Effects of Sugarcane Leaf Return and Fertilizer Reduction on Maize Growth, Yield and Soil Properties in Red Soil
Abstract
1. Introduction
2. Results
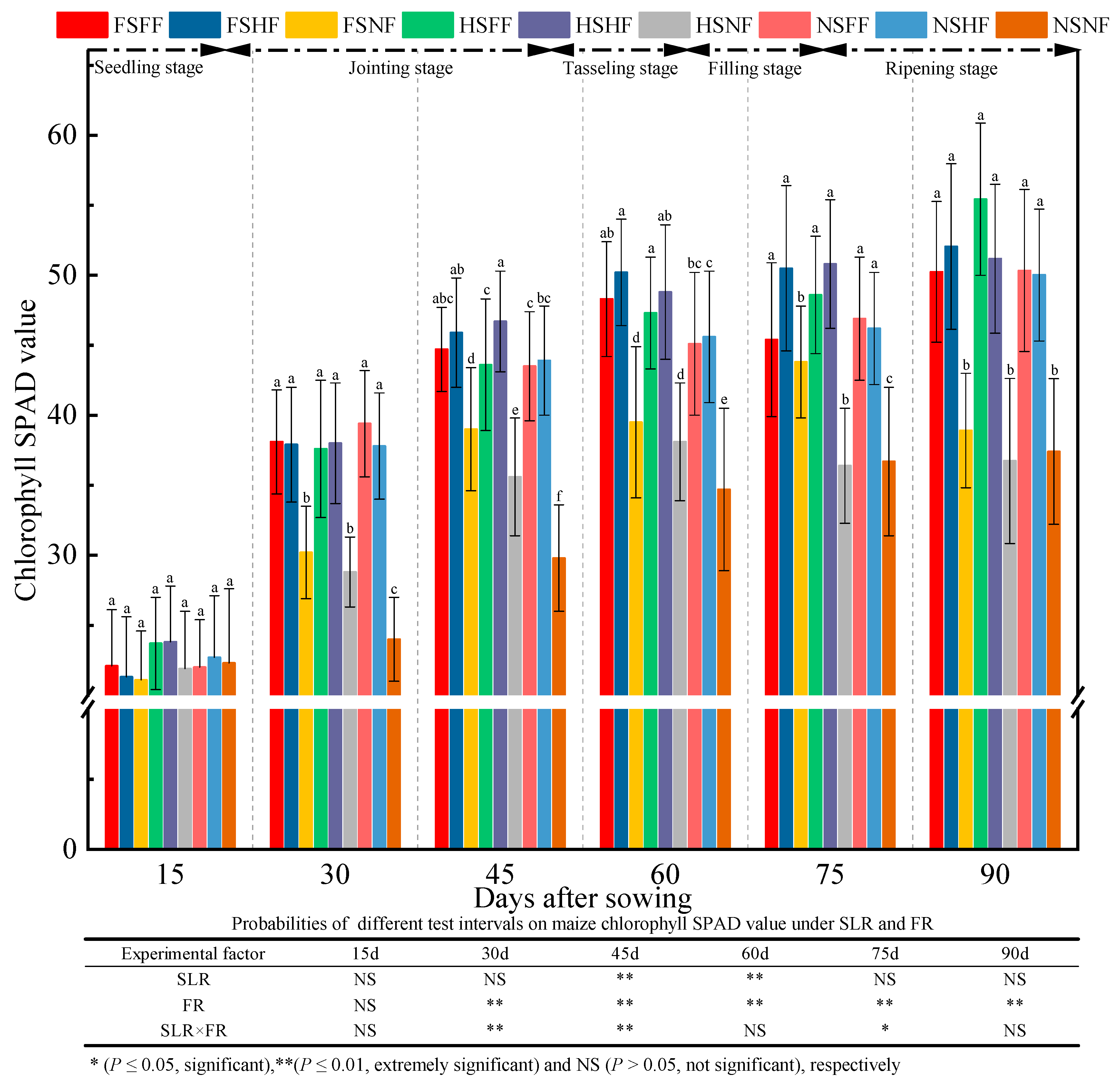
2.1. Maize Growth
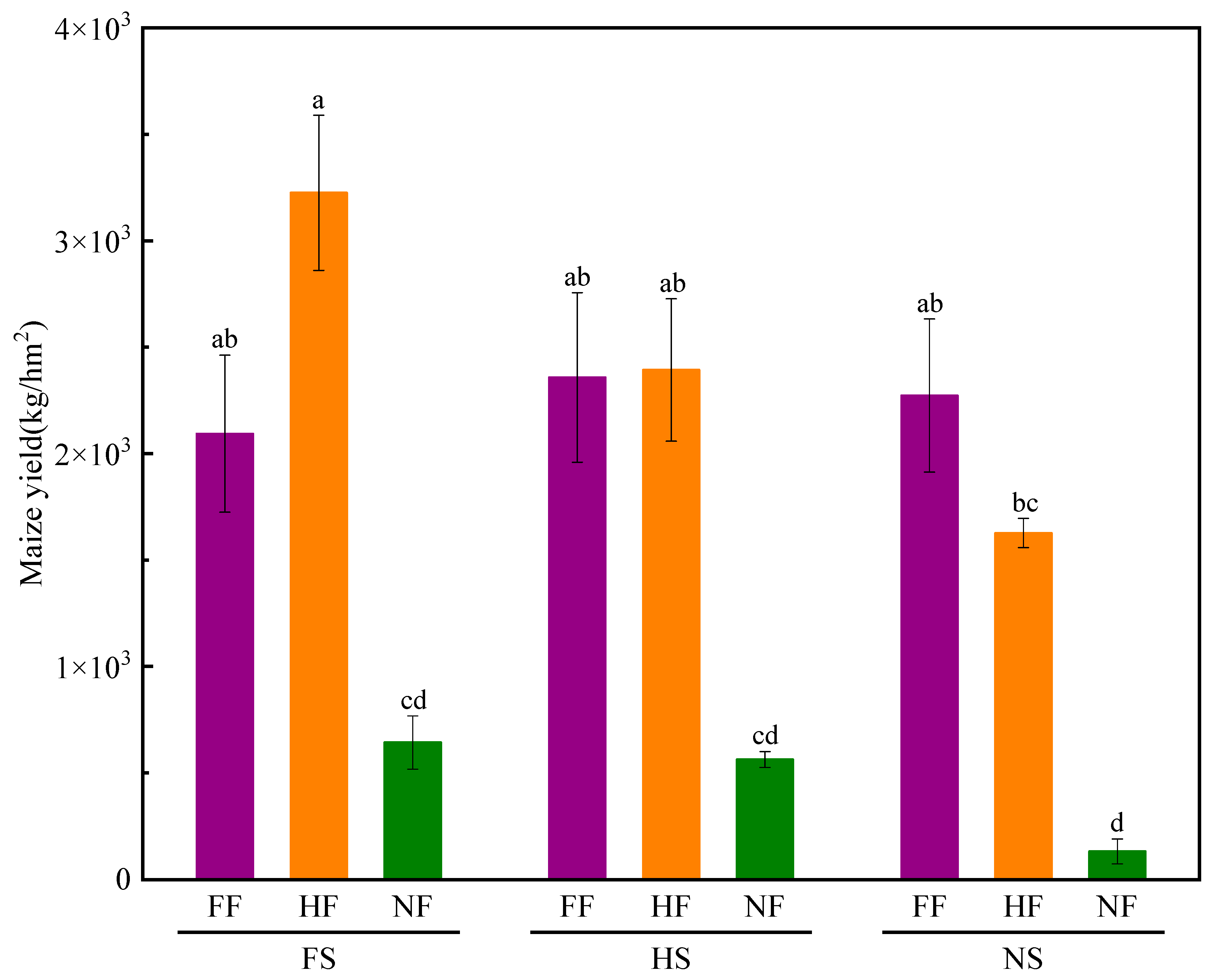
2.2. Maize Yield Component Factors and Yield
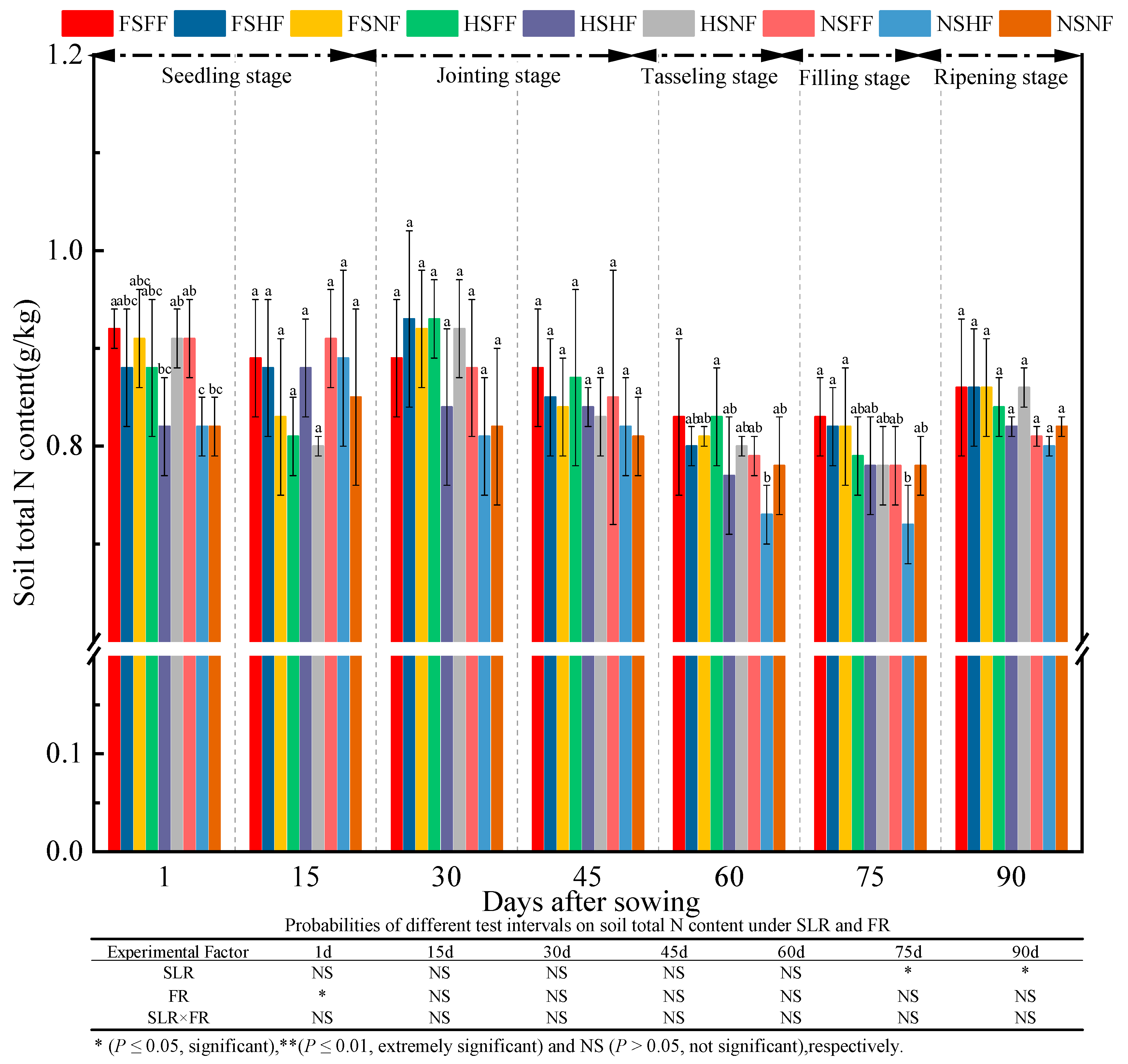
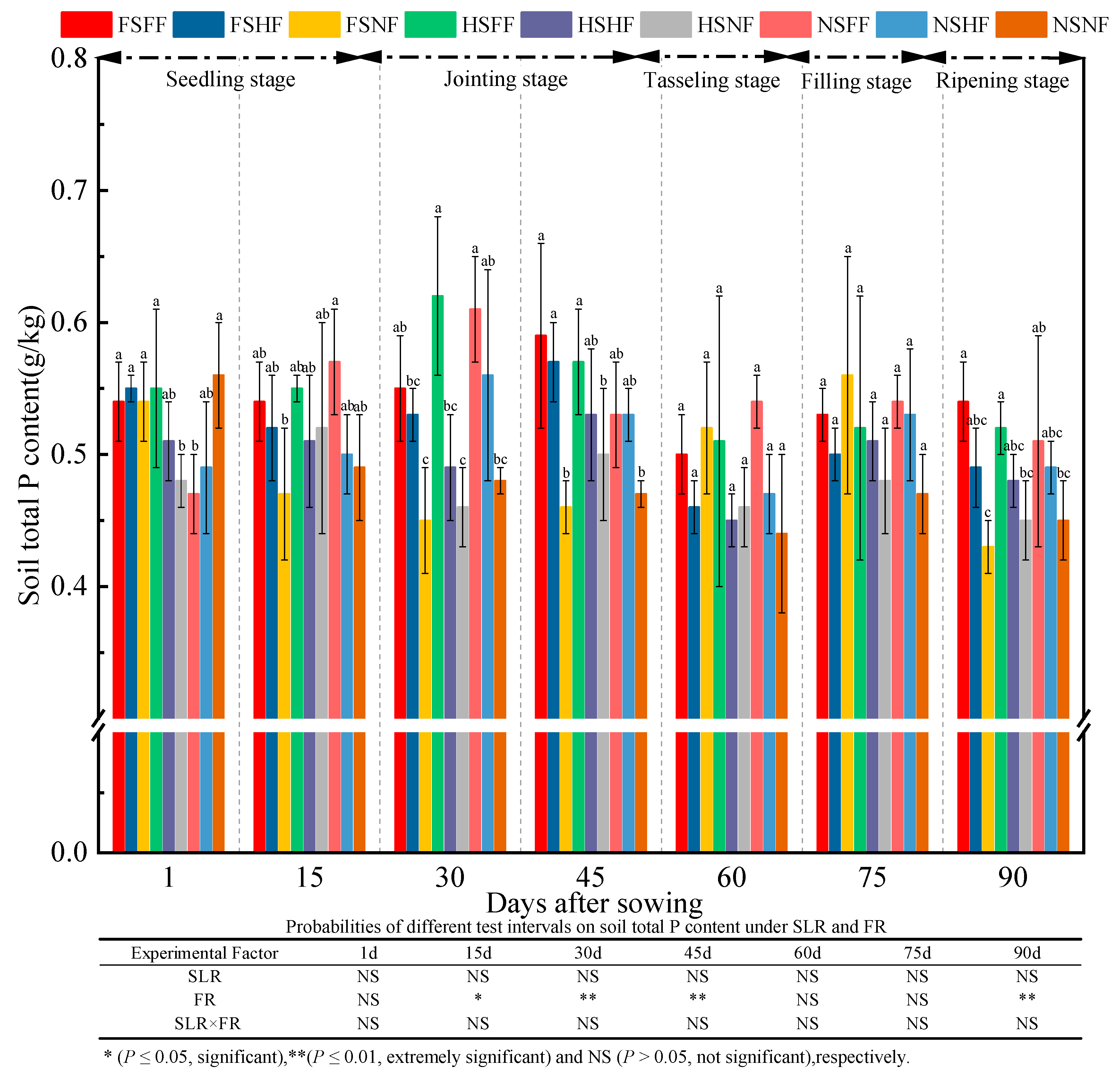
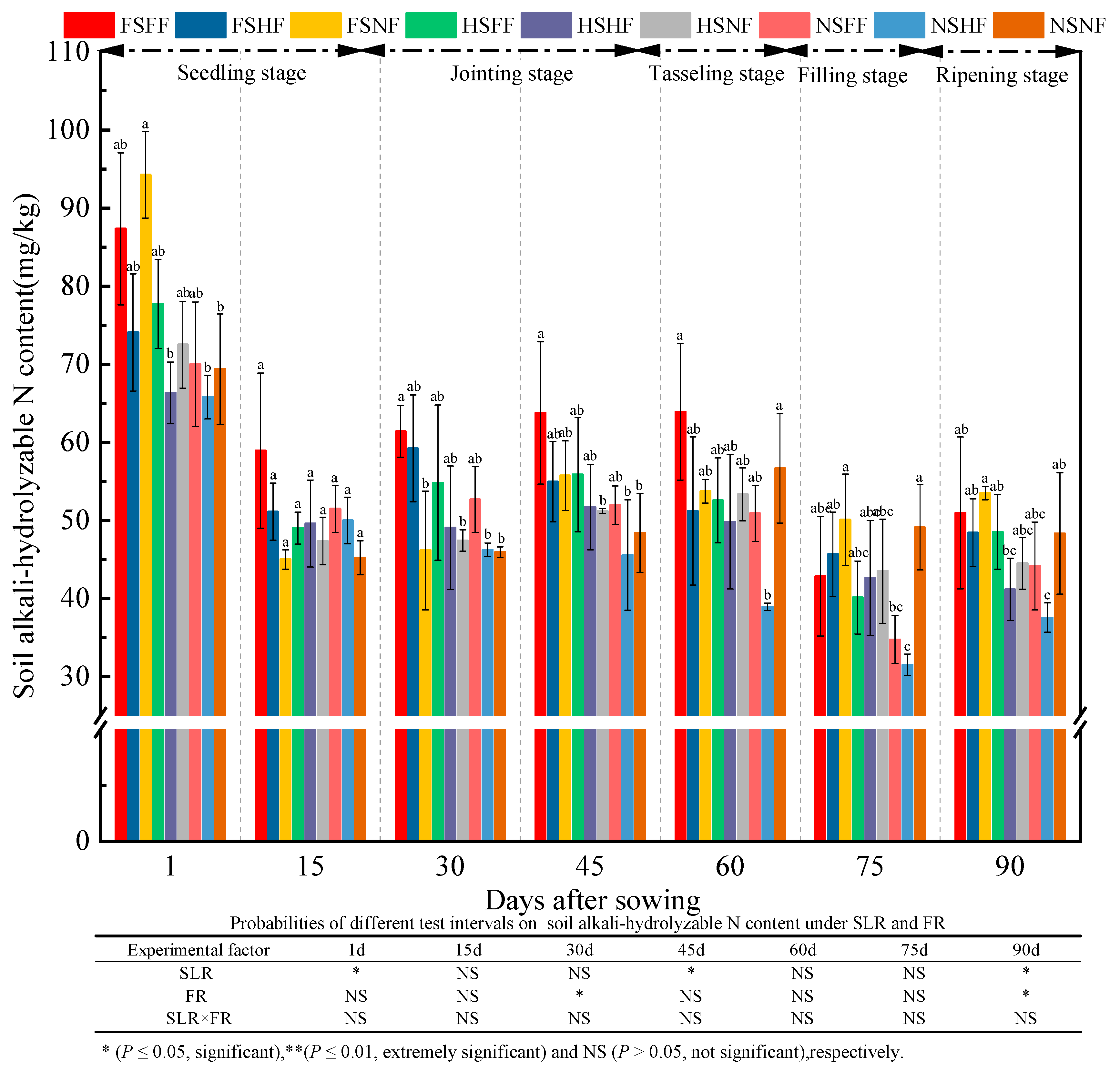
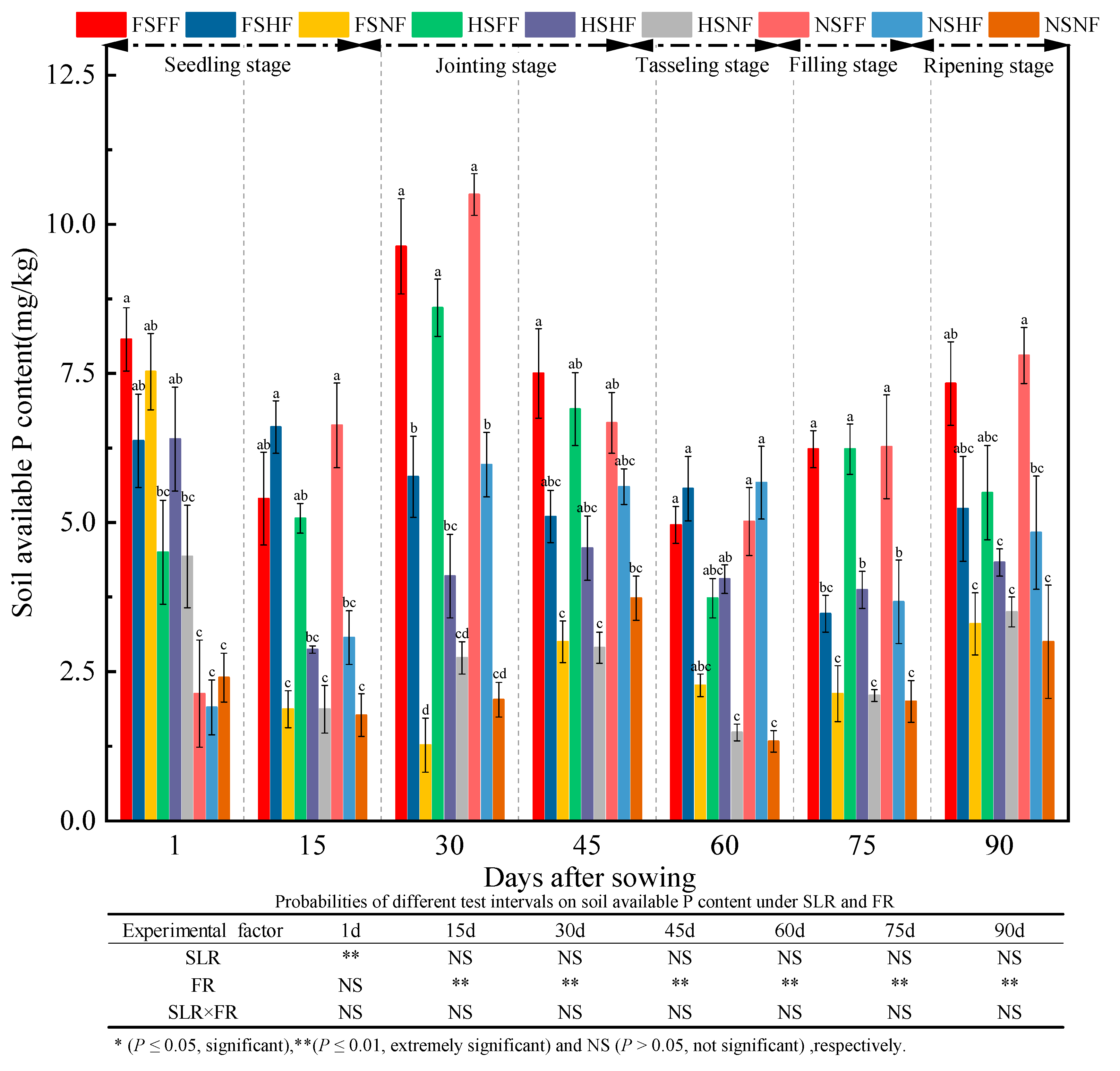
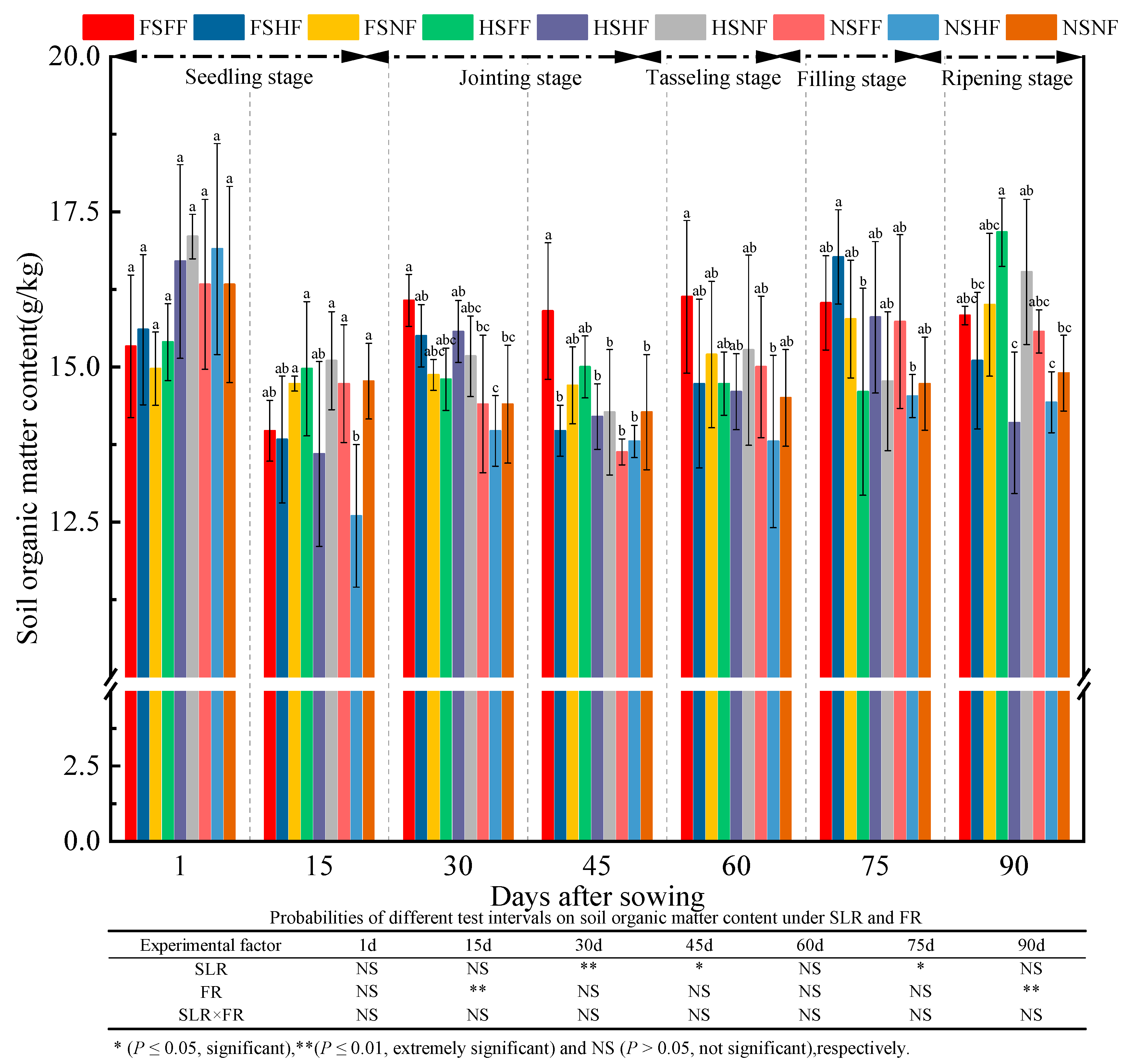
2.3. Soil Properties
2.4. Correlation Analysis between Maize Growth and Soil Properties
3. Discussion
3.1. Impacts of SLR and FR on Maize Growth
3.2. Impacts of SLR and FR on Maize Yield Component Factors and Yield
3.3. Impacts of SLR and FR on Soil Properties
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design
4.3. Plant Measurements
4.3.1. Maize Growth
4.3.2. Maize Yield Component Factors and Yield
4.4. Soil Sampling and Measurements
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Zhou, W.; Yang, J.; Ao, J.; Huang, Y.; Shen, D.; Jiang, Y.; Huang, Z.; Shen, H. Effects of Seaweed Extracts on the Growth, Physiological Activity, Cane Yield and Sucrose Content of Sugarcane in China. Front. Plant Sci. 2021, 12, 659130. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J. Discussion on Mechanization Technology of Sugarcane Production in Guangxi. Int. J. Manag. Educ. Hum. Dev. 2022, 2, 148–150. [Google Scholar]
- Chen, B.; He, Z.; Liang, Q.; Tang, X.; Lin, T.; Liu, Y. Comparative Analysis of Sugar Production Cost in Guangxi and World Major Producing Countries. Asian Agric. Res. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Qi-Zhan, T.; Gui-Fen, C.; Zhong, L.; Cheng-Yan, M.; Ming-Hua, G. Several sugarcane cultivars residues quantity and nutrient content of the residue. Soil Fertil. 2009, 2, 67–70. [Google Scholar]
- Li, Y.M.; Du, Y.X.; Zhou, Y.S.; Zhang, S.Y. Research Progress of Sugarcane Leaves. Food Ind. 2018, 39, 237–239. [Google Scholar]
- Gullett, B.K.; Touati, A.; Huwe, J.; Hakk, H. PCDD and PCDF Emissions from Simulated Sugarcane Field Burning. Environ. Sci. Technol. 2006, 40, 6228–6234. [Google Scholar] [CrossRef]
- Razafimbelo, T.; Barthes, B.; Larré-Larrouy, M.; De Luca, E.F.; Laurent, J.; Cerri, C.C.; Feller, C. Effect of sugarcane residue management (mulching versus burning) on organic matter in a clayey Oxisol from southern Brazil. Agric. Ecosyst. Environ. 2006, 115, 285–289. [Google Scholar] [CrossRef]
- Dai, H.; Wang, Y.; Wen, F.; Tang, Y. Analysis and Experiment of Flow Field in Sugarcane Leaf Cutting and Returning Machine. IOP Conf. Series Mater. Sci. Eng. 2020, 735, 12079. [Google Scholar] [CrossRef]
- Chen, L.; Sun, S.; Yao, B.; Peng, Y.; Gao, C.; Qin, T.; Zhou, Y.; Sun, C.; Quan, W. Effects of straw return and straw biochar on soil properties and crop growth: A review. Front. Plant Sci. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Sha, Z.; Ma, X.; Wang, J.; Lv, T.; Li, Q.; Misselbrook, T.; Liu, X. Effect of N stabilizers on fertilizer-N fate in the soil-crop system: A meta-analysis. Agric. Ecosyst. Environ. 2020, 290, 106763. [Google Scholar] [CrossRef]
