Comparison of Proanthocyanidin Content in Rabbiteye Blueberry (Vaccinium virgatum Aiton) Leaves and the Promotion of Apoptosis against HL-60 Promyelocytic Leukemia Cells Using ‘Kunisato 35 Gou’ Leaf Extract
Abstract
:1. Introduction
2. Results
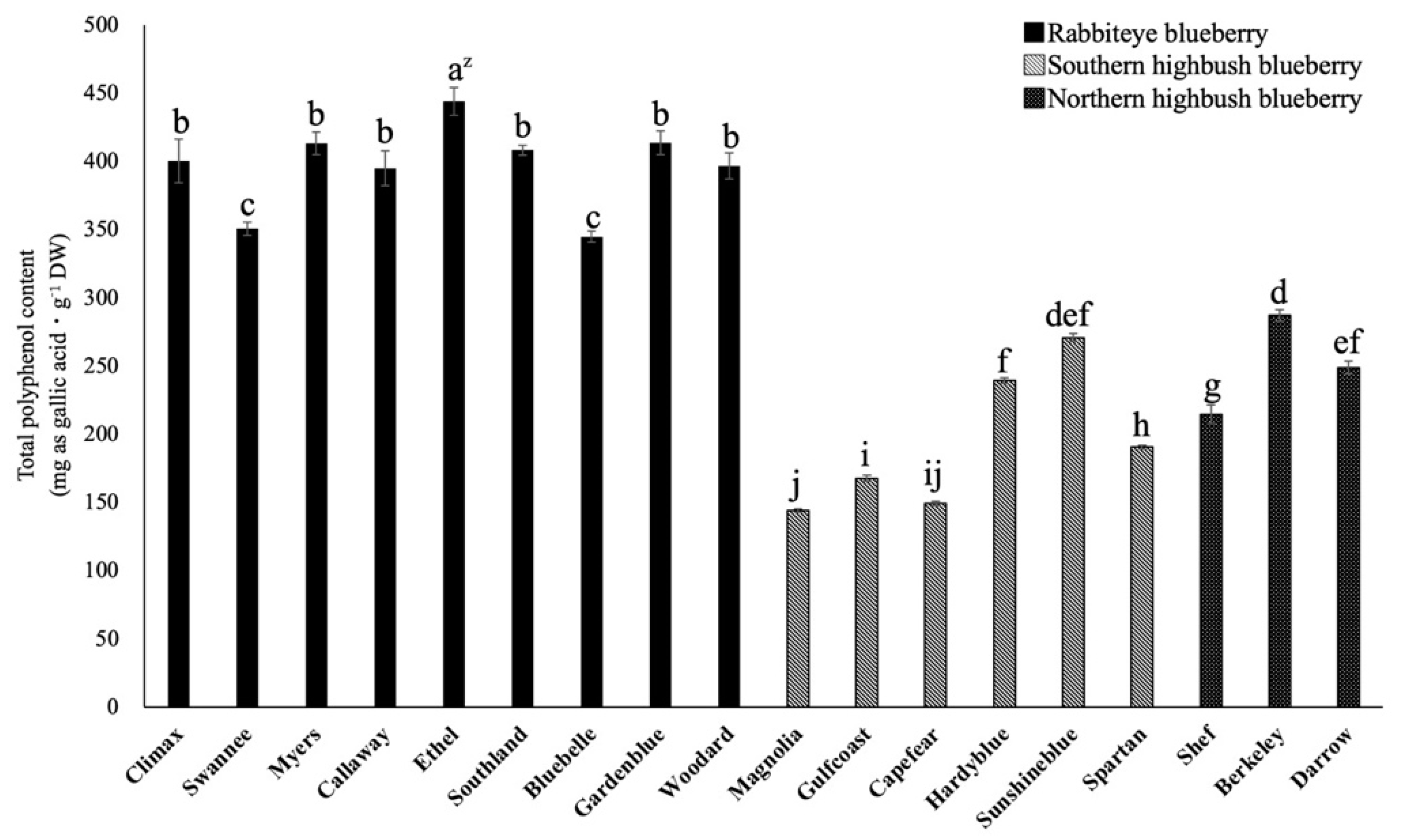
2.1. Comparison of Total Polyphenol Content among 18 Varieties
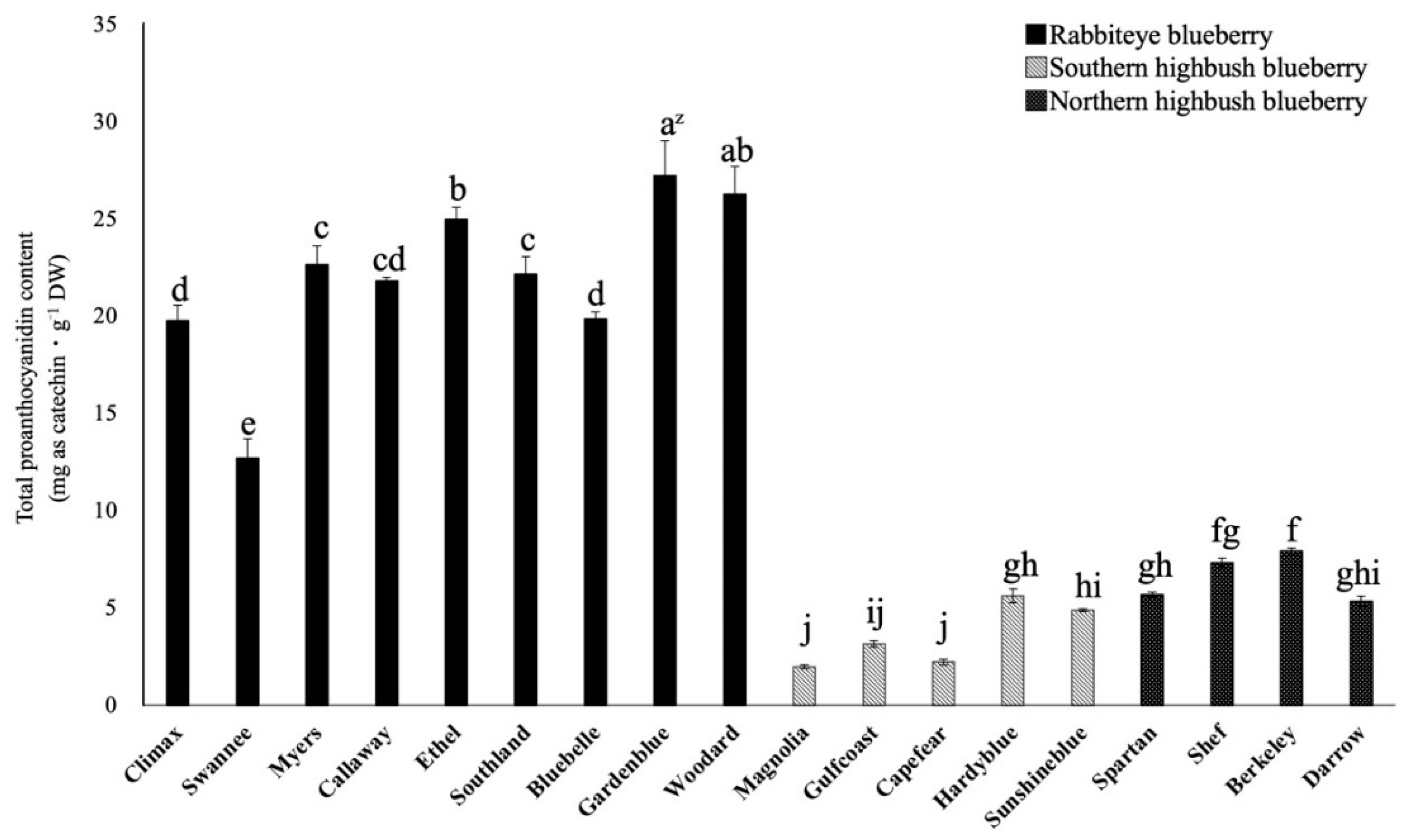
2.2. Comparison of Total Proanthocyanidin Content among 18 Varieties
2.3. Antioxidant Activity Analysis
2.4. Changes in the Total Polyphenol Content and the Total Proanthocyanidin Content of Rabbiteye Blueberry Leaves throughout the Growing Season
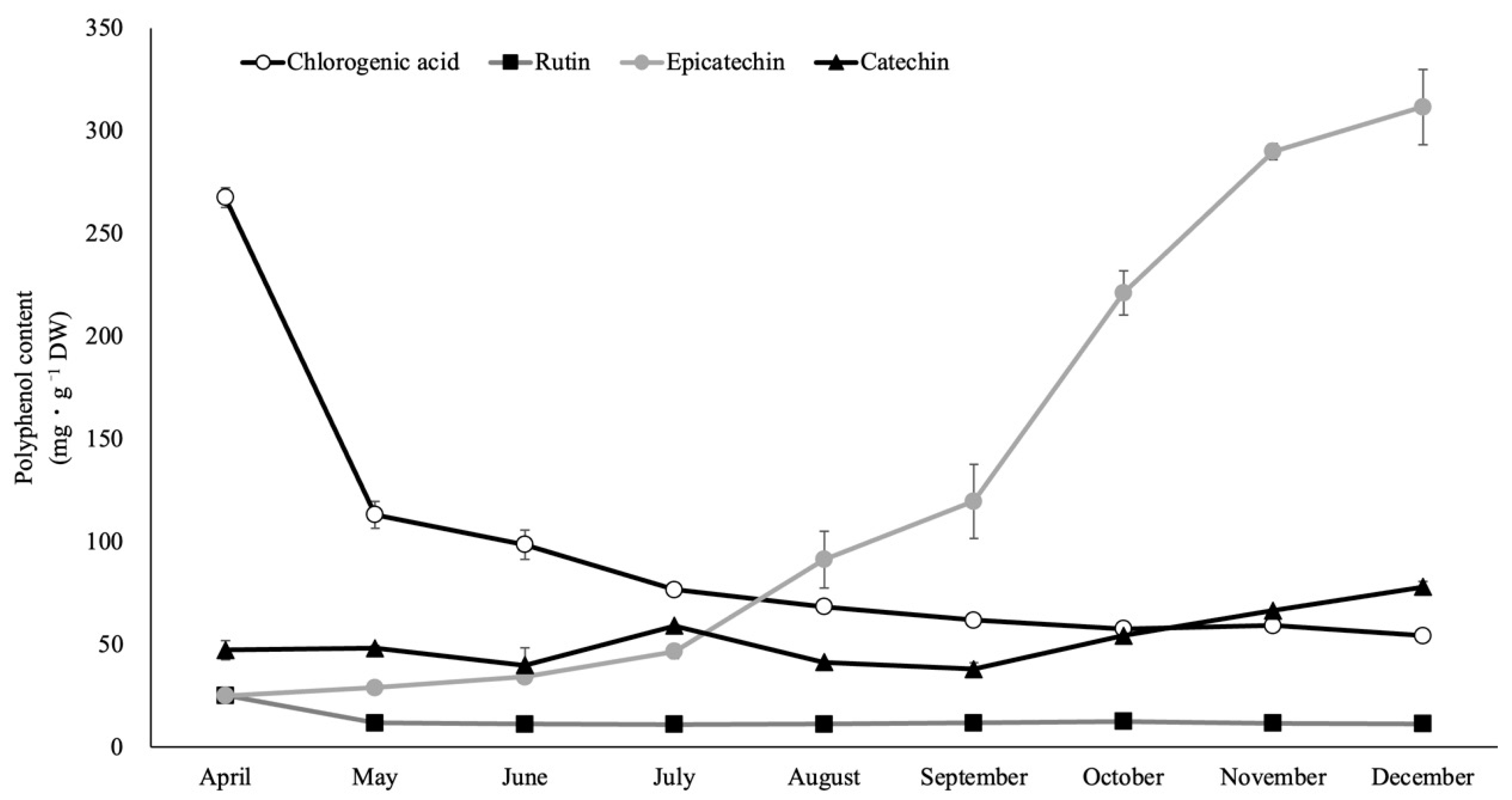
2.5. Polyphenol Components of Rabbiteye Blueberry Leaves throughout the Growing Season
2.6. Anti-Cancer Cell Proliferation Properties against HL-60 Cells
2.7. Anti-Cancer Cell Proliferation Properties against HL-60 Cells
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Polyphenol Analysis
4.3. Total Proanthocyanidin Analysis
4.4. Antioxidant Activity
4.5. Cell Line Used for the Analysis of the Anti-Cancer Cell Proliferation Properties and Flow Cytometric Propidium Iodide Annexin V Assay
4.6. Preparation of Extracts for Analysis of Anti-Cancer Cell Proliferation Properties
4.7. Flow Cytometric Propidium Iodide Annexin V Assay
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scherm, H.; Krewer, G. Blueberry Production in Georgia. Small Fruits Rev. 2003, 2, 83–91. [Google Scholar] [CrossRef]
- Dipayan, S.; Widya, A.; Floyd, W.; Elina, C.; Edgar, V.; Kalidas, S. In vitro screening and evaluation of phenolic antioxidant-linked anti-hyperglycemic functions of rabbit-eye blueberry (Vaccinium ashei) cultivars. J. Berry Res. 2017, 7, 163–177. [Google Scholar]
- Shibata, Y.; Ohara, K.; Matsumoto, K.; Hasegawa, T.; Akimoto, M. Total Anthocyanin Content, Total Phenolic Content, and Antioxidant Activity of Various Blueberry Cultivars Grown in Togane, Chiba Prefecture, Japan. J. Nutr. Sci. Vitaminol. 2021, 67, 201–209. [Google Scholar] [CrossRef]
