Simulation of Photosynthetic Quantum Efficiency and Energy Distribution Analysis Reveals Differential Drought Response Strategies in Two (Drought-Resistant and -Susceptible) Sugarcane Cultivars
Abstract
1. Introduction
2. Results
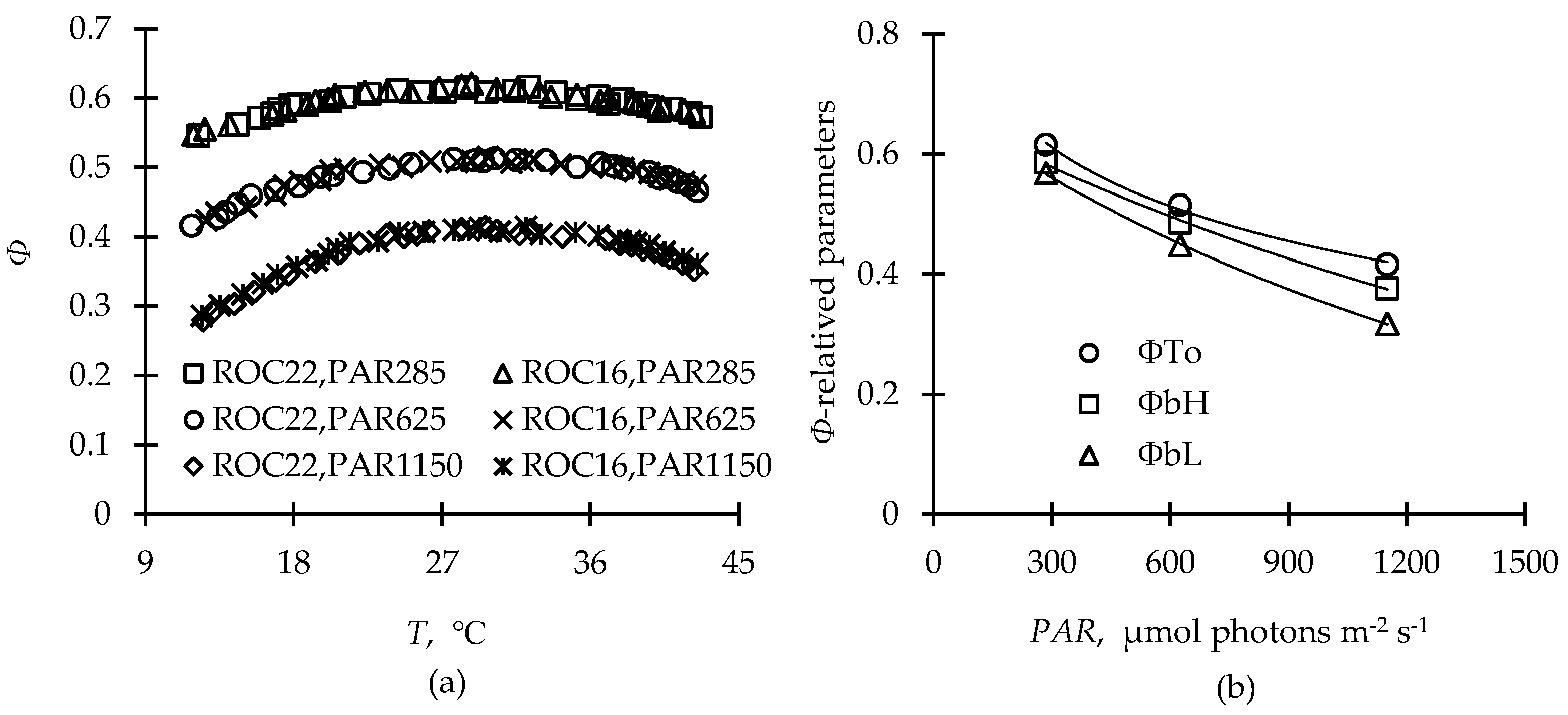
2.1. Photothermal Model Parameterization and Its Biological Significance
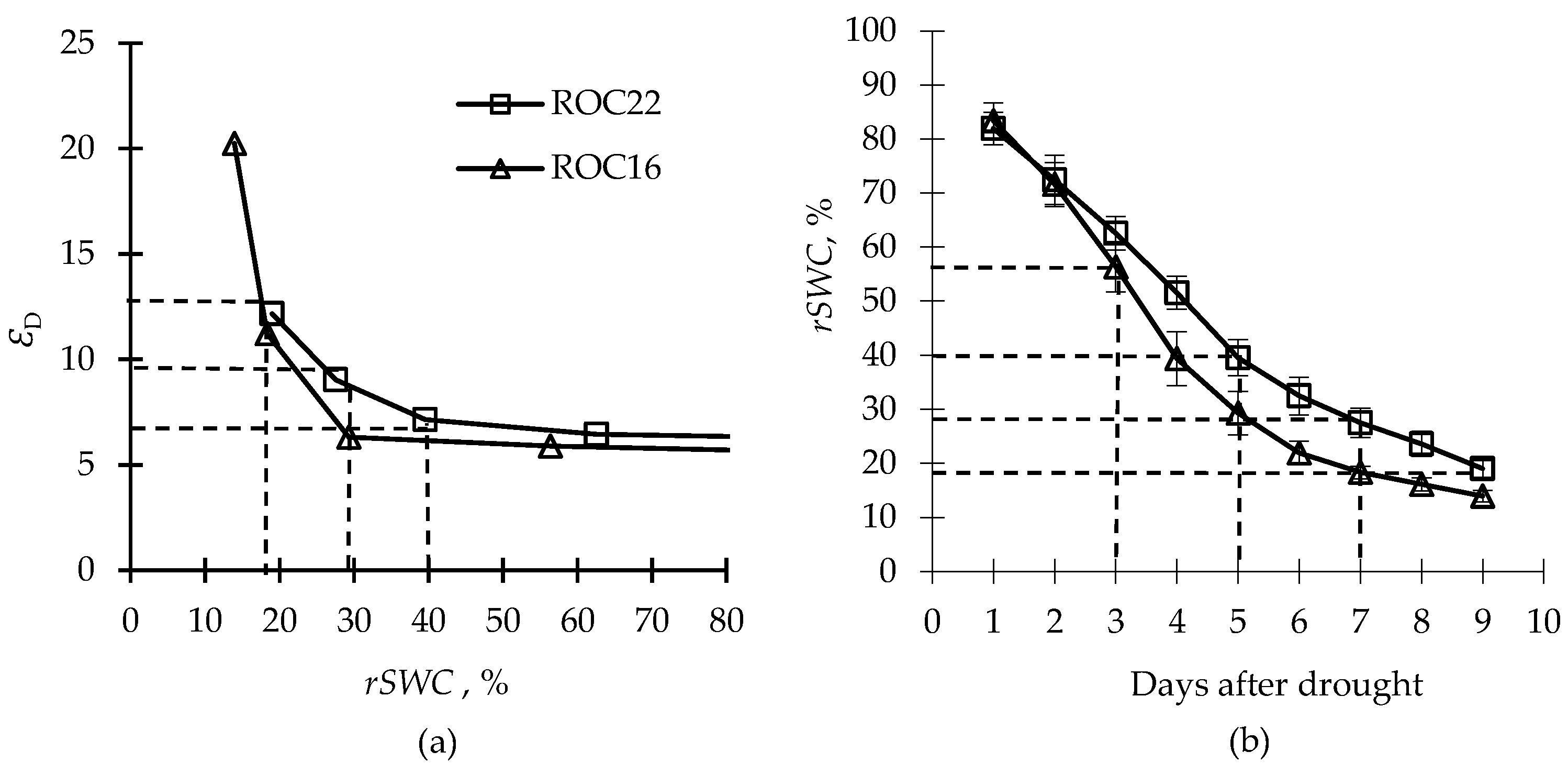
2.2. Response of the Estimated Parameter to Drought Treatment
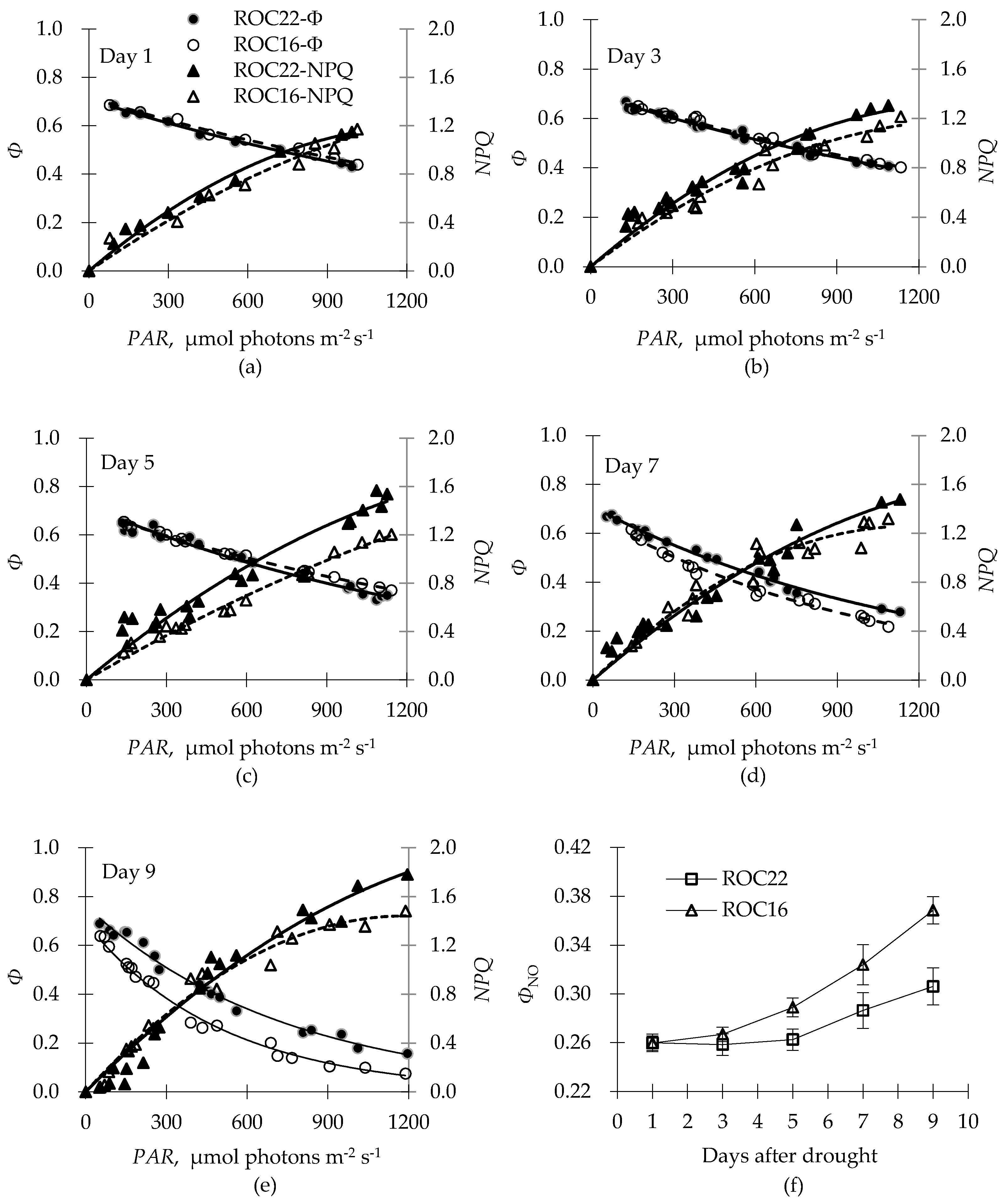
2.3. Energy Distributionin PSII in Response to Drought
2.4. Different Drought-Response Patterns for Two Cultivars
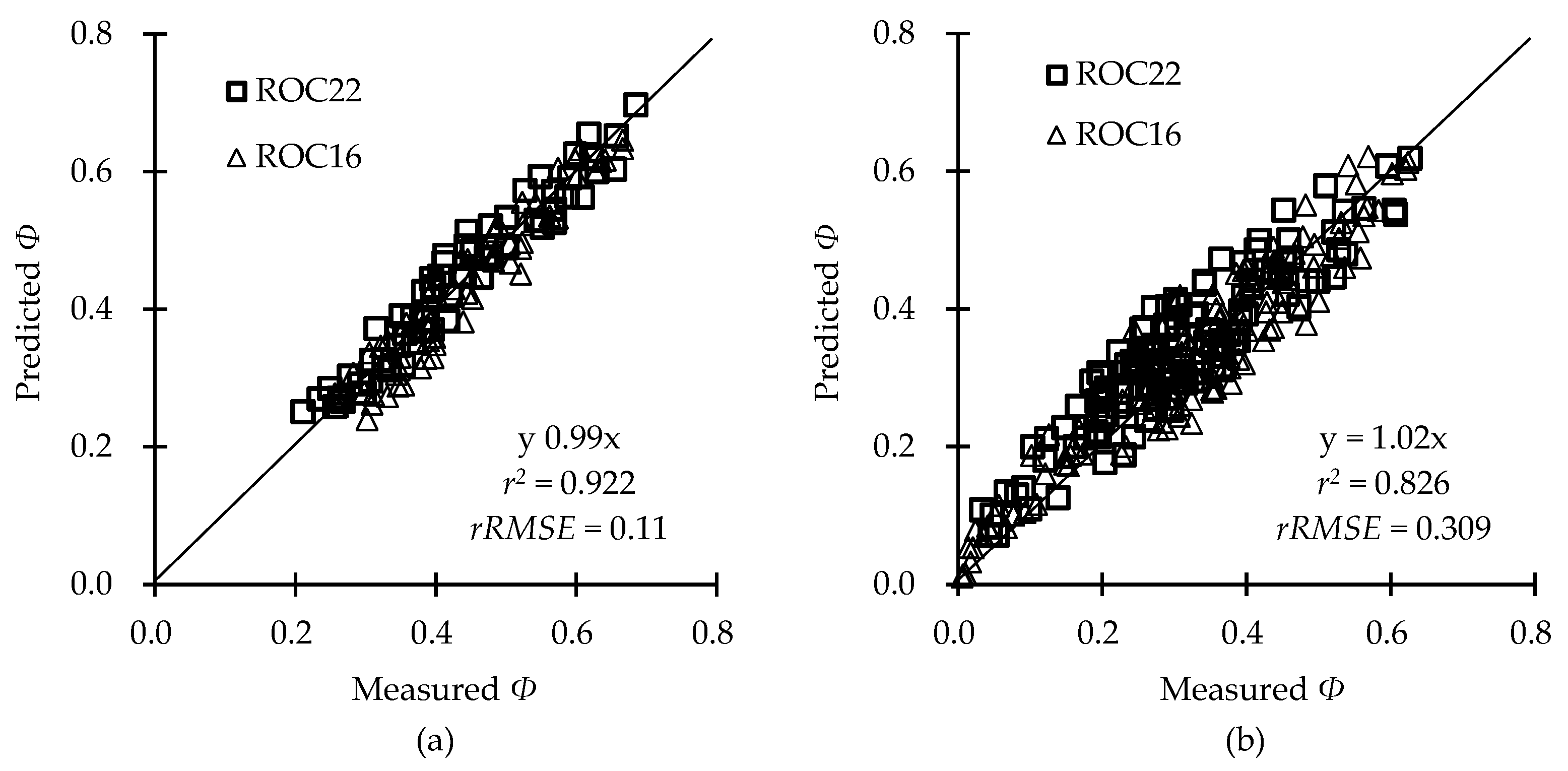
2.5. Model Validation
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Chlorophyll Fluorescence and Substrate Water Content Measurement
4.3. Model Construction
4.3.1. Estimation of Photosynthetic Quantum Efficiency (Φ) under Different Photothermal Conditions
4.3.2. Estimation of the Parameters under Drought Conditions
4.3.3. Model Validation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, M.T.; Seema, N.; Khan, I.A.; Yasmine, S. Applications and potential of sugarcane as an energy crop. Agric. Res. Updates 2017, 16, 1–23. [Google Scholar]
- Huang, W.; Sui, Y.; Yang, X.; Dai, S.; Qu, H.; Li, M. Spatio-temporal characteristics of crop drought in southern China based on drought index of continuous days without available precipitation. Trans. Chin. Soc. Agric. Eng. 2014, 30, 125–135. [Google Scholar]
- Khonghintaisong, J.; Songsri, P.; Toomsan, B.; Jongrungklang, N. Rooting and physiological trait responses to early drought stress of sugarcane cultivars. Sugar Tech 2018, 20, 396–406. [Google Scholar] [CrossRef]
- Misra, V.; Solomon, S.; Mall, A.K.; Prajapati, C.P.; Hashem, A.; Abd_Allah, E.F.; Ansari, M.I. Morphological assessment of water stressed sugarcane: A comparison of waterlogged and drought affected crop. Saudi. J. Biol. Sci. 2020, 27, 1228–1236. [Google Scholar] [CrossRef]
- Chapae, C.; Songsri, P.; Gonkhamdee, S.; Jongrungklang, N. Understanding drought responses of sugarcane cultivars controlled under low water potential conditions. Chil. J. Agric. Res. 2020, 80, 370–380. [Google Scholar] [CrossRef]
- Cia, M.C.; Guimarães, A.C.R.; Medici, L.O.; Chabregas, S.M.; Azevedo, R.A. Antioxidant responses to water deficit bydrought-tolerant and –sensitive sugarcane varieties. Ann. Appl. Biol. 2012, 161, 313–324. [Google Scholar] [CrossRef]
- Sales, C.R.G.; Marchiori, P.E.R.; Machado, R.S.; Fontenele, A.V.; Machado, E.C.; Silveira, J.A.G.; Ribeiro, R.V. Photosyntheticandantioxidantresponsestodroughtduringthesugarcaneripening. Photosynthetica 2015, 53, 547–554. [Google Scholar] [CrossRef]
