Seasonal and Geographic Variation in Alkaloid Content of Kratom (Mitragyna speciosa (Korth.) Havil.) from Thailand
Abstract
1. Introduction
2. Results
2.1. General Appearances and Macroscopic Characteristics
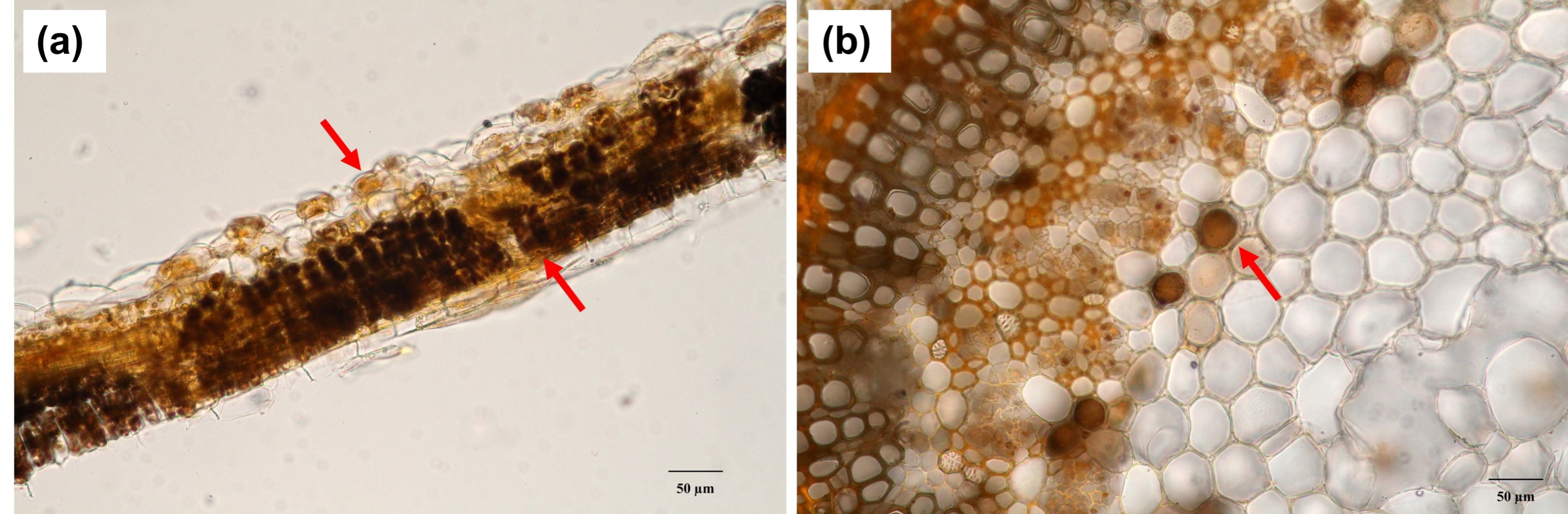
2.2. Histolocalization of Alkaloid
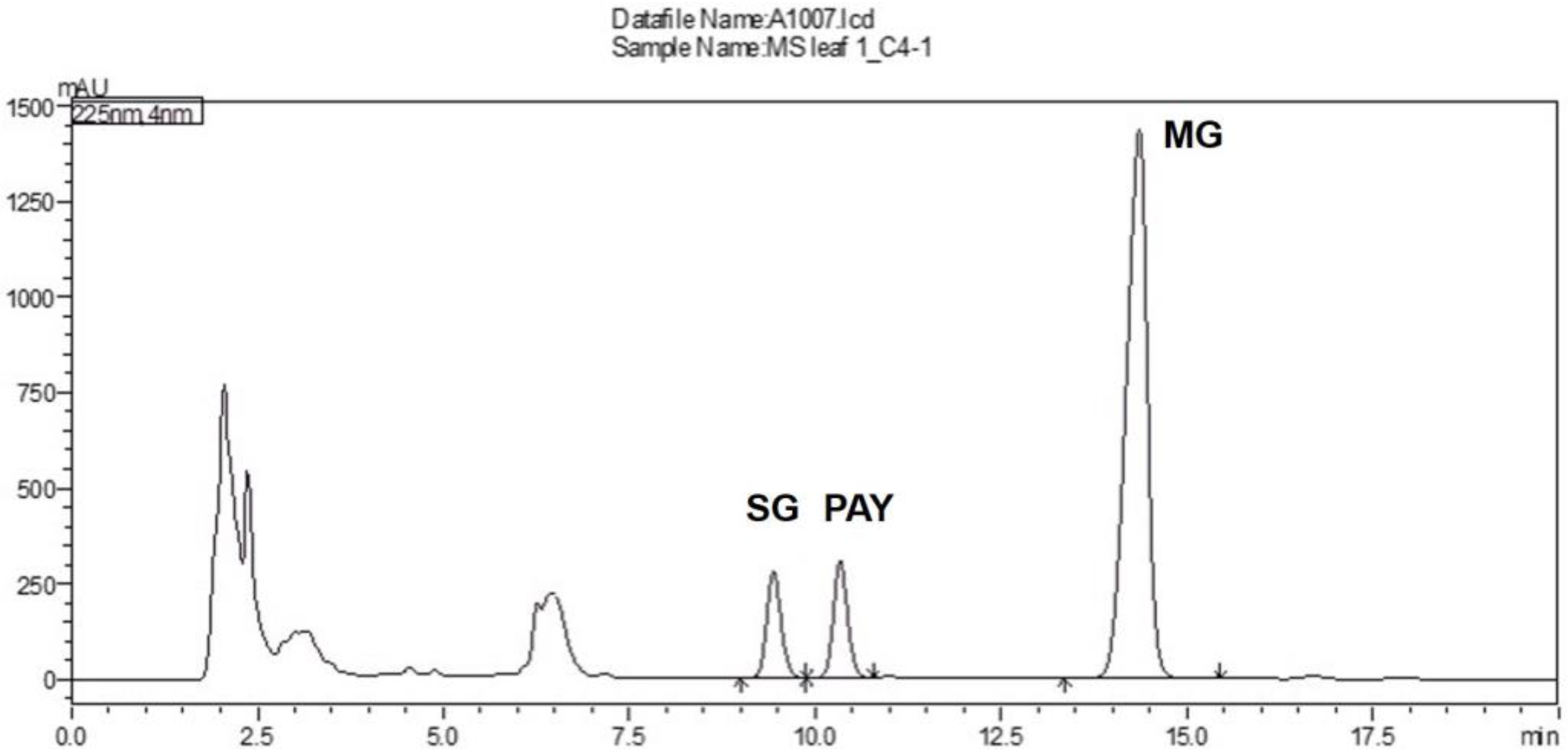