- Silva, A.G.B.; Lisboa, I.P.; Cherubin, M.R.; Cerri, C.E.P. How Much Sugarcane Straw is Needed for Covering the Soil? Bioenerg. Res. 2019, 12, 858–864. [Google Scholar] [CrossRef]
- Galdos, M.; Cavalett, O.; Seabra, J.E.; Nogueira, L.A.H.; Bonomi, A. Trends in global warming and human health impacts related to Brazilian sugarcane ethanol production considering black carbon emissions. Appl. Energy 2013, 104, 576–582. [Google Scholar] [CrossRef]
- Cerri, C.C.; Galdos, M.V.; Maia, S.M.F.; Bernoux, M.; Feigl, B.J.; Powlson, D.; Cerri, C.E.P. Effect of sugarcane harvesting systems on soil carbon stocks in Brazil: An examination of existing data. Eur. J. Soil Sci. 2011, 62, 23–28. [Google Scholar] [CrossRef]
- Leite, L.F.C.; Sagrilo, E.; de Araújo, A.S.F.; de Souza, H.A. Short-term effect of sugarcane straw on soil organic carbon pools. Embrapa Meio-Norte-Artig. Periódico Indexado 2018, 8, 405–416. [Google Scholar] [CrossRef]
- Gmach, M.R.; Scarpare, F.V.; Cherubin, M.R.; Lisboa, I.P.; Dos Santos, A.K.B.; Cerri, C.E.P.; Cerri, C.C. Sugarcane straw removal effects on soil water storage and drainage in southeastern Brazil. J. Soil Water Conserv. 2019, 74, 466–476. [Google Scholar] [CrossRef]
- Fortes, C.; Trivelin, P.C.O.; Vitti, A.C. Long-term decomposition of sugarcane harvest residues in Sao Paulo state, Brazil. Biomass Bioenergy 2012, 42, 189–198. [Google Scholar] [CrossRef]
- Adalberto, C.G.; Roberto, C.M.; Santos, M.; Martineli, S.G.; Oliveira, B.; Carneiro, B.L.; Junqueira, F.; Nunes, C. Soil physical quality response to sugarcane straw removal in Brazil: A multi-approach assessment. Soil Tillage Res. 2018, 184, 301–309. [Google Scholar]
- Rossetto, R.; Cantarella, H.; Dias, F.; Landell, M.; Vitti, A.C. Conservation management and nutrient recycling in sugarcane with a view to mechanical harvesting. Inf. Agronômicas 2008, 124, 8–13. [Google Scholar]
- Cardoso, T.D.F.; Cavalett, O.; Chagas, M.F.; Morais, E.R.D.; Carvalho, J.L.N.; Franco, H.C.J.; Galdos, M.V.; Scarpare, F.V.; Braunbeck, O.A.; Cortez, L.A.B.; et al. Technical and economic assessment of trash recovery in the sugarcane bioenergy production system. Sci. Agric. 2013, 70, 353–360. [Google Scholar] [CrossRef]
- Trivelin, P.C.O.; Franco, H.C.J.; Otto, R.; Ferreira, D.A.; Vitti, A.C.; Fortes, C.; Faroni, C.E.; Oliveira, E.C.A.; Cantarella, H. Impact of sugarcane trash on fertilizer requirements for São Paulo, Brazil. Sci. Agric. 2013, 70, 345–352. [Google Scholar] [CrossRef]
- Magalhães, P.; Nogueira, L.; Canatarella, H.; Rossetto, R.; Franco, H.; Braunbeck, O.A. Agro-industrial technological paths. In Sustainability of Sugarcane Bioenergy by Center of Strategic Studies and Management; CGEE: Brasília, Brazil, 2012; pp. 27–69. [Google Scholar]
- Berhane, M.; Xu, M.; Liang, Z.; Shi, J.; Wei, G.; Tian, X. Effects of long-term straw return on soil organic carbon storage and sequestration rate in North China upland crops: A meta-analysis. Glob. Chang. Biol. 2020, 26, 2686–2701. [Google Scholar] [CrossRef]