- Cha-um, S.; Wangmoon, S.; Mongkolsiriwatana, C.; Ashraf, M.; Kirdmanee, C. Evaluatingsugarcane (Saccharum sp.) cultivars for water deficit tolerance using some key physiological markers. Plant Biotechnol. 2012, 29, 431–439. [Google Scholar] [CrossRef]
- Silva, M.D.A.; Silva, J.A.G.D.; Enciso, J.; Sharma, V.; Jifon, J. Yield components as indicators of drought tolerance of sugarcane. Sci. Agric. 2008, 65, 620–627. [Google Scholar] [CrossRef]
- Priji, P.J.; Hemaprabha, G. Sugarcane specific drought responsive candidate genes belonging to ABA dependent pathway identified from basic species clones of Saccharum sp. and Erianthus sp. Sugar Tech 2014, 17, 130–137. [Google Scholar] [CrossRef]
- Liu, D.L.; Bull, T.A. Simulation of biomass and sugar accumulation in sugarcane using a process-based model. Ecol. Model. 2001, 144, 181–211. [Google Scholar] [CrossRef]
- Singels, A.; Bezuidenhout, C.N. A new method of simulating dry matter partitioning in the Canegro sugarcane model. Field Crops Res. 2002, 78, 151–164. [Google Scholar] [CrossRef]
- Singels, A.; Paraskevopoulos, A.L.; Mashabela, M.L. Farm level decision support for sugarcane irrigation management during drought. Agric. Water Manag. 2019, 222, 274–285. [Google Scholar] [CrossRef]
- Inman-Bamber, N.G.; Jackson, P.A.; Stokes, C.J.; Verrall, S.; Lakshmanan, P.; Basnayake, J. Sugarcane for water-limited environments: Enhanced capability of the APSIM sugarcane model for assessing traits for transpiration efficiency and root watersupply. Field Crops Res. 2016, 196, 112–123. [Google Scholar] [CrossRef]
- Lebourgeois, V.; Chopart, J.L.; Bégué, A.; Mézo, L.L. Towards using a thermal infrared index combined with water balance modeling to monitor sugarcane irrigation in a tropical environment. Agric. Water Manag. 2010, 97, 75–82. [Google Scholar] [CrossRef]
- Morel, J.; Bégué, A.; Todoroff, P.; Martiné, J.-F.; Lebourgeois, V.; Petit, M. Coupling asugarcane crop model with the remotely sensed timeseries of fIPAR to optimise the yield estimation. Eur.J.Agron. 2014, 61, 60–68. [Google Scholar] [CrossRef]
- Veysi, S.; Naseri, A.A.; Hamzeh, S.; Bartholomeus, H. A satellite based crop water stress index for irrigation scheduling in sugarcane fields. Agric. Water Manag. 2017, 189, 70–86. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. PlantBiol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Ismail, M.R.; Davies, W.J.; Awad, M.H. Leaf growth and stomatal sensitivity to ABA in droughted pepper plants. Sci. Hortic. 2002, 96, 313–327. [Google Scholar] [CrossRef]
