Suppression of Seedling Survival and Recruitment of the Invasive Tree Prosopis juliflora in Saudi Arabia through Its Own Leaf Litter: Greenhouse and Field Assessments
Abstract
1. Introduction
2. Results
2.1. Prosopis Juliflora Seed Characteristics
2.2. Greenhouse and Field Leaf Litter Experiment Conditions
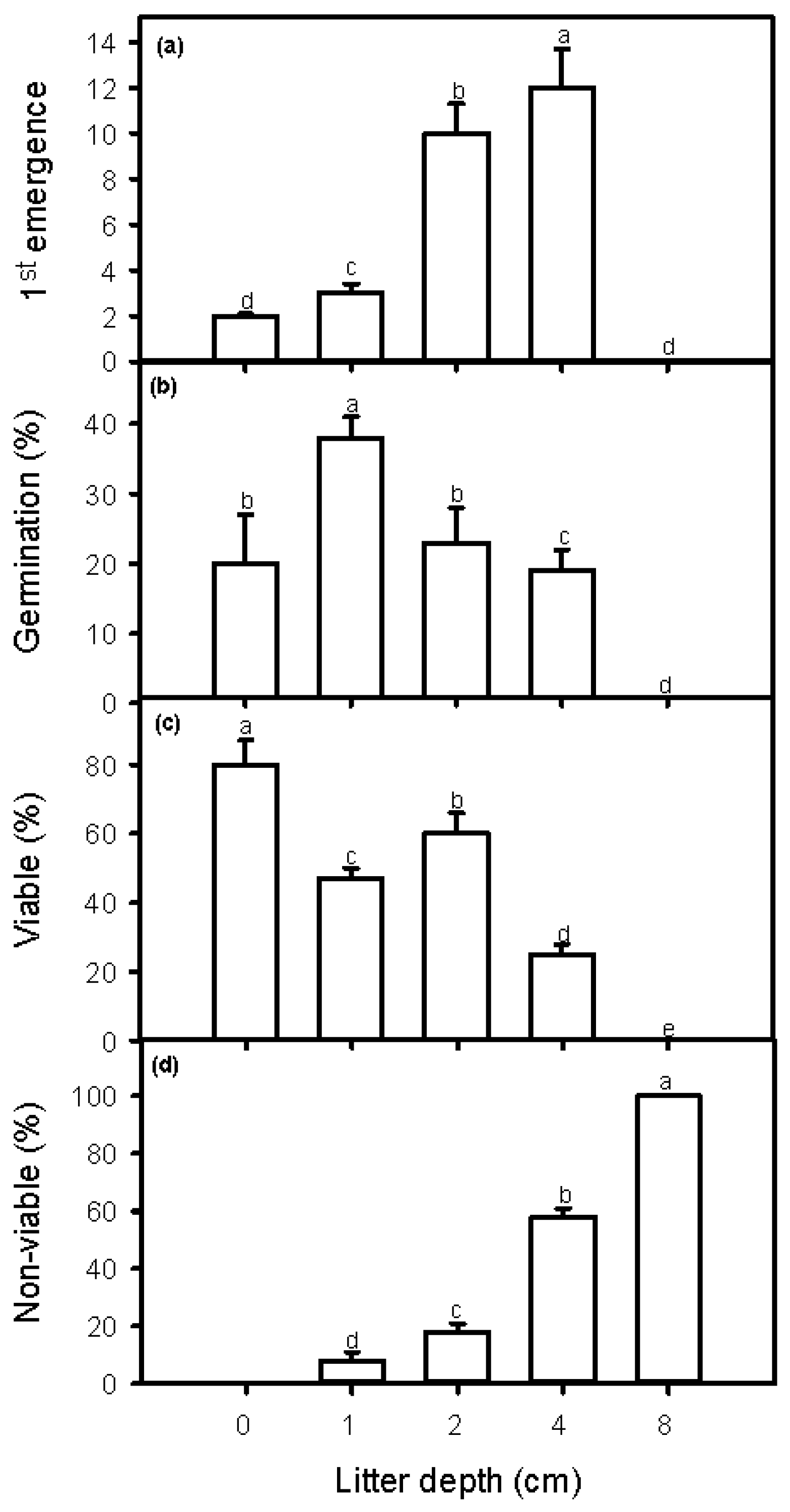
2.3. Seedling Emergence, Seed Germination, and Seed Viability Measurements
3. Discussion
4. Materials and Methods
4.1. Fruit and Leaf Litter Collection
4.2. Greenhouse Leaf Litter Experiment
4.3. Seedling Emergence, Seed Germination, and Seed Viability Measurements
4.4. Greenhouse Leaf Litter Environmental Conditions
4.5. Field Leaf Litter Experiment
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker, T.T.; Lockaby, B.G.; Conner, W.H.; Meier, C.E.; Stanturf, J.A.; Burke, M.K. Leaf Litter Decomposition and Nutrient Dynamics in Four Southern Forested Floodplain Communities. Soil Sci. Soc. Am. J. 2001, 65, 1334–1347. [Google Scholar] [CrossRef]
- Facelli, J.M.; Pickett, S.T.A. Plant litter: Its dynamics and effects on plant community structure. Bot. Rev. 1991, 57, 1–32. [Google Scholar] [CrossRef]
- Boyero, L.; Pearson, R.G.; Hui, C.; Gessner, M.O.; Pérez, J.; Alexandrou, M.A.; Graça, M.A.; Cardinale, B.J.; Albariño, R.J.; Arunachalam, M.; et al. Biotic and abiotic variables influencing plant litter breakdown in streams: A global study. Proc. Royal Soc. B Biol. Sci. 2016, 283, 20152664. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L. Population Biology of Plants; Academic Press: New York, NY, USA, 1977; p. 892. [Google Scholar]
- Zhang, R.; Hu, X.; Baskin, J.M.; Baskin, C.C.; Wang, Y. Effects of Litter on Seedling Emergence and Seed Persistence of Three Common Species on the Loess Plateau in Northwestern China. Front. Plant Sci. 2017, 8, 103. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, X.; Heděnec, P.; Yue, K.; Wei, X.; Yang, J.; Wu, F. Litter facilitates plant development but restricts seedling establishment during vegetation regeneration. Funct. Ecol. 2022, 36, 3134–3147. [Google Scholar] [CrossRef]
- Fowler, N.L. Microsite Requirements for Germination and Establishment of Three Grass Species. Am. Midl. Nat. 1986, 115, 131. [Google Scholar] [CrossRef]
- Facelli, J.M.; Pickett, S.T.A. Plant Litter: Light Interception and Effects on an Old-Field Plant Community. Ecology 1991, 72, 1024–1031. [Google Scholar] [CrossRef]
- Gross, K.L. Effects of Seed Size and Growth Form on Seedling Establishment of Six Monocarpic Perennial Plants. J. Ecol. 1984, 72, 369. [Google Scholar] [CrossRef]
- Wilsey, B.J.; Polley, H.W. Effects of seed additions and grazing history on diversity and productivity of subhumid grasslands. Ecology 2003, 84, 920–931. [Google Scholar] [CrossRef]
- Xiong, S.; Nilsson, C. The effects of plant litter on vegetation: A meta-analysis. J. Ecol. 1999, 87, 984–994. [Google Scholar] [CrossRef]
- Boeken, B.; Orenstein, D. The effect of plant litter on ecosystem properties in a Mediterranean semi-arid shrubland. J. Veg. Sci. 2001, 12, 825–832. [Google Scholar] [CrossRef]