- Yang, Z.C.; Zhao, N.; Huang, F.; Lv, Y.Z. Long-term effects of different organic and inorganic fertilizer treatments on soil organic carbon sequestration and crop yields on the North China Plain. Soil Tillage Res. 2015, 146, 47–52. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, A.; Jiang, W.; Chen, X.; Jin, R.; Zhao, P.; Tang, Z. Effects of wheat straw incorporation and nitrogen application on sweetpotato yield and quality and soil fertility. Arch. Acker Pflanzenbau Bodenkd. 2021, 67, 1822–1833. [Google Scholar] [CrossRef]
- Han, W.; He, M. The application of exogenous cellulase to improve soil fertility and plant growth due to acceleration of straw decomposition. Bioresour. Technol. 2010, 101, 3724–3731. [Google Scholar] [CrossRef]
- Shindo, H.; Nishio, T. Immobilization and remineralization of N following addition of wheat straw into soil: Determination of gross N transformation rates by 15N-ammonium isotope dilution technique. Soil Biol. Biochem. 2005, 37, 425–432. [Google Scholar] [CrossRef]
- Muhammad, W.; Vaughan, S.M.; Dalal, R.C.; Menzies, N.W. Crop residues and fertilizer nitrogen influence residue decomposition and nitrous oxide emission from a Vertisol. Biol. Fertil. Soils 2011, 47, 15–23. [Google Scholar] [CrossRef]
- Ludemann, C.I.; Gruere, A.; Heffer, P.; Dobermann, A. Global data on fertilizer use by crop and by country. Sci. Data 2022, 9, 501. [Google Scholar] [CrossRef]
- Shah, A.N.; Iqbal, J.; Tanveer, M.; Yang, G.; Hassan, W.; Fahad, S.; Yousaf, M.; Wu, Y. Nitrogen fertilization and conservation tillage: A review on growth, yield, and greenhouse gas emissions in cotton. Environ. Sci. Pollut. Res. 2017, 24, 2261–2272. [Google Scholar] [CrossRef]
- Tian, H.; Lu, C.; Melillo, J.; Ren, W.; Huang, Y.; Xu, X.; Liu, M.; Zhang, C.; Chen, G.; Pan, S.; et al. Food benefit and climate warming potential of nitrogen fertilizer uses in China. Environ. Res. Lett. 2012, 7, 44020. [Google Scholar] [CrossRef]
- Wang, Q.; Noor, H.; Sun, M.; Ren, A.; Feng, Y.; Qiao, P.; Zhang, J.; Gao, Z. Wide space sowing achieved high productivity and effective nitrogen use of irrigated wheat in South Shanxi, China. PeerJ 2022, 10, e13727. [Google Scholar] [CrossRef] [PubMed]
- van Wesenbeeck, C.F.A.; Keyzer, M.A.; van Veen, W.C.M.; Qiu, H. Can China’s overuse of fertilizer be reduced without threatening food security and farm incomes? Agric. Syst. 2021, 190, 103093. [Google Scholar] [CrossRef]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef]
- Sabir, M.S.; Shahzadi, F.; Ali, F.; Shakeela, Q.; Niaz, Z.; Ahmed, S. Comparative Effect of Fertilization Practices on Soil Microbial Diversity and Activity: An Overview. Curr. Microbiol. 2021, 78, 3644–3655. [Google Scholar] [CrossRef]
- Meng, H.; Xu, M.; Lv, J.; He, X.H.; Wang, B.; Cai, Z. Quantification of Anthropogenic Acidification Under Long-term Fertilization in the Upland Red Soil of South China. Soil Sci. 2014, 179, 486–494. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Zeng, L.; Xu, J.; Wu, L. Impacts of continuous excessive fertilization on soil potential nitrification activity and nitrifying microbial community dynamics in greenhouse system. J. Soil Sediment 2017, 17, 471–480. [Google Scholar] [CrossRef]
- Qiao, J.; Wang, J.; Zhao, D.; Zhu, N.; Tang, J.; Zhou, W.; Schwenke, G.; Yan, T.; Yang, L. Effect of continuous N fertilizer reduction on N losses and wheat yield in the Taihu Lake region, China. J. Clean. Prod. 2022, 364, 132475. [Google Scholar] [CrossRef]