- Tombesi, S.; Nardini, A.; Frioni, T.; Soccolini, M.; Zadra, C.; Farinelli, D.; Poni, S.; Palliotti, A. Stomatal closure is induced byhydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015, 5, 12449. [Google Scholar] [CrossRef]
- Dias, M.C.; Brüggemarm, W. Limitations of photosynthesis in Phaseolus vulgaris under drought stress: Gas exchange, chlorophyll fluorescence and Calvin cycle enzymes. Photosynthetica 2010, 48, 96–102. [Google Scholar] [CrossRef]
- Massacci, A.; Nabiev, S.M.; Pietrosanti, L.; Nematov, S.K.; Chernikova, T.N.; Thor, K.; Leipner, J. Response of the photosynthetic apparatus of cotton (Gossypium hirsutum) to the onset of drought stress under field conditions studied by gas-exchange analysis and chlorophyll fluorescence imaging. Plant Physiol. Biochem. 2008, 46, 189–195. [Google Scholar] [CrossRef]
- Zhu, L.; Xing, Y.; Yang, L.; Li, Y.; Yang, R.; Mo, L. Effects of waters tress on leaf water and chlorophyll fluorescence parameters of sugarcane seedling. Agric. Sci. Technol. 2010, 11, 17–21. [Google Scholar]
- An, D.; Cao, J.; Huang, X.; Zhou, J.; Dou, M. Application of Lake-model based indices from chlorophyll fluorescence on sugarcane seedling drought resistance study. Chin. J. Plant. Ecol. 2015, 39, 398–406. [Google Scholar]
- Silva, M.A.; Pincelli, R.P.; Barbosa, A.M. Water stress effects on chlorophyll fluorescence and chlorophyll content in sugarcane cultivars with contrasting tolerance. Biosci. J. 2018, 34, 75–87. [Google Scholar] [CrossRef]
- Krause, G.H.; Somersalo, S.; Zumbusch, E.; Weyers, B.; Laasch, H. On the mechanism of photoinhibition in chloroplasts. Relationship between changes in fluorescence and activity of PhotosystemII. J. Plant Physiol. 1990, 136, 472–479. [Google Scholar] [CrossRef]
- Naumann, J.C.; Young, D.R.; Anderson, J.E. Linking leaf chlorophyll fluorescence properties to physiological responses for detection of salt and drought stress in coastal plants pecies. Physiol. Plant. 2007, 131, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Yin, X.; Sun, Z.; Struik, P.C.; Putten, P.E.L.V.D.; Ieperen, W.V.; Harbinson, J. Using a biochemical C4 photosynthesis model and combined gas exchange and chlorophyll fluorescence measurements to estimate bundle-sheath conductance of maize leaves differing in age and nitrogen content. Plant Cell Environ. 2011, 34, 2183–2199. [Google Scholar] [CrossRef]
