Heteroblastic Inflorescence of Lamium amplexicaule L. in Egyptian Flora
Abstract
1. Introduction
2. Results
2.1. Species Morphology
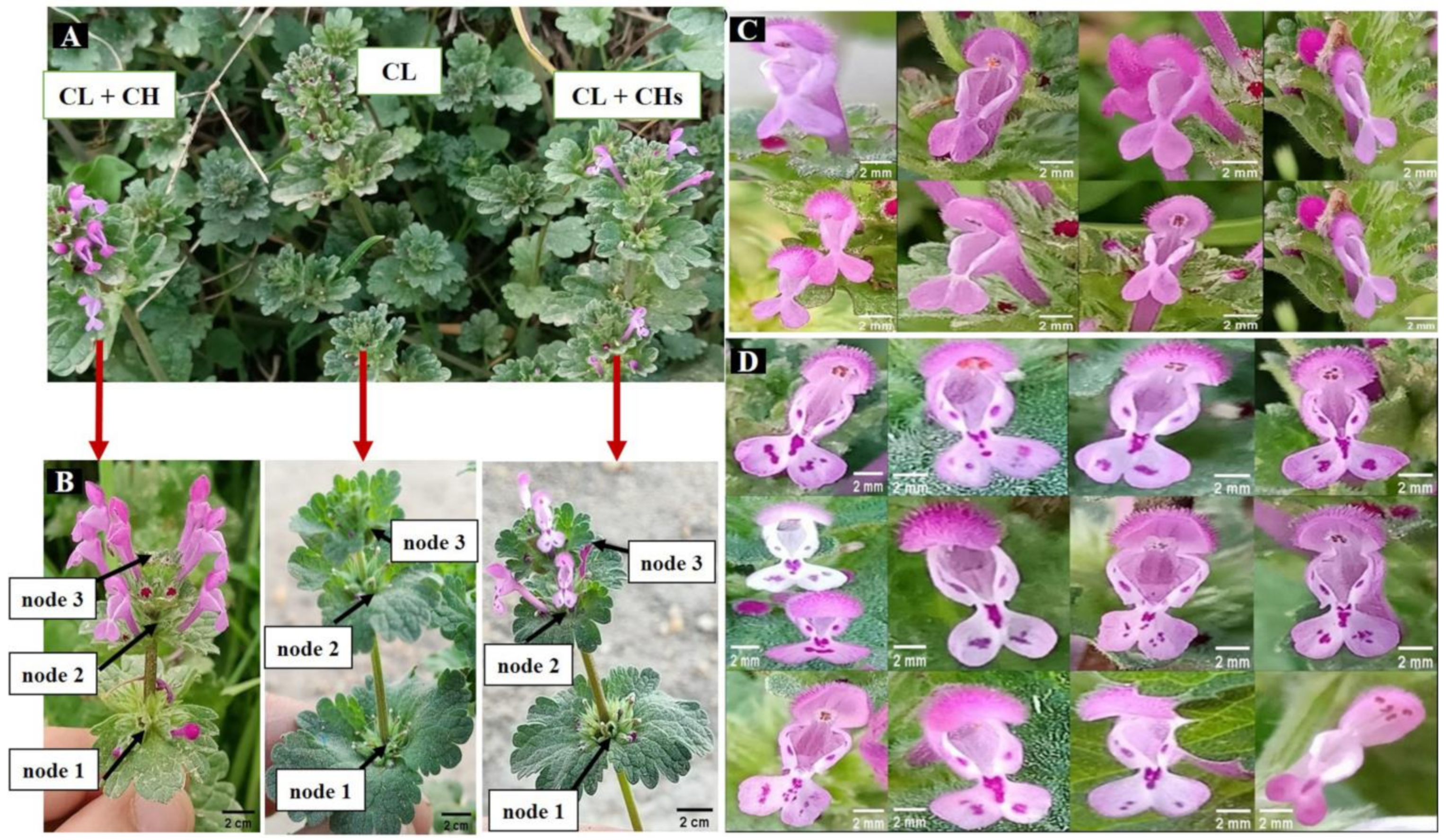
2.2. Inflorescence Heteroblasticity in the Egyptian Environment
2.3. Lamium Phenoplasticity among the Morphs Using Macromorphological Characteristics
- Cleistogamous morph (only CL flowers/individual); is characterized by ovate, non-segmented lowers leaves, short petiole (to 20 mm), and a larger inflorescence bract (27 mm × 30 mm) with shallow incised margin, and up to seven nodes with flowers/branches.
- Dimorphic-cleistogamous morph (CL + CH flowers/individual); its lower leaves are ovate-peltate, non-segmented, and have a longer petiole (to 27 mm). The inflorescence bract is similar to the CL morph in size, with a shallow-deeply incised margin and up to eight nodes with flowers/branches. The shape of the lower lip and the features of the lower corolla’s lateral lobes also showed variability (Figure 1C).
- Dimorphic-cleistogamous spotted morph (both CL + CHs flowers/individual with spotted petals) is characterized by a wide range of pink spotted lower lips (Figure 1D), and its lower leaves are ovate 3–5 segments. Petiole up to 30 mm long, the inflorescence bract is smaller than in the other morphs (its length to 20 mm) with shallow incised margin and up to ten nodes with flowers/branches.
