Luxury Zinc Supply Prevents the Depression of Grain Nitrogen Concentrations in Rice (Oryza sativa L.) Typically Induced by Elevated CO2
Abstract
1. Introduction
2. Results and Discussion
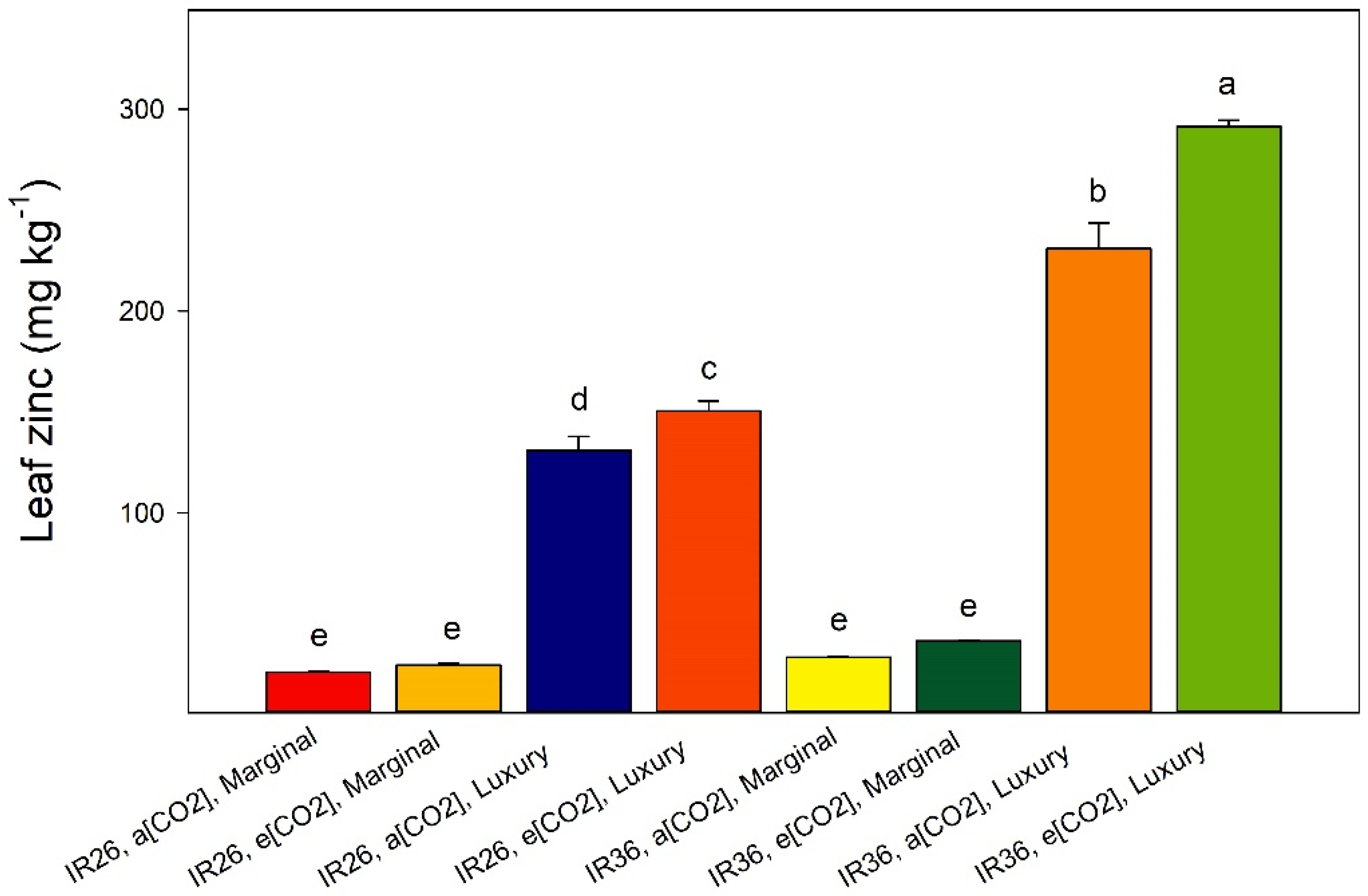
2.1. Nutrient Status at Panicle Emergence
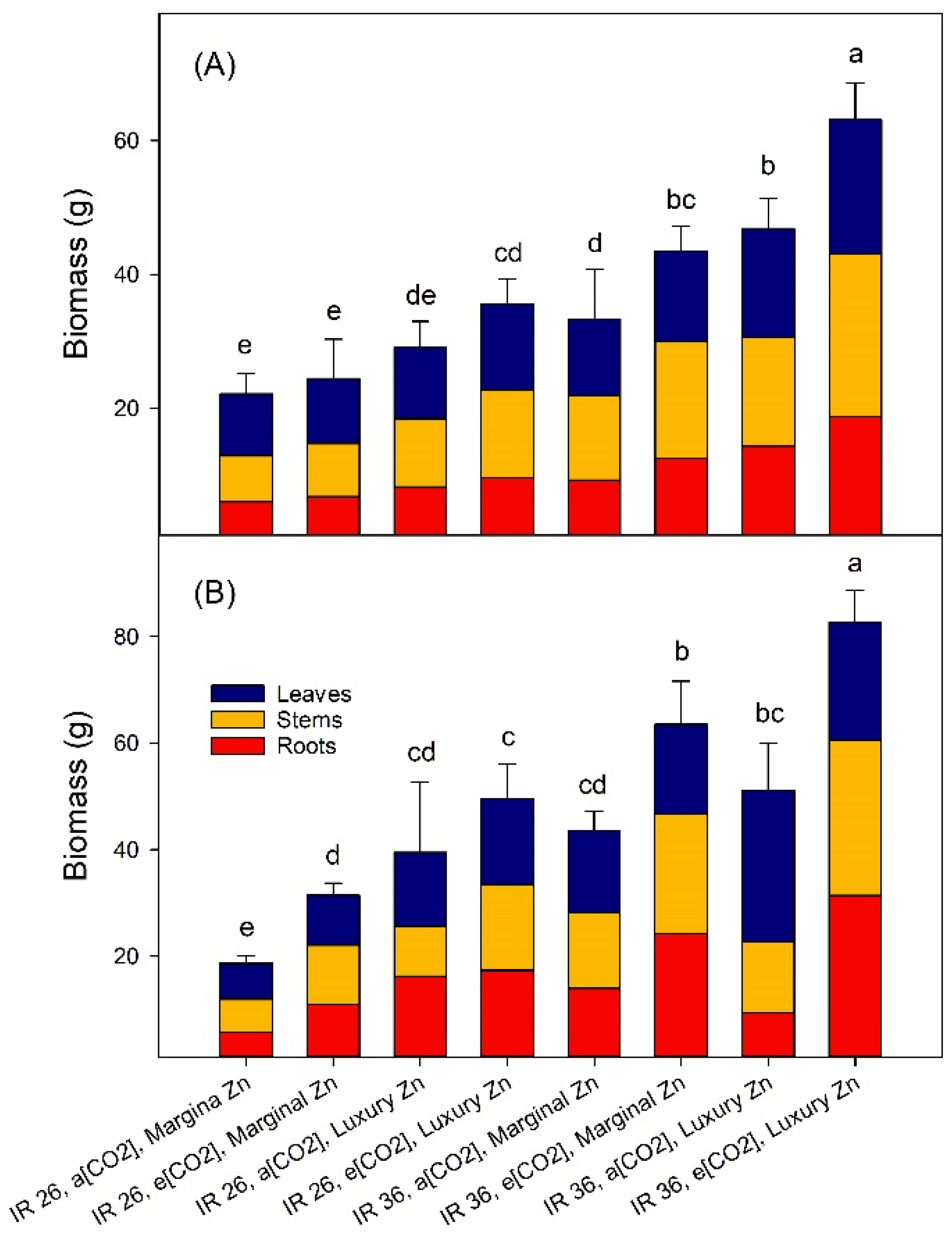
2.2. Vegetative Growth
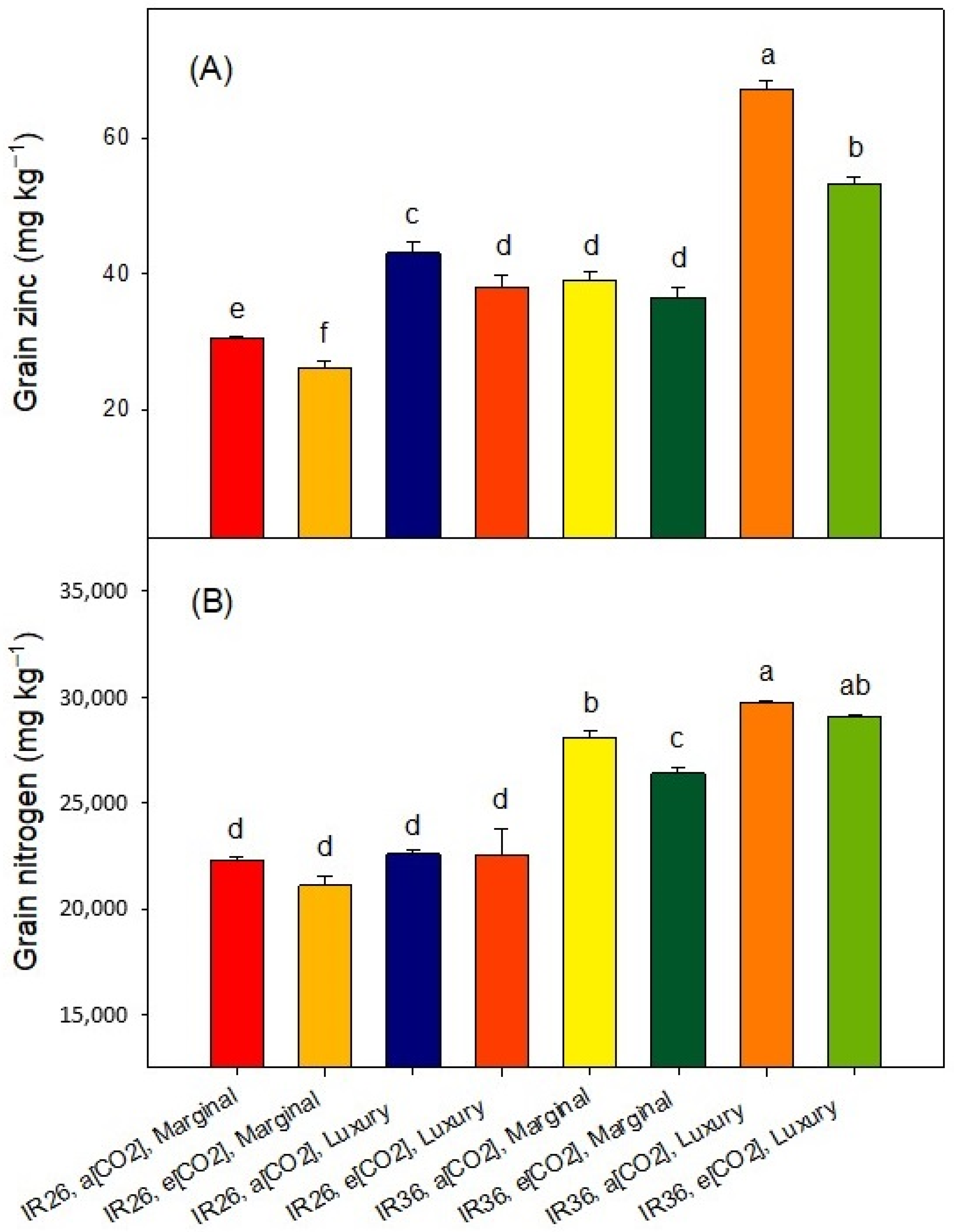
2.3. Grain Yield and Composition
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
3.2. Postharvest Treatments and Measurements
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fageria, N.K. Yield physiology of rice. J. Plant Nutr. 2007, 30, 843–879. [Google Scholar] [CrossRef]
- International Rice Genome Sequencing Project. The map-based sequence of the rice genome. Nature 2005, 436, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Tsioumani, E. The state of the world’s biodiversity for food and agriculture: A call to action? Environ. Policy Law 2019, 49, 110–112. [Google Scholar] [CrossRef]
- Brown, K.H.; Wuehler, S.E. (Eds.) Zinc and Human Health. In The Results of Recent Trials and Implications for Program Interventions and Research; International Research Development Centre: Ottawa, ON, USA, 2000. [Google Scholar]
- Impa, S.M.; Johnson-Beebout, S.E. Mitigating zinc deficiency and achieving high grain zinc in rice through integration of soil chemistry and plant physiology research. Plant Soil 2012, 361, 3–14. [Google Scholar] [CrossRef]