- Derks, A.; Schaven, K.; Bruce, D. Diverse mechanisms for photoprotection in photosynthesis. Dynamic regulation of photosystem II excitation in response to rapid environmental change. Biochim. Biophys. Acta 2015, 1847, 468–485. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, N.; Kaiser, E.; Li, G.; An, D.; Sun, Q.; Chen, W.; Liu, W.; Luo, W. Integrating chlorophyll fluorescence parameters into a crop model improves growth prediction under severe drought. Agric. For. Meteorol. 2021, 303, 108367. [Google Scholar] [CrossRef]
- Xu, R.; Dai, J.; Luo, W.; Yin, X.; Li, Y.; Tai, X.; Han, L.; Chen, Y.; Lin, L.; Li, G.; et al. A photothermal model of leaf area index for greenhouse crops. Agric. For. Meteorol. 2010, 150, 541–552. [Google Scholar] [CrossRef]
- Clarke, J.E.; Johnson, G.N. In vivo temperature dependence of cyclic and pseudocyclic electron transport in barley. Planta 2001, 212, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Quick, W.P.; Bungard, R.A.; Bailey, K.J.; Lea, P.J.; Leegood, R.C. The role of photorespiration during drought stress: Ananalysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant Cell Environ. 1999, 22, 361–373. [Google Scholar] [CrossRef]
- Sagun, J.V.; Badger, M.R.; Chow, W.S.; Ghannoum, O. Mehler reaction plays a role in C3 and C4 photosynthesis under shade and low CO2. Photosynth. Res. 2021, 149, 171–185. [Google Scholar] [CrossRef]
- Asada, K. The water-water cyclein chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Ort, D.R.; Baker, N.R. A photo-protective role for O2 as an alternative electron sink in photosynthesis? Curr. Opin. Plant Biol. 2002, 5, 193–198. [Google Scholar] [CrossRef]
- Flexas, J.; Briantais, J.; Cerovic, Z.; Medrano, H.; Moya, I. Steady-state and maximum chlorophyll fluorescence responses to water stress in grapevine leaves: A new remote sensing system. Remote Sens. Environ. 2000, 73, 283–297. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Chinthapalli, B.; Murmu, J.; Raghavendra, A.S. Dramatic difference in the responses of phosphoenolpyruvate carboxylase to temperature in leaves of C3 and C4 plants. J. Exp. Bot. 2003, 54, 707–714. [Google Scholar] [CrossRef]
- Wang, D.; Naidu, S.L.; Portis, A.R., Jr.; Moose, S.P.; Long, S.P. Can the cold tolerance of C4 photosynthesis in Miscanthus 9 giganteus relative to Zea mays be explained by differences in activities and thermal properties of Rubisco? J. Exp. Bot. 2008, 59, 1779–1787. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Li, X.; Niu, L.; Jameson, P.E.; Zhou, W. Transcription-associated metabolomic adjustments in maize occur during combined drought and cold stress. Plant Physiol. 