- Scalzo, J.; Stevenson, D.; Hedderley, D. Blueberry estimated harvest from seven new cultivars: Fruit and anthocyanins. Food Chem. 2013, 139, 44–50. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Okan, O.T.; Deniz, I.; Yayli, N.; Şat, I.G.; Öz, M.; Hatipoglu Serdar, G. Antioxidant Activity, Sugar Content and Phenolic Profiling of Blueberries Cultivars: A Comprehensive Comparison. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 639–652. [Google Scholar] [CrossRef] [Green Version]
- Akšic, M.F.; Tosti, T.; Sredojevic, M.; Milivojevic, J.; Meland, M.; Natic, M. Comparison of Sugar Profile between Leaves and Fruits of Blueberry and Strawberry Cultivars Grown in Organic and Integrated Production System. Plants 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Gómez, J.; García-Martínez, T.; Varo, M.A.; Mérida, J.; Serratosa, M.P. Phenolic compounds, antioxidant activity and color in the fermentation of mixed blueberry and grape juice with different yeasts. Food Sci. Technol. 2021, 146, 111661. [Google Scholar] [CrossRef]
- Gato, E.; Perez, A.; Rosalowska, A.; Celeiro, M.; Bou, G.; Lores, M. Multicomponent Polyphenolic Extracts from Vaccinium corymbosum at Lab and Pilot Scale. Characterization and Effectivity against Nosocomial Pathogens. Plants 2021, 10, 2801. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Blueberries and their anthocyanins: Factors affecting biosynthesis and properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Toyama, Y.; Toshima, S.; Hirano, T.; Yamasaki, M.; Kunitake, H. Polyphenol contents, antioxidant activities, and anti-cancer cell proliferation properties at each stage of fruit development in intersectional hybrids between highbush blueberry and shashanbo (Vaccinium bracteatum Thunb.). J. Berry Res. 2021, 11, 689–704. [Google Scholar] [CrossRef]
- Xie, G.; Xu, X.; Zhou, X.; Liu, Y.; Zhao, Z. Changes in phenolic profiles and antioxidant activity in rabbiteye blueberries during ripening. Int. J. Food Prop. 2019, 22, 320–329. [Google Scholar]
- Pico, J.; Yan, Y.; Gerbrandt, E.M.; Castellarin, S.D. Determination of free and bound phenolics in northern highbush blueberries by a validated HPLC/QTOF methodology. J. Food Compost. Anal. 2022, 108, 104412. [Google Scholar] [CrossRef]
