Persimmon Leaves: Nutritional, Pharmaceutical, and Industrial Potential—A Review
Abstract
1. Introduction
2. Distribution of PL
3. Traditional Uses and Industrial Potential
4. Proximate Composition of PL
5. Bioactive Compounds of PL
5.1. Phenolic Compounds
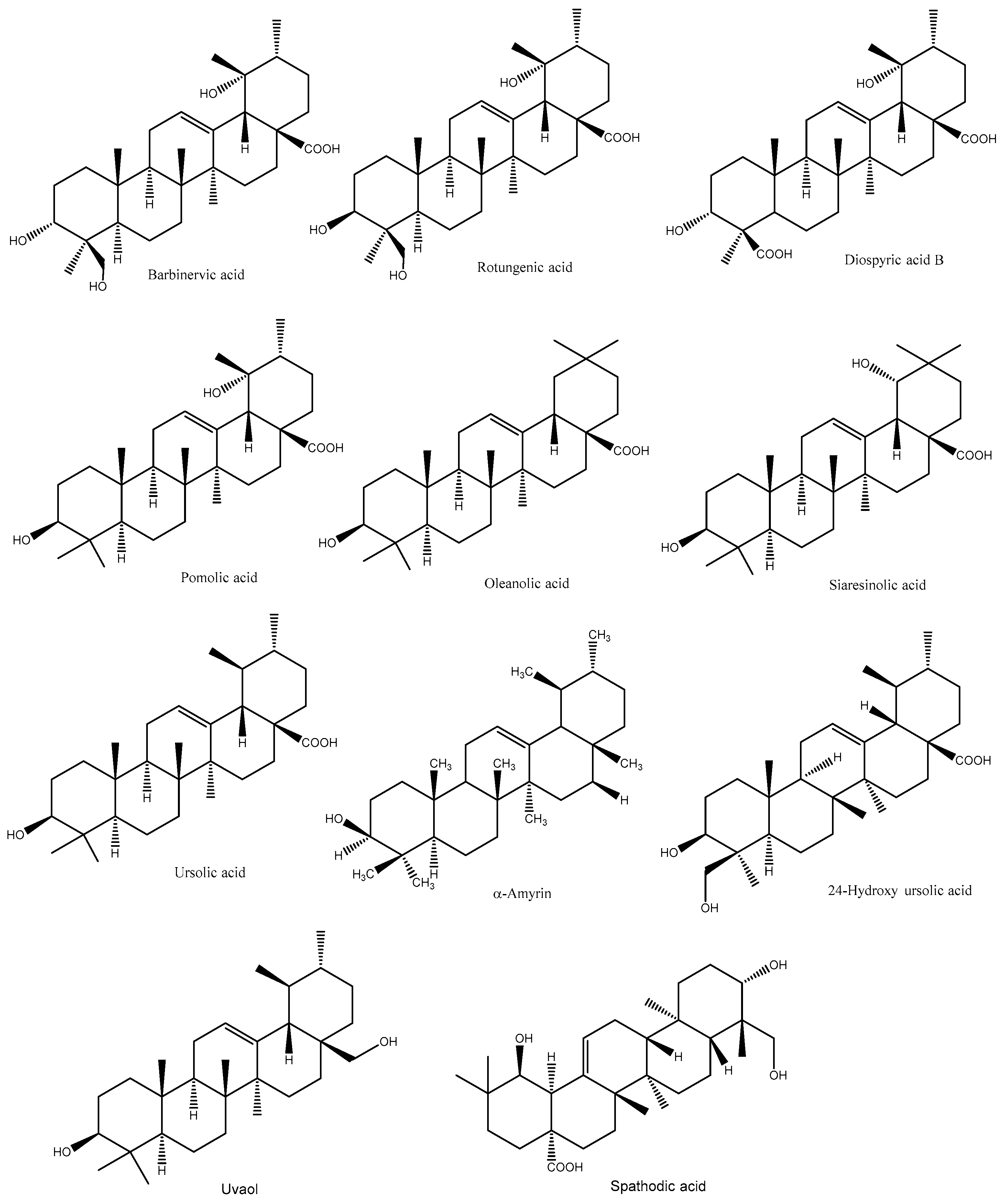
5.2. Terpenoids
5.3. Polysaccharides
5.4. Other Compounds
6. Effect of Seasonality and Geographical Location on the Chemical Composition of PL
7. Pharmacological Effects of PL
7.1. Antioxidant Effects
7.2. Anticancer and Antitumor Effects
7.3. Beneficial Effects on Eye-Related Diseases
7.4. Antidiabetic Effects
7.5. Antihyperlipidemic and Anti-Obesity Effects
7.6. Immunostimulatory Effects
7.7. Neuroprotective Effects
7.8. Antiallergic, Antiwrinkle, Anti-Inflammatory, Anti-Tyrosinase, and Antibacterial Effects
7.9. Other Effects
8. Bioavailability of PL
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hossain, A.; Moon, H.K.; Kim, J.K. Effect of drying and harvest time on the physicochemical properties of the most common Korean persimmon leaves. Korean J. Food Preserv. 2018, 25, 428–435. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Heredia, A.; Castelló, M.L.; Andrés, A. Influence of drying method and extraction variables on the antioxidant properties of persimmon leaves. Food Biosci. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Las Heras, R.M.; Quifer-Rada, P.; Andrés, A.; Lamuela-Raventós, R. Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). J. Funct. Foods 2016, 23, 370–377. [Google Scholar] [CrossRef]
- Hossain, A.; Moon, H.K.; Kim, J.-K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018, 27, 177–184. [Google Scholar] [CrossRef]
- Huang, S.W.; Wang, W.; Zhang, M.Y.; Liu, Q.B.; Luo, S.Y.; Peng, Y.; Sun, B.; Wu, D.L.; Song, S.J. The effect of ethyl acetate extract from persimmon leaves on Alzheimer’s disease and its underlying mechanism. Phytomedicine 2016, 23, 694–704. [Google Scholar] [CrossRef]
- Kashif, M.; Akhtar, N.; Mustafa, R. An overview of dermatological and cosmeceutical benefits of Diospyros kaki and its phytoconstituents. Rev. Bras. Farmacogn. 2017, 27, 650–662. [Google Scholar] [CrossRef]
- Khan, M.M.; Tran, B.Q.; Jang, Y.J.; Park, S.H.; Fondrie, W.E.; Chowdhury, K.; Yoon, S.H.; Goodlett, D.R.; Chae, S.W.; Chae, H.J.; et al. Assessment of the therapeutic potential of persimmon leaf extract on prediabetic subjects. Mol. Cells 2017, 40, 466–475. [Google Scholar]
- Lee, J.S.; Lee, M.K.; Ha, T.Y.; Bok, S.H.; Park, H.M.; Jeong, K.S.; Woo, M.N.; Do, G.M.; Yeo, J.Y.; Choi, M.S. Supplementation of whole persimmon leaf improves lipid profiles and suppresses body weight gain in rats fed high-fat diet. Food Chem. Toxicol. 2006, 44, 1875–1883. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Fang, K.; Ding, Y.; Zhang, L.; Zhang, Y. Flavonoids from persimmon (Diospyros kaki) leaves (FPL) attenuate H2O2-induced apoptosis in MC3T3-E1 cells via the NF-κB pathway. Food Funct. 2014, 5, 471–479. [Google Scholar] [CrossRef]
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240. [Google Scholar] [CrossRef]
- Park, H.R.; Hwang, D.; Do Hong, H.; Shin, K.S. Antitumor and antimetastatic activities of pectic polysaccharides isolated from persimmon leaves mediated by enhanced natural killer cell activity. J. Funct. Foods 2017, 37, 460–466. [Google Scholar] [CrossRef]
- Song, Y.R.; Han, A.R.; Lim, T.G.; Kang, J.H.; Hong, H. Do Discrimination of structural and immunological features of polysaccharides from persimmon leaves at different maturity stages. Molecules 2019, 24, 356. [Google Scholar] [CrossRef]
- Hossain, A.; Moon, H.K.; Kim, J.-K. Effect of pre-treatment and extraction conditions on the antioxidant properties of persimmon (Diospyros kaki) leaves. Biosci. Biotechnol. Biochem. 2017, 81, 2079–2085. [Google Scholar] [CrossRef]
- Hossain, A.; Rahman, M.J. Safety, Nutrition and Functionality of the Traditional Foods. In Traditional Foods-History, Preparation, Processing and Safety; Al-Khusaibi, M., Al-Habsi, N., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 219–238. [Google Scholar]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Kwon, J.; Park, J.E.; Lee, J.S.; Lee, J.H.; Hwang, H.; Jung, S.H.; Kwon, H.C.; Jang, D.S. Chemical constituents of the leaves of Diospyros kaki (Persimmon). Plants 2021, 10, 3032. [Google Scholar] [CrossRef]