- Donath, T.W.; Eckstein, R.L. Effects of bryophytes and grass litter on seedling emergence vary by vertical seed position and seed size. Plant Ecol. 2009, 207, 257–268. [Google Scholar] [CrossRef]
- Wellstein, C. Seed-litter-position drives seedling establishment in grassland species under recurrent drought. Plant Biol. 2012, 14, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Loydi, A.; Eckstein, R.L.; Otte, A.; Donath, T.W. Effects of litter on seedling establishment in natural and semi-natural grass-lands. J. Ecol. 2013, 101, 454–464. [Google Scholar] [CrossRef]
- Jensen, K.; Gutekunst, K. Effects of litter on establishment of grassland plant species: The role of seed size and successional status. Basic Appl. Ecol. 2003, 4, 579–587. [Google Scholar] [CrossRef]
- Eckstein, R.L.; Donath, T.W. Interactions between litter and water availability affect seedling emergence in four familial pairs of floodplain species. J. Ecol. 2005, 93, 807–816. [Google Scholar] [CrossRef]
- Deutsch, E.S.; Bork, E.W.; Willms, W.D. Separation of grassland litter and ecosite influences on seasonal soil moisture and plant growth dynamics. Plant Ecol. 2010, 209, 135–145. [Google Scholar] [CrossRef]
- Eckstein, R.L.; Ruch, D.; Otte, A.; Donath, T.W. Invasibility of a Nutrient-Poor Pasture through Resident and Non-Resident Herbs Is Controlled by Litter, Gap Size and Propagule Pressure. PLoS ONE 2012, 7, e41887. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.; Anthelme, F.; et al. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef]
- Goldberg, D.E.; Werner, P.A. The effects of size of opening in vegetation and litter cover on seedling establishment of goldenrods (Solidago spp.). Oecologia 1983, 60, 149–155. [Google Scholar] [CrossRef]
- Jankowska-Blaszczuk, M.; Daws, M.I. Impact of red:far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil. Funct. Ecol. 2007, 21, 1055–1062. [Google Scholar] [CrossRef]
- Rotundo, J.L.; Aguiar, M.R. Litter effects on plant regeneration in arid lands: A complex balance between seed retention, seed longevity and soil-seed contact. J. Ecol. 2005, 93, 829–838. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: New York, NY, USA, 2012; p. 412. [Google Scholar]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; John Wiley & Sons: New York, NY, USA, 2013; p. 444. [Google Scholar]
- Simberloff, D.; Martin, J.L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Sala, O.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sannwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Novak, S.J. The role of evolution in the invasion process. Proc. Nat. Acad. Sci. USA 2007, 104, 3671–3672. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Gandhi, K.J.K.; Herms, D.A. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biol. Invasions 2010, 12, 389–405. [Google Scholar] [CrossRef]
- Liao, C.; Luo, Y.; Jiang, L.; Zhou, X.; Wu, X.; Fang, C.; Chen, J.; Li, B. Invasion of Spartina alterniflora Enhanced Ecosystem Carbon and Nitrogen Stocks in the Yangtze Estuary, China. Ecosystems 2007, 10, 1351–1361. [Google Scholar] [CrossRef]