- Sezer, E.D.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Gunel, N.S.; Sözmen, E. Assessing Anticancer Potential of Blueberry Flavonoids, Quercetin, Kaempferol, and Gentisic Acid, through Oxidative Stress and Apoptosis Parameters on HCT-116 Cells. J. Med. Food 2019, 22, 1118–1126. [Google Scholar] [CrossRef]
- Yamane, T. Beneficial Effects of Anthocyanin from Natural Products on Lifestyle-Related Diseases through Inhibition of Protease Activities. Stud. Nat. Prod. Chem. 2018, 58, 245–264. [Google Scholar]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and leaves from wild blueberry plants contain diverse polyphenols and decrease neuroinflammatory responses in microglia. J. Funct. Foods 2020, 68, 103906. [Google Scholar] [CrossRef]
- Takeshita, M.; Ishida, Y.; Akamatsu, E.; Ohmori, Y.; Sudoh, M.; Uto, H.; Tsubouchi, H.; Kataoka, H. Proanthocyanidin from Blueberry Leaves Suppresses Expression of Subgenomic Hepatitis C Virus RNA. J. Biol. Chem. 2009, 284, 21165–21176. [Google Scholar] [CrossRef] [Green Version]
- Yuji, K.; Sakaida, H.; Kai, T.; Fukuda, N.; Yukizaki, C.; Sakai, M.; Tsubouchi, H.; Kataoka, H. Effect of dietary blueberry (Vaccinium ashei Reade) leaves on serum and hepatic lipid levels in rats. J. Oleo Sci. 2013, 62, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Sakaida, H.; Nagao, K.; Higa, K.; Shirouchi, B.; Inoue, N.; Hidaka, F.; Kai, T.; Yanagita, T. Effect of Vaccinium ashei reade Leaves on Angiotensin Converting Enzyme Activity in Vitro and on Systolic Blood Pressure of Spontaneously Hypertensive Rats in Vivo. Biosci. Biotechnol. Biochem. 2007, 71, 2335–2337. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Du, M.; Sanchez, K.; Betts, N.M.; Wu, M.; Aston, C.E.; Lyons, T.J. Blueberries Decrease Cardiovascular Risk Factors in Obese Men and Women with Metabolic Syndrome. J. Nutr. 2010, 140, 1582–1587. [Google Scholar] [CrossRef] [Green Version]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in Blueberries Improve Insulin