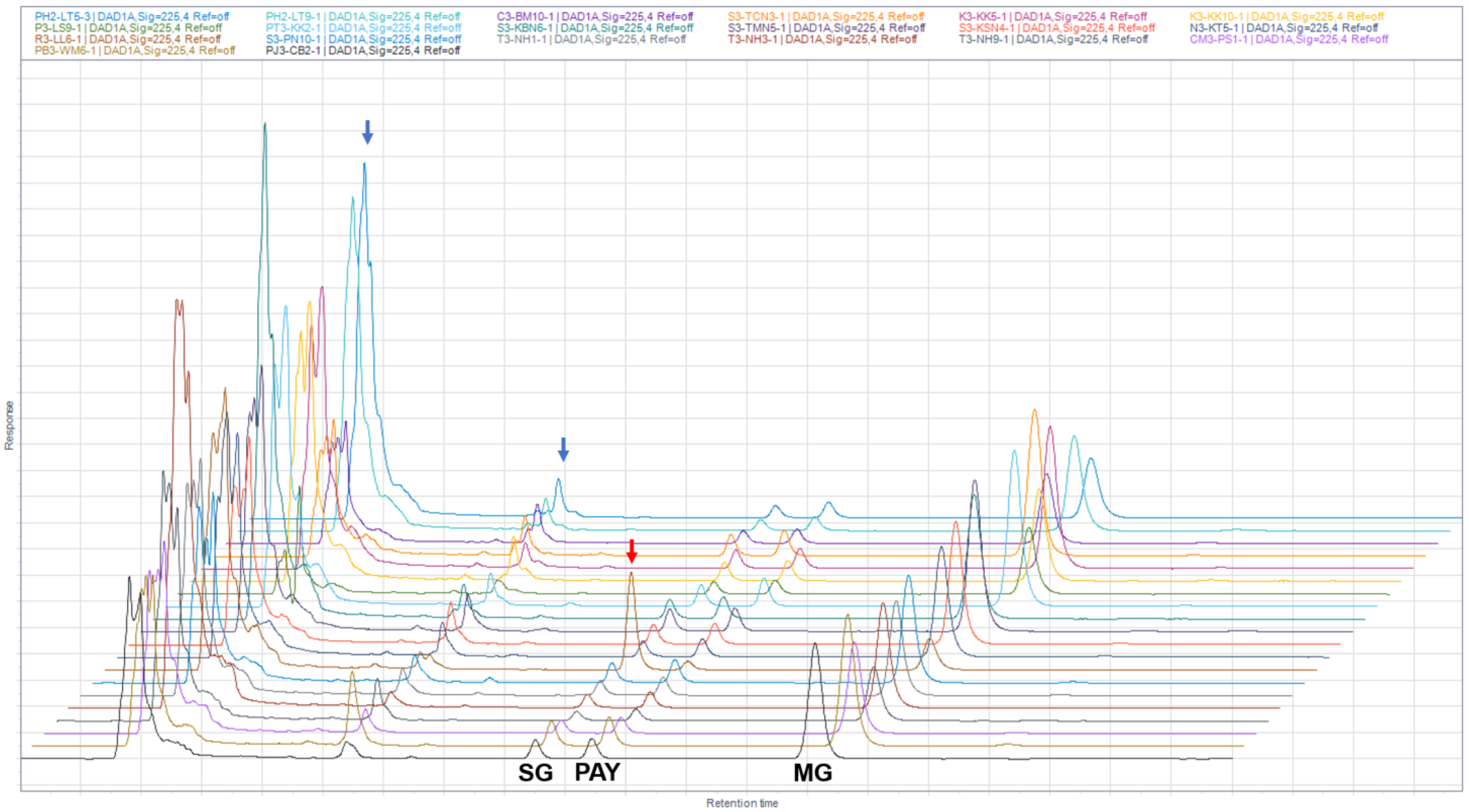
2.3. Establishment and Validation of HPLC Analysis
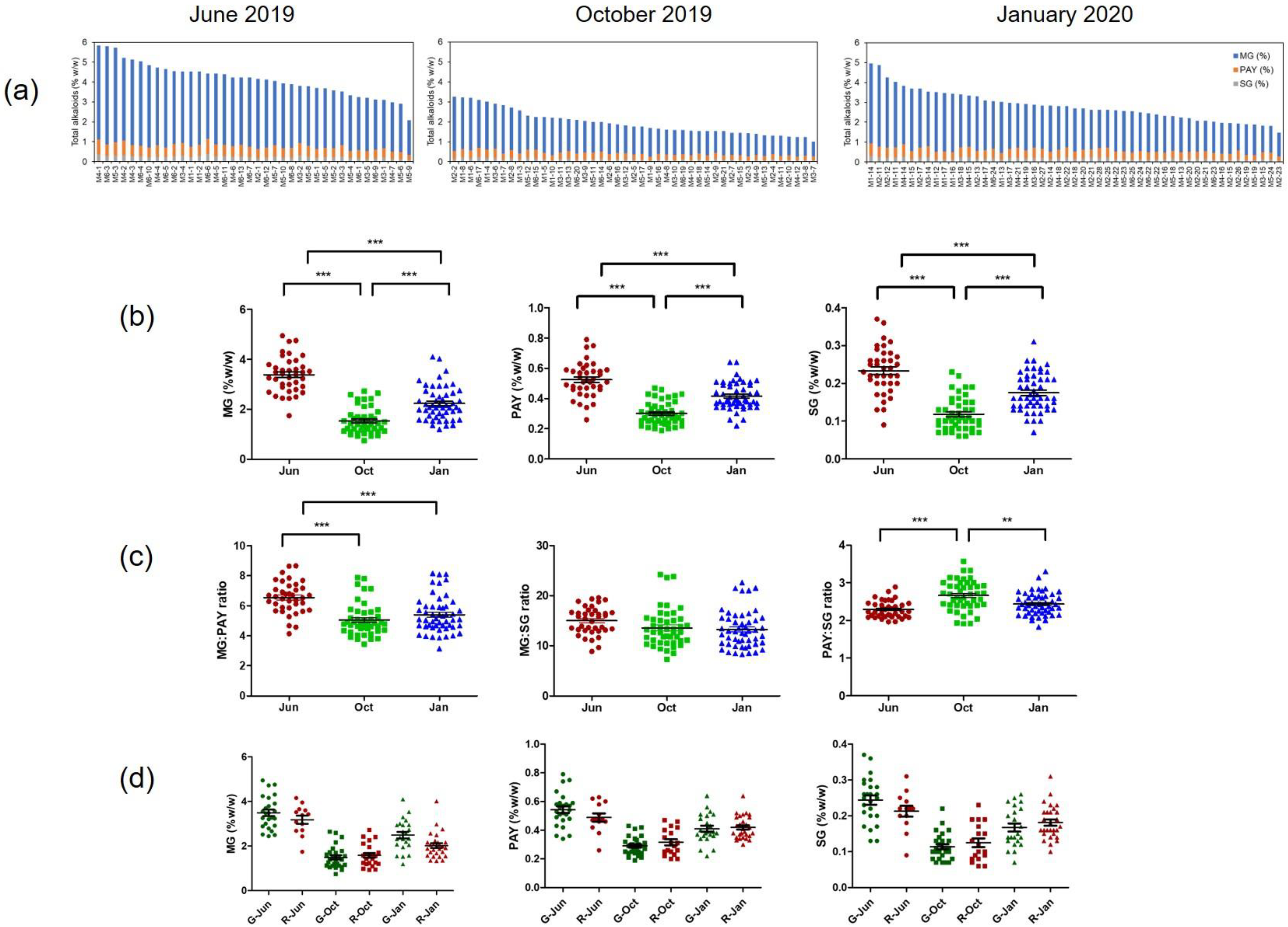
2.4. Distribution of Alkaloids in Kratom Grown in Nam Phu Subdistrict
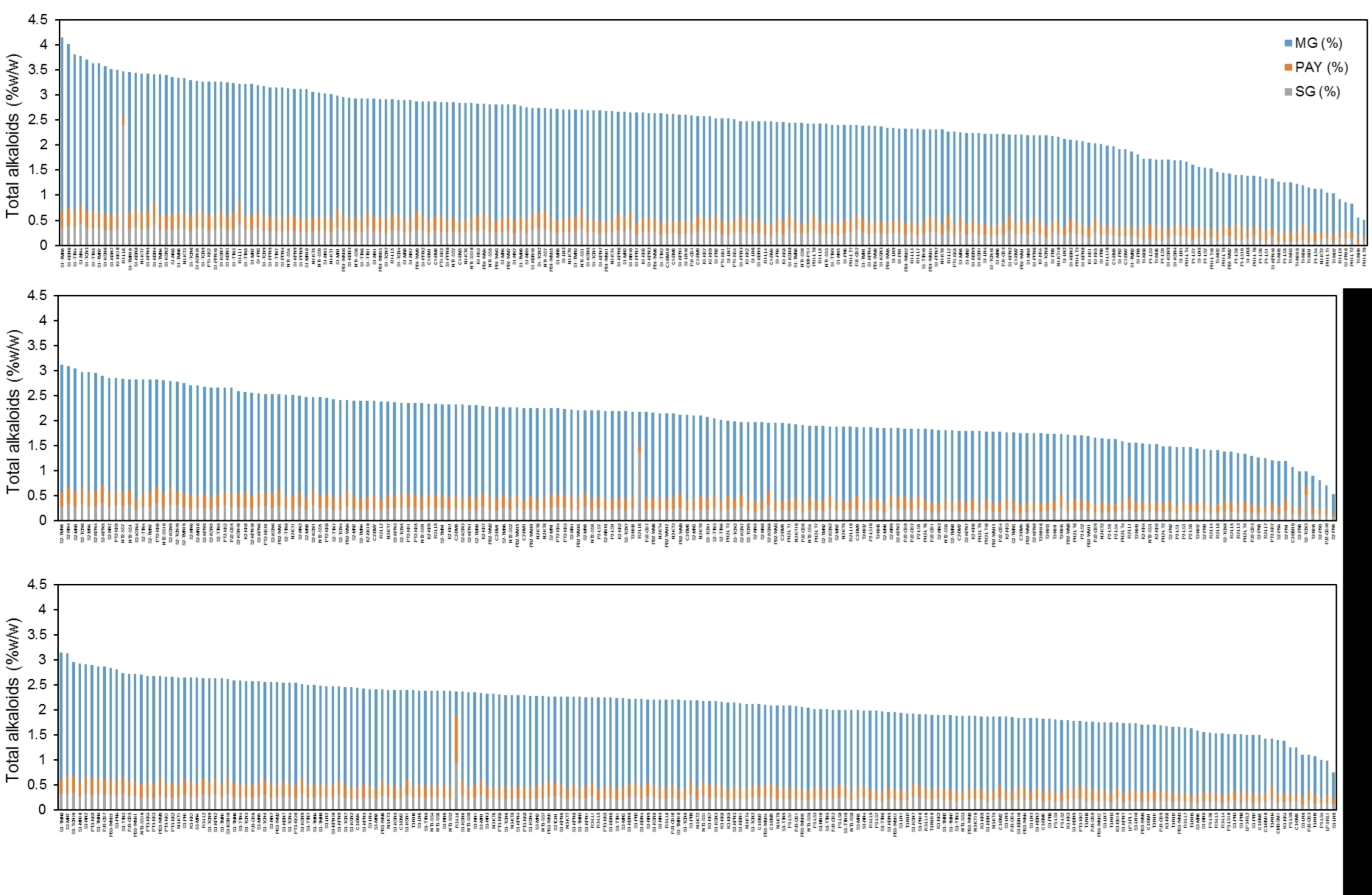
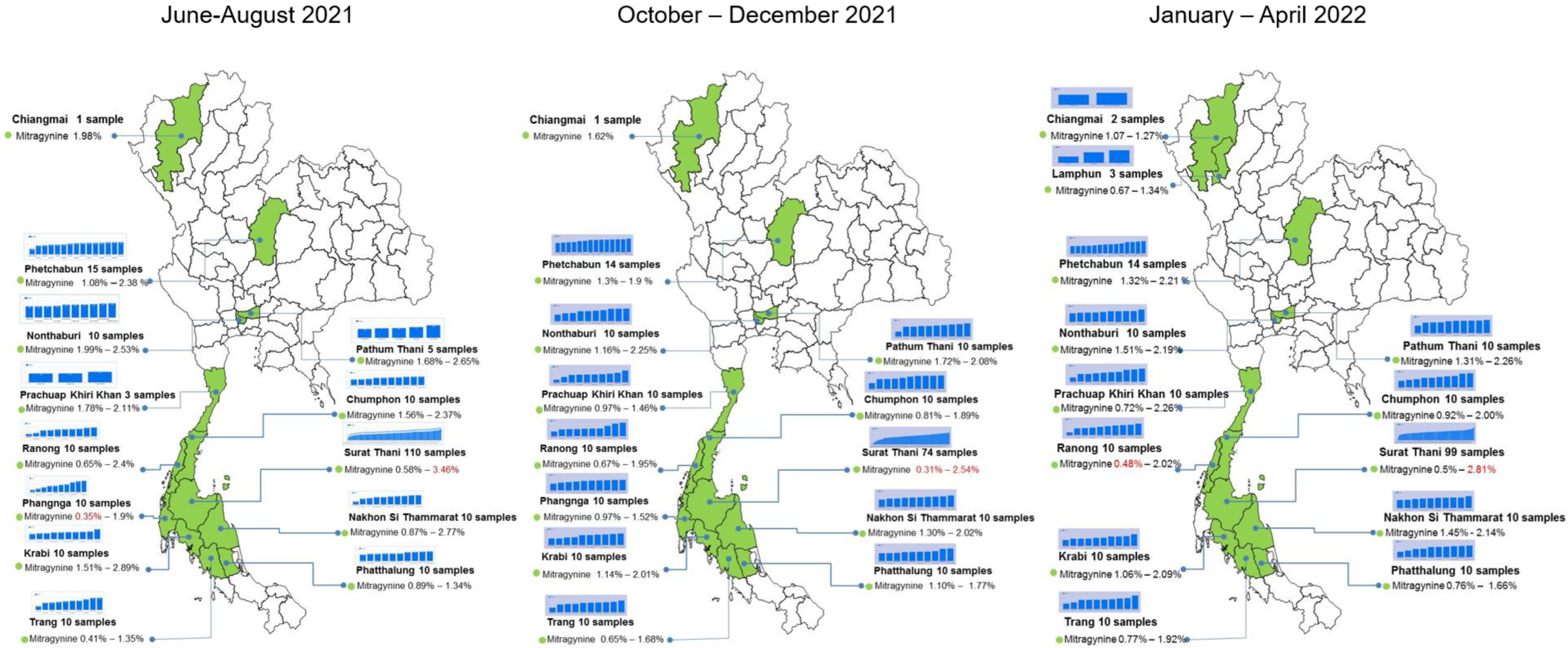
2.5. Distribution of Alkaloids in Kratom across Thailand
