The Hybridization Barrier between Herbaceous Medicago sativa and Woody M. arborea Is Weakened by Reproductive Abnormalities in M. sativa Seed Parents
Abstract
1. Introduction
2. Results and Discussion
2.1. Further Weakening of the Crossing Barrier
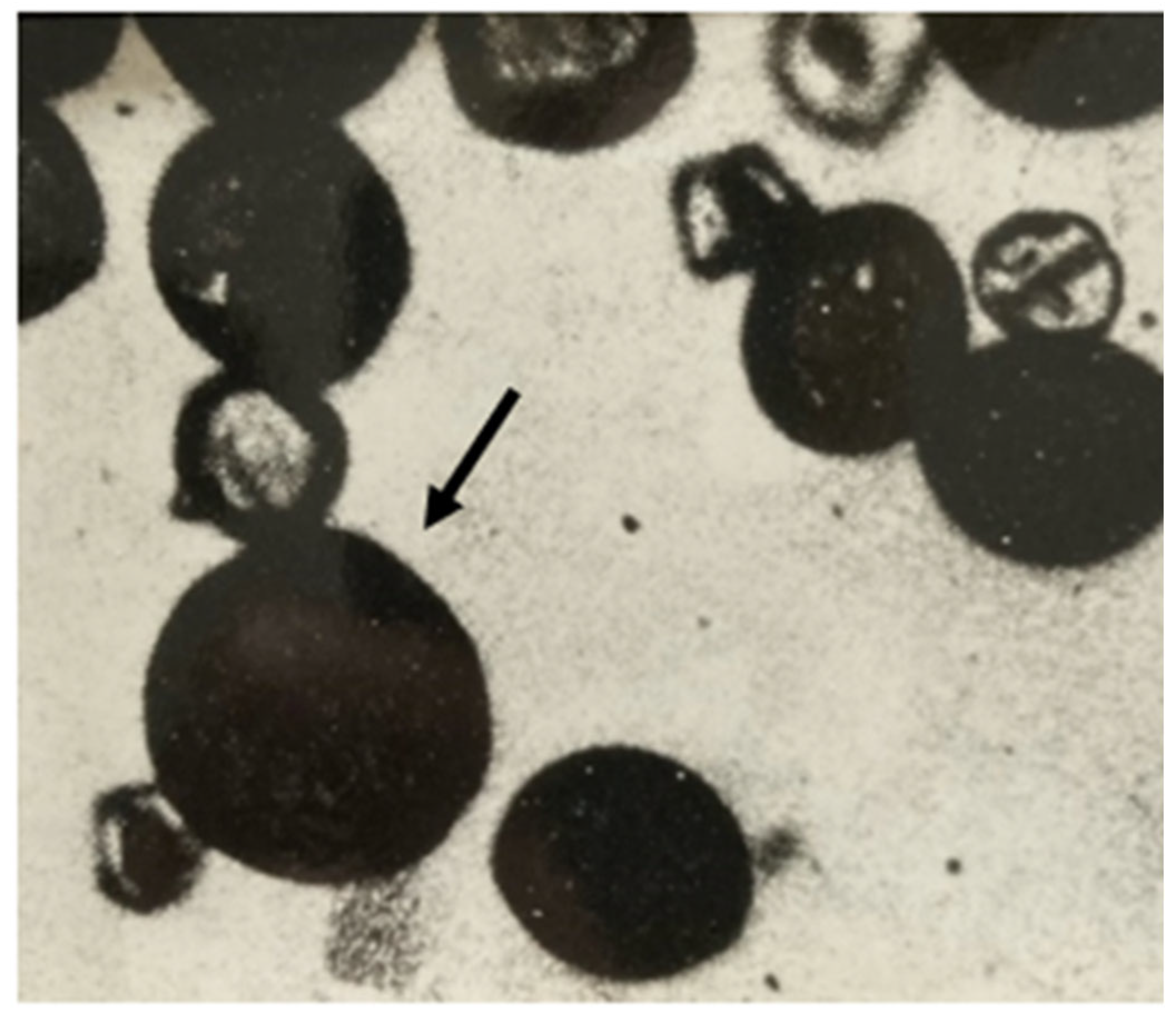
2.2. Evidence That 2n Eggs Enable Hybrid Production
2.3. Evidence That 2n Pollen Can Be Used to Screen for Plants That Produce 2n Eggs and Hybrids: An Experiment Performed in Reverse Order
3. Materials and Methods
3.1. Alfalfa Seed Parents 2n = 4x = 32
3.2. Special Clone M8 2n = 4x = 32
3.3. Medicago arborea Pollen Parents 2n = 4x = 32
3.4. Alborea Seed Parents 2n = 4x = 32, and near 32
3.5. Octoploid Alfalfa Pollen Parents 2n = 8x = 64 Used in 2n Egg Test
3.6. The Test for 2n Eggs in Alfalfa and Alborea Seed Parents
3.7. Test for 2n Pollen in Male Sterile and Self-Sterile Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bingham, E.; Armour, D.; Irwin, J. The hybridization barrier between herbaceous Medicago sativa and woody M. arborea is weakened by selection of seed parents. Plants 2013, 2, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.; Bingham, E. Genetic improvement for yield and fertility of alfalfa cultivars representing different eras of breeding. Crop Sci. 1994, 34, 953–957. [Google Scholar] [CrossRef]
- Irwin, J.; Lloyd, D.; Lowe, K. Lucerne biology and genetic improvement- an analysis of past activities and future goals in Australia. Aust. J. Agric. Res. 2001, 52, 699–712. [Google Scholar] [CrossRef]
- Lamb, J.; Shaeffer, C.; Rhodes, L.; Sule, R.; Undersander, D.; Brummer, E. Five decades of alfalfa cultivar improvement: Impact on forage yield, persistence, and nutritive value. Crop Sci. 2006, 46, 902–909. [Google Scholar] [CrossRef]
- Armour, D.; Mackie, J.; Musial, J.; Irwin, J. Transfer of anthracnose resistance and pod coiling traits from Medicago arborea to M. sativa by sexual reproduction. Theor. Appl. Genet. 2008, 117, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.; Armour, D.; Pepper, P.; Lowe, P. Heterosis in lucerne testcrosses with Medicago arborea introgressions and Omani landraces and their performance in synthetics. Crop Pasture Sci. 2010, 61, 450–463. [Google Scholar] [CrossRef]
- Inostraza, L.; Espinoza, S.; Barahona, V.; Gerding, M.; Humphries, A.; del Pozo, L.; Ovalle, C. Phenotypic diversity and productivity of Medicago sativa subspecies from drought prone environments in Mediterranean type environments. Plants 2021, 10, 862. [Google Scholar] [CrossRef]
