou, X.; Zhang, G. The Impact of Tartary Buckwheat Extract on the Nutritional Property of Starch in a Whole Grain Context. J. Cereal Sci. 2019, 89, 102798. [Google Scholar] [CrossRef]
- Ikeda, K.; Ikeda, S. Buckwheat in Japan. In Ethnobotany of Buckwheat; Kreft, I., Chang, K.J., Choi, Y.S., Park, C.H., Eds.; Jinsol Publishing Co.: Seoul, Republic of Korea, 2003; pp. 54–69. [Google Scholar]
- Vombergar, B.; Tašner, L.; Horvat, M.; Vorih, S.; Pem, N.; Golob, S.; Kovač, T. Buckwheat–Challenges in Nutrition and Technology. Fagopyrum 2022, 39, 33–42. [Google Scholar] [CrossRef]
- Vombergar, B.; Luthar, Z. Starting Points for the Study of the Effects of Flavonoids, Tannins and Crude Proteins in Grain Fractions of Common Buckwheat (Fagopyrum esculentum Moench) and Tartary Buckwheat (Fagopyrum tataricum Gaertn.). Folia Biol. Geol. 2018, 59, 101. [Google Scholar] [CrossRef]
- Ikeda, K.; Ishida, Y.; Ikeda, S.; Asami, Y.; Lin, R. Tartary, but Not Common, Buckwheat Inhibits α-Glucosidase Activity: Its Nutritional Implications. Fagopyrum 2017, 34, 13–18. [Google Scholar] [CrossRef]
- Kreft, M. Buckwheat Phenolic Metabolites in Health and Disease. Nutr. Res. Rev. 2016, 29, 30–39. [Google Scholar] [CrossRef]
- Gasmi, A.; Mujawdiya, P.K.; Lysiuk, R.; Shanaida, M.; Peana, M.; Gasmi Benahmed, A.; Beley, N.; Kovalska, N.; Bjørklund, G. Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2. Pharmaceuticals 2022, 15, 1049. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.; Pal, A.; Goswami, K.; Squitti, R.; Rongiolettie, M. Molecular Basis of Quercetin as a Plausible Common Denominator of Macrophage-Cholesterol-Fenofibrate Dependent Potential COVID-19 Treatment Axis. Results Chem. 2021, 3, 100148. [Google Scholar] [CrossRef] [PubMed]
- Vogrinčič, M.; Kreft, I.; Filipič, M.; Žegura, B. Antigenotoxic effect of Tartary (Fagopyrum tataricum) and common (Fagopyrum esculentum) buckwheat flour. J. Med. Food 2013, 16, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Kishida, N.; Kato, N. Consumption of a buckwheat protein extract retards 7,12-dimethylbenz[α]anthracene-induced mammary carcinogenesis in rats. Biosci. Biotechnol. Biochem. 1999, 63, 1837–1839. [Google Scholar] [CrossRef]
- Valido, E.; Stoyanov, J.; Gorreja, F.; Stojic, S.; Niehot, C.; Kiefte-de Jong, J.; Llanaj, E.; Muka, T.; Glisic, M. Systematic Review of Human and Animal Evidence on the Role of Buckwheat Consumption on Gastrointestinal Health. Nutrients 2023, 15, 1. [Google Scholar] [CrossRef]
- Dziedzic, K.; Górecka, D.; Szwengiel, A.; Olejnik, A.; Rychlik, J.; Kreft, I.; Drozdzynska, A.; Walkowiak, J. The cytotoxic effect of artificially digested buckwheat products on HT-29 colon cancer cells. J. Cereal Sci. 2018, 83, 68–73. [Google Scholar] [CrossRef]
- Zhou, X.-L.; Chen, Z.-D.; Zhou, Y.-M.; Shi, R.-H.; Li, Z.-J. The Effect of Tartary Buckwheat Flavonoids in Inhibiting the Proliferation of MGC80-3 Cells during Seed Germination. Molecules 2019, 24, 3092. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef]
- Mansi, K.; Kumar, R.; Narula, D.; Kumar Pandey, S.; Vinod Kumar, V.; Kulvinder Singh, K. Microwave-Induced CuO Nanorods: A Comparative Approach between Curcumin, Quercetin, and Rutin to Study Their Antioxidant, Antimicrobial, and Anticancer Effects against Normal Skin Cells and Human Breast Cancer Cell Lines MCF-7 and T-47D. ACS Appl. Bio. Mater. 2022, 5, 5762–5778. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).