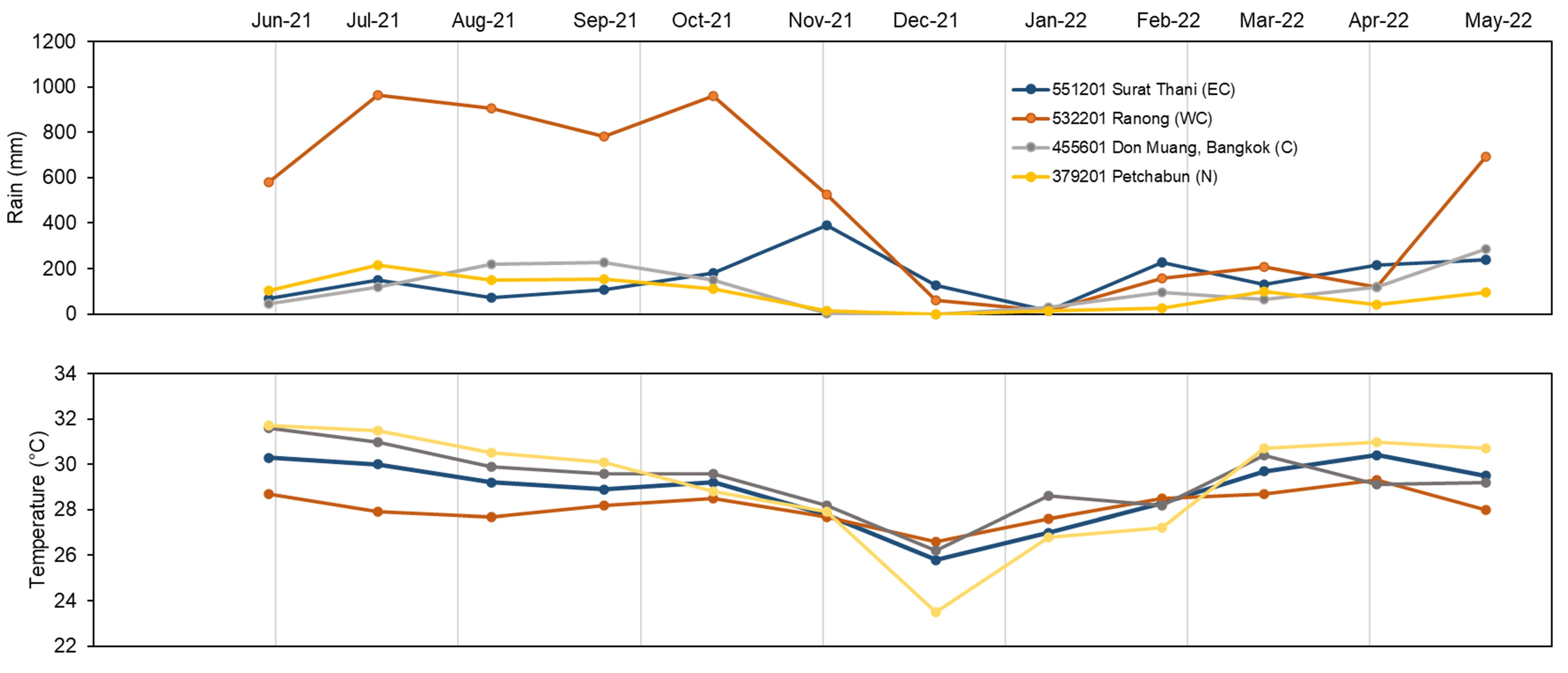
2.6. Meteorological Data and Geographic Variation in Alkaloid Content
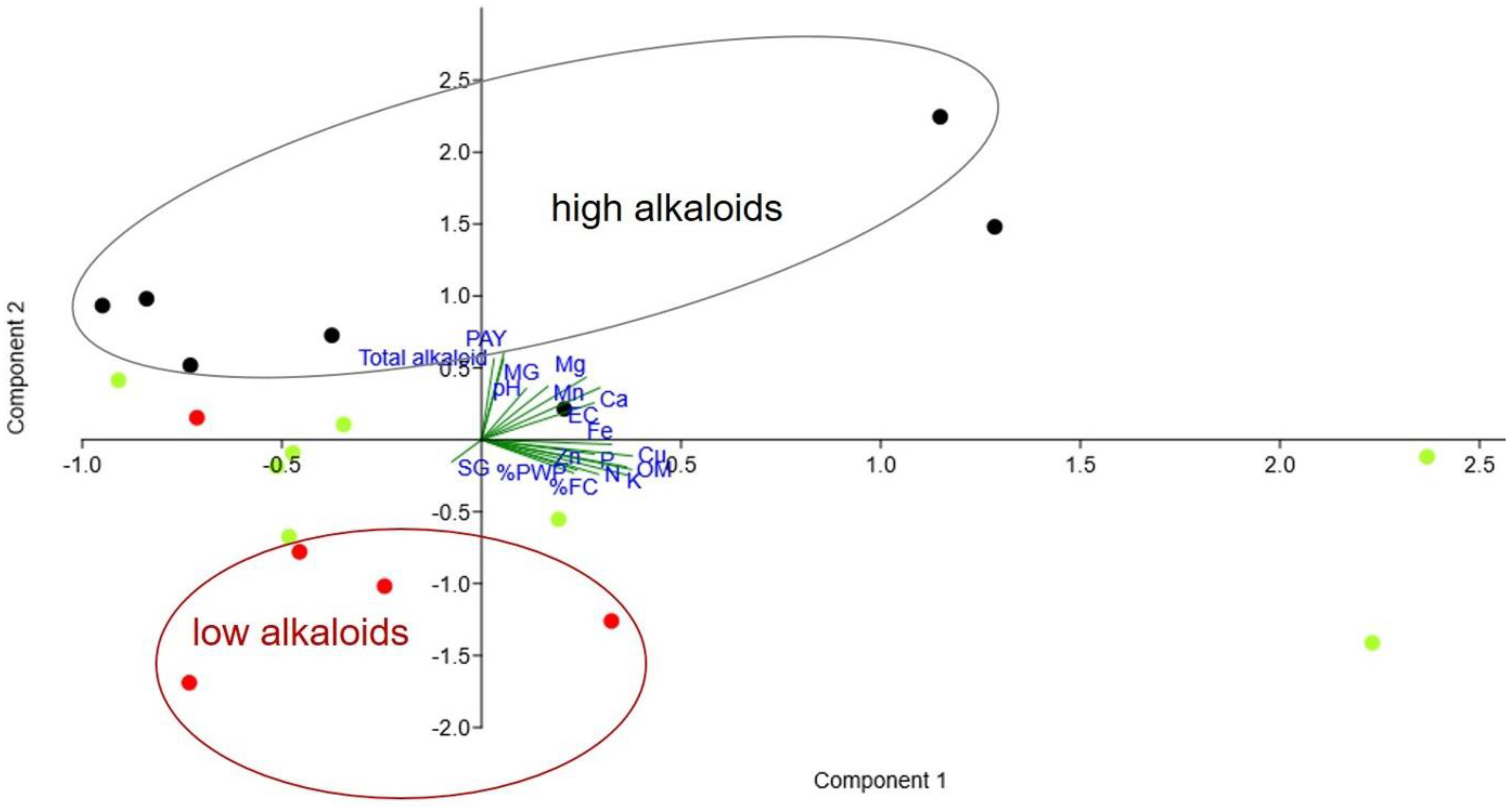
2.7. Effect of Soil Nutrients on Alkaloids Contents
3. Discussion
4. Materials and Methods
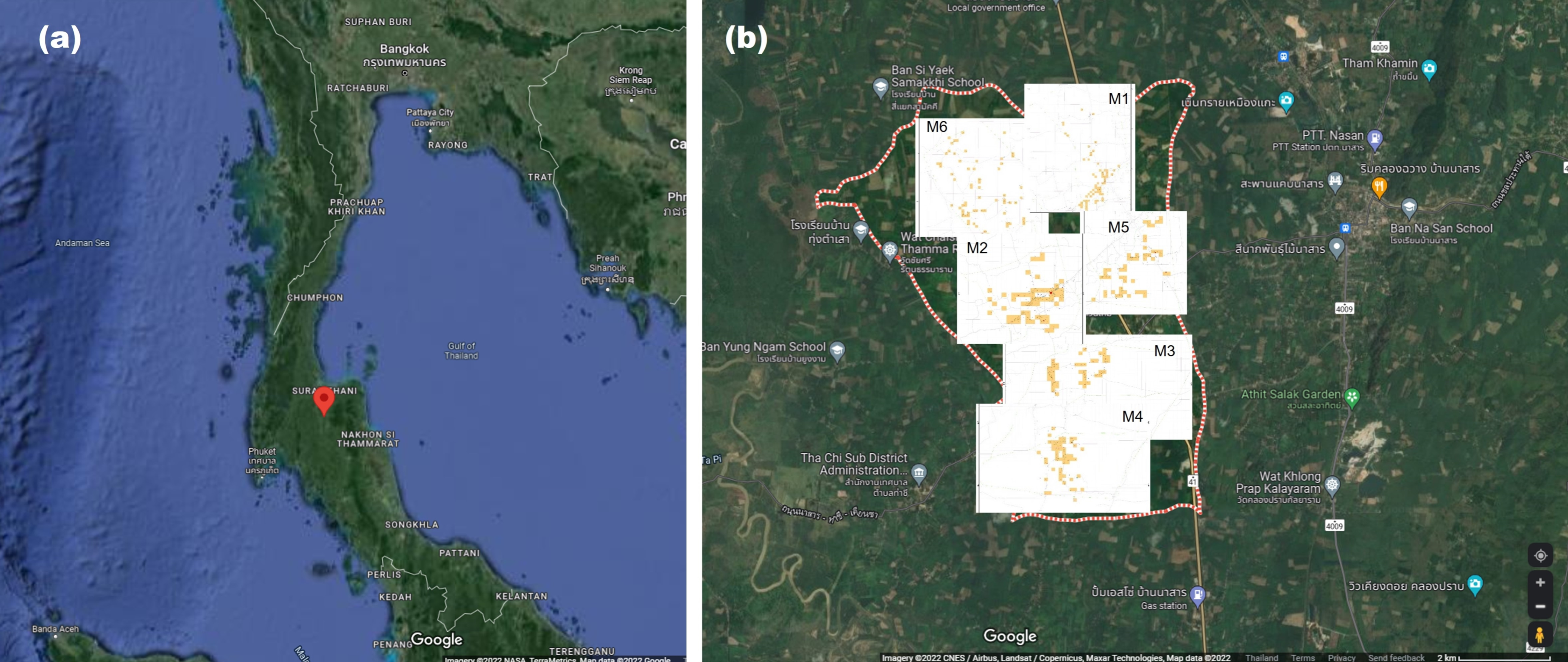
4.1. Plant Materials