2.4. Flower Phenoplasticity in the Different Morphs
2.4.1. Calyx
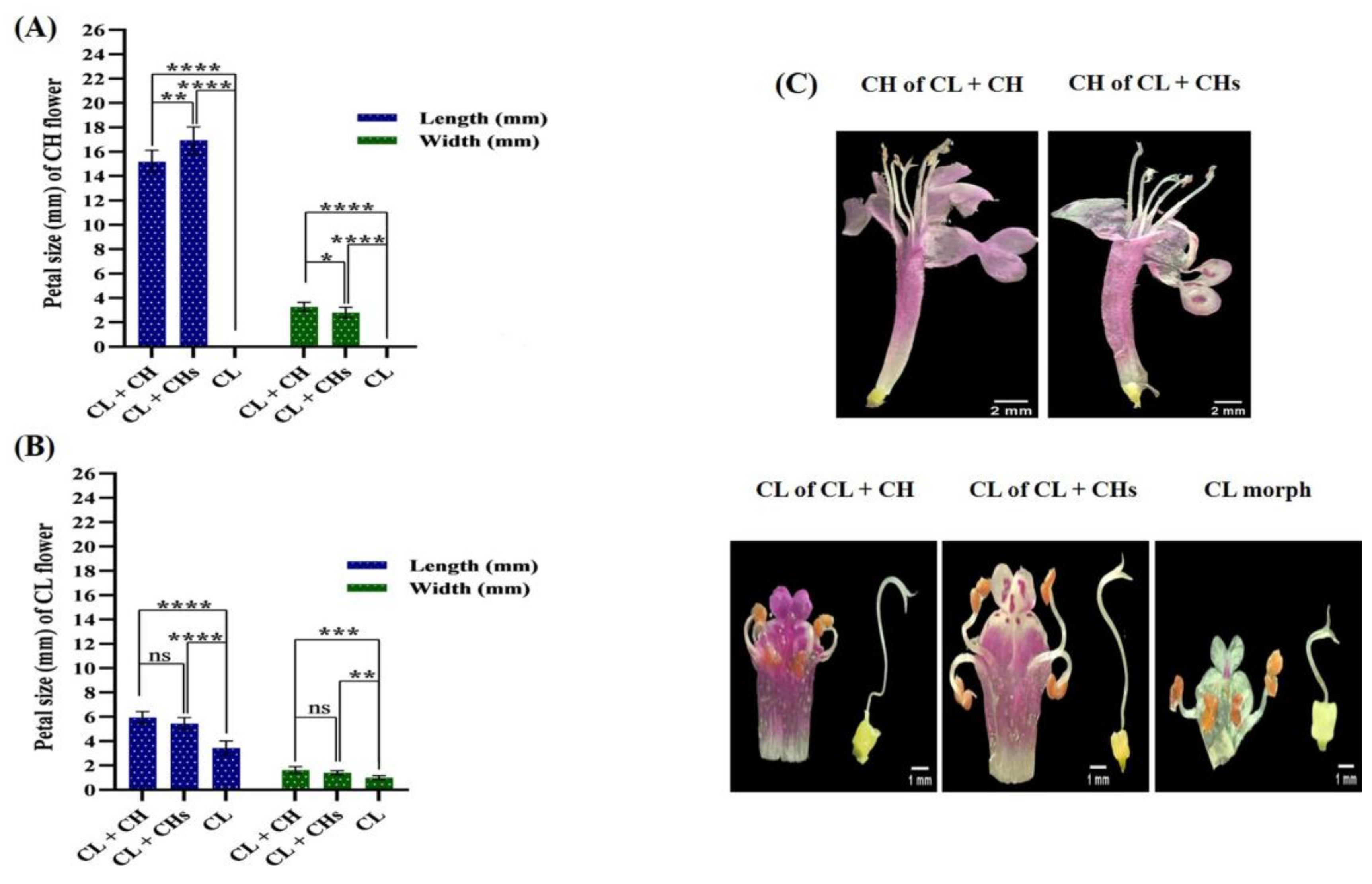
2.4.2. Corolla
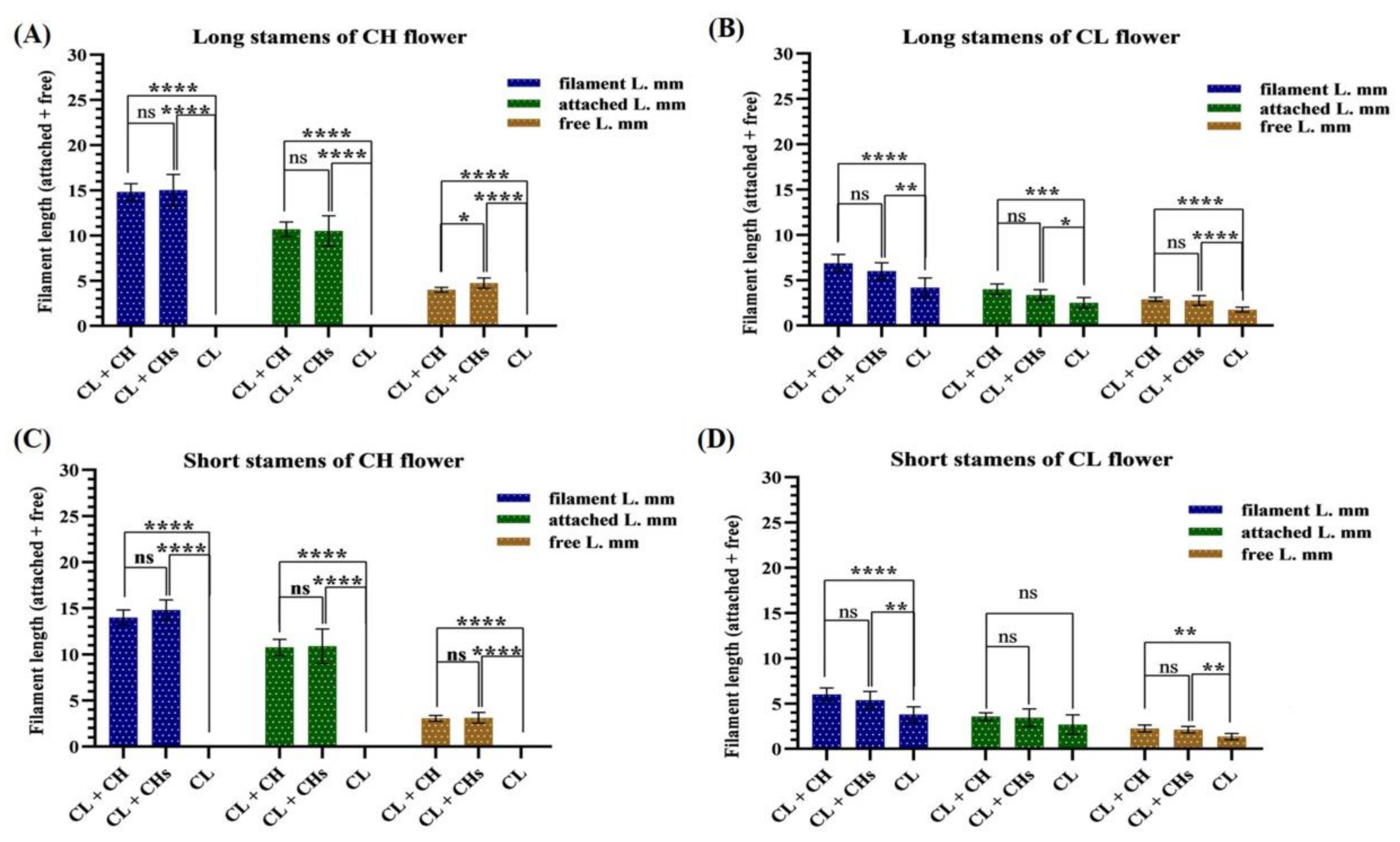
2.4.3. Anthers
2.4.4. Nectary Disk
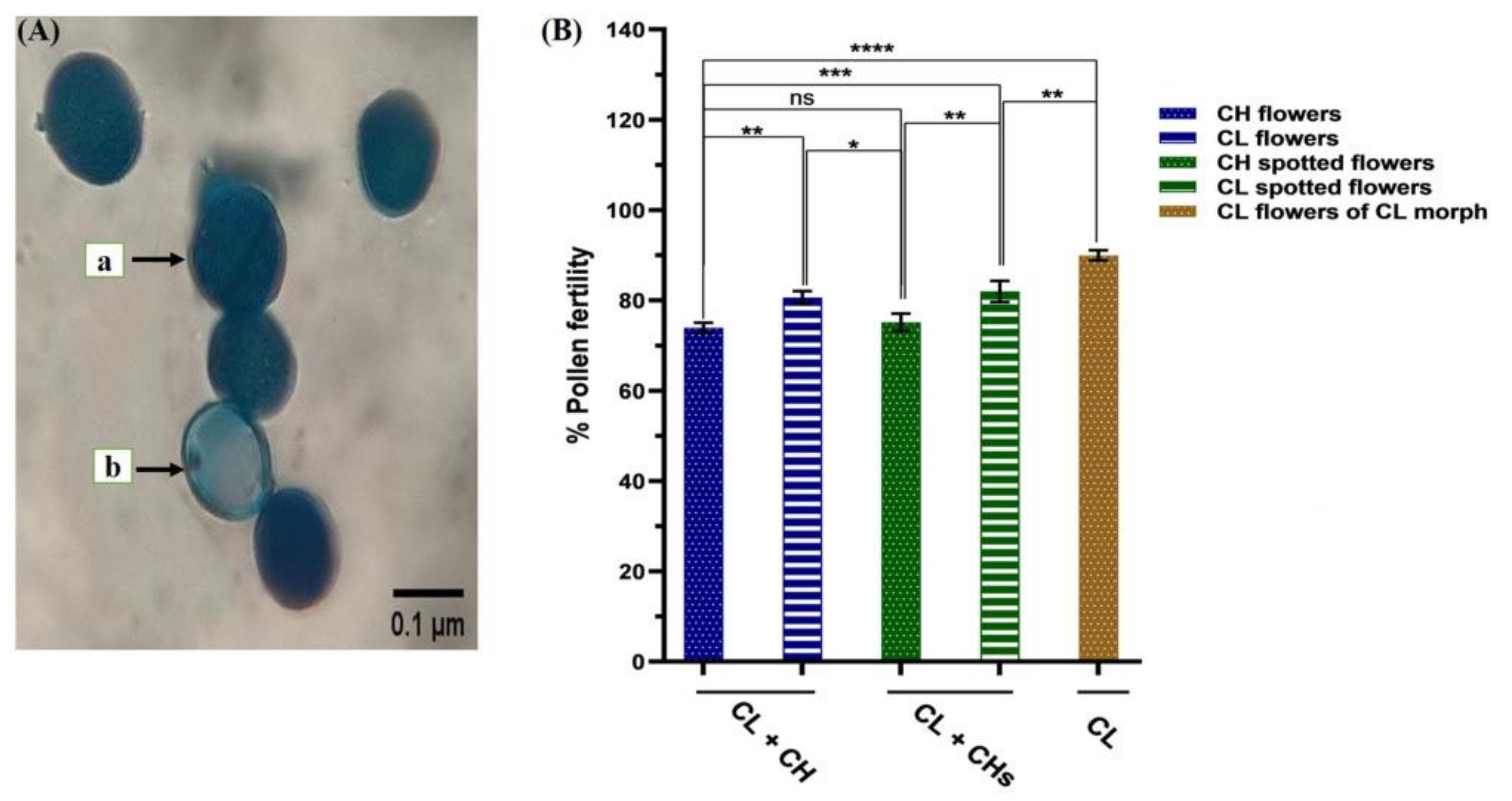
2.4.5. Pollen Fertility
2.4.6. Nutlets Sculpture
2.5. Trichomes
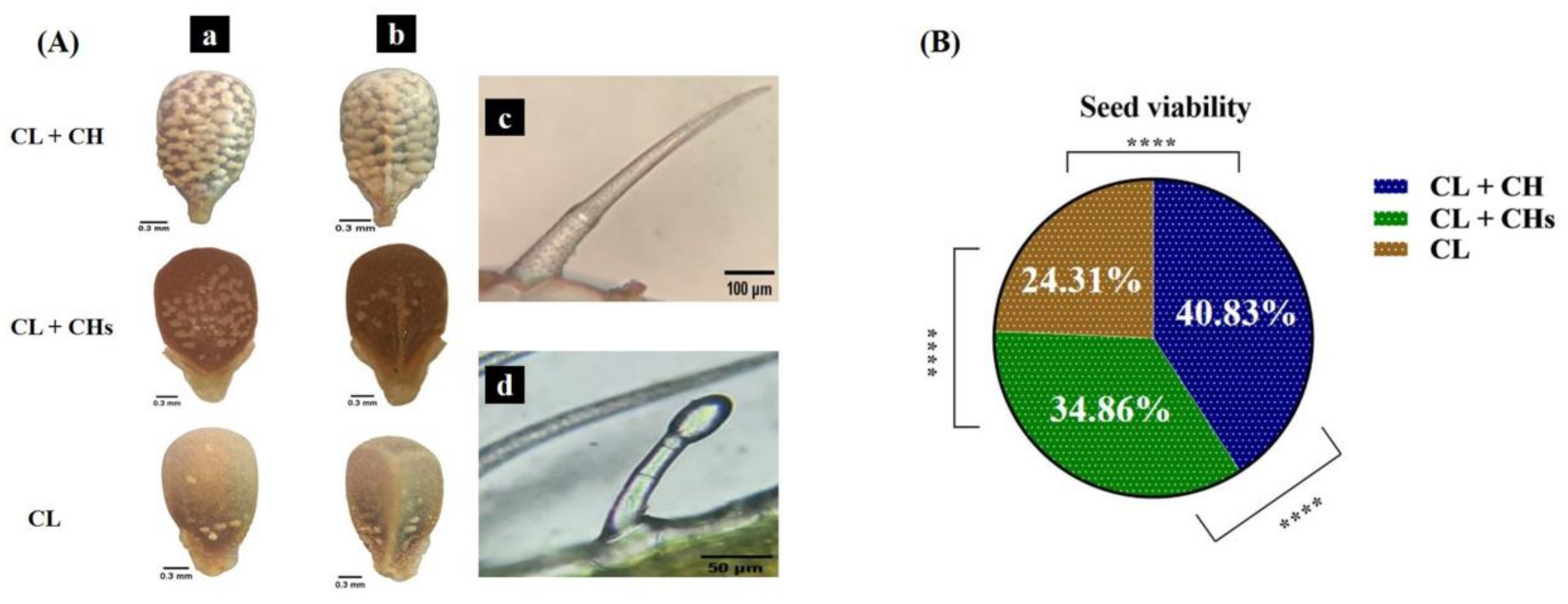
2.6. Seed Viability and Morph Resemblance to Its Parent Seeds
2.7. Variability in Flowering Timing among Morphs
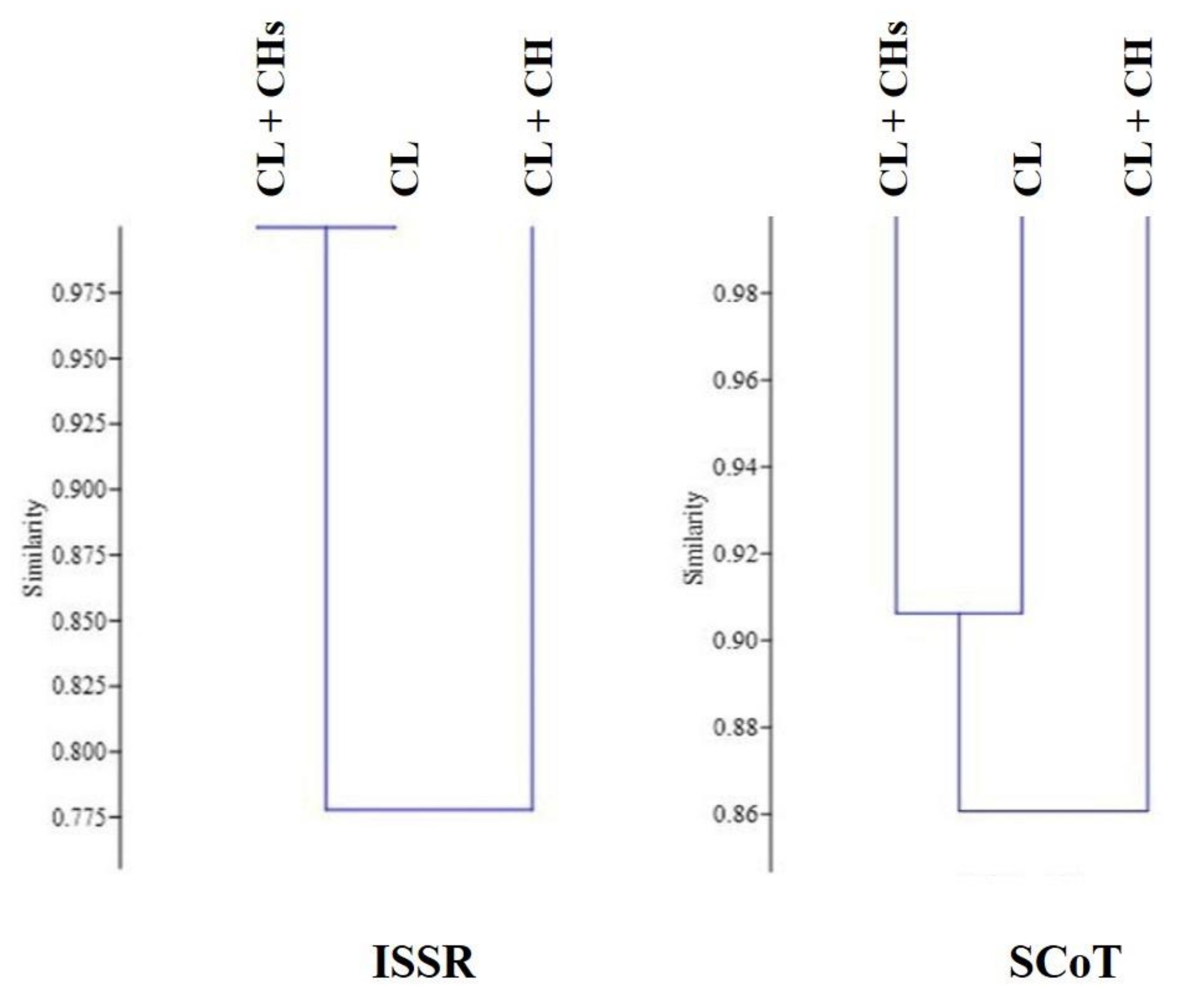
2.8. Genetic Diversity between the Studied Morphs
3. Discussion
3.1. Inflorescence Heteroblasticity in the Egyptian Environment
3.2. The Growing Season of L. amplexicaule
3.3. Predominant Morph in Egypt
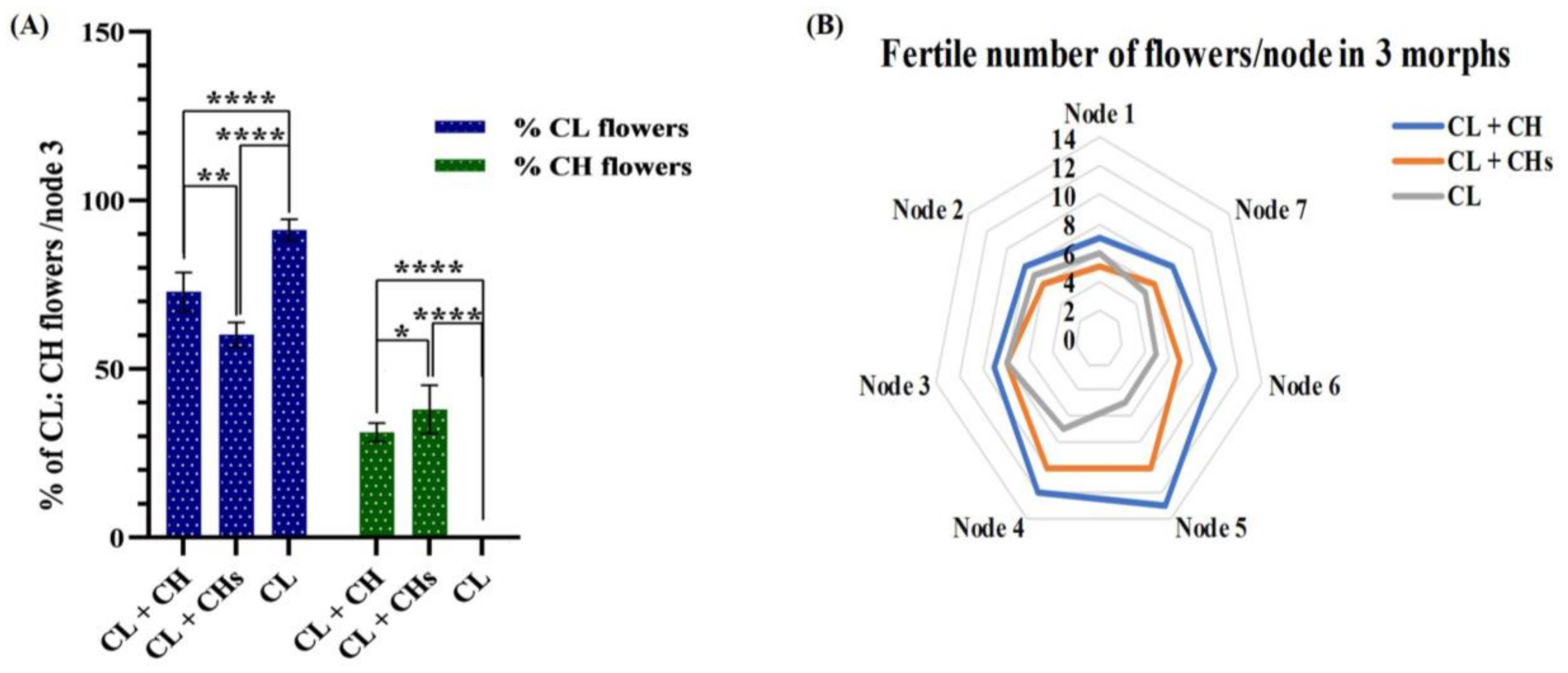
3.4. Proportions of the CH: CL Flowers in Different Morphs
3.5. Factors Controlling the Proportions of the CH Flowers
3.6. Flower Phenoplasticity in the Different Morphs
3.7. Pollen Grain Fertility
3.8. The Nutlet Sculpture and Seed Viability
3.9. Trichomes
3.10. Morph Resemblance to Its Parent Seeds
3.11. Flowering Time for the Studied Morphs
3.12. Genetic Diversity with and within the Studied Morphs
4. Materials and Methods
4.1. Plant Material
4.1.1. Morphological Investigations, Seed Viability, and Morph Resemblance to the Parental Seeds
4.1.2. Pollen Grain Fertility
4.1.3. Molecular Investigation
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Atalay, Z. Anatomy, palynology and floral diversity of the genus lamium L. (lamiaceae) in Turkey. Ph.D. Thesis, Department of Biological Sciences, The Graduate School of Natural and Applied Sciences of Middle East Technical University, Ankara, Türkiye, 2016; p. 208. [Google Scholar]
- Boulos, L. Flora of Egypt; (Verbenaceae—Compositae); Al-Hadara Publishing: Cairo, Egypt, 2002; Volume 3, pp. 24–25. [Google Scholar]
- Boulos, L. Flora of Egypt Checklist, Revised Annotated ed.; Al-Hadara Publishing: Cairo, Egypt, 2009; pp. 198–201. [Google Scholar]
- Azimishad, F.; Sheidai, M.; Talebi, S.M.; Noormohammadi, Z. Biosystematic study and population genetic analysis in Lamium amplexicaule L. (Lamioideae, Lamiaceae). Genetika 2018, 50, 533–548. [Google Scholar] [CrossRef]
- Tackholm, V.; Boulos, L. Students’ Flora of Egypt; Cairo University: Cairo, Egypt, 1974; p. 888. [Google Scholar]