- ACC/SCN; IFPRI. Fourth Report on the World Nutrition Situation. In Nutrition Throughout the Lifecycle; World Health Organisation: Geneva, Switzerland, 2000. [Google Scholar]
- Wairich, A.; Ricachenevsky, F.K.; Lee, S. A tale of two metals: Biofortification of rice grains with iron and zinc. Front. Plant Sci. 2022, 13, 944624. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D. The rise and fall of protein malnutrition in global health. Ann. Nutr. Metab. 2016, 69, 79–88. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.; Nösberger, J.; Ort, D.R. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef]
- Högy, P.; Fangmeier, A. Effects of elevated atmospheric CO2 on grain quality of wheat. J. Cereal Sci. 2008, 48, 580–591. [Google Scholar] [CrossRef]
- Pang, J.; Zhu, J.; Xie, Z.; Chen, G.; Liu, G.; Zhang, Y. Effects of elevated CO2 on nutrient uptake by rice and nutrient contents in rice grains. Chin. J. Rice Sci. 2005, 19, 350–354. [Google Scholar]
- Stafford, N. The other greenhouse effect. Nature 2007, 448, 526–528. [Google Scholar] [CrossRef]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef]
- Roy, P.; Ijiri, T.; Okadome, H.; Nei, D.; Orikasa, T.; Nakamura, N.; Shiina, T. Effect of processing conditions on overall energy consumption and quality of rice (Oryza sativa L.). J. Food Eng. 2008, 89, 343–348. [Google Scholar] [CrossRef]
- Perdon, A.A.; Siebenmorgen, T.J.; Mauromoustakos, A.; Griffin, V.K.; Johnson, E.R. Degree of milling effects on rice pasting properties. Cereal Chem. 2001, 78, 205–209. [Google Scholar] [CrossRef]