4.2. Chemicals and Instrumentations
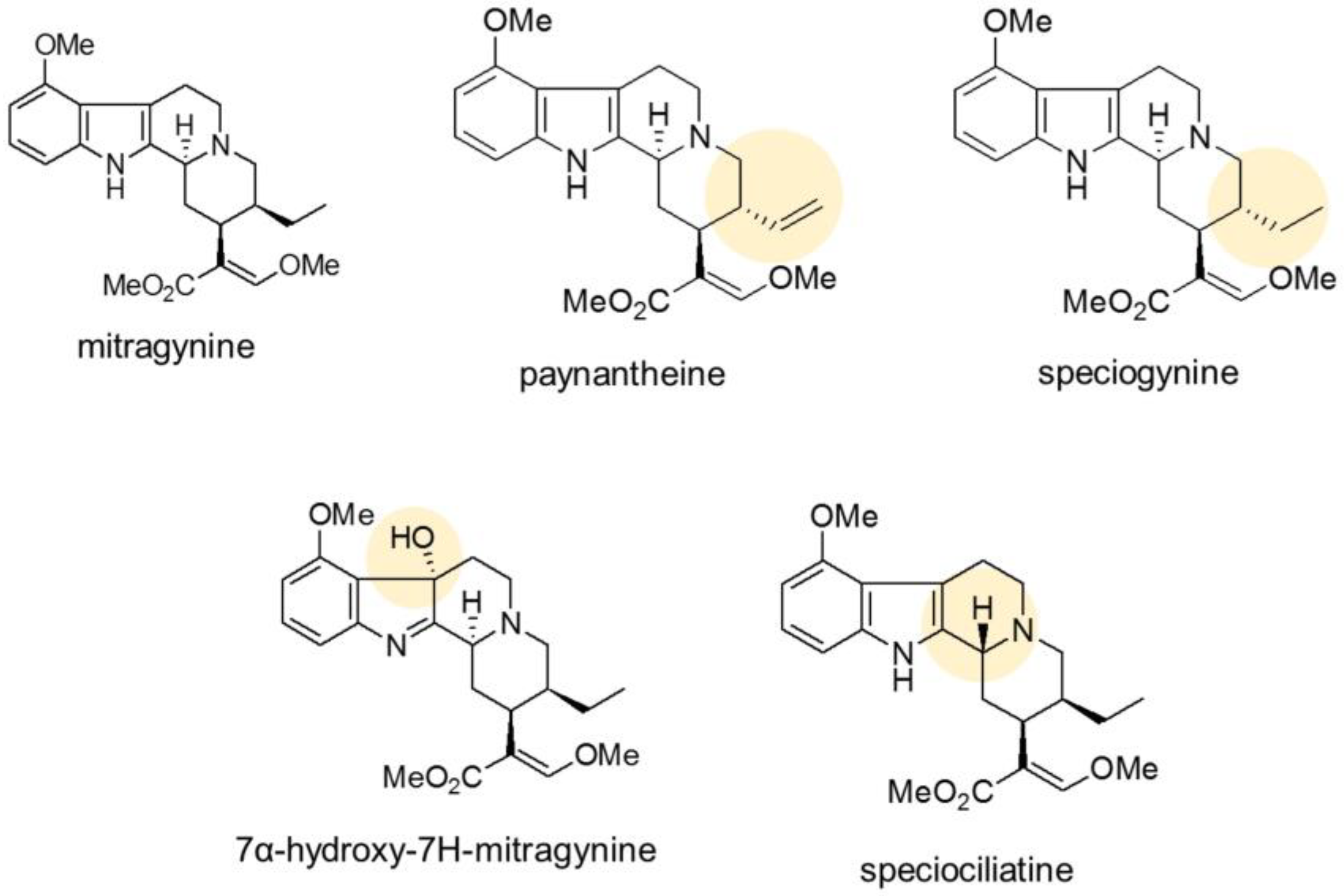
4.3. Isolation of Mitragynine, Paynantheine, and Speciogynine
4.4. Macroscopic Examinations
4.5. Histolocalization Examination
4.6. Quantification of Alkaloid Contents
4.6.1. Sample Preparation
4.6.2. Method Validation
Specificity
Linearity and Range
Accuracy
Precision
4.6.3. HPLC Analysis
4.7. Soil Collection and Nutrient Analysis
4.8. Meteorological Data
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smitinand, T. Thai Plant Names; Forest Herbarium: Department of National Parks, Wildlife and Plant Conservation: Bangkok, Thailand, 2014; p. 383.