- Woolam, B.C. Determining Seasonal Emergence, Growth Characteristics, and Control Programs for Henbit (Lamium amplexicaule L.); Louisiana State University and Agricultural & Mechanical College: Baton Rouge, LA, USA, 2016. [Google Scholar] [CrossRef]
- Stojanova, B.; Maurice, S.; Cheptou, P.-O. Is plasticity across seasons adaptive in the annual cleistogamous plant Lamium amplexicaule? Ann. Bot. 2016, 117, 681–691. [Google Scholar] [CrossRef]
- DeFelice, M.S. Henbit and the deadnettles, Lamium spp.—Archangels or demons? Weed Technol. 2005, 19, 768–774. [Google Scholar] [CrossRef]
- Turner, W.B.R. The biology and non-chemical control of Common Poppy (Papaver rhoeas L.). Organistion 2004, 21, 1–7. [Google Scholar]
- Lord, E. The development of cleistogamous and chasmogamous flowers in Lamium amplexicaule (Labiatae): An example of heteroblastic inflorescence development. Bot. Gaz. 1979, 140, 39–50. Available online: https://www.journals.uchicago.edu/doi/pdf/10.1086/337056 (accessed on 15 January 2021). [CrossRef]
- Sato, Y.; Takakura, K.-I.; Nishida, S.; Nishida, T. Dominant Occurrence of Cleistogamous Flowers of Lamium amplexicaule in relation to the Nearby Presence of an Alien Congener L. purpureum (Lamiaceae). Int. Sch. Res. Not. 2013, 2013, 476862. [Google Scholar] [CrossRef]
- Munguía-Rosas, M.A.; Campos-Navarrete, M.J.; Parra-Tabla, V. The effect of pollen source vs. flower type on progeny performance and seed predation under contrasting light environments in a cleistogamous herb. PLoS ONE 2013, 8, e80934. [Google Scholar] [CrossRef]
- Lord, E.M. Cleistogamy: A tool for the study of floral morphogenesis, function and evolution. Bot. Rev. 1981, 47, 421–449. [Google Scholar] [CrossRef]
- Stojanova, B.; Maurice, S.; Cheptou, P.O. Season-dependent effect of cleistogamy in Lamium amplexicaule: Flower type origin versus inbreeding status. Am. J. Bot. 2020, 107, 155–163. [Google Scholar] [CrossRef]
- Minaeifar, A.A.; Sheidai, M.; Attar, F.; Noormohammadi, Z.; Ghasemzadeh-Baraki, S. Biosystematic study in the genus Cousinia Cass. (Asteraceae), section Cousinia. Biochem. Syst. Ecol. 2016, 69, 252–260. [Google Scholar] [CrossRef]
- Muraseva, D.S.; Guseva, A.A. ISSR primer screening for analysis of genetic diversity among Scutellaria tuvensis (Lamiaceae) populations. BIO Web Conf. 2021, 38, 82. [Google Scholar] [CrossRef]