- Clark, K.L.; Skowronski, N.; Hom, J. Invasive insects impact forest carbon dynamics. Glob. Chang. Biol. 2010, 16, 88–101. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Vitousek, P.M. Biological Invasions by Exotic Grasses, the Grass/Fire Cycle, and Global Change. Annu. Rev. Ecol. Syst. 1992, 23, 63–87. [Google Scholar] [CrossRef]
- Balch, J.K.; Bradley, B.A.; D’Antonio, C.M.; Gómez-Dans, J. Introduced annual grass increases regional fire activity across the arid western USA (1980–2009). Global Change Biol. 2013, 19, 173–183. [Google Scholar] [CrossRef]
- Gaertner, M.; Biggs, R.; Beest, M.T.; Hui, C.; Molofsky, J.; Richardson, D.M. Invasive plants as drivers of regime shifts: Iden-tifying high-priority invaders that alter feedback relationships. Divers. Distrib. 2014, 20, 733–744. [Google Scholar] [CrossRef]
- Sadeghi, S.M.M.; Van Stan, J.J., II; Pypker, T.G.; Friesen, J. Canopy hydrometeorological dynamics across a chronosequence of a globally invasive species, Ailanthus altissma (Mill., tree of heaven). Agric. For. Meteo. 2017, 240–241, 10–17. [Google Scholar] [CrossRef]
- Young, S.L.; Kimball, S.; Novak, S.J. Invasion of plant communities. In Global Plant Invasions; Clements, D.R., Upadhyaya, M.K., Joshi, S., Shrestha, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 9–51. [Google Scholar]
- Heger, T.; Trepl, L. Predicting Biological Invasions. Biol. Invasions 2003, 5, 313–321. [Google Scholar] [CrossRef]
- Simberloff, D.; Gibbons, L. Now you see them, now you don’t!—Population crashes of established introduced species. Biol. Invasions 2004, 6, 161–172. [Google Scholar] [CrossRef]
- Pysek, P.; Jarosik, V.; Pergl, J.; Randall, R.; Chytry, M.; Kuhn, I.; Tichy, L.; Danihelka, J.; Jun, J.C.; Sadlo, J. The global invasion success of Central European plants is related to distribution characteristics in their native range and species traits. Div. Distrib. 2009, 15, 891–903. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Weber, E.; Fischer, M. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol. Lett. 2010, 13, 235–245. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Dawson, W.; Maurel, N. Characteristics of successful alien plants. Mol. Ecol. 2015, 24, 1954–1968. [Google Scholar] [CrossRef] [PubMed]
- Cooling, M.; Hoffmann, B.D. Here today, gone tomorrow: Declines and local extinctions of invasive ant populations in the absence of intervention. Biol. Invasions 2015, 17, 3351–3357. [Google Scholar] [CrossRef]
- Holdredge, C.; Bertness, M.D. Litter legacy increases the competitive advantage of invasive Phragmites australis in New England wetlands. Biol. Invasions 2010, 13, 423–433. [Google Scholar] [CrossRef]
- Kaproth, M.A.; Eppinga, M.B.; Molofsky, J. Leaf litter variation influences invasion dynamics in the invasive wetland grass Phalaris arundinacea . Biol. Invasions 2013, 15, 1819–1832. [Google Scholar] [CrossRef]