- Bei, W.; Peng, W.; Ma, Y.; Xu, A. Flavonoids from the leaves of Diospyros kaki reduce hydrogen peroxide-induced injury of NG108-15 cells. Life Sci. 2005, 76, 1975–1988. [Google Scholar] [CrossRef]
- Bei, W.J.; Peng, W.; Ma, Y.; Xu, A. NaoXinQing, an anti-stroke herbal medicine, reduces hydrogen peroxide-induced injury in NG108-15 cells. Neurosci. Lett. 2004, 363, 262–265. [Google Scholar] [CrossRef]
- Chung, H.S.; Park, H.S.; Kim, H.S.; Youn, K.S.; Moon, K.D. Physico-chemical properties of persimmon leaves brined in varying concentrations of sodium chloride. Int. J. Food Prop. 2020, 23, 599–608. [Google Scholar] [CrossRef]
- Shen, Q.; Ding, H.G.; Zhong, L. Characterization of the surface properties of persimmon leaves by FT-Raman spectroscopy and wicking technique. Colloids Surf. B Biointerfaces 2004, 37, 133–136. [Google Scholar] [CrossRef]
- Gerengi, H.; Uygur, I.; Solomon, M.; Yildiz, M.; Goksu, H. Evaluation of the inhibitive effect of Diospyros kaki (Persimmon) leaves extract on St37 steel corrosion in acid medium. Sustain. Chem. Pharm. 2016, 4, 57–66. [Google Scholar] [CrossRef]
- Go, E.J.; Song, K. Bin Capsosiphon fulvescens films containing persimmon (Diospyros kaki L.) leaf extract. Food Biosci. 2020, 37, 100723. [Google Scholar] [CrossRef]
- Lim, J.-H.; Kim, B.-K.; Park, C.-E.; Park, K.-J.; Kim, J.-C.; Jeong, J.-W.; Jeong, S.-W. Antioxidative and antimicrobial activities of persimmon leaf tea and green tea. J. East Asian Soc. Diet. Life 2008, 18, 797–804. [Google Scholar]
- Hassan, O.; Chang, T.; Hossain, A. Changes in the secondary compounds of persimmon leaves as a defense against circular leaf spot caused by Plurivorosphaerella nawae. PLoS ONE 2020, 15, e0230286. [Google Scholar] [CrossRef]
- Jung, K.-M.; Kang, G.-H.; Kwon, M.-K.; Song, I.-K.; Cho, D.-H.; Chou, Y.-D. Chemical components and antioxidant activity of persimmon leaves. Korean J. Food Preserv. 2004, 11, 175–181. [Google Scholar]
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant potential of sea cucumbers and their beneficial effects on human health. Mar. Drugs 2022, 20, 521. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Effect of High-Pressure Processing (HPP) on Phenolics of North Atlantic Sea Cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2022, 70, 3489–3501. [Google Scholar] [CrossRef]
- Hossain, A.; Yeo, J.D.; Dave, D.; Shahidi, F. Phenolic compounds and antioxidant capacity of sea cucumber (Cucumaria frondosa) processing discards as affected by high-pressure processing (HPP). Antioxidants 2022, 11, 337. [Google Scholar] [CrossRef]
- Hossain, A.; Senadheera, T.R.L.; Dave, D.; Shahidi, F. Phenolic profiles of Atlantic sea cucumber (Cucumaria frondosa) tentacles and their biological properties. Food Res. Int. 2023, 163, 112262. [Google Scholar] [CrossRef]
- An, B.-J.; Choi, H.-J.; Son, J.-H.; Woo, H.-S.; Han, H.-S.; Park, J.-H.; Son, G.-M.; Son, G.-M.; Choi, C. Identification of biologically effect and chemical structure of polyphenol compounds from the leaves of Korea persimmon (Diospyrus kaki L. Folium). J. Korean Soc. Food Cult. 2003, 18, 443–456. [Google Scholar]
- Kim, K.A.; Kang, S.W.; Ahn, H.R.; Song, Y.; Yang, S.J.; Jung, S.H. Leaves of persimmon (Diospyros kaki Thunb.) ameliorate N-methyl-N-nitrosourea (MNU)-induced retinal degeneration in mice. J. Agric. Food Chem. 2015, 63, 7750–7759. [Google Scholar] [CrossRef]
- Choi, S.; Kang, W.; Chung, S.; Cheon, S. Antioxidative activity of flavonoids in persimmon leaves. Foods Biotechnol. 1996, 5, 119–123. [Google Scholar]
- Kawakami, K.; Aketa, S.; Nakanami, M.; Iizuka, S.; Hirayama, M. Major water-soluble polyphenols, proanthocyanidins, in leaves of persimmon (Diospyros kaki) and their α-amylase inhibitory activity. Biosci. Biotechnol. Biochem. 2010, 74, 1380–1385. [Google Scholar] [CrossRef]
- Xue, Y.L.; Miyakawa, T.; Hayashi, Y.; Okamoto, K.; Hu, F.; Mitani, N.; Furihata, K.; Sawano, Y.; Tanokura, M. Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. J. Agric. Food Chem. 2011, 59, 6011–6017. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, M.; Li, H. Method development and validation for simultaneous determination of six flavonoids in rat eyes after oral administration of Diospyros kaki leaves extract by UPLC-MS/MS. Chem. Pharm. Bull. 2021, 69, 218–221. [Google Scholar] [CrossRef]