- Mosaferi, S.; Sheidai, M.; Keshavarzi, M.; Noormohammdai, Z. Genetic diversity and morphological variability in Polygonum aviculare sl (Polygonaceae) of Iran. Phytotaxa 2015, 233, 166–178. [Google Scholar] [CrossRef]
- Abdelhameed, A.; Amer, W.; Hassan, W.; Aboellil, A. Auto-taxonomy of Brassica tournefortii Gouan. (Brassicaceae) in Egypt. Bangladesh J. Plant Taxon. 2020, 27, 233–250. [Google Scholar] [CrossRef]
- Collard, B.C.; Mackill, D.J. Start codon targeted (SCoT) polymorphism: A simple, novel DNA marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Report. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Guo, D.-L.; Zhang, J.-Y.; Liu, C.-H. Genetic diversity in some grape varieties revealed by SCoT analyses. Mol. Biol. Rep. 2012, 39, 5307–5313. [Google Scholar] [CrossRef]
- Chai, X.; Dong, R.; Liu, W.; Wang, Y.; Liu, Z. Optimizing sample size to assess the genetic diversity in common vetch (Vicia sativa L.) populations using start codon targeted (SCoT) markers. Molecules 2017, 22, 567. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; He, X.; Luo, C.; Gao, M.; Zhu, J. The optimization of SCoT-PCR system of Longan (Dimocarpus longan). Genomics Appl. Biol. 2009, 28, 970–974. [Google Scholar]
- Lord, E.M. Effect of daylength on open flower production in the cleistogamous species Lamium amplexicaule L. Ann. Bot. 1982, 49, 261–263. [Google Scholar] [CrossRef]
- Azimishad, F.; Sheidai, M.; Talebi, S.M.; Noormohammadi, Z. Species relationship and genetic diversity in some Iranian Lamium L. species using ISSR markers. Biodivers. J. Biol. Divers. 2019, 20, 1963–1972. [Google Scholar] [CrossRef]
- Culley, T.M.; Klooster, M.R. The cleistogamous breeding system: A review of its frequency, evolution, and ecology in angiosperms. Bot. Rev. 2007, 73, 1–30. [Google Scholar] [CrossRef]
- Zinger, E.; Gueijman, A.; Obolski, U.; Ram, Y.; Ruby, E.; Binder, M.; Yechieli, N.; Ohad, N.; Hadany, L. Less fit Lamium amplexicaule plants produce more dispersible seeds. Sci. Rep. 2019, 9, 6299. [Google Scholar] [CrossRef] [PubMed]
- Solbrig, O.T. Studies on the population biology of the genus Viola. II. The effect of plant size on fitness in Viola sororia. Evolution 1981, 35, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Clay, K. Environmental and genetic determinants of cleistogamy in a natural population of the grass Danthonia spicata. Evolution 1982, 36, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Macnair, M. Pollen tube competition as a mechanism of prezygotic reproductive isolation between Mimulus nasutus and its presumed progenitor M. guttatus. New Phytol. 1999, 144, 471–478. [Google Scholar] [CrossRef]