- Irwin, J.; Sewell, J.; Woodfield, D.; Bingham, E. Restructuring lucerne (Medicago sativa) through introgression of the Medicago arborea genome. Agric. Sci. 2016, 28, 40–47. [Google Scholar]
- Bingham, E.; Irwin, J. Evidence that 2n eggs explain partial hybrids between Medicago sativa and Medicago arborea. Plants 2022, 11, 1380. [Google Scholar] [CrossRef]
- De Wet, J.; Newell, C.; Brink, D. Counterfeit hybrids between Tripsacum and Zea. Am. J. Bot. 1984, 71, 245–251. [Google Scholar] [CrossRef]
- Cheng, B.; Seguin-Swartz, G.; Somers, D. Cytogenetic and molecular characterization of intergeneric hybrids between Brassica napus and Orychophragmus violaceus. Genome 2002, 45, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Fedak, G. Wide crosses in Hordeum. In Barley Agronomy Monograph; Rasmussen, D., Ed.; ASA-CSSA-SSSA: Madison, WI, USA, 1985; pp. 155–186. [Google Scholar]
- Pfeiffer, T.; Bingham, E. Abnormal meiosis in alfalfa, Medicago sativa: Cytology of 2N egg and 4N pollen formation. Can. J. Genet. Cytol. 1983, 25, 107–112. [Google Scholar] [CrossRef]
- Morgensen, H. The hows and whys of cytoplasmic inheritance in seed plants. Am. J. Bot. 1996, 83, 383–404. [Google Scholar] [CrossRef]
- Bingham, E. Backcrossing tetraploid into diploid Medicago falcata using 2n eggs. Crop Sci. 1990, 30, 1353–1354. [Google Scholar] [CrossRef]
- Bingham, E. Registration of WISFAL alfalfa (Medicago sativa subspecies falcata) tetraploid germplasm derived from diploids. Crop Sci. 1993, 33, 217–218. [Google Scholar] [CrossRef]
- Bingham, E. Research on the genetic transfer from Medicago arborea to alfalfa. Medicago Genet. Rep. 2010, 10, 1–4. [Google Scholar]
- Small, E.; Jomphe, M. A synopsis of the genus Medicago. Can. J. Bot. 1989, 67, 3260–3294. [Google Scholar] [CrossRef]
- Bingham, E.; Saunders, J. Chromosome manipulations in alfalfa: Scaling cultivated alfalfa to seven ploidy levels. Crop Sci. 1974, 14, 474–477. [Google Scholar] [CrossRef]
- Brink, R.; Cooper, D. The endosperm in seed development. Bot. Rev. 1947, 13, 423–477. [Google Scholar] [CrossRef]
- Dilkova, M.; Bingham, E. Microsporogenesis of alfalfa cultivars and selected genotypes. Medicago Genet. Rep. 2017, 17, 1–16. [Google Scholar]
| Year | |||
|---|---|---|---|
| 2003–2013 | 2013–2022 | ||
| Alfalfa Parents | Hybrids–Florets Pollinated–Florets per Hybrid | Alfalfa Parents | Hybrids–Florets Pollinated–Florets per Hybrid |
| Queensland | Queensland | ||
| WA2071 | 5–2100–420 | WA2570 (M8 S1) | 13–500–38 |
| WA2625 (Both MBms derived) | 0–600 | cv. Sequel (4 plants) | 0–2000 |
| Wisconsin | Wisconsin | ||