- Chang, Y.L.; Lin, J.T.; Lin, H.L.; Liao, P.L.; Wu, P.J.; Yang, D.J. Phenolic compositions and antioxidant properties of leaves of eight persimmon varieties harvested in different periods. Food Chem. 2019, 289, 74–83. [Google Scholar] [CrossRef]
- Martínez-Las Heras, R.; Pinazo, A.; Heredia, A.; Andrés, A. Evaluation studies of persimmon plant (Diospyros kaki) for physiological benefits and bioaccessibility of antioxidants by in vitro simulated gastrointestinal digestion. Food Chem. 2017, 214, 478–485. [Google Scholar] [CrossRef]
- Tao, W.; Pan, H.; Jiang, H.; Wang, M.; Ye, X.; Chen, S. Extraction and identification of proanthocyanidins from the leaves of persimmon and loquat. Food Chem. 2022, 372, 130780. [Google Scholar] [CrossRef]
- Cho, Y.J.; An, B.J.; Kim, J.H. Application of isolated tyrosinase inhibitory compounds from persimmon leaves. J. Life Sci. 2011, 21, 976–984. [Google Scholar] [CrossRef]
- Bei, W.; Zang, L.; Guo, J.; Peng, W.; Xu, A.; Good, D.A.; Hu, Y.; Wu, W.; Hu, D.; Zhu, X.; et al. Neuroprotective effects of a standardized flavonoid extract from Diospyros kaki leaves. J. Ethnopharmacol. 2009, 126, 134–142. [Google Scholar] [CrossRef]
- Bae, U.J.; Park, S.H.; Jung, S.Y.; Park, B.H.; Chae, S.W. Hypoglycemic effects of aqueous persimmon leaf extract in a murine model of diabetes. Mol. Med. Rep. 2015, 12, 2547–2554. [Google Scholar] [CrossRef]
- Kazzem, M.; Sun, Y.T.; Low, M.; Seto, S.W.; Chang, D.; Lee, S.; Suresh, H.; Khoo, C.S.; Bensoussan, A.; Kiat, H. Chromatographic analysis and anti-oxidative property of Naoxinqing tablet, a proprietary preparation of Diospyros kaki leaves. Molecules 2019, 24, 1101. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.L.; Rasmussen, S.K.; Wang, M.H. Vomifoliol 9-O-α-arabinofuranosyl (1→6)-β-D-glucopyranoside from the leaves of Diospyros kaki stimulates the glucose uptake in HepG2 and 3T3-L1 cells. Carbohydr. Res. 2011, 346, 1212–1216. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossein, A. Importance of insoluble-bound phenolics to the antioxidant potential is dictated by source material. Antioxidants 2023, 12, 203. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018, 3, 8–75. [Google Scholar] [CrossRef]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef]
- Chen, G.; Lu, H.; Wang, C.; Yamashita, K.; Manabe, M.; Meng, Z.; Xu, S.; Kodama, H. Effect of five flavonoid compounds isolated from leaves of Diospyros kaki on stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin. Chim. Acta 2002, 326, 169–175. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.Q.; Jia, J.M. Three minor novel triterpenoids from the leaves of Diospyros kaki. Chem. Pharm. Bull. 2009, 57, 532–535. [Google Scholar] [CrossRef]
- Chen, G.; Ren, H.; Yu, C. A new 18,19-secoursane triterpene from the leaves of Diospyros kaki. Chem. Nat. Compd. 2012, 47, 805–806. [Google Scholar] [CrossRef]
- Phuong, T.T.; Chul, H.L.; Trong, T.D.; Phi, H.N.; Wan, G.K.; Sang, J.L.; Won, K.O. Triterpenoids from the leaves of Diospyros kaki (Persimmon) and their inhibitory effects on protein tyrosine phosphatase 1B. J. Nat. Prod. 2008, 71, 1775–1778. [Google Scholar]
- Park, J.Y.; Shin, M.S. Inhibitory effects of pectic polysaccharide isolated from Diospyros kaki leaves on tumor cell angiogenesis via VEGF and MMP-9 regulation. Polymers 2021, 13, 64. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Ha, H.; Kim, R.; Cho, C.W.; Song, Y.R.; Do Hong, H.; Kim, T. Anti-osteoporotic effects of polysaccharides isolated from persimmon leaves via osteoclastogenesis inhibition. Nutrients 2018, 10, 901. [Google Scholar] [CrossRef]
- Lee, S.G.; Jung, J.Y.; Shin, J.S.; Shin, K.S.; Cho, C.W.; Rhee, Y.K.; Do Hong, H.; Lee, K.T. Immunostimulatory polysaccharide isolated from the leaves of Diospyros kaki Thumb modulate macrophage via TLR2. Int. J. Biol. Macromol. 2015, 79, 971–982. [Google Scholar] [CrossRef]
- Shin, Y.-A.; Park, H.-R.; Hong, H.-D.; Shin, K.-S. Immuno-stimulating activities of polysaccharide fractions isolated from persimmon leaves. Korean J. Food Nutr. 2012, 25, 941–950. [Google Scholar] [CrossRef]
- Huang, S.W.; Qiao, J.W.; Sun, X.; Gao, P.Y.; Li, L.Z.; Liu, Q.B.; Sun, B.; Wu, D.L.; Song, S.J. Secoiridoids and lignans from the leaves of Diospyros kaki Thunb. with antioxidant and neuroprotective activities. J. Funct. Foods 2016, 24, 183–195. [Google Scholar] [CrossRef]