- Via, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarosik, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pysek, P. Ecological im-pacts of invasive alien plants: A meta-analysis of their effects on species, communities, and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar]
- Bartuszevige, A.M.; Hrenko, R.L.; Gorchov, D.L. Effects of leaf litter on establishment, growth and survival of invasive seedlings in a decisuous forest. Am. Mid. Nat. 2007, 158, 472–477. [Google Scholar] [CrossRef]
- Daly, E.; McCarthy, N.; O’Halloran, J.; Irwin, S.; Rathaille, M.O. Effects of forest litter depth on seed germination efficiency of Rhododendron ponticum . Ir. For. 2014, 71, 50–62. [Google Scholar]
- Dai, Z.-C.; Wang, X.-Y.; Qi, S.-S.; Cai, H.-H.; Sun, J.-F.; Huang, P.; Du, D.-L. Effects of leaf litter on inter-specific competitive ability of the invasive plant Wedelia trilobata. Ecol. Res. 2016, 31, 367–374. [Google Scholar] [CrossRef]
- Asrat, G.; Seid, A. Allelopathic effect of meskit (Prosopis juliflora (Sw.) DC) aqueous extracts on tropical crops tested under laboratory conditions. Momona Ethiop. J. Sci. 2017, 9, 32. [Google Scholar] [CrossRef]
- Benitez, L.; Kendig, A.E.; Adkihari, A.; Clay, K.; Harmon, P.F.; Holt, R.D.; Goss, E.M.; Flory, S.L. Invasive grass litter supresses a native grass species and promotes disease. Ecosphere 2022, 13, e3907. [Google Scholar] [CrossRef]
- Hierro, J.L.; Callaway, R.M. Allelopathy and exotic plant invasion. Plant Soil 2003, 256, 29–39. [Google Scholar] [CrossRef]
- Hierro, J.L.; Maron, J.L.; Callaway, R.M. A biogeographical approach to plant invasions: The importance of studying exotics in their introduced and native range. J. Ecol. 2005, 93, 5–15. [Google Scholar] [CrossRef]
- Abbas, A.M.; Soliman, W.S.; Ahmed, M.E.; Ibraheim, N.H.; Mohamed, M.; Mahmoud, F.Y.; Monsour, H.M.; Mohamed, A. Predicting the spatial spread of invasive Prosopis juliflora (SW.) DC along environmental gradient in Gebel Elba National Park, Egypt. Int. J. Sci. Engin. Res. 2016, 7, 596–599. [Google Scholar]
- Muturi, G.M.; Poorter, L.; Bala, P.; Mohren, G.M. Unleached Prosopis litter inhibits germination but leached stimulates seedling growth of dry woodland species. J. Arid. Environ. 2017, 138, 44–50. [Google Scholar] [CrossRef]
- Abbas, A.M.; Mancilla-Leytón, J.M.; Castillo, J.M. Can camels disperse seeds of the invasive tree Prosopis juliflora . Weed Res. 2018, 58, 221–228. [Google Scholar] [CrossRef]
- Abbas, A.M.; Figueroa, M.E.; Castillo, J.M. Burial effects on seed germination and seedling establishment of Prosopis juliflora (SW.) DC. Arid. Land Res. Manag. 2018, 33, 55–69. [Google Scholar] [CrossRef]
- Abbas, A.M.; Mahfouz, L.; Ahmed, M.K.; Al-Kahtani, M.A.; Ruxton, G.D.; Lambet, A.M. Effects of ingestion by sheep on the germination of seeds of the invasive Prosopis juliflora tree. Small Rumin. Res. 2020, 188, 106098. [Google Scholar] [CrossRef]
- Goncalves, G.S.; de Andrade, L.A.; Xavier, K.R.F.; da Silva, J.F. Control methods of Prosopis juliflora (Sw.) DC. (Fabaceae) in invaded areas in the semiarid region of Brazil. Ciência Florest. St. Maria 2015, 25, 645–653. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Le Maitre, D.C.; Richardson, D.M. Prosopis invasions in South Africa: Population structures and impacts on native tree population stability. J. Arid. Environ. 2015, 114, 70–78. [Google Scholar] [CrossRef]
