Antimalarial and Cytotoxic Activity of Native Plants Used in Cabo Verde Traditional Medicine
Abstract
1. Introduction
2. Results
2.1. Percentage Yield of Crude Plant Material
2.2. Antiplasmodial Activity
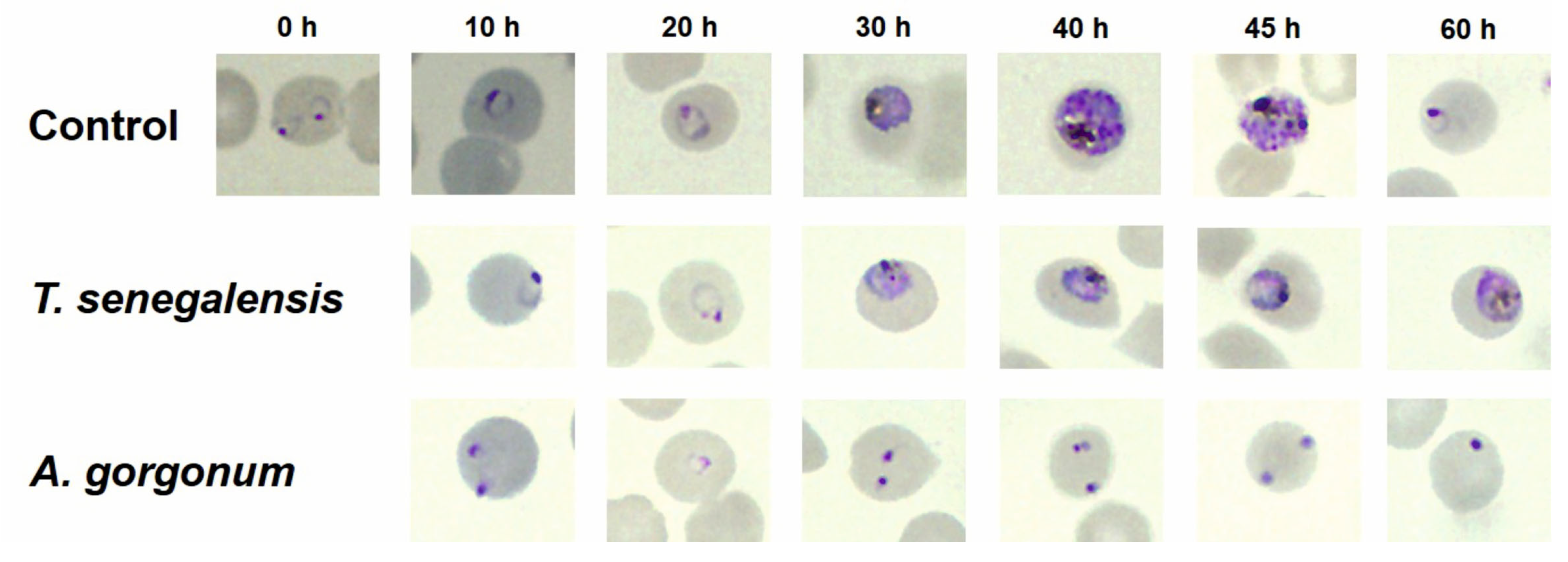
2.3. In Vitro Effect of A. gorgonum and T. senegalensis on P. falciparum Morphology
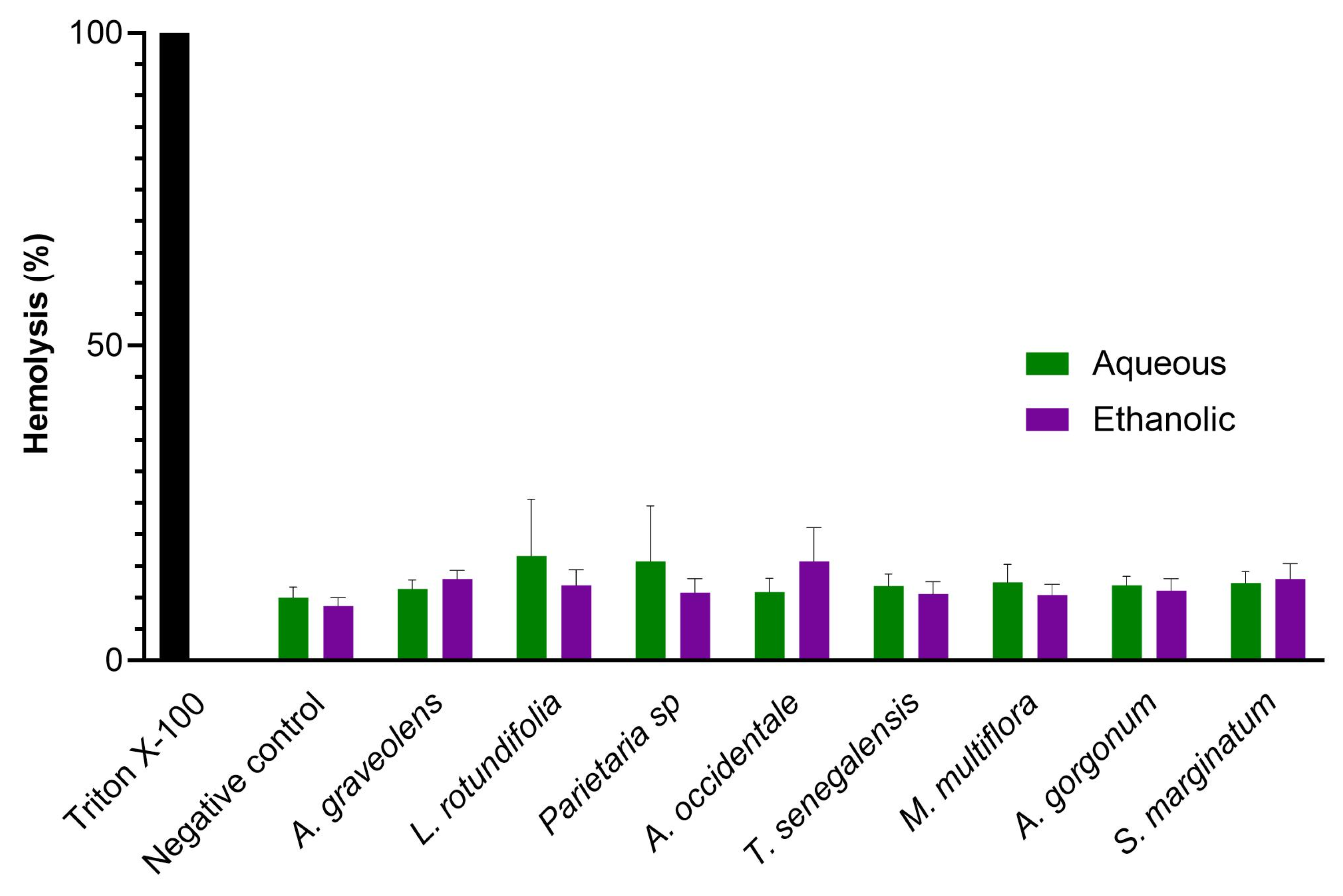
2.4. In Vitro Hemolysis Assay and Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Plant Powder Preparation
4.3. Crude Ethanolic Extracts
4.4. Preparation of the Infusions
4.5. Determination of Percentage Yield (%)
4.6. Plasmodium Culture
4.7. In Vitro Antiplasmodial Assay
4.8. Morphological Changes in Parasites by Microscopy
4.9. In Vitro Cytotoxic Activity
4.10. In Vitro Hemolysis Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badmos, A.O.; Alaran, A.J.; Adebisi, Y.A.; Bouaddi, O.; Onibon, Z.; Dada, A.; Lin, X.; Lucero-Prisno, D.E. What sub-Saharan African countries can learn from malaria elimination in China. Trop. Med. Health 2021, 49, 86. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Potential Impact of Health Service Disruptions on the Burden of Malaria: A Modelling Analysis for Countries in Sub-Saharan Africa; WHO: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/331845/9789240004641-eng.pdf (accessed on 1 July 2022).