- Chen, G.; Xue, J.; Xu, S.X.; Zhang, R.Q. Chemical constituents of the leaves of Diospyros kaki and their cytotoxic effects. J. Asian Nat. Prod. Res. 2007, 9, 347–353. [Google Scholar] [CrossRef]
- Kim, J.; Lee, W. Changes of chemical components in persimmon leaves (Diospyros kaki Thunberg) during growth. East Asian Soc. Diet. Life 2002, 12, 32–37. [Google Scholar]
- Clark, C.G.; Smith, G.S. Seasonal Changes in the Mineral Nutrient Content of Persimmon Leaves. Sci. Hortic. 1990, 42, 85–97. [Google Scholar] [CrossRef]
- Jeong, S.I.; Cho, J.K.; Mok, J.Y.; Kim, S.J.; Park, J.M.; Jeon, I.H.; Kim, H.S.; Jang, S.I. Antioxidant activity of persimmon leaves during growth. Korean J. Pharmacogn. 2010, 41, 255–263. [Google Scholar]
- Jung, W.; Jeong, J. Change of antioxidative activity at different harvest time and improvement of atopic dermatitis effects for persimmon leaf extract. Korea J. Herbol. 2012, 27, 41–49. [Google Scholar] [CrossRef]
- Kawakami, K.; Shibukura, Y.; Kanno, T.; Furuki, T.; Aketa, S.; Hirayama, M. Identification of 2 -galloylated flavonol 3-O-glycosides accumulating in in developing leaves of persimmon. Phytochem. Anal. 2011, 22, 403–410. [Google Scholar] [CrossRef]
- Kim, J.-K.; Kang, W.; Kim, G.; Moon, H.-K. Changes of flavor compounds in persimmon leaves (Diospyros kaki) during growth. J. East Asian Soc. Diet. Life 2001, 11, 472–478. [Google Scholar]
- Afzal, R.; Hwang, H. Bin Persimmon leaves (Diospyros kaki) extract enhances the viability of human corneal endothelial cells by improving Na+-K+-ATPase activity. Pharmaceuticals 2022, 15, 72. [Google Scholar] [CrossRef]
- Ryul Ahn, H.; Kim, K.A.; Kang, S.W.; Lee, J.Y.; Kim, T.J.; Jung, S.H. Persimmon leaves (Diospyros kaki) extract protects optic nerve crush-induced retinal degeneration. Sci. Rep. 2017, 7, 46449. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.R.; Yang, J.W.; Kim, J.Y.; Lee, C.Y.; Kim, T.J.; Jung, S.H. The intraocular pressure-lowering effect of persimmon leaves (Diospyros kaki) in a mouse model of glaucoma. Int. J. Mol. Sci. 2019, 20, 5268. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Kim, H.J.; Choi, Y.H.; Lee, I.S. Physiological activities of dried persimmon, fresh persimmon and persimmon leaves. J. Korean Soc. Food Sci. Nutr. 2008, 37, 957–964. [Google Scholar] [CrossRef]
- Kawakami, K.; Aketa, S.; Sakai, H.; Watanabe, Y.; Nishida, H.; Hirayama, M. Antihypertensive and vasorelaxant effects of water-soluble proanthocyanidins from persimmon leaf tea in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2011, 75, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.H.; Kang, H.J.; Lee, H.S.; Shin, J.H.; Park, Y.G.; Jeong, S.I.; Jang, S.I. Antioxidant and anti-inflammatory activities of water-soluble extracts from different parts of kojongsi persimmon (Diospyros kaki L.). Korean J. Food Sci. Technol. 2014, 46, 505–510. [Google Scholar] [CrossRef]
- Jung, U.J.; Park, Y.B.; Kim, S.R.; Choi, M.S. Supplementation of persimmon leaf ameliorates hyperglycemia, dyslipidemia and hepatic fat accumulation in type 2 diabetic mice. PLoS ONE 2012, 7, e49030. [Google Scholar] [CrossRef]
- Kim, H.S.; Suh, J.S.; Jang, Y.K.; Ahn, S.H.; Raja, G.; Kim, J.C.; Jung, Y.; Jung, S.H.; Kim, T.J. Anti-cancer potential of persimmon (Diospyros kaki) leaves via the PDGFR-Rac-JNK pathway. Sci. Rep. 2020, 10, 18119. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef]
- Ding, Y.; Ren, K.; Dong, H.; Song, F.; Chen, J.; Guo, Y.; Liu, Y.; Tao, W.; Zhang, Y. Flavonoids from persimmon (Diospyros kaki L.) leaves inhibit proliferation and induce apoptosis in PC-3 cells by activation of oxidative stress and mitochondrial apoptosis. Chem. Biol. Interact. 2017, 275, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.C.; Choi, J.W.; Song, N.E.; Cho, C.W.; Rhee, Y.K.; Hong, H. Do Polysaccharide isolated from persimmon leaves (Diospyros kaki Thunb.) suppresses TGF-β1-induced epithelial-to-mesenchymal transition in A549 cells. Int. J. Biol. Macromol. 2020, 164, 3835–3845. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Lee, J.-S.; Bok, S.-H.; Choi, M.S. Effects of extracts of persimmon leaf, buckwheat leaf, and chinese matrimony vine leaf on body fat and lipid metabolism in rats. J. Korean Soc. Food Sci. Nutr. 2011, 40, 1215–1226. [Google Scholar] [CrossRef]