- Mayers, A.M.; Lord, E.M. Comparative flower development in the cleistogamous species Viola odorata. I. A growth rate study. Am. J. Bot. 1983, 70, 1548–1555. [Google Scholar] [CrossRef]
- Le Corff, J. Effects of light and nutrient availability on chasmogamy and cleistogamy in an understory tropical herb, Calathea micans (Marantaceae). Am. J. Bot. 1993, 80, 1392–1399. [Google Scholar] [CrossRef]
- Lord, E.M. Floral morphogenesis in Lamium amplexicaule L. (Labiatae) with a model for the evolution of the cleistogamous flower. Bot. Gaz. 1982, 143, 63–72. [Google Scholar] [CrossRef]
- El-Gazzar, A.; El-Saeid, A.; Khattab, A.; Elkady, A.; ElGhamery, A. Computer-generated keys to the flora of Egypt. 8. The Lamiaceae. Egypt. J. Bot. 2019, 59, 209–232. [Google Scholar] [CrossRef]
- Furukawa, T.; Itagaki, T.; Murakoshi, N.; Sakai, S. Inherited dimorphism in cleistogamous flower production in Portulaca oleracea: A comparison of 16 populations growing under different environmental conditions. Ann. Bot. 2020, 125, 423–431. [Google Scholar] [CrossRef]
- Wąsowicz, K.; Szczecińska, M.; Sawicki, J. The reasons for low intrapopulation genetic variation in Willd. Acta Musei Sil. Sci. Nat. 2011, 60, 79–84. [Google Scholar] [CrossRef]
- Stojanova, B.; Dubois, M.P.; Maurice, S.; Cheptou, P.O. Isolation and characterization of microsatellite markers for the cleistogamous species Lamium amplexicaule (Lamiaceae). Appl. Plant Sci. 2013, 1, 1200259. [Google Scholar] [CrossRef]
- Culley, T.M.; Wolfe, A.D. Population genetic structure of the cleistogamous plant species Viola pubescens Aiton (Violaceae), as indicated by allozyme and ISSR molecular markers. Heredity 2001, 86, 545–556. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar] [CrossRef]
- Waller, D.M. Differences in fitness between seedlings derived from cleistogamous and chasmogamous flowers in Impatiens capensis. Evolution 1984, 38, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, K.; Głowacka, K. Nutlet micromorphology and its taxonomic utility in Lamium L. (Lamiaceae). Plant Syst. Evol. 2015, 301, 1863–1874. [Google Scholar] [CrossRef]