- Blasco, B.; Leroy, D.; Fidock, D.A. Antimalarial drug resistance: Linking Plasmodium falciparum parasite biology to the clinic. Nat. Med. 2017, 23, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Zoh, M.G.; Bonneville, J.M.; Tutagata, J.; Laporte, F.; Fodjo, B.K.; Mouhamadou, C.S.; Sadia, C.G.; McBeath, J.; Schmitt, F.; Horstmann, S.; et al. Experimental evolution supports the potential of neonicotinoid-pyrethroid combination for managing insecticide resistance in malaria vectors. Sci. Rep. 2021, 11, 19501. [Google Scholar] [CrossRef] [PubMed]
- Gebremeskel, A.T.; Out, A.; Abimbola, S.; Yaya, S. Building resilient health systems in Africa beyond the COVID-19 pandemic response. BMJ Glob. Health 2021, 6, e006108. [Google Scholar] [CrossRef]
- Catarino, L.; Havik, P.J.; Romeiras, M.M. Medicinal plants of Guinea-Bissau: Therapeutic applications, ethnic diversity and knowledge transfer. J. Ethnopharmacol. 2016, 183, 71–94. [Google Scholar] [CrossRef]
- Soares-Bezerra, R.J.; Calheiros, A.S.; da Silva Ferreira, N.C.; da Silva Frutuoso, V.; Alves, L.A. Natural products as a source for new anti-inflammatory and analgesic compounds through the inhibition of purinergic P2X receptors. Pharmaceuticals 2013, 6, 650–658. [Google Scholar] [CrossRef]
- Jalali, H.; Nejad, A.M.; Ebadi, A.G.; Laey, G. Ethnobotany and folk pharmaceutical properties of major trees or shrubs in northeast of Iran. Asian J. Chem. 2009, 21, 5632. [Google Scholar]
- Bahmani, M.; Rafieian-Kopaei, M.; Naghdi, N.; Nejad, A.S.M.; Afsordeh, O. Physalis alkekengi: A review of its therapeutic effects. J. Chem. Pharm. Sci. 2016, 9, 1472–1485. [Google Scholar]
- Romeiras, M.M.; Catarino, L.; Torrão, M.M.; Duarte, M.C. Diversity and origin of medicinal exotic flora in Cape Verde Islands. Plant Ecol. Evol. 2011, 144, 214–225. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Duarte, M.C.; Santos-Guerra, A.; Carine, M.; Francisco-Ortega, J. Botanical exploration of the Cape Verde Islands: From the pre-Linnaean records and collections to late 18th century floristic accounts and expeditions. Taxon 2014, 63, 625–640. [Google Scholar] [CrossRef]
- Duarte, M.C.; Gomes, I.; Catarino, S.; Brilhante, M.; Gomes, S.; Rendall, A.; Moreno, Â.; Fortes, A.R.; Ferreira, V.S.; Baptista, I.; et al. Diversity of Useful Plants in Cabo Verde Islands: A Biogeographic and Conservation Perspective. Plants 2022, 11, 1313. [Google Scholar] [CrossRef] [PubMed]
- DePina, A.J.; Stresman, G.; Barros, H.S.B.; Moreira, A.L.; Dia, A.K.; Furtado, U.D.; Faye, O.; Seck, I.; Niang, E.H.A. Updates on malaria epidemiology and profile in Cabo Verde from 2010 to 2019, the goal of elimination. Malar. J. 2020, 19, 380. [Google Scholar] [CrossRef] [PubMed]
- DePina, A.J.; Dia, A.K.; de Ascenção Soares, M.A.; Ferreira, M.C.; Moreira, A.L.; Leal, S.V.; Pires, C.M.; Moreira, J.M.G.; Tavares, M.F.; Da Moura, A.J.F.; et al. Knowledge, attitudes and practices about malaria in Cabo Verde: A country in the pre-elimination context. BMC Public Health 2019, 19, 850. [Google Scholar] [CrossRef]