- Hu, S.; Wu, Y.; Yi, N.; Zhang, S.; Zhang, Y.; Xin, X. Chemical properties of dissolved organic matter derived from sugarcane rind and the impacts on copper adsorption onto red soil. Environ. Sci. Pollut. Res. 2017, 24, 21750–21760. [Google Scholar] [CrossRef]
- Huang, J.; Duan, Y.; Xu, M.; Zhai, L.; Zhang, X.; Wang, B.; Zhang, Y.; Gao, S.; Sun, N. Nitrogen mobility, ammonia volatilization, and estimated leaching loss from long-term manure incorporation in red soil. J. Integr. Agric. 2017, 16, 2082–2092. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, G.; Shi, W.; Shi, J.; Yang, B.; Lu, S.; Zhao, Q. Sustainable development of crop farming in Lingchuan County of Guangxi. Acta Ecol. Sin. 2014, 34, 5164–5172. [Google Scholar] [CrossRef]
- Wei, D.; Zhao, X.; Wei, J.; Hu, G.; Wu, X.; Cheng, M.; Wen, J.; Mo, Y. Effects of Planting Dates on Growth and Yield of Sugarcane in Red Soil. J. Anhui Agric. Sci. 2022, 50, 30–32. [Google Scholar] [CrossRef]
- Thuy, N.H.; Shan, Y.; Bijay-Singh; Wang, K.; Cai, Z.; Yadvinder-Singh; Buresh, R.J. Nitrogen Supply in Rice-Based Cropping Systems as Affected by Crop Residue Management. Soil Sci. Soc. Am. J. 2008, 72, 514–523. [Google Scholar] [CrossRef]
- Liu, S.; Huang, D.; Chen, A.; Wei, W.; Brookes, P.C.; Li, Y.; Wu, J. Differential responses of crop yields and soil organic carbon stock to fertilization and rice straw incorporation in three cropping systems in the subtropics. Agric. Ecosyst. Environ. 2014, 184, 51–58. [Google Scholar] [CrossRef]
- Fan, Y.; Gao, J.; Sun, J.; Liu, J.; Su, Z.; Wang, Z.; Yu, X.; Hu, S. Effects of straw returning and potassium fertilizer application on root characteristics and yield of spring maize in China inner Mongolia. Agron. J. 2021, 113, 4369–4385. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, J.; Sun, L.; Wang, H.; Lv, Y.; Li, Y.; Hameed, F. Effects of straw returning on rice growth and yield under water-saving irrigation. Chil. J. Agric. Res. 2019, 79, 66–74. [Google Scholar] [CrossRef]
- Yang, S.; Yaliang, L.; Weihang, X.; Lili, F.; Yihan, Z.; Linliang, L.; Xiwen, S.; Zhian, Z. Effects of Rice Straw Application to Soda-Salinization Paddy Soil on Rice Growth and Grain Yield. Crops 2012, 4, 49–52. [Google Scholar]
- Fang, F.F. Study on the Effect of Wheat Straw Returning to the Field on the Early Growth of Rice and Its Mechanism. Master’s Thesis, Yangzhou University, Yangzhou, China, 2018. [Google Scholar]
- Geng, Y.; Cao, G.; Wang, L.; Wang, S. Effects of equal chemical fertilizer substitutions with organic manure on yield, dry matter, and nitrogen uptake of spring maize and soil nitrogen distribution. PLoS ONE 2019, 14, e219512. [Google Scholar] [CrossRef]
- Han, J.; Dong, Y.; Zhang, M. Chemical fertilizer reduction with organic fertilizer effectively improve soil fertility and microbial community from newly cultivated land in the Loess Plateau of China. Appl. Soil Ecol. 2021, 165, 103966. [Google Scholar] [CrossRef]
- Bell, J.M.; Schwartz, R.; McInnes, K.J.; Howell, T.; Morgan, C.L.S. Deficit irrigation effects on yield and yield components of grain sorghum. Agric. Water Manag. 2018, 203, 289–296. [Google Scholar] [CrossRef]