Sensitivity in Obese, Insulin-Resistant Men and Women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Wang, T.; Wang, H. Ginsenoside Rb2 enhances the anti-inflammatory effect of ω-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression. Acta Pharmacol. Sin. 2017, 38, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, M.; Matsuyama, Y.; Hayasegawa, R.; Hamada, K.; Nishiyama, K.; Kai, T.; Kamenaga, K.; Arakawa, T.; Tari, H.; Shimizu, Y.; et al. Blueberry (Vaccinium virgatum Aiton) Leaf Infusion Ameliorates Insulin Resistance in Mice Fed a High-fat, High-sucrose Diet. Food Sci. Technol. Res. 2015, 21, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Yamasaki, K.; Sugamoto, K.; Arakawa, T.; Nishiyama, K.; Yamasaki, M. Chronic intake of high-dose of blueberry leaf extract does not augment the harmful effects of ethanol in rats. PeerJ 2019, 7, e6989. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.; Yamasaki, M.; Kunitake, H. Effects of Dietary Blueberry (Vaccinium ashei Reade) Leaves on Mildly Postprandial Hypertriglyceridemia. J. Oleo Sci. 2020, 69, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichikawa, T.; Sugamoto, K.; Matsuura, Y.; Kunitake, H.; Shimoda, K.; Morishita, K. Inhibition of adult T-cell leukemia cell proliferation by polymerized proanthocyanidin from blueberry leaves through JAK proteolysis. Cancer Sci. 2022, 113, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Sugamoto, K.; Tanaka, Y.L.; Saito, A.; Goto, Y.; Nakayama, T.; Okabayashi, T.; Kunitake, H.; Morishita, K. Highly polymerized proanthocyanidins (PAC) components from blueberry leaf and stem significantly inhibit SARS-CoV-2 infection via inhibition of ACE2 and viral 3CLpro enzymes. Biochem. Biophys. Res. Commun. 2022, 615, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Chakraborty, S. Study of dietary polyphenols from natural herbal sources for providing protection against human degenerative disorders. Biocatal. Agric. Biotechnol. 2021, 33, 101956. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Kumar, M.; Prakash, S.; Radha; Kumari, N.; Pundir, A.; Punia, S.; Saurabh, V.; Choudhary, P.; Changan, S.; Dhumal, S.; et al. Beneficial Role of Antioxidant Secondary Metabolites from Medicinal Plants in Maintaining Oral Health. Antioxidants 2021, 10, 1061. [Google Scholar] [CrossRef]