- Ngernsaengsaruay, C.; Leksungnoen, N.; Boonthasak, W.; Utharatsamee, S.; Racharak, P.; Leetanasakskul, K.; Pongamorn, P.; Saengbuapuean, A. Additional knowledge on the genus Mitragyna (Rubiaceae) in Thailand. Thai For. Bull. (Bot.) 2022, 50, 20–39. [Google Scholar] [CrossRef]
- Saingam, D.; Assanangkornchai, S.; Geater, A.F.; Balthip, Q. Pattern and consequences of krathom (Mitragyna speciosa Korth.) use among male villagers in southern Thailand: A qualitative study. Int. J. Drug. Policy 2013, 24, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, O.; Hendrickson, R.G.; Greenberg, M.I. Kratom: History, current user trends, adverse health effects and potential benefits. Dis. Mon. 2022, 19, 101442. [Google Scholar] [CrossRef] [PubMed]
- Shellard, E.J.; Lees, M.D. The Mitragyna species of Asia. V. The anatomy of the leaves of Mitragyna speciosa Korth. Planta Med. 1965, 13, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Nakaphan, T.; Teerachaisakul, M.; Puttum, S.; Sompimai, K.; Nootim, P. Traditional use of kratom (Mitragyna speciosa Korth.) among folk healers in Southern Thailand. J. Thai Trad. Alt. Med. 2016, 14, 274–284. [Google Scholar]
- Sukrong, S.; Zhu, S.; Ruangrungsi, N.; Phadungcharoen, T.; Palanuvej, C.; Komatsu, K. Molecular analysis of the genus Mitragyna existing in Thailand based on rDNA ITS sequences and its application to identify a narcotic species: Mitragyna speciosa. Biol. Pharm. Bull. 2007, 30, 1284–1288. [Google Scholar] [CrossRef]
- Tungphatthong, C.; Urumarudappa, S.K.J.; Awachai, S.; Sooksawate, T.; Sukrong, S. Differentiation of Mitragyna speciosa, a narcotic plant, from allied Mitragyna species using DNA barcoding-high-resolution melting (Bar-HRM) analysis. Sci. Rep. 2021, 11, 6738. [Google Scholar] [CrossRef]
- Cinosi, E.; Martinotti, G.; Simonato, P.; Singh, D.; Demetrovics, Z.; Roman-Urrestarazu, A.; Bersani, F.S.; Vicknasingam, B.; Piazzon, G.; Li, J.H.; et al. Following “the roots” of kratom (Mitragyna speciosa): The evolution of an enhancer from a traditional use to increase work and productivity in southeast Asia to a recreational psychoactive drug in western countries. Biomed. Res. Int. 2015, 2015, 968786. [Google Scholar] [CrossRef]
- Takayama, H. Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa. Chem. Pharm. Bull. 2004, 52, 916–928. [Google Scholar] [CrossRef]
- Nagakura, N.; Rueffer, M.; Zenk, M.H. The biosynthesis of monoterpenoid indole alkaloids from strictosidine. J. Chem. Soc. Perkin Trans. I 1979, 2308–2312. [Google Scholar] [CrossRef]
- Rueffer, M.; Nagakura, N.; Zenk, M.H. Strictosidine, the common precursor for monoterpenoid indole alkaloids with 3α and 2β confi guration. Tetrahedron. Lett. 1978, 18, 1593–1596. [Google Scholar] [CrossRef]
- Charoonratana, T.; Wungsintaweekul, J.; Pathompak, P.; Georgiev, M.I.; Choi, Y.H.; Verpoorte, R. Limitation of mitragynine biosynthesis in Mitragyna speciosa (Roxb.) Korth. through tryptamine availability. Z. Naturforsch. C J. Biosci. 2013, 68, 394–405. [Google Scholar] [CrossRef]
- Hassan, Z.; Muzaimi, M.; Navaratnam, V.; Yusoff, N.H.; Suhaimi, F.W.; Vadivelu, R.; Vicknasingam, B.K.; Amato, D.; von Hörsten, S.; Ismail, N.I.; et al. From kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neurosci. Biobehav. Rev. 2013, 37, 138–151. [Google Scholar] [CrossRef]
- Hinou, J.; Harvala, C. Polyphenolic compounds from the leaves of Mitragyna speciosa. Fitoterapia 1988, 59, 156. [Google Scholar]
- León, F.; Habib, E.; Adkins, J.E.; Furr, E.B.; McCurdy, C.R.; Cutler, S.J. Phytochemical characterization of the leaves of Mitragyna speciosa grown in U.S.A. Nat. Prod. Commun. 2009, 4, 907–910. [Google Scholar] [PubMed]
- Matsumoto, K.; Mizowaki, M.; Suchitra, T.; Takayama, H.; Sakai, S.; Aimi, N.; Watanabe, H. Antinociceptive action of mitragynine in mice: Evidence for the involvement of supraspinal opioid receptors. Life Sci. 1996, 59, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Thongpradichote, S.; Matsumoto, K.; Tohda, M.; Takayama, H.; Aimi, N.; Sakai, S.; Watanabe, H. Identification of opioid receptor subtypes in antinociceptive actions of supraspinally-administered mitragynine in mice. Life Sci. 1998, 62, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Kruegel, A.; Gassaway, M.M.; Kapoor, A.; Váradi, A.; Majumdar, S.; Filizola, M.; Javitch, J.A.; Sames, D. Synthetic and receptor signaling explorations of the Mitragyna alkaloids: Mitragynine as an atypical molecular framework for opioid receptor modulators. J. Am. Chem. Soc. 2016, 138, 6754–6764. [Google Scholar] [CrossRef] [PubMed]
- Chittrakarn, S.; Sawangjaroen, K.; Prasettho, S.; Janchawee, B.; Keawpradub, N. Inhibitory effects of kratom leaf extract (Mitragyna speciosa Korth.) on the rat gastrointestinal tract. J. Ethnopharmacol. 2008, 116, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Purintrapiban, J.; Keawpradub, N.; Kansenalak, S.; Chittrakarn, S.; Janchawee, B.; Sawanjaroen, K. Study on glucose transport in muscle cells by extracts from Mitragyna speciosa (Korth.) and mitragynine. Nat. Prod. Res. 2011, 25, 1379–1387. [Google Scholar] [CrossRef]
- Tanguay, P. Kratom in Thailand: Decriminalisation and community control? Transnatl. Inst. Ser. Legis. Reform Drug Policies Nr. 2011, 13, 1–16. [Google Scholar]
- Charoenratana, S.; Anukul, C.; Aramrattana, A. Attitudes towards kratom use, decriminalization and the development of a community-based kratom control mechanism in southern Thailand. Int. J. Drug. Policy 2021, 95, 103197. [Google Scholar] [CrossRef] [PubMed]
- Assanangkornchai, S.; Muekthong, A.; Sam-angsri, N.; Pattanasattayawong, U. The use of Mitragyna speciosa (“krathom”), an addictive plant, in Thailand. Subst. Use Misuse 2007, 42, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization’s (WHO). Pre-review report: Kratom (Mitragyna speciosa), mitragynine, 7-hydroxymitragynine. In Proceedings of the Expert Committee on Drug Dependence Forty-fourth Meeting, Geneva, Switzerland, 11–15 October 2021. [Google Scholar]
- Erowid: Kratom Legal Status. Available online: https://erowid.org/plants/kratom/kratom_law.shtml (accessed on 6 January 2023).