- GhassemiSahebi, F.; Mohammadrezapour, O.; Delbari, M.; KhasheiSiuki, A.; Ritzema, H.; Cherati, A. Effect of utilization of treated wastewater and seawater with Clinoptilolite-Zeolite on yield and yield components of sorghum. Agric. Water Manag. 2020, 234, 106117. [Google Scholar] [CrossRef]
- Chen, Y.; Xin, L.; Liu, J.; Yuan, M.; Liu, S.; Jiang, W.; Chen, J. Changes in bacterial community of soil induced by long-term straw returning. Sci. Agric. 2017, 74, 349–356. [Google Scholar] [CrossRef]
- Mubarak, A.R.; Rosenani, A.B.; Anuar, A.R.; Zauyah, S. Decomposition and nutrient release of maize stover and groundnut haulm under tropical field conditions of malaysia. Commun. Soil Sci. Plant Anal. 2002, 33, 609–622. [Google Scholar] [CrossRef]
- Pu, J.; Jiang, N.; Zhang, Y.; Guo, L.; Huang, W.; Chen, L. Effects of various straw incorporation strategies on soil phosphorus fractions and transformations. Gcb Bioenergy 2022, 15, 88–98. [Google Scholar] [CrossRef]
- Wei, K.; Chen, Z.; Jiang, N.; Zhang, Y.; Feng, J.; Tian, J.; Chen, X.; Lou, C.; Chen, L. Effects of mineral phosphorus fertilizer reduction and maize straw incorporation on soil phosphorus availability, acid phosphatase activity, and maize grain yield in northeast China. Arch. Acker Pflanzenbau Bodenkd. 2021, 67, 66–78. [Google Scholar] [CrossRef]
- Damon, P.M.; Bowden, B.; Rose, T.; Rengel, Z. Crop residue contributions to phosphorus pools in agricultural soils: A review. Soil Biol. Biochem. 2014, 74, 127–137. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Shao, M. Earthworm inoculation and straw return decrease the phosphorus adsorption capacity of soils in the Loess region, China. J. Environ. Manag. 2022, 312, 114921. [Google Scholar] [CrossRef] [PubMed]
- Meier, E.A.; Thorburn, P.J.; Wegener, M.K.; Basford, K.E. The availability of nitrogen from sugarcane trash on contrasting soils in the wet tropics of North Queensland. Nutr. Cycl. Agroecosystems 2006, 75, 101–114. [Google Scholar] [CrossRef]
- Yan, Y.; Ji, W.; Li, B.; Wang, G.; Chen, S.; Zhu, D.; Liu, Z. Quantification of the effects of long-term straw return on soil organic matter spatiotemporal variation: A case study in typical black soil region. EGUsphere 2022, 2022, 1–17. [Google Scholar] [CrossRef]
- Yan, Y.; Ji, W.; Li, B.; Wang, G.; Hu, B.; Zhang, C.; Mouazen, A.M. Effects of Long-Term Straw Return and Environmental Factors on the Spatiotemporal Variability of Soil Organic Matter in the Black Soil Region: A Case Study. Agronomy 2022, 12, 2532. [Google Scholar] [CrossRef]
- Li, Z.; Li, D.; Ma, L.; Yu, Y.; Zhao, B.; Zhang, J. Effects of straw management and nitrogen application rate on soil organic matter fractions and microbial properties in North China Plain. J. Soils Sediments 2019, 19, 618–628. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, H.; Hua, K.; Wang, D.; He, C. Long-term straw incorporation benefits the elevation of soil phosphorus availability and use efficiency in the agroecosystem. Span. J. Agric. Res. 2018, 16, e1101. [Google Scholar] [CrossRef]