- Kedrina-Okutan, O.; Novello, V.; Hoffmann, T.; Hadersdorfer, J.; Occhipinti, A.; Schwab, W.; Ferrandino, A. Constitutive Polyphenols in Blades and Veins of Grapevine (Vitis vinifera L.) Healthy Leaves. J. Agric. Food Chem. 2018, 66, 10977–10990. [Google Scholar] [CrossRef]
- Matsuo, Y.; Fujita, Y.; Ohnishi, S.; Tanaka, T.; Hirabaru, H.; Kai, T.; Sakaida, H.; Nishizono, S.; Kouno, I. Chemical constituents of the leaves of rabbiteye blueberry (Vaccinium ashei) and characterisation of polymeric proanthocyanidins containing phenylpropanoid units and A-type linkages. Food Chem. 2010, 121, 1073–1079. [Google Scholar] [CrossRef] [Green Version]
- Shoji, T.; Masumoto, S.; Moriichi, N.; Ohtake, Y.; Kanda, T. Administration of Apple Polyphenol Supplements for Skin Conditions in Healthy Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2020, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, S.; Kamon, Y.; Xu, L.; Hashidzume, A. Synthesis of Dense 1,2,3-Triazole Polymers Soluble in Common Organic Solvents. Polymers 2021, 13, 1627. [Google Scholar] [CrossRef]
- Fujii, Y.; Hiyoshi, M.; Imamura, N.; Yano, K.; Hamada, T.; Wada, T. Factors Affecting Nonalcoholic Fatty Liver Disease After Pancreatic Head Resection. Pancreas 2019, 48, e75–e77. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Tanaka, Y. Host genetic variations associated with disease progression in chronic hepatitis C virus infection. Hepatol. Res. 2018, 48, 127–133. [Google Scholar] [CrossRef]
- Nagahama, K.; Eto, N.; Sakakibara, Y.; Matsusita, Y.; Sugamoto, K.; Morishita, K.; Suiko, M. Oligomeric proanthocyanidins from rabbiteye blueberry leaves inhibits the proliferation of human T-cell lymphotropic virus type 1-associated cell lines via apoptosis and cell cycle arrest. J. Funct. Foods 2014, 6, 356–366. [Google Scholar] [CrossRef] [Green Version]