- Zhang, M.; Sharma, A.; León, F.; Avery, B.; Kjelgren, R.; McCurdy, C.R.; Pearson, B.J. Effects of nutrient fertility on growth and alkaloidal content in Mitragyna speciosa (Kratom). Front. Plant Sci. 2020, 11, 597696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sharma, A.; León, F.; Avery, B.; Kjelgren, R.; McCurdy, C.R.; Pearson, B.J. Plant growth and phytoactive alkaloid synthesis in kratom [Mitragyna speciosa (Korth.)] in response to varying radiance. PLoS ONE 2022, 17, e0259326. [Google Scholar] [CrossRef] [PubMed]
- Leksungnoen, N.; Andriyas, T.; Ngernsaengsaruay, C.; Uthairatsamee, S.; Racharak, P.; Sonjaroon, W.; Kjelgren, R.; Pearson, B.J.; McCurdy, C.R.; Sharma, A. Variations in mitragynine content in the naturally growing kratom (Mitragyna speciosa) population of Thailand. Front. Plant Sci. 2022, 13, 1028547. [Google Scholar] [CrossRef]
- Limsuwanchote, S.; Wungsintaweekul, J.; Keawpradub, N.; Putalun, W.; Morimoto, S.; Tanaka, H. Development of indirect competitive ELISA for quantification of mitragynine in kratom (Mitragyna speciosa (Roxb.) Korth.). Forensic. Sci. Int. 2014, 244, 70–77. [Google Scholar] [CrossRef]
- Chear, N.J.; León, F.; Sharma, A.; Kanumuri, S.R.R.; Zwolinski, G.; Abboud, K.A.; Singh, D.; Restrepo, L.F.; Patel, A.; Hiranita, T.; et al. Exploring the chemistry of alkaloids from Malaysian Mitragyna speciosa (Kratom) and the role of oxindoles on human opioid receptors. J. Nat. Prod. 2021, 84, 1034–1043. [Google Scholar] [CrossRef]
- Lalangi, C.A.; Arfiyani, E.; Ningtias, W.; Maulida, E.N. Mitragynine percentages of various kratom variants seized in Indonesia: A quantitative analysis using liquid chromatography-photo diode array detector. Int. J. Appl. Pharm. 2021, 13, 252–256. [Google Scholar] [CrossRef]
- Pratiwi, D.; Sulistyaningsih, Y.; Ratnadewi, D. Localization of alkaloid and other secondary metabolites in Cinchona ledgeriana Moens: Anatomical and histochemical studies on fresh tissues and cultured cells. HAYATI J. Biosci. 2020, 27, 1–7. [Google Scholar] [CrossRef]
- Pootakham, W.; Yoocha, T.; Jomchai, N.; Kongkachana, W.; Naktang, C.; Sonthirod, C.; Chowpongpang, S.; Aumpuchin, P.; Tangphatsornruang, S.A. Chromosome-scale genome assembly of Mitragyna speciosa (kratom) and the assessment of its genetic diversity in Thailand. Biology 2022, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.A.; Kellogg, J.J.; Wallace, E.D.; Khin, M.; Flores-Bocanegra, L.; Tanna, R.S.; McIntosh, S.; Raja, H.A.; Graf, T.N.; Hemby, S.E.; et al. Chemical composition and biological effects of kratom (Mitragyna speciosa): In vitro studies with implications for efficacy and drug interactions. Sci. Rep. 2020, 10, 19158. [Google Scholar] [CrossRef] [PubMed]
- Nukitram, J.; Cheaha, D.; Sengnon, N.; Wungsintaweekul, J.; Limsuwanchote, S.; Kumarnsit, E. Ameliorative effects of alkaloid extract from Mitragyna speciosa (Korth.) Havil. Leaves on methamphetamine conditioned place preference in mice. J. Ethnopharmacol. 2022, 284, 114824. [Google Scholar] [CrossRef] [PubMed]
- Keawpradub, N. Alkaloids from the Fresh Leaves of Mitragyna speciosa. Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 1990. [Google Scholar]
- Department of Medical Sciences, Ministry of Public Health. Thai Herbal Pharmacopoeia 2021; Keawjawjom Printing & Publishing: Bangkok, Thailand, 2021; Volume I & II, p. 849.
- Limsuwanchote, S.; Putalun, W.; Tanaka, H.; Morimoto, S.; Keawpradub, N.; Wungsintaweekul, J. Development of an immunochromatographic strip incorporating anti-mitragynine monoclonal antibody conjugated to colloidal gold for kratom alkaloids detection. Drug Test. Anal. 2018, 10, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- ICH Harmonised Tripatite Guideline. In Validation of Analytical Guideline: Text and Methodology Q2(R1); European Union: Maastricht, The Netherlands, 2005.
- Jone, B., Jr. Laboratory Guide for Conducting Soil Tests and Plant Analysis; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Day, P.R. Particle Fractionation and Particle Size Analysis. In Methods of Soil Analysis; Agronomy No.9, Part 1; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]




| Parameters 1 | MG | PAY | SG |
|---|---|---|---|
| Linearity Range (µg/mL) | 5–200 | 2–80 | 2.5–100 |
| R2 | 0.9991 | 0.9990 | 0.9994 |
| Precision (%RSD) | |||
| Inter-day | |||
| Low level; n = 3 | 2.939 | 6.321 | 5.826 |
| Medium level; n = 6 | 2.592 | 3.227 | 3.628 |
| High level; n = 3 | 3.331 | 3.132 | 3.446 |
| Intra-day | |||
| Medium level; n = 6 | 3.212 | 2.911 | 3.074 |
| Accuracy (%Recovery) | |||
| Low level; n = 3 | 94.27 ± 3.66 | 96.86 ± 3.90 | 94.61 ± 3.56 |
| Medium level; n = 3 | 97.30 ± 1.12 | 104.54 ± 0.88 | 98.06 ± 0.97 |
| High level; n = 3 | 96.48 ± 1.50 | 100.42 ± 1.80 | 98.04 ± 1.58 |
| LOD (µg/mL)2 | 0.075 | 0.075 | 0.078 |
| LOQ (µg/mL)2 | 0.2 | 0.2 | 0.2 |
| Code | Sample Code | Alkaloid Contents (% w/w) | pH (1:5) | EC (dS/m) | Macronutrients | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MG | PAY | SG | Total Alkaloid | N (g/kg) | P (mg/kg) | K (mg/kg) | Ca (mg/kg) | Mg (mg/kg) | ||||
| 1 | S3-PN10 | 1.43 | 0.25 | 0.23 | 1.91 | 5.78 | 0.036 | 0.73 | 67.7 | 38 | 265.9 | 13.8 |