- Li, X.G.; Jia, B.; Lv, J.; Ma, Q.; Kuzyakov, Y.; Li, F. Nitrogen fertilization decreases the decomposition of soil organic matter and plant residues in planted soils. Soil Biol. Biochem. 2017, 112, 47–55. [Google Scholar] [CrossRef]
- Pittelkow, C.M.; Liang, X.; Linquist, B.A.; van Groenigen, K.J.; Lee, J.; Lundy, M.E.; van Gestel, N.; Six, J.; Venterea, R.T.; van Kessel, C. Productivity limits and potentials of the principles of conservation agriculture. Nature 2015, 517, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Poeplau, C.; Kätterer, T.; Bolinder, M.A.; Börjesson, G.; Berti, A.; Lugato, E. Low stabilization of aboveground crop residue carbon in sandy soils of Swedish long-term experiments. Geoderma 2015, 237-238, 246–255. [Google Scholar] [CrossRef]
- Powlson, D.S.; Glendining, M.J.; Coleman, K.; Whitmore, A.P. Implications for Soil Properties of Removing Cereal Straw: Results from Long-Term Studies1. Agron. J. 2011, 103, 279–287. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Pu, X.; Zhang, P.; Ou, Y. Influence of Stalk Residue Retention and Fertilization on the Rhizospheric Effect with Drip-Irrigated Cotton. Agron. J. 2019, 111, 1028–1038. [Google Scholar] [CrossRef]
- Ran, C.; Gao, D.; Liu, W.; Guo, L.; Bai, T.; Shao, X.; Geng, Y. Straw and nitrogen amendments improve soil, rice yield, and roots in a saline sodic soil. Rhizosphere 2022, 24, 100606. [Google Scholar] [CrossRef]
- Song, X.; Sun, R.; Chen, W.; Wang, M. Effects of surface straw mulching and buried straw layer on soil water content and salinity dynamics in saline soils. Can. J. Soil. Sci. 2020, 100, 58–68. [Google Scholar] [CrossRef]
- Tan, Y.; Li, H.; Xu, S.; Liu, B.; Jiang, Z.; Wei, G. Effects of Cane Leave Residue on the Soil Fertility. Sugar Crops China 2010, 1, 1–4. [Google Scholar] [CrossRef]
- Liao, Q.; Wei, G.; Chen, G.; Liu, B.; Huang, D.; Li, Y. Effect of Trash Addition to the Soil on Microbial Communities and Physico-Chemical Properties of Soils and Growth of Sugarcane Plants. Sugar Tech 2014, 16, 400–404. [Google Scholar] [CrossRef]
- Wuest, S.B. Correction of Bulk Density and Sampling Method Biases Using Soil Mass per Unit Area. Soil Sci. Soc. Am. J. 2009, 73, 312–316. [Google Scholar] [CrossRef]
- Mckee, G.W. A Coefficient for Computing Leaf Area in Hybrid Corn1. Agron. J. 1964, 56, 240–241. [Google Scholar] [CrossRef]
- Bu, L.; Liu, J.; Zhu, L.; Luo, S.; Chen, X.; Li, S.; Lee Hill, R.; Zhao, Y. The effects of mulching on maize growth, yield and water use in a semi-arid region. Agric. Water Manag. 2013, 123, 71–78. [Google Scholar] [CrossRef]
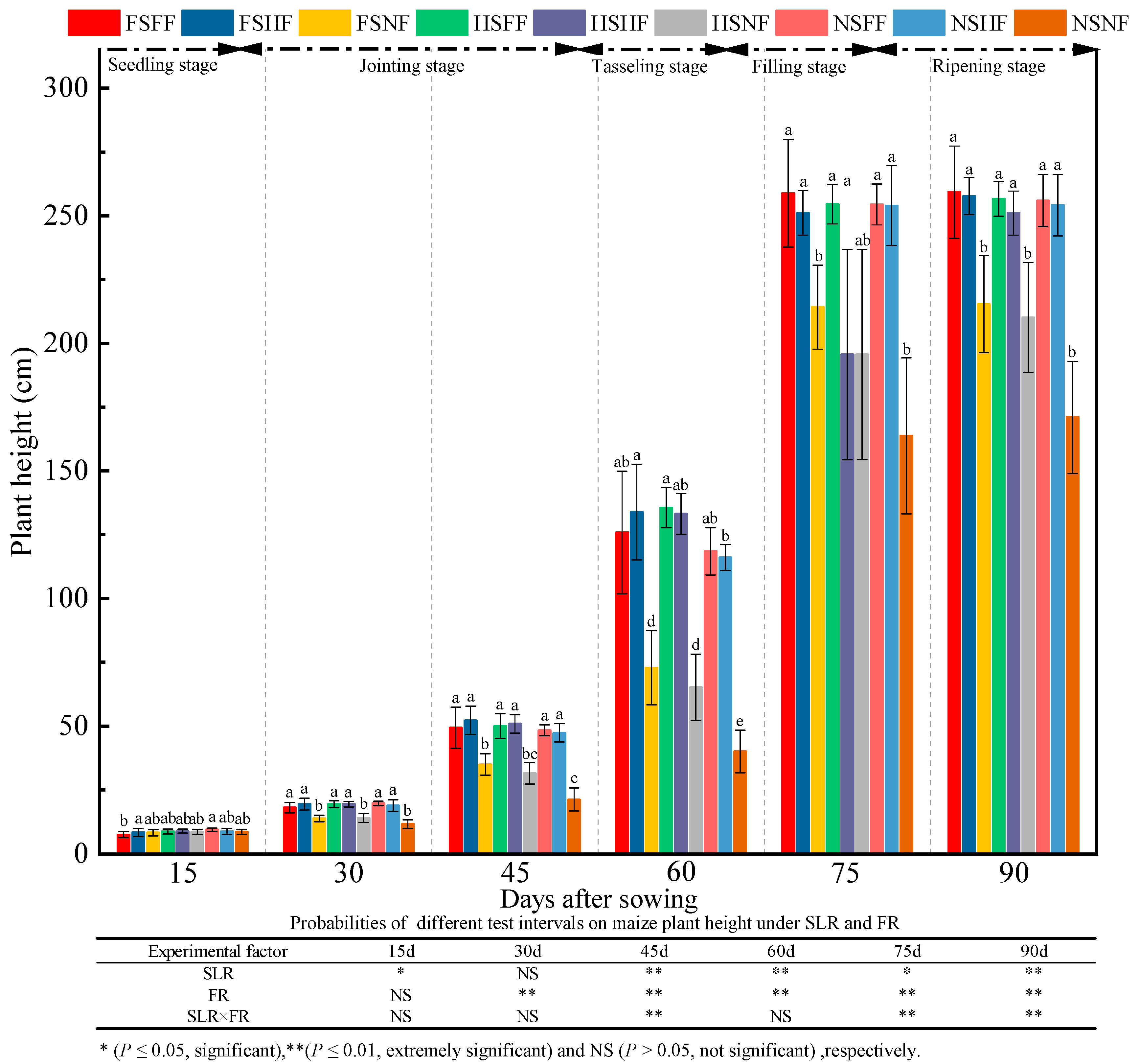
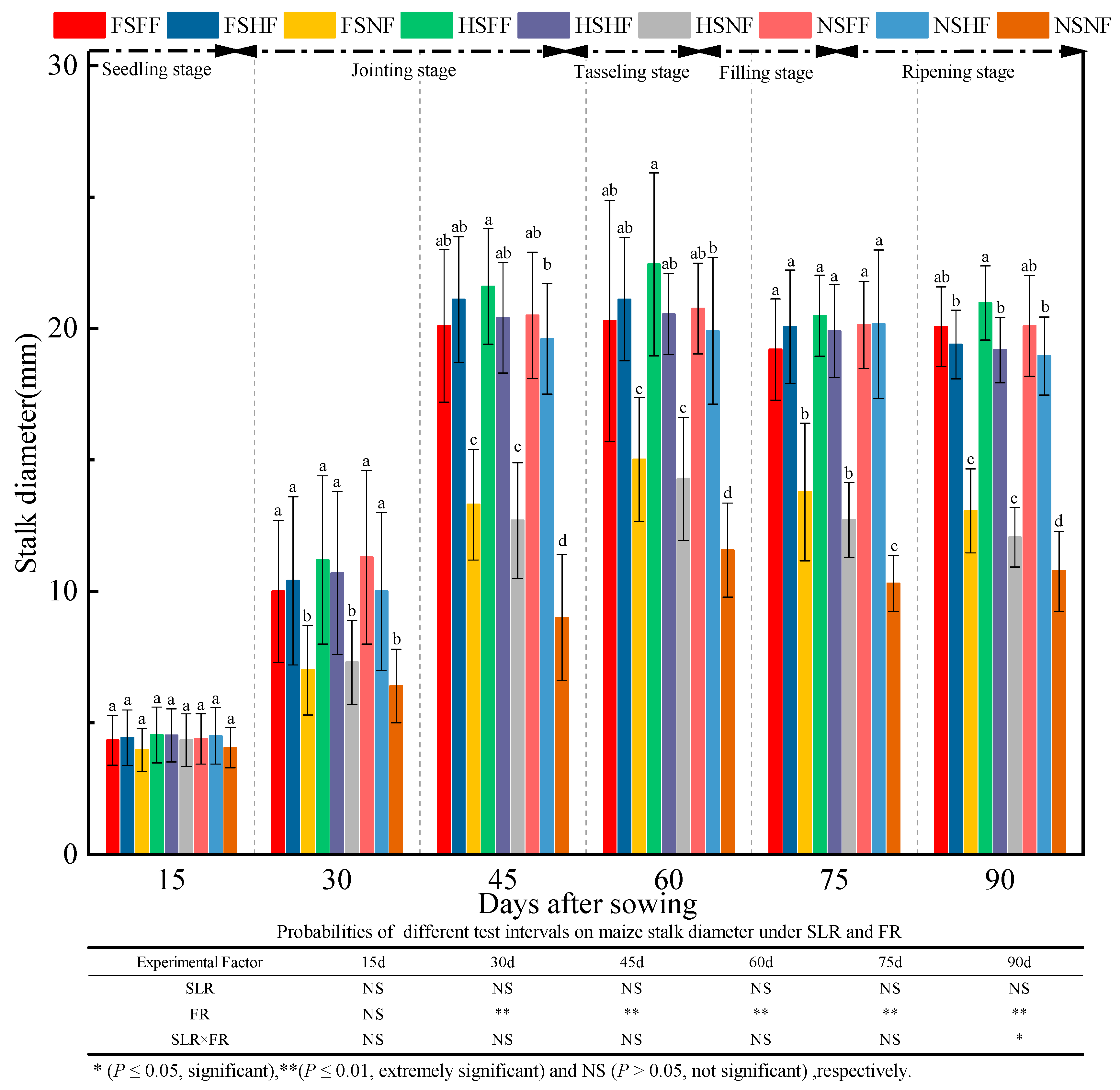
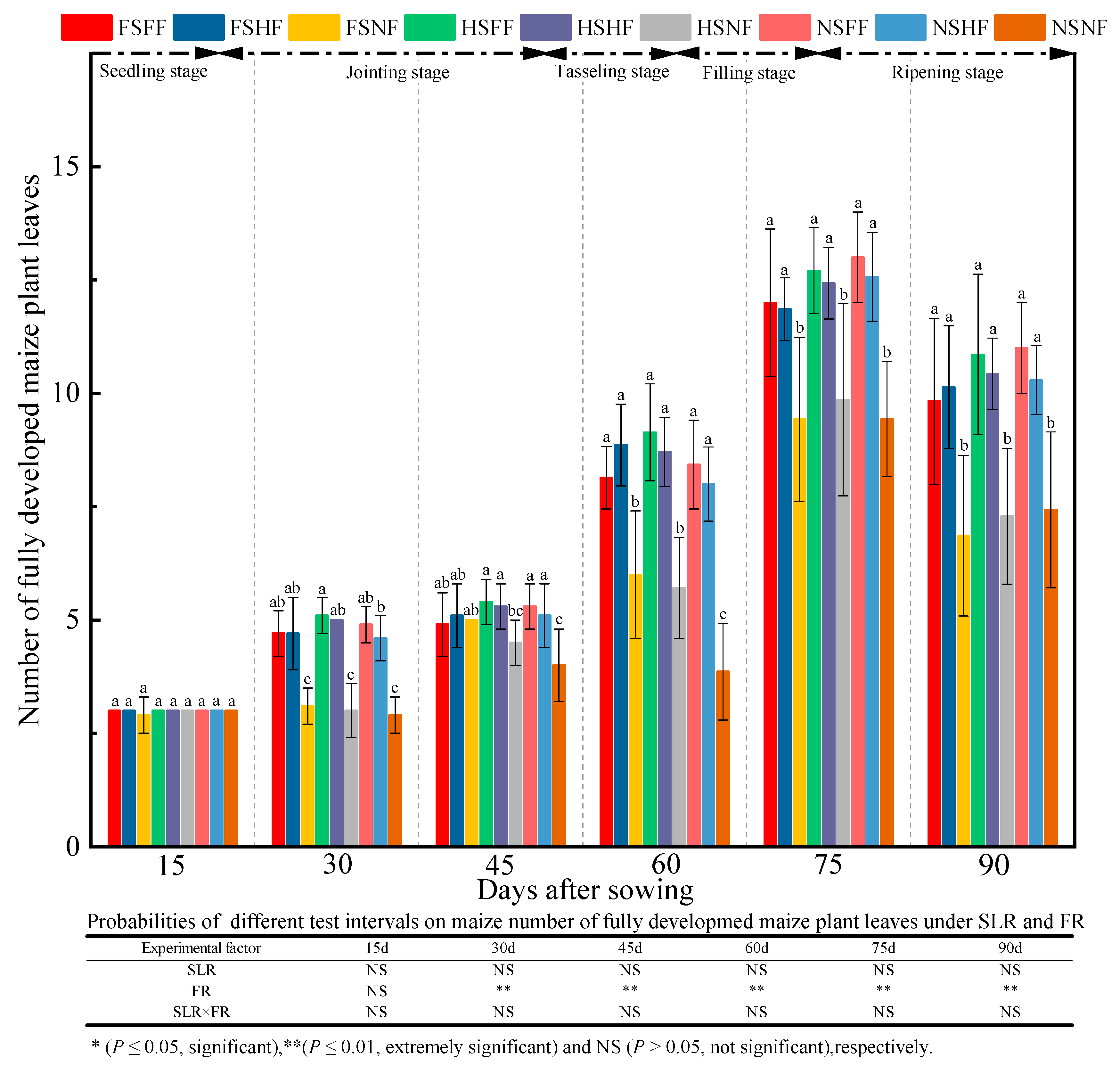
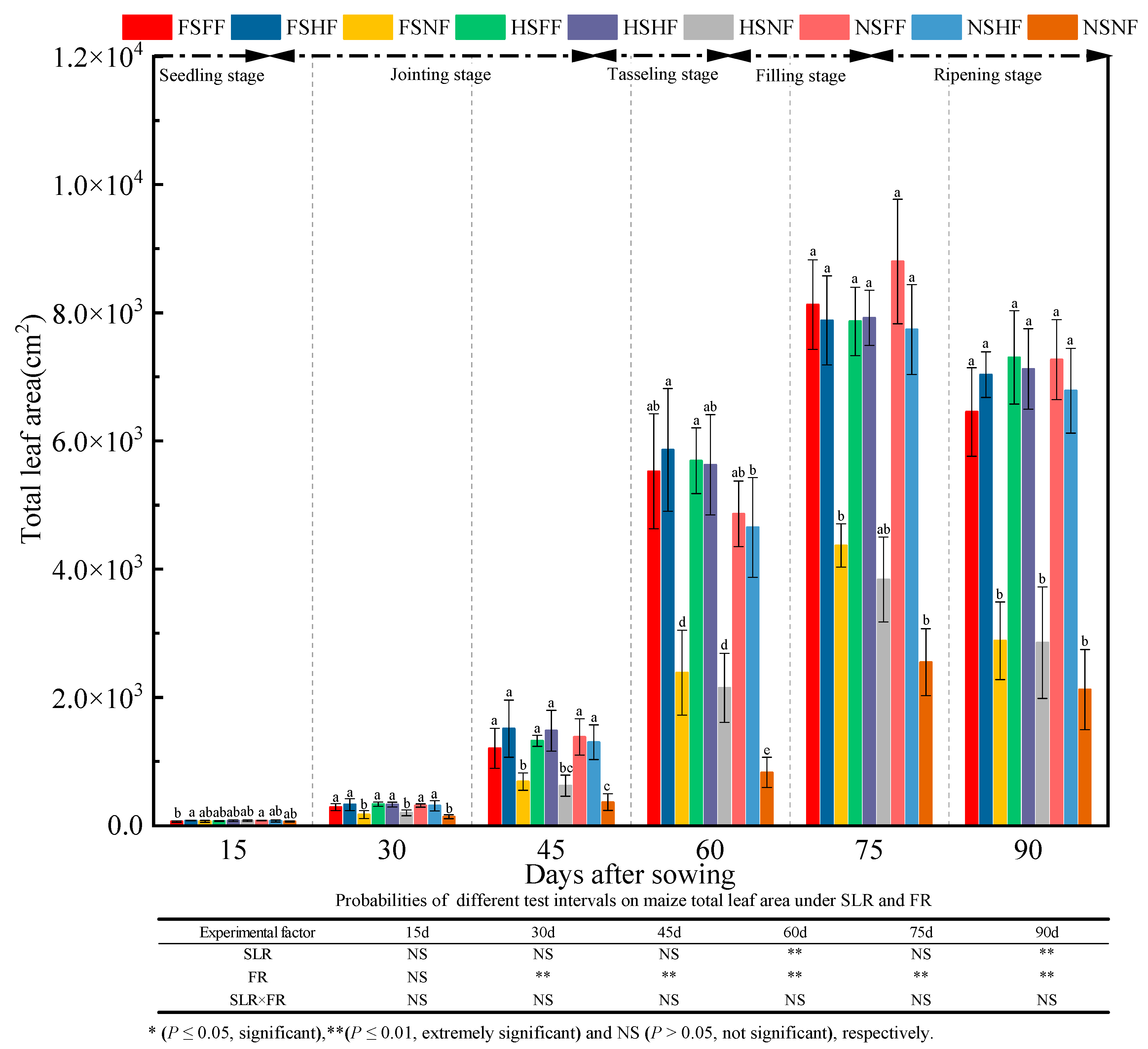




| Experimental Treatment | Ear Weight per Plant (g) | 1000 Kernel Weight (g) | Number of Productive Ears | Rows per Ear | Ear Diameter (mm) | Ear Length (cm) | Plant Air-Dried Weight (g) | Ear Height (cm) | Grain Yield Rate (%) | Harvest Index (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| FSFF | 198.87 ± 17.41 a | 299.21 ± 16.43 ab | 1.14 ± 0.18 ab | 13.75 ± 1.67 ab | 41.93 ± 6.29 a | 18.86 ± 2.78 a | 530.50 ± 58.45 ab | 106.13 ± 18.58 a | 54.36 ± 8.55 b | 40.3 ± 5.7 a |