2021, 186, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, H.; Ballester, A.; Muñoz, R.; Quiles, M.J. Chlororespiration and tolerance to drought, heat and high illumination. J. Plant Physiol. 2010, 167, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheem, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shu, S.; Li, S.; He, L.; Li, H.; Du, N.; Sun, J.; Guo, S. Effects of exogenous putrescine on chlorophyll fluorescence imaging and heat dissipation capacity in cucumber (Cucumis sativus L.) under salt stress. J. Plant Growth Regul. 2014, 33, 798–808. [Google Scholar] [CrossRef]
- Wei, H.; He, W.; Li, Z.; Ge, L.; Zhang, J.; Liu, T. Salt-tolerant endophytic bacterium Enterobacter ludwigiiB30 enhance bermuda grass growth under salt stress by modulating plant physiology and changing rhizosphere and root bacterial community. Front. Plant Sci. 2022, 13, 959427. [Google Scholar] [CrossRef] [PubMed]
- Suárez, J.C.; Vanegas, J.I.; Contreras, A.T.; Anzola, J.A.; Urban, M.O.; Beebe, S.E.; Rao, I.M. Chlorophyll Fluorescence Imaging as a Tool for evaluating disease resistance of common bean lines in the western amazon region of colombia. Plants 2022, 11, 1371. [Google Scholar] [CrossRef]
- Ruban, A.V.; Johnson, M.P.; Duffy, C.D.P. The photoprotective molecular switch in the photosystem II antenna. Biochim. Biophys. Acta 2012, 1817, 167–181. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, S.; Liu, S.; Wang, W.; Sui, N. Energy dissipation and antioxidant enzyme system protect photosystem II of sweet sorghum under drought stress. Photosynthetica 2018, 56, 861–872. [Google Scholar] [CrossRef]
- Liu, M.; Qi, H.; Zhang, Z.; Song, Z.; Kou, T.; Zhang, W.; Yu, Z. Response of photosynthesis and chlorophyll fluorescence to drought stress in two maize cultivars. Afr. J. Agric. Res. 2012, 7, 4751–4760. [Google Scholar]
- Zhang, F.; Zhu, K.; Wang, Y.; Zhang, Z.; Lu, F.; Yu, H.; Zou, J. Changes in photosynthetic and chlorophyll fluorescence characteristics of sorghum under drought and water logging stress. Photosynthetica 2019, 57, 1156–1164. [Google Scholar] [CrossRef]
- Conn, P.F.; Schalch, W.; Truscott, T.G. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. B Biol. 1991, 11, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.M.; Kettunen, R.; Tyystjärvi, E. ATP and light regulate Dl protein modification and degradation role of Dl* inphotoinhibition. FEBS Lett. 1992, 297, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Moinuddin, M.; Gulzar, S.; Hameed, A.; Gul, B.; Khan, M.A.; Edwards, G.E. Differences in photosynthetic syndromes of four halophytic marsh grasses in Pakistan. Photosynth. Res. 2017, 131, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, H.; Zhou, L.; Xu, Z.; Zhou, G. Tracking chlorophyll fluorescence as an indicator of drought and rewatering across the entire leaf life span in a maize field. Agric. Water Manag. 2019, 211, 190–201. [Google Scholar] [CrossRef]