- Chen, L.; Ma, X.B.; Liang, Y.H.; Pei, S.C.; Feng, Y.P.; Wei, M. Effects of persimmon leaf Total flavonoid on enzyme of lipoprotein metabolism and antioxidation in hyperlipidemia rats. Chin. J. Nat. Med. 2011, 9, 74–77. [Google Scholar] [CrossRef]
- Shin, M.S.; Lee, H.; Do Hong, H.; Shin, K.S. Characterization of immunostimulatory pectic polysaccharide isolated from leaves of Diospyros kaki Thumb. (persimmon). J. Funct. Foods 2016, 26, 319–329. [Google Scholar] [CrossRef]
- Sa, Y.S.; Kim, S.J.; Choi, H.S. The anticoagulant fraction from the leaves of Diospyros kaki L. has an antithrombotic activity. Arch. Pharm. Res. 2005, 28, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Zhang, M.; Gu, L.; Liu, Z.; Jia, J.; Chen, X. Effects of phospholipid complexes of total flavonoids from Persimmon (Diospyros kaki L.) leaves on experimental atherosclerosis rats. J. Ethnopharmacol. 2016, 191, 245–253. [Google Scholar] [CrossRef]
- Yu, H.; Shao, S.; Xu, J.; Guo, H.; Zhong, Z.; Xu, J. Persimmon leaf extract alleviates chronic social defeat stress-induced depressive-like behaviors by preventing dendritic spine loss via inhibition of serotonin reuptake in mice. Chin. Med. 2022, 17, 65. [Google Scholar] [CrossRef]
- Yoo, K.H.; Jeong, J.M. Antioxidative and antiallergic effect of persimmon leaf extracts. J. Korean Soc. Food Sci. Nutr. 2009, 38, 1691–1698. [Google Scholar] [CrossRef]
- An, B.J.; Kwak, J.H.; Park, J.M.; Lee, J.Y.; Park, T.S.; Lee, J.T.; Son, J.H.; Jo, C.; Byun, M.W. Inhibition of enzyme activities and the antiwrinkle effect of polyphenol isolated from the persimmon leaf (Diospyros kaki folium) on human skin. Am. Soc. Dermatol. Surg. 2005, 31, 848–855. [Google Scholar] [CrossRef]
- Moon, S.-H.; Lee, M.-K.; Chae, K.-S. Inhibitory effects of the solvent fractions from persimmon leaves on xanthine oxidase activity. Korean J. Food Nutr. 2001, 14, 120–125. [Google Scholar]
- Kotani, M.; Matsumoto, M.; Fujita, A.; Higa, S.; Wang, W.; Suemura, M.; Kishimoto, T.; Tanaka, T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/NGa mice. J. Allergy Clin. Immunol. 2000, 106, 159–166. [Google Scholar] [CrossRef]
- Moon, S.-H.; Park, K.-Y. Antioxidative effect of persimmon leaves. Korean J. Food Nutr. 2000, 13, 53–58. [Google Scholar]
- Ashry, O.M.; Hussein, E.M.; Abd El-Azime, A.S.H. Restorative role of persimmon leaf (Diospyros kaki) to gamma irradiation-induced oxidative stress and tissue injury in rats. Int. J. Radiat. Biol. 2017, 93, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Nishida, H.; Tatewaki, N.; Nakajima, Y.; Konishi, T.; Hirayama, M. Persimmon leaf extract inhibits the ATM activity during DNA damage response induced by doxorubicin in A549 lung adenocarcinoma cells. Biosci. Biotechnol. Biochem. 2011, 75, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, D.S.; Kim, D.C.; Yoon, C.S.; Ko, W.; Oh, H.; Kim, Y.C. Anti-inflammatory effects and mechanisms of action of coussaric and betulinic acids isolated from Diospyros kaki in lipopolysaccharide-stimulated RAW 264.7 macrophages. Molecules 2016, 21, 1206. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Takasaki, M.; Tajima, N.; Fukamachi, H.; Igarashi, T. Antibacterial activities of persimmon extracts relate with their hydrogen peroxide concentration. Biol. Pharm. Bull. 2014, 37, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yi, S.; Wang, Z.; Chen, S.; Xin, S.; Xie, J.; Zhao, C. Self-nanoemulsifying drug delivery system of persimmon leaf extract: Optimization and bioavailability studies. Int. J. Pharm. 2011, 420, 161–171. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, Y.Z.; Meng, F.Y.; Li, Y.L.; Li, C.X.; Duan, F.P.; Wang, Q.; Zhang, X.T.; Zhang, C.N. Studies of the microbial metabolism of flavonoids extracted from the leaves of Diospyros kaki by intestinal bacteria. Arch. Pharm. Res. 2015, 38, 614–619. [Google Scholar] [CrossRef]

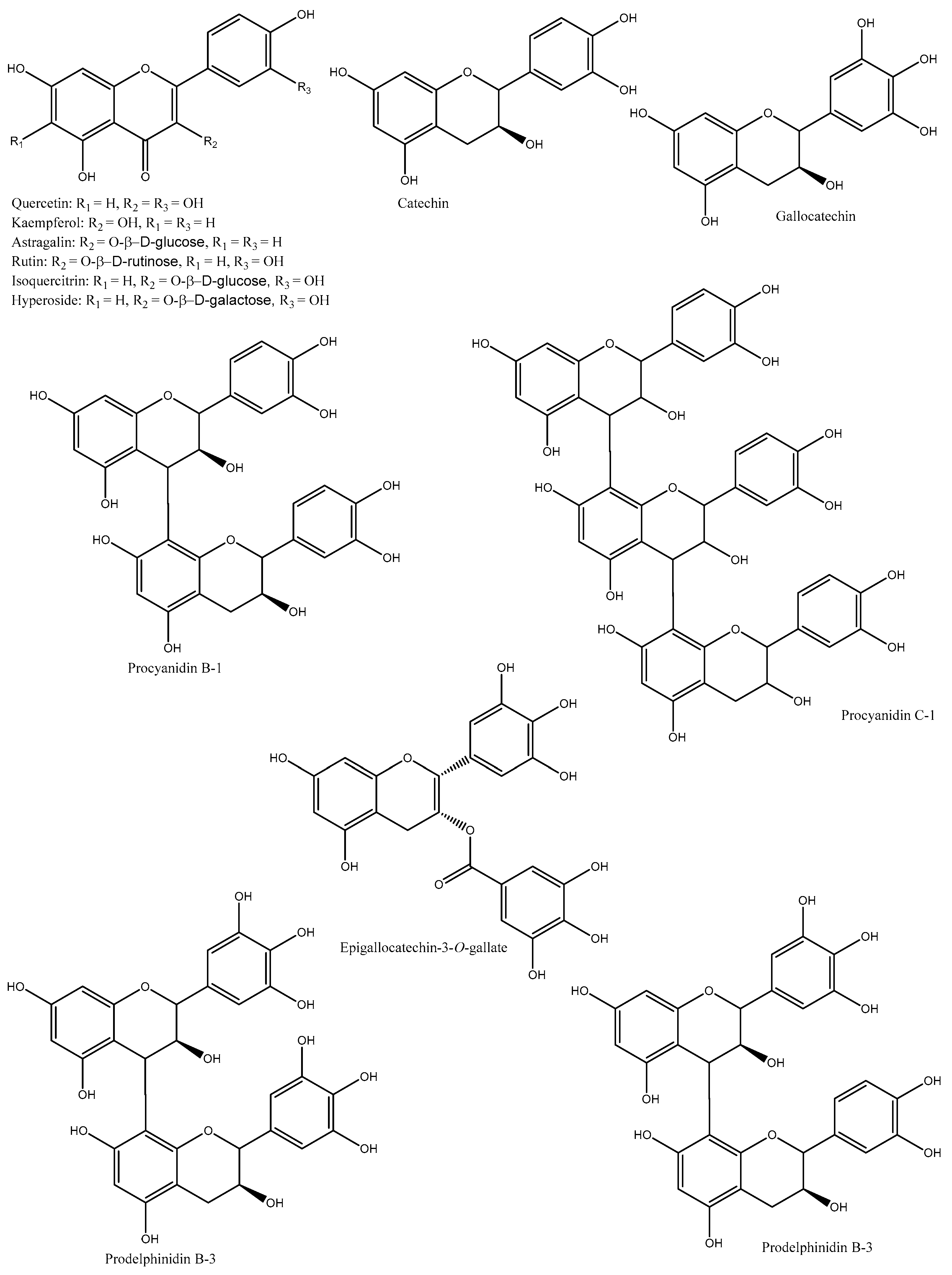



| Species/Cultivars | Origin | TPC (mg GAE/g) | TFC (mg CE/g) | TTC (mg CE/g) | Individual Compounds (µg/g) | References |
|---|---|---|---|---|---|---|
| D. kaki (Sangju dungsi) | Korea | 90.41 | 30.67 | 47 | NA | [13] |
| D. kaki (Sangju-dungsi, Sangamdungsi, Gabjubaekmok, Cheongdobansi, and Suhong) | Korea | 72.59–112.09 | 30.27–37.83 | 28.67–81.33 | NA | [4] |
| D. lotus (Dongsi) | Korea | 58.01–58.16 | 14.16–15.83 | 32.38–35.46 | NA | [24] |