| FSHF | 247.56 ± 17.45 a | 281.57 ± 14.29 b | 1.29 ± 0.29 a | 14.00 ± 1.33 ab | 40.75 ± 8.71 ab | 18.26 ± 4.46 a | 502.86 ± 62.26 ab | 108.57 ± 12.07 a | 53.32 ± 9.33 b | 48.9 ± 7.9 a |
| FSNF | 54.21 ± 7.94 cd | 255.97 ± 22.33 b | 0.71 ± 0.19 bc | 12.00 ± 1.15 c | 34.95 ± 8.52 bc | 13.86 ± 2.70 b | 162.86 ± 49.57 c | 69.64 ± 14.47 b | 47.30 ± 8.46 c | 50.7 ± 8.9 a |
| HSFF | 184.10 ± 17.34 a | 383.62 ± 23.82 a | 0.86 ± 0.18 abc | 14.00 ± 2.53 ab | 42.09 ± 7.05 a | 19.93 ± 1.13 a | 596.29 ± 28.85 a | 108.07 ± 15.58 a | 58.34 ± 9.66 ab | 33.5 ± 2.9 a |
| HSHF | 229.80 ± 15.05 a | 292.69 ± 23.47 ab | 1.14 ± 0.18 ab | 14.50 ± 1.77 a | 42.96 ± 6.50 a | 19.81 ± 2.28 a | 505.71 ± 25.07 ab | 109.14 ± 8.17 a | 66.75 ± 9.32 a | 45.5 ± 5.5 a |
| HSNF | 76.85 ± 6.82 bcd | 251.19 ± 26.87 b | 0.57 ± 0.13 c | 12.57 ± 0.98 bc | 38.14 ± 1.46 ab | 12.79 ± 2.04 b | 163.33 ± 34.45 c | 72.86 ± 6.94 b | 40.43 ± 7.35 cd | 46.8 ± 9.4 a |
| NSFF | 158.09 ± 12.14 ab | 341.46 ± 21.52 ab | 1.29 ± 0.19 a | 14.00 ± 1.41 ab | 41.54 ± 8.08 a | 18.50 ± 2.14 a | 560.00 ± 80.00 ab | 104.14 ± 10.39 a | 51.72 ± 7.21 b | 36.0 ± 2.2 a |
| NSHF | 146.94 ± 19.16 abc | 304.43 ± 26.92 ab | 1.00 ± 0.00 abc | 14.25 ± 1.28 ab | 40.56 ± 6.53 ab | 18.93 ± 0.98 a | 468.57 ± 66.19 b | 107.64 ± 6.18 a | 60.33 ± 6.09 ab | 33.3 ± 5.9 a |
| NSNF | 35.12 ± 6.83 d | 247.30 ± 24.22 b | 0.57 ± 0.13 c | 12.57 ± 1.51 bc | 29.49 ± 4.44 c | 13.93 ± 3.89 b | 120.00 ± 30.9 c | 44.79 ± 12.03 c | 40.96 ± 9.01 cd | 35.5 ± 4.6 a |
| SLR | NS | NS | NS | NS | NS | NS | NS | <0.01 | NS | NS |
| FR | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | NS | NS |
| SLR×FR | NS | NS | NS | NS | NS | NS | NS | <0.01 | NS | NS |
| Yield Component Factor | Treatment Acronyms | Maximum Value | Unit | FF/NF (%) | HF/NF (%) | Multiple Comparison Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FS | HS | NS | FS | HS | NS | FS/NS (%) | HS/NS (%) | FF/NF (%) | HF/NF (%) | ||||
| Ear weight per plant | FSHF | 247.56 | g | 266.85 | 139.56 | 350.14 | 356.67 | 199.02 | 318.39 | 40.10 | 47.90 | 285.77 | 211.6 |
| 1000 kernel weight | HSFF | 383.62 | g | 16.89 | 52.72 | 38.08 | 10.00 | 16.52 | 23.10 | −8.44 | −0.05 | 34.73 | 15.51 |
| Number of productive ears | HSFF | 1.29 | – | 60.56 | 50.88 | 126.32 | 81.69 | 100.00 | 75.44 | 22.09 | 10.47 | 77.42 | 83.87 |
| Rows per ear | HSHF | 14.50 | – | 14.58 | 11.38 | 11.38 | 16.67 | 15.35 | 13.37 | −2.24 | 0.35 | 14.94 | 12.37 |
| Ear diameter | HSHF | 43.96 | mm | 19.97 | 10.36 | 40.86 | 16.60 | 12.64 | 37.54 | 3.92 | 8.60 | 22.21 | 20.88 |
| Ear length | HSFF | 19.93 | cm | 36.08 | 55.82 | 32.81 | 31.75 | 54.89 | 35.59 | −0.48 | 1.91 | 41.00 | 40.01 |
| Plant air-dried weight | HSFF | 596.29 | G | 225.74 | 265.08 | 366.67 | 208.77 | 209.62 | 290.48 | −0.97 | 9.78 | 277.24 | 229.41 |
| Ear height | HSHF | 109.14 | cm | 52.40 | 48.33 | 132.51 | 55.90 | 49.79 | 140.32 | 10.16 | 13.06 | 69.98 | 73.72 |
| Grain yield rate | HSHF | 66.75 | % | 14.93 | 44.30 | 26.27 | 12.73 | 65.10 | 47.29 | 25.16 | 4.29 | 55.55 | 21.42 |
| Harvest index | FSNF | 50.7 | % | −20.51 | −28.42 | 1.41 | −3.55 | −2.78 | −6.20 | 34.85 | 19.52 | −21.76 | −1.52 |