- Zhang, J.; Tardieu, F. Relative Contribution of apices and mature tissues to ABA synthesisin droughted maize root systems. Plant Cell Physiol. 1996, 37, 598–605. [Google Scholar] [CrossRef]
- Campalans, A.; Messeguer, R.; Goday, A.; Pagès, M. Plant responses to drought, from ABA signal transduction events to the action of the induced proteins. Plant Physiol. Biochem. 1999, 37, 327–340. [Google Scholar] [CrossRef]
- Jin, W.; Yang, L.; Ying, P.; Yang, J.; Luo, C.; Li, Y. Eco-physiological responses of different sugarcane varieties to drought and re-watering. J. South. Agric. 2012, 43, 1945–1951. [Google Scholar]
- Wei, J.; Zhou, H.; Li, H.; Mao, L.; Song, X.; Lei, J.; Gui, Y.; Zhang, R.; Li, Y.; Liu, X. Introduction and utilization of sugarcane germplasm resources in Guangxi in the past 40 years. J. South. Agric. 2021, 52, 280–287. [Google Scholar]
- Yao, Y.; Liu, Y.; Su, J.; Lin, X.H. The effects of photosynthetic characters and water use efficiency under drought stress in sugarcane materials. Sugarcane Canesugar 2013, 1, 14–18. [Google Scholar]
- Khalil, F.; Xiao, N.; Tayyab, M.; Chen, P. Screening of EMS-induced drought-tolerant sugarcane mutants employing physiological, molecular and enzymatic approaches. Agronomy 2018, 8, 226. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components-calculation of qP and Fv/Fm without measuring Fo′. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- An, D.S. Chlorophyll Fluorescence Based Water Stress Diagnosis for Cut Lilium Grown in Greenhouse; Nanjing Agriculture University: Nanjing, China, 2011. [Google Scholar]
- Zhang, M.Q.; Lv, J.L.; Chen, R.K. Diurnal variation of photosynthetic rate in sugarcane and its responses to light and temperature. J. Fujian Agric. Univ. 1998, 27, 398–401. [Google Scholar]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Sales, C.R.G.; Ribeiro, R.V.; Silveira, J.A.G.; Machado, E.C.; Martins, M.O.; Lagôa, A.M.M.A. Superoxide dismutase and ascorbate peroxidase improve the recovery of photosynthesis in sugarcane plants subjected to water deficit and low substrate temperature. Plant Physiol. Biochem. 2013, 73, 326–336. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Definition | Unit | Value |