| D. kaki | Korea | NA | NA | NA | Catechin, gallocatechin, pyrocyanidin C-1, procyanidin B-1, prodelphinidin B-3, procyanidin B-7-3-O-gallate, gallocatechin-(4α→8)-catechin, procyanidin C-1-3′-3″-3″-O-trigallate, and epigallocatechin-(4β→8)-epigallocatechin-(4β→8)-catechin | [30] |
| D. kaki | Korea | NA | NA | NA | Quercetin, quercetin-3-O-β-glucoside, quercetin-3-O-β-galactoside, quercetin-3-O-β-2″-galloylglucoside, kaempferol, kaempferol-3-O-β-glucoside, kaempferol-3-O-β-galactoside, and kaempferol-3-O-β-2″-galloylglucoside | [31] |
| D. kaki | Korea | NA | NA | NA | Isoquercetin, quercetin 3-O-β-D-glucopyranoside-2″-gallate, kaempferol 3-O-β-D-glucopyranoside-2″-gallate, and astragalin | [32] |
| D. kaki | Korea | NA | NA | NA | Catechin, hyperoside, quercetin, isoquercitrin, trifolin, astragalin, quercetin-3-O-β-2″-galloylgalactoside, quercetin-3-O-β-2″-galloylglucoside, kaempferol, kaempferol-3-O-β-D-2″-coumaroylgalactoside, kaempferol-3-O-β-2″-galloylgalactoside, kaempferol-3-O-α-arabinoside, and scopoletin | [16] |
| D. kaki (Hiratanenashi and Tonewase) | Japan | 26–27.7 | NA | NA | Proanthocyanidins (catechin, epigallocatechin, epigallocatechin-3-O-gallate, epicatechin, epicatechin-3-O-gallate, and prodelphinidin) | [33] |
| D. kaki | Japan | 112 | 58.4 | NA | NA | [15] |
| D. kaki (Fuyu, Jiro, Kinsyu, Tanrei, Yotsumizo, and Saijo) | Japan | NA | NA | NA | Isoquercitrin, hyperoside, trifolin, chrysontemin, astragalin, kaempferol-3-O-(2″-O-galloyl-β-D-glucopyranoside), and quercetin-3-O-(2″-O-galloyl-β-D-glucopyranoside) | [34] |
| D. kaki | China | NA | NA | NA | Quercetin-3-O-β-glucoside, quercetin-3-O-β-galactoside, quercetin-3-(2-galloylglucoside), kaempferol-3-O-β-glucoside, kaempferol-3-O-β-galactoside, and kaempferol-3-(2-galloylglucoside) | [35] |
| D. kaki (Tonewase, Fuyu, Aoso, Hachiya, Diamond Bull Heart, and Bull Heart) | Taiwan | 69.27–149.59 | 40.78–90.62 | 12.58–19.23 | Protocatechuic acid, gallic acid, p-hydroxybenzoic acid, vanillic acid, chlorogenic acid, caffeic acid, p-coumaric acid, sinapic acid, catechin, epicatechin, myricetin-3-O-glucoside, myricetin-3-O-rhamnoside, rutin, quercetin-3-O-glucoside, quercetin-3-O-galactoside, quercitrin, quercetin-3-O-arabinoside, kaempferol-3-O-rutinoside, kaempferol-3-O-glucoside, myricetin, naringin, kaempferol-3-O-rhamnoside, isorhamnetin-3-O-rutinoside, naringenin-7-O-glucoside, isorhamnetin-3-O-glucoside, quercetin, kamempferol, apigenin, and isorhamnetin. | [36] |
| D. kaki (Rojo brillante) | Spain | 86 | 22.9 | NA | [37] | |
| D. kaki (Rojo brillante) | Spain | Gallic acid-O-hexoside (10.3), gallic acid (32.5), gallocatechin (442.2), catechin-O-hexoside I (19), procyanidin B1 (203.71), procyanidin dimer I (54.6), catechin (435.2), procyanidin dimer II (22.6), prodelphinidin dimer B3 (24.4), myricetin-O-hexoside I (304.8), myricetin-O-hexoside II (563.4), isoquercetin (247.4), quercetin-O-hexoside (348.8), quercetin-O-pentoside I (31.9), quercetin-O-pentoside II (52), kaempferol-3-O-glucoside (165.3), kaempferol-O-hexoside I (176.8), myricetin (44.7), quercetin (354.7), kaempferol (206.2), and isorhamnetin (42.8) | [3] | |||
| D. kaki | China | Astragalin, trifolin, annulatin, myricetin, myricetin-3-O-glucopyranoside, quercetin, vitexin, hyperoside, quercetin-3-O-galloylglucoside, isorhamnetin-3-β-D-glucopyranoside, isorhamnetin-3-β-D-galactoside, kaempferol, kaempferol-3-O-galloylglucoside, kaempferol-3-O-galloylgalactoside, and salvianolicacid D | [5] |
| Health Effects | Species/Cultivars | Origin | Responsible Compounds/Extracts | Results/Mechanisms | References |
|---|---|---|---|---|---|
| Beneficial actions against eye-related diseases | Diospyros kaki | Korea | Ethanolic extracts (flavonoids) | Showed the potential to be an effective agent against corneal edema and related corneal disorders | [63] |
| D. kaki | Korea | Ethanolic extracts | Exhibited protective properties against retinal degeneration (e.g., glaucoma) in vitro and in vivo | [64] | |
| D. kaki | Korea | Ethanolic extracts (catechin, kaempferol, and quercetin) | Reduced elevated intraocular pressure in mouse models of glaucoma | [65] | |
| D. kaki | Korea | Ethanolic extracts (quercetin) | Showed the potential to prevent degenerative retinal diseases (retinitis pigmentosa and age-related macular degeneration) | [31] | |