- Abdullateef, R.A.; Zakaria, N.H.; Hasali, N.H.; Osman, M. Studies on pollen viability and germinability in accessions of Stevia rebaudiana Bertoni. Int. J. Biol. 2012, 4, 72. [Google Scholar] [CrossRef]
- Saarela, J. Taxonomic synopsis of invasive and native Spartina (Poaceae, Chloridoideae) in the Pacific Northwest (British Columbia, Washington and Oregon), including the first report of Spartina× townsendii for British Columbia, Canada. PhytoKeys 2012, 10, 25–82. [Google Scholar] [CrossRef]
- Tanaka, N. High stability in chromosomal traits of Chamaelirium japonicum and C. koidzuminum (Melanthiaceae) with holocentric chromosomes. Cytologia 2020, 85, 33–40. [Google Scholar] [CrossRef]
- Mohamed, S.; Shoaib, R.; Gadalla, N. Selection of some seedling apricot strains at Al-Amar Region. J. Appl. Sci. 2015, 15, 195–204. [Google Scholar] [CrossRef]
- Sawant, S.V.; Singh, P.K.; Gupta, S.K.; Madnala, R.; Tuli, R. Conserved nucleotide sequences in highly expressed genes in plants. J. Genet. 1999, 78, 123–131. [Google Scholar] [CrossRef]
- Fathi, M.; Hussein, S.; Mohamed, S. Horticultural and molecular genetic evaluation of some peach selected strains cultivated under kalubiah governorate conditions. J. Amer. Sci. 2013, 9, 12–23. [Google Scholar]
- Xiong, F.; Zhong, R.; Han, Z.; Jiang, J.; He, L.; Zhuang, W.; Tang, R. Start codon targeted polymorphism for evaluation of functional genetic variation and relationships in cultivated peanut (Arachis hypogaea L.) genotypes. Mol. Biol. Rep. 2011, 38, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, S.; Saha, S.; Bandyopadhyay, T.K.; Ghosh, P. Efficiency of ISSR marker for characterization of Cymbopogon germplasms and their suitability in molecular barcoding. Plant Syst. Evol. 2015, 301, 439–450. [Google Scholar] [CrossRef]

| Using SCoT Results | |||
|---|---|---|---|
| CL + CH | CL + CHs | CL | |
| CL + CH | 1.000 | 0.903 | 0.818 |
| CL + CHs | 0.903 | 1.000 | 0.906 |
| CL | 0.818 | 0.906 | 1.000 |
| Using ISSR results | |||
| CL + CH | 1.000 | 0.778 | 0.778 |
| CL + CHs | 0.778 | 1.000 | 1.000 |
| CL | 0.778 | 1.000 | 1.000 |
| Locality | GPS Coordinates | Date of Collection | |
|---|---|---|---|
| N° | E° | ||
| Metobus-Kafr el Shiek * | 31°29′75″ | 30°52′33″ | 20 February 2021 & 9 March 2022 |
| Barrage-Damietta | 31°24′18″ | 31°47′17″ | 7 January 1927 |