|---|---|---|---|
| aL, bL | Empirical coefficient describing the change of ΦbL with PAR | — | 0.688, 6.77 × 10−4 for both cultivars |
| aH, bH | Empirical coefficient describing the change of ΦbH with PAR | — | 0.676, 5.16 × 10−4 for both cultivars |
| c, d | Empirical coefficient describing the change of ΕD with rSWC when rSWC was below rSWCc | — | 39.63, 10.01 for ROC22 286.78, 21.53 for ROC16 |
| Fv/Fm | Maximum quantum efficiency of PSII photochemistry | — | 0.769 for both cultivars without water deficit |
| NPQ | Nonphotochemical quenching | — | — |
| PAR | Photosynthetically active radiation | μmol photons m−2 s−1 | — |
| rSWC | Relative substrate water content calculated from VWC/VWCs | % | — |
| rSWCc | Critical value of rSWC | % | 40 for ROC22 29 for ROC16 |
| T | Temperature | °C | — |
| Tmin | Minimum temperature for sugarcane photosynthesis and growth | °C | 15 |
| To | Optimum temperature for sugarcane photosynthesis and growth | °C | 30 |
| Tmax | Maximum temperature for sugarcane photosynthesis and growth | °C | 40 |
| VWC | Volumetric water content of substrate | % | — |
| VWCs | Saturated VWC | % | 38 (0.66) for Exp1~3 38.3 (1.11) for Exp4~5 |
| Ε | The amplitude decrease in ΦTo with increasing PAR | — | 5.91 × 10−4 for both cultivars |
| ΕD | Ε varied with rSWC to describe the drought-stress degree | — | — |
| Φ | Photosynthetic quantum efficiency | mol e−1 (mol photon)−1 | — |
| ΦbL | Basic Φ under low temperature, varied with PAR | mol e−1 (mol photon)−1 | — |
| ΦbH | Basic Φ under high temperature, varied with PAR | mol e−1 (mol photon)−1 | — |
| ΦTo | Optimum Φ under To, varied with PAR | mol e−1 (mol photon)−1 | — |
| ΦNO | Yield for other energy losses (indicate non-regulated energy dissipation) | mol e−1 (mol photon)−1 | — |
| Exp1 aM, bV | Exp2 aM, bV | Exp3 aM, bV | Exp4 aV, bV | Exp5 aV, bM | ||
|---|---|---|---|---|---|---|
| Experimental and Environmental Conditions | ||||||
| Photothermal Experiment | Start date | 9 April 2020 | 11 July 2020 | 30 November 2020 | 20 May 2021 | 23 October 2021 |
| Finish date | 11 April 2020 | 13 July 2020 | 2 December 2020 | 22 May 2021 | 25 October 2021 | |
| Average daily high temp (°C) | 24.3 ± 2 | 36.2 ± 1.3 | 21.7 ± 1.8 | 35.2 ± 1.7 | 23.3 ± 4 | |
| Average daily low temp (°C) | 19.4 ± 1.4 | 28.6 ± 0.2 | 11.4 ± 3.3 | 27.5 ± 0.1 | 16.7 ± 1.1 | |
| Average daily PARave (μmol photons m−2 s−1) | 233 ± 129 | 575 ± 61 | 364 ± 27 | 523 ± 139 | 314.6 ± 165 | |
| Average daily PARmax (μmol photons m−2 s−1) | 1477 ± 623 | 2119 ± 66 | 1573 ± 231 | 2085 ± 253 | 1474 ± 647 | |