| D. kaki | China | Ethanolic extracts (flavonoids) | Potential effect in lowering the degeneration of retina | [35] | |
| Antihypertensive | D. kaki | Korea | Prodelphinidin B-3, procyanidin B-7-3-O-gallate, procyanidin C-1-3′-3″-3″-O-trigallate, and epigallocatechin-(4β→8)-epigallocatechin-(4β→8)-catechin) | Showed angiotensin-converting enzyme (ACE), xanthine oxidase, and tyrosinase inhibitory activities | [30] |
| D. kaki | Korea | Extracts | Showed ACE inhibitory activity | [23,30,66] | |
| D. kaki | Japan | Proanthocyanidins | Showed activity via an endothelium-dependent nitric oxide/cGMP pathway | [67] | |
| Anti-inflammatory | D. kaki | Korea | Water extracts | Suppressed the production of inflammatory mediators and pro-inflammatory cytokines | [68] |
| Neuroprotective | D. kaki | China | Flavonoid extracts | Showed the potential to prevent and treat ischemia/reperfusion injury and other related neurodegenerative diseases | [17,40] |
| D. kaki | China | Secoiridoids and lignans | Showed potential neuroprotective activity | [55] | |
| Antidiabetic | D. kaki | Korea | Aqueous extracts | Exhibited activity via α-glucosidase inhibition and maintenance of functional β-cells | [41] |
| D. kaki | Korea | Extracts (quercetin 3-O-2″galloylglucoside and kaempferol 3-O-2″galloylglucoside) | Showed therapeutic potentials in diabetes amelioration | [7] | |
| D. kaki | Korea | Methanolic extracts | Showed α-glucosidase and α-amylase inhibition | [66] | |
| D. kaki | Korea | PL powder enriched with phenolic compounds | Improved hyperglycemia by alterations in activity and/or mRNA expression of hepatic enzymes linked in glucose utilization and production | [69] | |
| D. kaki | Japan | Proanthocyanidins (mainly epigallocatechin-3-O-gallate) | Inhibited α-amylase and decreased blood glucose level in Wistar rats | [33] | |
| D. kaki | Korea | Vomifoliol 9-O-α-arabinofuranosyl (1→6)-β-D-glucopyranoside | Stimulated the glucose uptake in HepG2 and 3T3-L1 cells | [43] | |
| Anti-tyrosinase | D. kaki | Japan | Chrysontemin | Exhibited activity against tyrosinase for oxidation of levodopa | [34] |
| D. kaki | Korea | Ethanolic extracts (prodelphinidin B-3 and (+)-gallocatechin) | Showed tyrosinase inhibitory activity | [39] | |
| D. kaki | Korea | Triterpenoids | Inhibited protein tyrosine phosphatase 1B activity | [50] | |
| Anticancer and antitumor | D. kaki | Korea | Ethanolic extracts (mainly quercetin and kaempferol) | Triggered PDGFR-Rac-JNK signaling cascade in live cells, causing cancer cell death | [70] |
| D. kaki | China | Flavonoids | Decreased the level of reactive oxygen species (ROS) and malondialdehyde (MDA) in MC3T3-E1 cells | [71] | |
| D. kaki | China | Flavonoids | Reduced H2O2-induced apoptosis in MC3T3-E1 cells via the NF-kB pathway | [9] | |
| D. kaki | China | Flavonoids | Induced apoptosis in PC-3 cells by activation of oxidative stress and mitochondrial apoptosis | [72] | |
| D. kaki | Korea | Phenolic compounds | Exhibited protective effect against ultraviolet B (UVB)-induced cell cytotoxicity | [59] | |
| D. kaki | Korea | Pectic polysaccharides (mainly acidic sugars, rhamnose, arabinose, and galactose) | Inhibited vascular endothelial growth factor and matrix metalloproteinase (MMP-9) expression in human umbilical vein endothelial cells via regulation of PI3K/AKT, p38, JNK, and NF-kB p65 signaling pathways | [51] | |
| D. kaki | Korea | Pectic polysaccharides | Increased levels of IL-6 and IL-12 produced by peritoneal macrophages | [11] | |
| D. kaki | Korea | Polysaccharides | Suppressed TGF-β1-induced epithelial-to-mesenchymal transition in A549 cells | [73] | |
| D. kaki | Korea | Polysaccharides (mainly neutral sugars and uronic acid) | Up-regulated the expressions of iNOS, TNF-α, IL-1β, and IL-6 genes by activating TLR2-mediated NF-kB activations | [53] | |
| Antihyperlipidemic and anti-obesity | D. kaki | Korea | PL extracts | Lowered body fat weight and improved plasma and hepatic lipid profiles in high-fat diet (HFD)-fed rats | [74] |
| D. kaki | Korea | PL powder enriched with phenolic compounds | Improved plasma and hepatic lipid levels profile via the increased fecal lipids in HFD rats | [8] | |
| D. kaki | China | Flavonoids | Improved lipid metabolic disorder in hyperlipidemic rats | [75] | |