- Meroni, M.; Ng, W.-T.; Rembold, F.; Leonardi, U.; Atzberger, C.; Gadain, H.; Shaiye, M. Mapping Prosopis juliflora in west Somliland with Landsat 8 satellite imagery and ground information. Land Degrad. Develop. 2017, 28, 494–506. [Google Scholar] [CrossRef]
- Thomas, J.; El-Sheikh, M.A.; Alfarhan, A.H.; Alatar, A.A.; Sivadasan, M.; Basahi, M.; Al-Obaid, S.; Rajakrishnan, R. Impact of alien invasive species on habitats and species richness in Saudi Arabia. J. Arid. Environ. 2016, 127, 53–65. [Google Scholar] [CrossRef]
- Kumar, S.; Mathur, M. Impacts of invasive Prosopis juliflora on plant communities in arid grazing lands. Trop. Ecol. 2014, 55, 33–47. [Google Scholar]
- Mahmood, K.; Chughtai, M.I.; Awan, A.R.; Wahood, R.A. Biomass production of some salt tolerant tree species grown in different ecological zones in Pakistan. Pak. J. Bot. 2016, 48, 89–96. [Google Scholar]
- Gallaher, M.; Merlin, M. Biology and impacts of pacific island invasive species. 6. Prosopis pallida and Prosopis juliflora (algarroba, mesquite, kiawe) (Fabaceae). Pac. Sci. 2010, 64, 489–526. [Google Scholar] [CrossRef]
- Pasiecznik, N.M.; Felker, P.; Harris, P.J.C.; Harsh, L.N.; Cruz, G.; Tewari, J.C.; Cadoret, K.; Maldonado, L.J. The Prosopis juli-flora—Prosopis pallida Complex: A Monograph; HDRA: Coventry, UK, 2001; p. 162. [Google Scholar]
- Shackleton, R.T.; Le Maitre, D.C.; Pasiecznik, N.M.; Richardson, D.M. Prosopis: A global assessment of the biogeography, benefits, impacts, and management of one of the world’s worst woody invasive plant taxa. AoB Plants 2014, 6, plu027. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Shackleton, R.T.; El-Keblawy, A.; Del Mar Trigo Pérez, M.; González, L. Invasive Mesquite (Prosopis juliflora), an Allergy and Health Challenge. Plants 2020, 9, 141. [Google Scholar] [CrossRef]
- Warrag, M. Autotoxic potential of foliage on seed germination and early growth of mesquite (Prosopis juliflora). J. Arid. Environ. 1995, 31, 415–421. [Google Scholar] [CrossRef]
- Finzi, A.C.; Canham, C.D.; Van Breemen, N. Canopy tree–soil interactions within temperate forests: Species effects on pH and cations. Ecol. Appl. 1998, 8, 447–454. [Google Scholar]
- Khan, D.; Ahmad, R.; Ismail, S. Germination, growth, and ion regulation in Prosopis juliflora (Sw.) DC under saline conditions. Pak. J. Bot. 1987, 19, 131–138. [Google Scholar]
- El-Keblawy, A.; Al-Rawai, A. Effects of salinity, temperature and light on germination of invasive Prosopis juliflora (Sw.) D.C. J. Arid. Environ. 2005, 61, 555–565. [Google Scholar] [CrossRef]
- Nascimento, C.E.d.S.; Da Silva, C.A.D.; Leal, I.R.; Tavares, W.dS.; Serrao, J.E.; Zamuncio, J.C.; Tabarelli, M. Seed germination and early seedling survival of the invasive species Prosopis juliflora (Fabaceae) depend on habitat and seed dispersal mode in the Caatinga dry forest. PeerJ 2020, 8, e9607. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Yang, W.; Wu, F.; Tan, B. Effects of light intensity on litter decomposition in a subtropical region. Ecosphere 2017, 8, e01770. [Google Scholar] [CrossRef]