| 2 | N3-KT5 | 1.48 | 0.19 | 0.18 | 1.85 | 6.01 | 0.023 | 0.68 | 75.4 | 33.9 | 1090.5 | 41.4 |
| 3 | S3-KBN6 | 1.76 | 0.24 | 0.24 | 2.24 | 5.50 | 0.081 | 1.08 | 11.9 | 50.5 | 309.5 | 55.1 |
| 4 | CM3-PS1 | 1.27 | 0.18 | 0.17 | 1.62 | 5.59 | 0.083 | 0.95 | 119.8 | 49.6 | 1418.1 | 45.0 |
| 5 | C3-BM10 | 1.06 | 0.18 | 0.18 | 1.42 | 6.17 | 0.014 | 0.62 | 0.9 | 20.9 | 815.1 | 33.0 |
| 6 | R3-LL6 | 0.48 | 0.11 | 0.94 | 1.53 | 5.37 | 0.027 | 0.74 | 3.7 | 52.0 | 14.2 | 5.5 |
| 7 | PJ3-CB2 | 1.56 | 0.22 | 0.22 | 2.00 | 5.41 | 0.033 | 0.67 | 22.6 | 79.4 | 693.2 | 295.7 |
| 8 | PB3-WM6 | 1.81 | 0.31 | 0.28 | 2.40 | 6.48 | 0.137 | 1.47 | 26.1 | 42.1 | 7079.5 | 1235.4 |
| 9 | K3-KK5 | 1.74 | 0.20 | 0.20 | 2.14 | 5.55 | 0.030 | 0.60 | 6.1 | 42.6 | 2214.3 | 99.9 |
| 10 | PH3-LT5 | 0.95 | 0.19 | 0.17 | 1.31 | 5.53 | 0.028 | 0.71 | 8.8 | 89.2 | 404.3 | 39.9 |
| 11 | S3-TMN5 | 2.00 | 0.25 | 0.25 | 2.50 | 5.46 | 0.036 | 0.65 | 188.6 | 39.1 | 608.5 | 99.9 |
| 12 | S3-TCN3 | 1.04 | 0.28 | 0.26 | 1.58 | 5.33 | 0.045 | 0.90 | 119.3 | 62.9 | 558.9 | 84.4 |
| 13 | T3-NH1 | 1.31 | 0.20 | 0.18 | 1.69 | 5.11 | 0.031 | 1.09 | 3.9 | 139.4 | 157.9 | 24.7 |
| 14 | K3-KK10 | 2.03 | 0.27 | 0.23 | 2.53 | 4.91 | 0.073 | 0.60 | 18.8 | 48.4 | 557.3 | 46.0 |
| 15 | T3-NH9 | 0.77 | 0.13 | 0.13 | 1.03 | 5.50 | 0.047 | 1.14 | 37.2 | 81.3 | 2340.9 | 229.3 |
| 16 | S3-KSN4 | 1.67 | 0.27 | 0.22 | 2.16 | 6.00 | 0.682 | 1.04 | 17.6 | 187.1 | 3848.9 | 617.6 |
| 17 | P3-LS9 | 0.93 | 0.16 | 0.15 | 1.24 | 4.89 | 0.030 | 1.62 | 11.7 | 201.2 | 727.5 | 78.2 |
| 18 | PT3-KK2 | 1.46 | 0.22 | 0.22 | 1.90 | 5.61 | 0.424 | 1.87 | 419.2 | 446.6 | 4562.1 | 609.6 |
| 19 | T3-NH3 | 1.40 | 0.18 | 0.16 | 1.74 | 5.51 | 0.093 | 3.49 | 349.3 | 290.6 | 1958.3 | 88.3 |
| 20 | PH3-LT9 | 1.43 | 0.17 | 0.15 | 1.75 | 5.73 | 0.048 | 1.28 | 7.1 | 53.7 | 752.2 | 131.8 |
| Code | Sample Code | Micronutrients | OM (g/kg) | Soil Type | Water Holding Capacity | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fe (mg/kg) | Mn (mg/kg) | Zn (mg/kg) | Cu (mg/kg) | FC (%) | PWP (%) | ||||
| 1 | S3-PN10 | 13.26 | 2.31 | 0.72 | 0.18 | 9.02 | sandy clay loam | 7.66 | 5.04 |
| 2 | N3-KT5 | 49.22 | 3.40 | 7.40 | 1.87 | 4.77 | clay loam | 14.29 | 7.97 |
| 3 | S3-KBN6 | 33.03 | 22.81 | 12.10 | 3.89 | 8.31 | clay | 25.87 | 19.09 |
| 4 | CM3-PS1 | 20.34 | 3.97 | 4.07 | 1.00 | 9.62 | sandy clay loam | 11.34 | 4.98 |
| 5 | C3-BM10 | 45.16 | 31.10 | 2.12 | 0.32 | 2.47 | sandy clay loam | 11.61 | 7.55 |
| 6 | R3-LL6 | 15.16 | 0.53 | 0.45 | 0.15 | 10.08 | clay | 34.08 | 29.89 |
| 7 | PJ3-CB2 | 50.47 | 42.37 | 1.16 | 0.90 | 3.39 | clay loam | 16.15 | 8.68 |
| 8 | PB3-WM6 | 31.66 | 37.93 | 1.30 | 2.58 | 12.88 | clay | 30.73 | 26.15 |
| 9 | K3-KK5 | 13.99 | 4.58 | 0.47 | 0.38 | 5.49 | clay | 18.35 | 13.16 |
| 10 | PH3-LT5 | 44.02 | 0.56 | 0.13 | 0.41 | 5.32 | clay | 30.15 | 24.88 |
| 11 | S3-TMN5 | 25.99 | 0.74 | 1.08 | 0.18 | 2.83 | sandy clay loam | 5.37 | 4.46 |
| 12 | S3-TCN3 | 53.11 | 2.14 | 1.51 | 0.20 | 7.66 | sandy clay | 13.25 | 11.46 |
| 13 | T3-NH1 | 11.77 | 0.58 | 0.12 | 0.21 | 5.71 | silty clay | 33.25 | 30.28 |
| 14 | K3-KK10 | 21.81 | 4.76 | 3.09 | 0.34 | 2.93 | sandy clay loam | 7.41 | 5.28 |
| 15 | T3-NH9 | 26.48 | 2.38 | 4.45 | 1.35 | 10.04 | silty clay | 17.16 | 16.43 |
| 16 | S3-KSN4 | 63.42 | 41.32 | 1.07 | 1.34 | 16.16 | clay | 31.67 | 24.51 |
| 17 | P3-LS9 | 44.72 | 21.82 | 3.76 | 3.73 | 13.60 | clay | 24.80 | 19.12 |
| 18 | PT3-KK2 | 70.54 | 11.11 | 6.00 | 5.77 | 16.95 | clay | 34.76 | 25.77 |
| 19 | T3-NH3 | 73.54 | 10.82 | 14.55 | 4.50 | 30.73 | silty clay loam | 35.03 | 21.40 |
| 20 | PH3-LT9 | 42.92 | 4.27 | 5.60 | 2.28 | 10.39 | silty clay | 35.75 | 28.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengnon, N.; Vonghirundecha, P.; Chaichan, W.; Juengwatanatrakul, T.; Onthong, J.; Kitprasong, P.; Sriwiriyajan, S.; Chittrakarn, S.; Limsuwanchote, S.; Wungsintaweekul, J. Seasonal and Geographic Variation in Alkaloid Content of Kratom (Mitragyna speciosa (Korth.) Havil.) from Thailand. Plants 2023, 12, 949. https://doi.org/10.3390/plants12040949
Sengnon N, Vonghirundecha P, Chaichan W, Juengwatanatrakul T, Onthong J, Kitprasong P, Sriwiriyajan S, Chittrakarn S, Limsuwanchote S, Wungsintaweekul J. Seasonal and Geographic Variation in Alkaloid Content of Kratom (Mitragyna speciosa (Korth.) Havil.) from Thailand. Plants. 2023; 12(4):949. https://doi.org/10.3390/plants12040949
Chicago/Turabian StyleSengnon, Narumon, Phanita Vonghirundecha, Wiraphon Chaichan, Thaweesak Juengwatanatrakul, Jumpen Onthong, Pongmanat Kitprasong, Somchai Sriwiriyajan, Somsmorn Chittrakarn, Supattra Limsuwanchote, and Juraithip Wungsintaweekul. 2023. "Seasonal and Geographic Variation in Alkaloid Content of Kratom (Mitragyna speciosa (Korth.) Havil.) from Thailand" Plants 12, no. 4: 949. https://doi.org/10.3390/plants12040949
APA StyleSengnon, N., Vonghirundecha, P., Chaichan, W., Juengwatanatrakul, T., Onthong, J., Kitprasong, P., Sriwiriyajan, S., Chittrakarn, S., Limsuwanchote, S., & Wungsintaweekul, J. (2023). Seasonal and Geographic Variation in Alkaloid Content of Kratom (Mitragyna speciosa (Korth.) Havil.) from Thailand. Plants, 12(4), 949. https://doi.org/10.3390/plants12040949