| Immunostimulatory | D. kaki | Korea | Pectic polysaccharides (neutral sugars and uronic acid) | Stimulated the immune activity (IL-6/IL-12 and TNF-α production) of peritoneal macrophages cells | [76] |
| D. kaki | Korea | Polysaccharides | Exhibited immuno-stimulating activity | [54] | |
| D. kaki | Japan | Triterpenoids | Induced superoxide generation and tyrosyl phosphorylation in human polymorphonuclear leukocytes | [47] | |
| Anti Alzheimer’s | D. kaki | China | Ethyl acetate extract (flavonoids and triterpenoids) | Showed a potent protective effect on cognitive deficits induced by Aβ in rats | [5] |
| Anticoagulant | D. kaki | Korea | PL extracts | Delayed thrombin time (TT), activated partial thromboplastin time (APTT), and prothrombin time (PT) in human plasma | [77] |
| Anti-osteoporotic | D. kaki | Korea | Polysaccharides, mainly neutral sugars and uronic acid | Improved ovariectomy-induced trabecular bone loss by suppressing osteoclast activity | [52] |
| Anti-atherosclerotic | D. kaki | China | Phospholipid complexes flavonoids | Improved the bioavailability in vivo and anti-atherosclerotic properties in atherosclerosis rats | [78] |
| Antidepressant | D. kaki | China | PL extracts | Showed antidepressant-like effect in chronic social defeat stress-subjected mice and improved neurogenesis | [79] |
| Antiallergic and antiwrinkle | D. kaki | Korea | Ethanolic extracts (prodelphinidin B-3 and (+)-gallocatechin) | Showed inhibitory activity against tyrosinase and melanin biosynthesis in melanoma cell | [39] |
| D. kaki | Korea | Phenolic extracts | Exhibited antiallergic effect | [80] | |
| D. kaki | Korea | Ethanolic extracts (flavonoids) | Showed xanthine oxidase, tyrosinase, and elastase inhibitory activities | [81] | |
| D. kaki | Korea | Ethyl acetate extracts | Inhibited xanthine oxidase | [82] | |
| D. kaki | Japan | Flavonoids (astragalin) | Inhibited histamine release from KU812 cell in response to cross-linkage of FcεRI (high-affinity IgE receptor) | [83] | |
| Antimicrobial | D. kaki | Korea | Phenolic extracts | Showed inhibitory activity against Listeria monocytogenes, Staphylococcus aureus, Escherichia coil, and Salmonella typhimurium | [23] |
| D. kaki | Korea | Methanolic extracts (polyphenols) | Exhibited inhibition against E. coli O157:H7 | [66] |
| Species/Cultivars | Origin | Responsible Compounds | DPPH RSA (%) | ABTS RSA (%) | Hydroxyl RSA (EC50 µg/mL) | TEAC (µmol TE/g) | Reducing Power (EC50 µg/mL) | References |
|---|---|---|---|---|---|---|---|---|
| D. kaki | Korea | Phenolic compounds | 48.86 | 88.17 | NA | NA | NA | [13] |
| D. kaki | Korea | Phenolic compounds | 48.19–54.09 | 73.85–94.66 | NA | NA | NA | [4] |
| D. kaki | Korea | Phenolic compounds | 64.47 (IC50 µg/mL) | NA | NA | NA | NA | [66] |
| D. lotus | Korea | Phenolic compounds | 26.5–27.22 | 75.24–75.37 | NA | NA | NA | [24] |
| D. kaki | Spain | Phenolic compounds | NA | NA | NA | 122 (mg TE/g) | NA | [37] |
| D. kaki | Spain | Phenolic compounds | 105–190 (mg TE/g) | NA | NA | NA | NA | [2] |
| D. kaki | Taiwan | Phenolic compounds | 56.74–98.84 (EC50 µg/mL) | NA | NA | 647.14–951.1 | 278.86–441.41 | [36] |
| D. kaki | China | Flavonoids | 96.36 (EC50 µg/mL) | NA | 111.23 | NA | NA | [71] |
| D. kaki | China | Naoxinqing tablet (astragalin, isoquercitin, quercetin, kaempfero, and 3,4-dihydroxybenzoic acid) | 119–181 (EC50 µM) | 68–350 (EC50 µM) | NA | NA | NA | [42] |
| D. kaki | China | Secoiridoids and lignans | 31.2–109.9 (IC50 µg/mL) | 3.6–22.9 (IC50 µg/mL) | NA | NA | NA | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, A.; Shahidi, F. Persimmon Leaves: Nutritional, Pharmaceutical, and Industrial Potential—A Review. Plants 2023, 12, 937. https://doi.org/10.3390/plants12040937
Hossain A, Shahidi F. Persimmon Leaves: Nutritional, Pharmaceutical, and Industrial Potential—A Review. Plants. 2023; 12(4):937. https://doi.org/10.3390/plants12040937
Chicago/Turabian StyleHossain, Abul, and Fereidoon Shahidi. 2023. "Persimmon Leaves: Nutritional, Pharmaceutical, and Industrial Potential—A Review" Plants 12, no. 4: 937. https://doi.org/10.3390/plants12040937
APA StyleHossain, A., & Shahidi, F. (2023). Persimmon Leaves: Nutritional, Pharmaceutical, and Industrial Potential—A Review. Plants, 12(4), 937. https://doi.org/10.3390/plants12040937