| WI 6–4 ms | 0–5000 | WI 6–4 ms | 0–500 |
| WI (5 ms plants) | 0–5000 | WI M8 | 4–350–88 |
| WI MBms | 11–2700–250 | ||
| WI M8 | 16–1350–85 | Alborea Parents | |
| WA Alborea 2554 | 1–1000 | ||
| WA Alborea 2973 X | 5–500–100 | ||
| Sequel | |||
| WI Alborea 101 | 3–40–13 | ||
| WI Alborea 301a | 2–50–25 | ||
| WI Alborea 301b | 1–50 | ||
| Alfalfa Parents | Seed–Florets | Progeny 8x | Progeny 4x | Low Frequency of 2n Pollen * |
|---|---|---|---|---|
| 6–4 ms | 0–500 | - | - | No |
| 2 K–1 ms–1 | 6–100 | 2 | 3 | Yes |
| 2 K–1 ms–3 | 3–50 | 2 | - | Yes |
| MBms | 7–100 | 5 | 2 | Yes |
| M8 | 12–100 | 9 | 2 | Yes |
| Alborea Parents | ||||
| Alborea 101 | 25–50 | 2 | 21 | Yes |
| Alborea 301a | 34–50 | 5 | 25 | Yes |
| Alborea 301b | 20–50 | 7 | 11 | Yes |
| Alborea 301c | 55–50 | 12 | 36 | Yes |
| Alborea 301d | 34–50 | 5 | 25 | Yes |
| Subspecies | Description |
|---|---|
| falcata-1 | Tall, robust, fall dormant, dark yellow flowers. |
| sativa-2 | From Saudi-Arabian cultivar Wadi-Qura, winter active, purple flowers. |
| falcata-3 | Like falcata-1, except thicker main stem that resists lodging. |
| coerulea-4 | Purple flowered diploid, doubled with colchicine, used as a tetraploid. |
| falcata-5 | Like falcata-1, except larger seed. |
| sativa-6 | From USA cultivar Columbia 2000, fall dormant, purple flowered. |
| The crossing strategy was as follows: 2-way crosses 1 X 2 and 4 X 5. Followed by 3-way crosses (1 X 2) X 3 and (4 X 5) X 6. Finally, 3-way X 3-way cross involving the above. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bingham, E.; Irwin, J. The Hybridization Barrier between Herbaceous Medicago sativa and Woody M. arborea Is Weakened by Reproductive Abnormalities in M. sativa Seed Parents. Plants 2023, 12, 962. https://doi.org/10.3390/plants12040962
Bingham E, Irwin J. The Hybridization Barrier between Herbaceous Medicago sativa and Woody M. arborea Is Weakened by Reproductive Abnormalities in M. sativa Seed Parents. Plants. 2023; 12(4):962. https://doi.org/10.3390/plants12040962
Chicago/Turabian StyleBingham, Edwin, and John Irwin. 2023. "The Hybridization Barrier between Herbaceous Medicago sativa and Woody M. arborea Is Weakened by Reproductive Abnormalities in M. sativa Seed Parents" Plants 12, no. 4: 962. https://doi.org/10.3390/plants12040962
APA StyleBingham, E., & Irwin, J. (2023). The Hybridization Barrier between Herbaceous Medicago sativa and Woody M. arborea Is Weakened by Reproductive Abnormalities in M. sativa Seed Parents. Plants, 12(4), 962. https://doi.org/10.3390/plants12040962