- Kai, H.; Fuse, T.; Kunitake, H.; Morishita, K.; Matsuno, K. Comparison of Cultivars and Seasonal Variation in Blueberry (Vaccinium Species) Leaf Extract on Adult T-Cell Leukemia Cell Line Growth Suppression. Medicines 2014, 1, 3–11. [Google Scholar] [CrossRef]
- Wang, Y.; Nambeesan, S.U. Full-length fruit transcriptomes of southern highbush (Vaccinium sp.) and rabbiteye (V. virgatum Ait.) blueberry. BMC Genom. 2022, 23, 733. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, S.; Fujikawa, M.; Yamane, H.; Shirasawa, K.; Babiker, E.; Tao, R. Genomic insight into the developmental history of southern highbush blueberry populations. Heredity 2021, 126, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Balcerczak, E.; Świechowski, R.; Dubicka, M.; Olszewsk, M.A. Seasonal variation in phenylpropanoid biosynthesis and in vitro antioxidant activity of Sorbus domestica leaves: Harvesting time optimisation for medicinal application. Ind. Crops Prod. 2020, 156, 112858. [Google Scholar] [CrossRef]
- Chen, H.; Wu, H.; Yan, B.; Zhao, H.; Liu, F.; Zhang, H.; Sheng, Q.; Miao, F.; Liang, Z. Core Microbiome of Medicinal Plant Salvia miltiorrhiza Seed: A Rich Reservoir of Beneficial Microbes for Secondary Metabolism? Int. J. Mol. Sci. 2018, 19, 672. [Google Scholar] [CrossRef] [Green Version]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Smolensky, D.; Rhodes, D.; McVey, S.; Fawver, Z.; Perumal, R.; Herald, T.; Noronha, L. High-Polyphenol Sorghum Bran Extract Inhibits Cancer Cell Growth through ROS Induction, Cell Cycle Arrest, and Apoptosis. J. Med. Food 2018, 21, 990–998. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Ashita, S.; Mandeep, K.; Jatinder, K.K.; Kaur, N.A. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar]
- Song, W.; Zhao, Y.Y.; Ren, Y.J.; Liu, L.L.; Wei, S.D.; Yang, H.B. Proanthocyanidins isolated from the leaves of Photinia × fraseri block the cell cycle and induce apoptosis by inhibiting tyrosinase activity in melanoma cells. Food Funct. 2021, 12, 3978–3991. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.; Mohamed, S.; Rahman, H.S.; Rosli, R. Epicatechin and scopoletin-rich Morinda citrifolia leaf ameliorated leukemia via anti-inflammatory, anti-angiogenesis, and apoptosis pathways in vitro and in vivo. J. Food Biochem. 2019, 43, e12868. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Suda, E. Antioxidant function, measurement of DPPH radical scavenging ability with spectrophotometer. Food Funct. Res. Method 2000, 8, 219–220. (In Japanese) [Google Scholar]
- Terasaka, H.; Kadoma, Y.; Sakagami, H.; Fujisawa, S. Cytotoxicity and apoptosis-inducing activity of bisphenol A and hydroquinone in HL-60 Cells. Anticancer Res. 2005, 25, 2241–2247. [Google Scholar] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyama, Y.; Fujita, Y.; Toshima, S.; Hirano, T.; Yamasaki, M.; Kunitake, H. Comparison of Proanthocyanidin Content in Rabbiteye Blueberry (Vaccinium virgatum Aiton) Leaves and the Promotion of Apoptosis against HL-60 Promyelocytic Leukemia Cells Using ‘Kunisato 35 Gou’ Leaf Extract. Plants 2023, 12, 948. https://doi.org/10.3390/plants12040948
Toyama Y, Fujita Y, Toshima S, Hirano T, Yamasaki M, Kunitake H. Comparison of Proanthocyanidin Content in Rabbiteye Blueberry (Vaccinium virgatum Aiton) Leaves and the Promotion of Apoptosis against HL-60 Promyelocytic Leukemia Cells Using ‘Kunisato 35 Gou’ Leaf Extract. Plants. 2023; 12(4):948. https://doi.org/10.3390/plants12040948
Chicago/Turabian StyleToyama, Yuki, Yoko Fujita, Saki Toshima, Tomonari Hirano, Masao Yamasaki, and Hisato Kunitake. 2023. "Comparison of Proanthocyanidin Content in Rabbiteye Blueberry (Vaccinium virgatum Aiton) Leaves and the Promotion of Apoptosis against HL-60 Promyelocytic Leukemia Cells Using ‘Kunisato 35 Gou’ Leaf Extract" Plants 12, no. 4: 948. https://doi.org/10.3390/plants12040948
APA StyleToyama, Y., Fujita, Y., Toshima, S., Hirano, T., Yamasaki, M., & Kunitake, H. (2023). Comparison of Proanthocyanidin Content in Rabbiteye Blueberry (Vaccinium virgatum Aiton) Leaves and the Promotion of Apoptosis against HL-60 Promyelocytic Leukemia Cells Using ‘Kunisato 35 Gou’ Leaf Extract. Plants, 12(4), 948. https://doi.org/10.3390/plants12040948