| Barrage-Damietta | 31°23′18″ | 31°47′17″ | 2 April 1950 & 30 March 1956 |
| Behiera Province | 31°24′03″ | 30°25′00″ | 25 April 1987 |
| Samouha-Alexandria | 31°12′56″ | 29°56′30″ | 23 March 1956 |
| Dikirnis-Dakahlia | 31°06′52″ | 31°38′55″ | 21 May 1967 |
| Mansoura | 31°02′29″ | 31°22′41″ | 19 March 1974 |
| Tanta | 30°47′11″ | 31°00′01″ | 12 March 1968 |
| El-Salhiya, Sharkiya | 30°44′51″ | 32°00′18″ | 1 October 1983 |
| Shubrakhit-Behiera | 30°41′17″ | 30°14′28″ | 5 March 1988 |
| Ismailia | 30°35′17″ | 32°16′26″ | 18 March 1927 |
| Mit Kinana-Qaliubia | 30°23′08″ | 31°15′42″ | 14 February 1969 |
| Abu-Zaabal-Cairo | 30°14′27″ | 31°21′10″ | 9 April 1954 |
| Mattaria-Cairo | 30°07′35″ | 31°19′02″ | February 1952 |
| Mattaria-Cairo | 30°07′34″ | 31°19′03″ | 1 May 1949 |
| Abu Sleem El-Menoufia * | 30°06′45″ | 31°12′54″ | 30 January 2022 & 27 January 2022 |
| Zaafran palace-Cairo | 30°04′31″ | 31°17′03″ | 24 March 1926 |
| Orman Garden-Cairo | 30°01′45″ | 31°12′46″ | 5 April 1928 |
| Cairo University-Giza | 30°01′39″ | 31°12′27″ | 7 January 1952 |
| Cairo University Garden-Giza | 30°01′37″ | 31°12′33″ | 1971 |
| Faculty Agriculture-Giza | 30°01′09″ | 31°12′38″ | 5 April 1971 |
| Giza spring garden-Giza | 30°00′53″ | 31°12′32″ | 1962 |
| El Saf-Giza * | 29°34′57″ | 31°15′17″ | 15 November 2021 & 10 January 2022 |
| El-Siliene spring-Faiyum * | 29°24′48″ | 30°51′27″ | 2 December 2021 & 18 January 2022 |
| Faiyum fields-Faiyum | 29°18′27″ | 30°36′29″ | 1952 |
| Qoshesha-Benisuef | 29°17′53″ | 31°10′37″ | 19 March 1982 |
| Belyfa-Beniseuf * | 29°07′27″ | 31°02′56″ | 20 January 2021 & 18 February 2022 |
| El-sheik Awad-S. Sinai | 28°52′01″ | 33°00′14″ | 20 February 2018 |
| Deir el Arba’-S. Sinai | 28°47′25″ | 33°35′23″ | 12 May 1956 |
| Deir el Rabba-S. Sinai S | 28°33′25″ | 33°58′23″ | 25 April 1961 & 24 May 1961 |
| Saint Catherine Monastery-S. Sinai | 28°33′25″ | 33°58′23″ | 11 April 1967 |
| Tell El Amarna-Minya | 27°38′41″ | 30°53′55″ | 22 January 1968 |
| Hurghada-Red Sea | 27°13′36″ | 33°46′07″ | 10 February 1961 |
| Dakhla Oasis-Western desert | 25°32′30″ | 28°55′47″ | 11 February 1931 |
| Kharga Oasis-Western desert | 25°26′30″ | 30°33′47″ | 13 February 1952 |