- Razak, M.F.B.A.; Yong, P.K.; Shah, Z.M.; Abdullah, L.C.; Yee, S.S.; Yaw, I.T.C.S. The effects of varying solvent polarity on extraction yield of Orthosiphon stamineus Leaves. J. Appl. Sci. 2012, 12, 1207–1210. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Braz. J. Pharm. Sci. 2020, 56. [Google Scholar] [CrossRef]
- Abubakar, A.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1. [Google Scholar] [CrossRef]
- Wakeel, A.; Jan, S.A.; Ullah, I.; Shinwari, Z.K.; Xu, M. Solvent polarity mediates phytochemical yield and antioxidant capacity of Isatis Tinctoria. PeerJ 2019, 7, e7857. [Google Scholar] [CrossRef]
- Essoh, A.P.; Liberal, Â.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Mandim, F.; Moldão-Martins, M.; Cravo, P.; Duarte, M.P.; Moura, M.; et al. Evaluation of the polyphenolic composition and bioactivities of three native Cabo Verde medicinal plants. Pharmaceuticals 2022, 15, 1162. [Google Scholar] [CrossRef]
- Pink, R.; Hudson, A.; Mouriès, M.-A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–740. [Google Scholar] [CrossRef]
- Kumari, P.; Sahal, D.; Jain, S.K.; Chauhan, V.S. Bioactivity guided fractionation of leaves extract of Nyctanthes arbor tristis (Harshringar) against P. falciparum. PLoS ONE 2012, 7, e51714. [Google Scholar] [CrossRef]
- Ortet, R.; Prado, S.; Regalado, E.L.; Valeriote, F.A.; Media, J.; Mendiola, J.; Thomas, O.P. Furfuran lignans and a flavone from Artemisia gorgonum Webb and their in vitro activity against Plasmodium falciparum. J. Ethnopharmacol. 2011, 138, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, L.F.; da Silva Pinto, A.C.; Pohlit, A.M.; Quignard, E.L.J.; Vieira, P.P.R.; Tadei, W.P.; Chaves, F.C.M.; Samonek, J.F.; Lima, C.A.J.; Costa, M.R.F.; et al. In Vivo and In Vitro Antimalarial Activity of 4-Nerolidylcatechol. Phytother. Res. 2011, 25, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Mignon, R.; Bastos, M.; Batista, D.; Neng, N.R.; Nogueira, J.M.F.; Vizetto-Duarte, C.; Custódio, L.; Varela, J.; Rauter, A.P. In vitro antitumoral activity of compounds isolated from Artemisia gorgonum Webb. Phytother. Res. 2014, 28, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Almeida, A.; Silva, H.; Pinto, D.; Seca, A.; Pereira, M.L. Potential Anti-inflammatory Effects of Artemisia gorgonum on rat liver injury induced by CCl4. Microsc. Microanal. 2016, 22, 26–27. [Google Scholar] [CrossRef]
- MacKinnon, S.; Durst, T.; Arnason, J.T.; Angerhofer, C.; Pezzuto, J.; Sanchez-Vindas, P.E.; Poveda, L.J.; Gbeassor, M. Antimalarial Activity of Tropical Meliaceae Extracts and Gedunin Derivatives. J. Nat. Prod. 1997, 60, 336–341. [Google Scholar] [CrossRef]
- Braga, T.M.; Rocha, L.; Chung, T.Y.; Oliveira, R.F.; Pinho, C.; Oliveira, A.I.; Morgado, J.; Cruz, A. Biological Activities of Gedunin—A Limonoid from the Meliaceae Family. Molecules 2020, 25, 493. [Google Scholar] [CrossRef]
- Kane, N.F.; Kyama, M.C.; Nganga, J.K.; Hassanali, A.; Diallo, M.; Kimani, F. Comparison of phytochemical profiles and antimalarial activities of Artemisia afra plant collected from five countries in Africa. S. Afr. J. Bot. 2019, 125, 126–133. [Google Scholar] [CrossRef]