| Drought Experiment | Start date | 13 April 2020 | 14 July 2020 | 3 December 2020 | 23 May 2021 | 26 October 2021 |
| Finish date | 22 April 2020 | 20 July 2020 | 14 December 2020 | 2 June 2021 | 4 November 2021 | |
| Average daily high temp (°C) | 26.6 ± 2.5 | 35.3 ± 1.2 | 23 ± 1.3 | 34.8 ± 1.2 | 28.2 ± 0.8 | |
| Average daily low temp (°C) | 20 ± 4.7 | 27.1 ± 1 | 15.3 ± 2.8 | 26.9 ± 1.1 | 21.4 ± 2.1 | |
| Average daily PARave (μmol photons m−2·s−1) | 341 ± 124 | 535 ± 60 | 262 ± 93 | 498 ± 74 | 321 ± 81 | |
| Average daily PARmax (μmol photons m−2 s−1) | 1896 ± 388 | 2313 ± 138 | 1441 ± 322 | 2412 ± 208 | 2012 ± 311 | |
| Physicochemical Properties of the Substrate | ||||||
| Bulk density (g·cm−3) | 1.19 | 1.19 | 1.19 | 1.21 | 1.21 | |
| Saturated volumetric water content (%) | 38 | 38 | 38 | 38.3 | 38.3 | |
| Organic C (g·kg−1) | 17.51 | 17.51 | 17.51 | 19.22 | 19.22 | |
| Avaliable N (mg·kg−1) | 36.34 | 36.34 | 36.34 | 33.88 | 33.88 | |
| Avaliable P (mg·kg−1) | 15.62 | 15.62 | 15.62 | 12.96 | 12.96 | |
| Avaliable K (mg·kg−1) | 44.70 | 44.70 | 44.70 | 46.16 | 46.16 | |
| pH | 4.82 | 4.82 | 4.82 | 5.07 | 5.07 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, D.; Zhao, B.; Liu, Y.; Xu, Z.; Kong, R.; Yan, C.; Su, J. Simulation of Photosynthetic Quantum Efficiency and Energy Distribution Analysis Reveals Differential Drought Response Strategies in Two (Drought-Resistant and -Susceptible) Sugarcane Cultivars. Plants 2023, 12, 1042. https://doi.org/10.3390/plants12051042
An D, Zhao B, Liu Y, Xu Z, Kong R, Yan C, Su J. Simulation of Photosynthetic Quantum Efficiency and Energy Distribution Analysis Reveals Differential Drought Response Strategies in Two (Drought-Resistant and -Susceptible) Sugarcane Cultivars. Plants. 2023; 12(5):1042. https://doi.org/10.3390/plants12051042
Chicago/Turabian StyleAn, Dongsheng, Baoshan Zhao, Yang Liu, Zhijun Xu, Ran Kong, Chengming Yan, and Junbo Su. 2023. "Simulation of Photosynthetic Quantum Efficiency and Energy Distribution Analysis Reveals Differential Drought Response Strategies in Two (Drought-Resistant and -Susceptible) Sugarcane Cultivars" Plants 12, no. 5: 1042. https://doi.org/10.3390/plants12051042
APA StyleAn, D., Zhao, B., Liu, Y., Xu, Z., Kong, R., Yan, C., & Su, J. (2023). Simulation of Photosynthetic Quantum Efficiency and Energy Distribution Analysis Reveals Differential Drought Response Strategies in Two (Drought-Resistant and -Susceptible) Sugarcane Cultivars. Plants, 12(5), 1042. https://doi.org/10.3390/plants12051042