- Hansen, T.H.; Lombi, E.; Fitzgerald, M.; Laursen, K.H.; Frydenvang, J.; Husted, S.; Boualaphanh, C.; Resurreccion, A.; Howard, D.L.; Jonge, M.D.; et al. Losses of essential mineral nutrients by polishing of rice differ among genotypes due to contrasting grain hardness and mineral distribution. J. Cereal Sci. 2012, 56, 307–315. [Google Scholar] [CrossRef]
- Huang, S.; Benchamas, G.; Huang, G. Whole processing and use of rice polishings. Innovative Food Sci. Emerg. Technol. 2020, 63, 102373. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Anuradha, K.; Agarwa, S.; Batchu, A.K.; Prasad Babu, A.; Mallikarjuna Swamy, B.P.; Longvah, T.; Sarla, N. Evaluating rice germplasm for iron and zinc concentration in brown rice and seed dimensions. J. Phytol. 2012, 4, 19–25. [Google Scholar]
- Zhu, C.W.; Kobayashi, K.; Loladze, I.; Zhu, J.G.; Jiang, Q.; Xu, X.; Liu, G.; Seneweera, S.; Ebi, K.L.; Drewnowski, A.; et al. Carbon dioxide levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018, 4, eaaq1012. [Google Scholar] [CrossRef]
- Yoshida, S. Mineral Nutrition and Zinc. In Fundamentals of Rice Crop Science; The International Rice Research Institute: Laguna, The Philippines, 1981; 269p. [Google Scholar]
- Das, S.; Green, A. Importance of zinc in crops and human health. J. SAT Agric. Res. 2013, 11, 1–7. [Google Scholar]