| Primer Code | Primer Sequence | No. of Total Amplified Bands | No. of Polymorphic Bands | No. of Unique Bands | Polymorphism%/Primer | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CL + CH | CL + CHs | CL | CL + CH | CL + CHs | CL | CL + CH | CL + CHs | CL | CL + CH | CL + CHs | CL | ||
| SCoT primers | |||||||||||||
| SCoT 2 | ACC ATG GCT ACC ACC GGC | 2 | 3 | 5 | 0 | 1 | 3 | 0 | 0 | 2 | 0 | 33.33 | 60 |
| SCoT 3 | ACG ACA TGG CGA CCC ACA | 6 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SCoT 4 | ACC ATG GCT ACC ACC GCA | 4 | 4 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 25 | 25 | 0 |
| SCoT 6 | CAA TGG CTA CCA CTA CAG | 1 | 2 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 50 | 50 |
| SCoT 8 | ACA ATG GCT ACC ACT GAG | 3 | 2 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 33.33 | 0 | 0 |
| SCoT 10 | ACA ATG GCT ACC ACC AGC | 5 | 5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| SCoT 14 | ACC ATG GCT ACC AGC GCG | 8 | 8 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ISSR primers | |||||||||||||
| 49 A | 5′ CAC ACA CAC ACA AG 3′ | 3 | 3 | 3 | 1 | 1 | 1 | 1 | 0 | 0 | 33.33 | 33.33 | 33.33 |
| 89 A | 5′ CAC ACA CAC ACA CA 3′ | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 89 B | 5′ CAC ACA CAC ACA GT 3′ | 4 | 3 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 25 | 0 | 0 |
| HB-10 | 5′ GAG AGA GAG AGA CC 3′ | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HB-11 | 5′ GTG TGT GTG TGT TGT CC 3′ | 2 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 50 | 0 | 0 |
| HB-12 | 5′ CAC CAC CAC GC 3′ | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, W.M.; Al Shaye, N.A.; Hassan, M.O.; Khalaf, M.H. Heteroblastic Inflorescence of Lamium amplexicaule L. in Egyptian Flora. Plants 2023, 12, 1028. https://doi.org/10.3390/plants12051028
Amer WM, Al Shaye NA, Hassan MO, Khalaf MH. Heteroblastic Inflorescence of Lamium amplexicaule L. in Egyptian Flora. Plants. 2023; 12(5):1028. https://doi.org/10.3390/plants12051028
Chicago/Turabian StyleAmer, Wafaa M., Najla A. Al Shaye, Mahmoud O. Hassan, and Maha H. Khalaf. 2023. "Heteroblastic Inflorescence of Lamium amplexicaule L. in Egyptian Flora" Plants 12, no. 5: 1028. https://doi.org/10.3390/plants12051028
APA StyleAmer, W. M., Al Shaye, N. A., Hassan, M. O., & Khalaf, M. H. (2023). Heteroblastic Inflorescence of Lamium amplexicaule L. in Egyptian Flora. Plants, 12(5), 1028. https://doi.org/10.3390/plants12051028