- Slate, M.L.; Tsombou, F.M.; Callaway, R.M.; Inderjit; El-Keblawy, A.A. Exotic Prosopis juliflora suppresses understory diversity and promotes agricultural weeds more than a native congener. Plant Ecol. 2020, 221, 659–669. [Google Scholar] [CrossRef]
- Basavaraja, P.K.; Sharma, S.D.; Badrinath, M.S.; Sridhara, S.; Hareesh, G.R. Prosopis juliflora—An efficient tree species for rec-lamation of salt affected soils. Karnataka J. Agric. Sci. 2007, 20, 727–731. [Google Scholar]


| Length (mm) | Width (mm) | Height (mm) | Volume (mm3) | Mass (g) |
|---|---|---|---|---|
| 6.3 ± 0.05 | 3.9 ± 0.03 | 2.2 ± 0.02 | 56.7 ± 0.63 | 0.041 ± 0.001 |
| Litter Depth (cm) | Litter Load (g·m−2) | pH | Electrical Conductivity (mS·cm−1) | Daily Soil Temperature (°C) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Green House | Field | ||||||||
| Maximum | Minimum | Max–Min | Maximum | Minimum | Max–Min | ||||
| 0 | 0 ± 0 e | 6.25 ± 0.10 e | 0.44 ± 0.01 a | 35.4 ± 0.4 | 21.5 ± 4.3 a | 13.9 ± 4.6 a | 46.8 ± 0.5 a | 24.3 ± 2.1 a | 22.5 ± 0.5 a |
| 1 | 1093 ± 37 d | 6.58 ± 0.13 d | 0.41 ± 0.01 b | 33.5 ± 0.3 b | 20.4 ± 1.8 a | 13.1 ± 3.4 a | 37.5 ± 0.5 b | 22.8 ± 0.4 a | 14.7 ± 0.5 b |
| 2 | 2503 ± 20 c | 6.83 ± 0.05 c | 0.34 ± 0.01 c | 30.5 ± 0.2 c | 18.0 ± 1.1 b | 12.5 ± 2.7 a | 35.6 ± 0.2 c | 21.3 ± 0.5 a,b | 14.4 ± 0.7 b |
| 4 | 3230 ± 92 b | 7.30 ± 0.13 b | 0.29 ± 0.02 d | 27.7 ± 0.7 d | 17.5 ± 1.4 c | 10.2 ± 2.8 b | 29.5 ± 0.6 d | 18.0 ± 1.4 b | 11.5 ± 1.9 c |
| 8 | 8875 ± 68 a | 7.83 ± 0.09 a | 0.17 ± 0.01 e | 24.3 ± 0.3 e | 17.3 ± 1.5 c | 7.0 ± 1.9 c | 22.6 ± 0.7 e | 14.5 ± 1.4 c | 8.1 ± 2.1 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.M.; Alomran, M.M.; Alharbi, N.K.; Novak, S.J. Suppression of Seedling Survival and Recruitment of the Invasive Tree Prosopis juliflora in Saudi Arabia through Its Own Leaf Litter: Greenhouse and Field Assessments. Plants 2023, 12, 959. https://doi.org/10.3390/plants12040959
Abbas AM, Alomran MM, Alharbi NK, Novak SJ. Suppression of Seedling Survival and Recruitment of the Invasive Tree Prosopis juliflora in Saudi Arabia through Its Own Leaf Litter: Greenhouse and Field Assessments. Plants. 2023; 12(4):959. https://doi.org/10.3390/plants12040959
Chicago/Turabian StyleAbbas, Ahmed M., Maryam M. Alomran, Nada K. Alharbi, and Stephen J. Novak. 2023. "Suppression of Seedling Survival and Recruitment of the Invasive Tree Prosopis juliflora in Saudi Arabia through Its Own Leaf Litter: Greenhouse and Field Assessments" Plants 12, no. 4: 959. https://doi.org/10.3390/plants12040959
APA StyleAbbas, A. M., Alomran, M. M., Alharbi, N. K., & Novak, S. J. (2023). Suppression of Seedling Survival and Recruitment of the Invasive Tree Prosopis juliflora in Saudi Arabia through Its Own Leaf Litter: Greenhouse and Field Assessments. Plants, 12(4), 959. https://doi.org/10.3390/plants12040959