- FAO. The State of Food Security and Nutrition in the World. In Transforming Food Systems for Food Security and Improved Nutrition and Affordable Healthy Diets for All; Food and Agriculture Organisation: Rome, Italy, 2021. [Google Scholar]
- Chen, G.; Wang, R.; Alfread, Q.; Yang, X. Physiological characterization of Zn efficient rice (Oryza sativa) genotypes at different Zn2+ levels. Rice Sci. 2003, 11, 47–51. [Google Scholar]
- Nakandalage, N.; Nicolas, M.; Norton, R.M.; Hirotsu, N.; Milham, P.J.; Seneweera, S. Improving rice zinc biofortification success rates through genetic and crop management approaches in a changing environment. Front. Plant Sci. 2016, 7, 00764. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Zhang, P.P.; Chen, Y.L.; Wang, C.Y.; Ma, G.; Lü, J.J.; Liu, J.B.; Guo, T.C. Distribution and accumulation of zinc and nitrogen in wheat grain pearling fractions in response to foliar zinc and soil nitrogen applications. J. Integr. Agric. 2021, 20, 2–13. [Google Scholar] [CrossRef]
- Reuter, D.J.; Robinson, J.B. Plant Analysis an Interpretation Manual; CSIRO Publishing: Collingwood, Australia, 1997. [Google Scholar]
- Bryson, G.M.; Mills, H.A. Plant Analysis Handbook IV; Macro-Micro Publishing Inc.: Athens, GA, USA, 2014. [Google Scholar]
- Defiani, M.R.; Conroy, J.P.; Milham, P.J. Dry mass and Zn nutrition of rice (Oryza sativa cvv. IR8 and Jarrah) grown at 36 and 70 Pa CO2 in hydroponics with a range of Zn supplies. J. Biol. 2003, 7, 6–10. [Google Scholar]