- Kraft, C.; Jenett-Siems, K.; Siems, K.; Jakupovic, J.; Mavi, S.; Bienzle, U.; Eich, E. In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. Phytother. Res. 2003, 17, 123–128. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Kalkhorani, M.; Abbas Zaidi, S.M.; Farzaei, M.H.; Rahimi, R. The genus Tamarix: Traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2020, 246, 112245. [Google Scholar] [CrossRef]
- Romeiras, M.M.; Catarino, S.; Gomes, I.; Fernandes, C.; Costa, J.C.; Caujapé-Castells, J.; Duarte, M.C. IUCN Red List assessment of the Cape Verde endemic flora: Towards a global strategy for plant conservation in Macaronesia. Bot. J. Linn. Soc. 2016, 180, 413–425. [Google Scholar] [CrossRef]
- Varela, D.; Romeiras, M.M.; Silva, L. Implications of climate change on the distribution and conservation of Cabo Verde endemic trees. Glob. Ecol. Conserv. 2022, 34, e02025. [Google Scholar] [CrossRef]
- Barbosa, L.A.G. Subsídios para um dicionário utilitário e glossário dos nomes vernáculos das plantas de arquipélago de Cabo Verde. Garcia Orta 1961, 9, 37–91. [Google Scholar]
- Gomes, I.; Gomes, S.; Vera-Cruz, M.T.; Kilian, N.; Leyens, T.; Lobin, W. Plantas Endémicas e Árvores Indígenas de Cabo Verde; INIDA: Praia, Cape Verde; GTZ: Eschborn, Germany, 1995; pp. 1–36. [Google Scholar]
- Varela, J.M. Contribuição para a utilização terapêutica de plantas medicinais de Cabo Verde & de plantas medicinais comuns a Angola, Cabo Verde, Guiné-Bissau e Moçambique. An. AECCOM 2001, 1, 57–74. Available online: http://memoria-africa.ua.pt/Library/ShowImage.aspx?q=/JoaoVario/AECCOM-Anais-V3N1&p=60 (accessed on 15 July 2022).
- Brochmann, C.; Rustan, Ø.H.; Lobin, W.; Kilian, N. The endemic vascular plants of the Cape Verde Islands, W Africa. Sommerfeltia 1997, 24, 1–356. [Google Scholar] [CrossRef]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 1 June 2022).
- Trager, W.; Jensen, J. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, C.L.; Ahmed, A.O.A.; Cooper, R.A.; Stedman, T.T. SYBR Green I®-based parasite growth inhibition assay for measurement of antimalarial drug susceptibility in Plasmodium falciparum. Methods Malar. Res. 2013, 122–129. [Google Scholar]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- De Sena Pereira, V.S.; Silva de Oliveira, C.B.; Fumagalli, F.; Emery, F.D.S.; da Silva, N.B.; de Andrade-Neto, V.F. Cytotoxicity, hemolysis and in vivo acute toxicity of 2-hydroxy-3-anilino-1,4-naphthoquinone derivatives. Toxicol. Rep. 2016, 3, 756–762. [Google Scholar] [CrossRef]

| Scientific Name (Plant Part Used) | Extraction Yield (%) H2O | Concentration (μg/mL) H2O | Extraction Yield (%) EtOH | Concentration (μg/mL) EtOH |
|---|---|---|---|---|
| Artemisia gorgonum (leaves and stem) | 20 | 100.3 | 7.3 | 36.5 |
| Lavandula rotundifolia (leaves) | 18 | 90.1 | 4.9 | 24.5 |
| Sideroxylon marginatum (leaves) | 18 | 90.3 | 5.7 | 28.5 |
| Tamarix senegalensis (leaves) | 8 | 40.4 | 2.8 | 14.0 |
| Scientific Name | 3D7 | Dd2 | ||
|---|---|---|---|---|
| EC50 (μg/mL) H2O | EC50 (μg/mL) EtOH | EC50 (μg/mL) H2O | EC50 (μg/mL) EtOH | |