| Maize yield | FSHF | 3225.08 | kg/hm2 | 225.38 | 318.63 | 1608.33 | 401.26 | 325.01 | 1122.90 | −3.14 | −29.42 | 941.32 | 695.04 |
| Index | pH | EC | TN | TP | TK | AN | AP | AK | SOM |
|---|---|---|---|---|---|---|---|---|---|
| Plant height | −0.18 * | −ns | −0.24 ** | ns | 0.52 ** | −ns | 0.40 ** | −ns | 0.20 ** |
| Stalk diameter | ns | −0.41 ** | −0.34 ** | ns | 0.35 ** | −0.39 ** | 0.21 ** | −0.16 * | 0.31 ** |
| Number of fully developed maize plant leaves | −ns | −0.42 ** | −0.36 ** | ns | 0.41 ** | −0.36 ** | 0.25 ** | −0.22 ** | 0.34 ** |
| Total leaf area | ns | ns | 0.21 ** | ns | −0.47 ** | ns | −ns | 0.25 ** | −0.18 * |
| SPAD | 0.24 ** | −ns | ns | −ns | −0.33 ** | −ns | −0.43 ** | −ns | −0.20 ** |
| Treatment Combination Name | Treatment Acronyms | SLR Content per Pot (g) | FR Content per Pot (g) (Fertilization Type/Weight) | Nutrient Input (g) | |||||
|---|---|---|---|---|---|---|---|---|---|
| SLR/pot | FR/pot | ||||||||
| N | P2O5 | K2O | N | P2O5 | K2O | ||||
| Full sugarcane leaf return + Full fertilizer | FSFF | 120.0 | CO(NH2)2/9.78 + Ca(H2PO4)2/25.00 + KCl/7.18 | 0.68 | 0.15 | 0.56 | 4.50 | 3.00 | 4.50 |
| Full sugarcane leaf return + Half fertilizer | FSHF | 120.0 | CO(NH2)2/4.89 + Ca(H2PO4)2/12.50 + KCl/3.59 | 0.68 | 0.15 | 0.56 | 2.25 | 1.50 | 2.25 |
| Full sugarcane leaf return + No fertilizer | FSNF | 120.0 | CO(NH2)2/0 + Ca(H2PO4)2/0 + KCl/0 | 0.68 | 0.15 | 0.56 | 0 | 0 | 0 |
| Half sugarcane leaf return + Full fertilizer | HSFF | 60.0 | CO(NH2)2/9.78 + Ca(H2PO4)2/25.00 + KCl/7.18 | 0.34 | 0.07 | 0.28 | 4.50 | 3.00 | 4.50 |
| Half sugarcane leaf return + Half fertilizer | HSHF | 60.0 | CO(NH2)2/4.89 + Ca(H2PO4)2/12.50 + KCl/3.59 | 0.34 | 0.07 | 0.28 | 2.25 | 1.50 | 2.25 |
| Half sugarcane leaf return + No fertilizer | HSNF | 60.0 | CO(NH2)2/0 + Ca(H2PO4)2/0 + KCl/0 | 0.34 | 0.07 | 0.28 | 0 | 0 | 0 |
| No sugarcane leaf return + Full fertilizer | NSFF | 0 | CO(NH2)2/9.78 + Ca(H2PO4)2/25.00 + KCl/7.18 | 0 | 0 | 0 | 4.50 | 3.00 | 4.50 |
| No sugarcane leaf return + Half fertilizer | NSHF | 0 | CO(NH2)2/4.89 + Ca(H2PO4)2/12.50 + KCl/3.59 | 0 | 0 | 0 | 2.25 | 1.50 | 2.25 |
| No sugarcane leaf return + No fertilizer (CK) | NSNF | 0 | CO(NH2)2/0 + Ca(H2PO4)2//0 + KCl/0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tan, Y.; Liang, D.; Pei, C.; Zhang, Z. Effects of Sugarcane Leaf Return and Fertilizer Reduction on Maize Growth, Yield and Soil Properties in Red Soil. Plants 2023, 12, 1029. https://doi.org/10.3390/plants12051029
Liu Y, Tan Y, Liang D, Pei C, Zhang Z. Effects of Sugarcane Leaf Return and Fertilizer Reduction on Maize Growth, Yield and Soil Properties in Red Soil. Plants. 2023; 12(5):1029. https://doi.org/10.3390/plants12051029
Chicago/Turabian StyleLiu, Yufeng, Yumo Tan, Dan Liang, Chengruo Pei, and Zhenhua Zhang. 2023. "Effects of Sugarcane Leaf Return and Fertilizer Reduction on Maize Growth, Yield and Soil Properties in Red Soil" Plants 12, no. 5: 1029. https://doi.org/10.3390/plants12051029
APA StyleLiu, Y., Tan, Y., Liang, D., Pei, C., & Zhang, Z. (2023). Effects of Sugarcane Leaf Return and Fertilizer Reduction on Maize Growth, Yield and Soil Properties in Red Soil. Plants, 12(5), 1029. https://doi.org/10.3390/plants12051029