| Artemisia gorgonum | 49.1 ± 12.3 * | 1.7 ± 0.5 | 52.1 ± 11.3 * | 3.3 ± 0.6 |
| Lavandula rotundifolia | 95.4 ± 13.2 * | 26.3 ± 3.2 | 83.7 ± 19.6 * | 32.0 ± 7.8 |
| Sideroxylon marginatum | 48.6 ± 5.6 * | 25.6 ± 2.2 | 46.0 ± 8.7 * | 32.0 ± 5.8 |
| Tamarix senegalensis | 4.7 ± 0.6 * | 11.4 ± 2.1 | 5.4 ± 1.2 * | 13.8 ± 3.3 |
| Cell Line | Solvent of Extraction | Tamarix senegalensis (GI50 (µg/mL)) | SI | Artemisia gorgonum (GI50 (µg/mL)) | SI |
|---|---|---|---|---|---|
| Caco-2 | Infusions | >400 | >85 | 181 ± 10 * | 3.7 |
| Ethanolic | 125 ± 4 | 11 | 17.3 ± 0.2 | 10 | |
| PLP2 | Infusions | >400 | >85 | >400 | >8 |
| Ethanolic | 178 ± 3 | 15 | >400 | >235 |
| Species (Family) | Common Names | Native Status | Medicinal Applications | Parts Used | Preparation and Administration | Sample Voucher |
|---|---|---|---|---|---|---|
| Artemisia gorgonum Webb (Asteraceae) | Losna, absinto | Endemic | Intestinal & stomach problems, cough and respiratory diseases, fevers, malaria, diarrhea, and others | Leaves **, stems ** | Infusion, vaporization, and smoked as a cigarette | Silva 2 (LISC Herbarium) |
| Lavandula rotundifolia Benth. (Lamiaceae) | Aipo, alfazema-brava, alpo-rotcha | Endemic | Pains, stomach and kidney problems, cough and respiratory diseases, fever, malaria, diarrhea, and others | Leaves *, flowers | Infusion, bath, and oral administration | Silva 9 (LISC Herbarium) |
| Sideroxylon marginatum (Decne. ex Webb) Cout. (Sapotaceae) | Marmulano | Endemic | Pains, fever and flu-like illness, rheumatism, bones | Leaves *, bark | Infusion, maceration, oral, and dermal administration | Silva 8 (LISC Herbarium) |
| Tamarix senegalensis DC. (Tamaricaceae) | Tarrafe, tamargueira | Native | Cough and respiratory diseases, fever, and flu-like illness | Leaves *, fruits | Infusion, bath, and oral administration | Silva 10 (LISC Herbarium) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Essoh, A.P.; Cassiano, G.C.; Mandim, F.; Barros, L.; Gomes, I.; Medeiros, M.M.; Moura, M.; Cravo, P.V.L.; Romeiras, M.M. Antimalarial and Cytotoxic Activity of Native Plants Used in Cabo Verde Traditional Medicine. Plants 2023, 12, 963. https://doi.org/10.3390/plants12040963
Essoh AP, Cassiano GC, Mandim F, Barros L, Gomes I, Medeiros MM, Moura M, Cravo PVL, Romeiras MM. Antimalarial and Cytotoxic Activity of Native Plants Used in Cabo Verde Traditional Medicine. Plants. 2023; 12(4):963. https://doi.org/10.3390/plants12040963
Chicago/Turabian StyleEssoh, Anyse P., Gustavo Capatti Cassiano, Filipa Mandim, Lillian Barros, Isildo Gomes, Márcia Melo Medeiros, Mónica Moura, Pedro Vitor Lemos Cravo, and Maria M. Romeiras. 2023. "Antimalarial and Cytotoxic Activity of Native Plants Used in Cabo Verde Traditional Medicine" Plants 12, no. 4: 963. https://doi.org/10.3390/plants12040963
APA StyleEssoh, A. P., Cassiano, G. C., Mandim, F., Barros, L., Gomes, I., Medeiros, M. M., Moura, M., Cravo, P. V. L., & Romeiras, M. M. (2023). Antimalarial and Cytotoxic Activity of Native Plants Used in Cabo Verde Traditional Medicine. Plants, 12(4), 963. https://doi.org/10.3390/plants12040963