- Neue, H.U.; Quijano, C.; Senadhira, D.; Setter, T. Strategies for dealing with micronutrient disorders and salinity in lowland rice systems. Field Crops Res. 1998, 56, 139–155. [Google Scholar] [CrossRef]
- Dobermann, A.; Fairhurst, T.H. Rice: Nutritional Disorders and Nutrient Management; Phosphate Institute of Canada and the International Rice Research Institute: Laguna, The Philippines, 2000. [Google Scholar]
- Begum, M.C.; Islam, M.; Sarkar, M.R.; Azad, M.A.S.; Huda, A.N.; Kabir, A.H. Auxin signaling is closely associated with Zn efficiency in rice (Oryza sativa L.). J. Plant Interact. 2016, 11, 124–129. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: A review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Jiang, W.; Struik, P.C.; Lingna, J.; Van Keulen, H.; Ming, Z.; Stomph, T.J. Uptake and distribution of root-applied or foliar-applied 65Zn after flowering in aerobic rice. Ann. Appl. Biol. 2007, 150, 383–391. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Bahuguna, R.N.; Djanaguiraman, M.; Gamuyao, R.; Prasad, P.V.V.; Craufurd, P.Q. Implications of high temperature and elevated CO2 on flowering time in plants. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Waha, K.; Dietrich, J.P.; Portmann, F.T.; Siebert, S.; Thornton, P.K.; Bondeau, A.; Herrero, M. Multiple cropping systems of the world and the potential for increasing cropping intensity. Glob. Environ. Chang. 2020, 64, 102131. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.X.; Feng, X.M.; Hu, X.Y.; Tian, G.L.; Ling, N.; Wang, J.H.; Shen, Q.R.; Guo, S.W. Effects of soil zinc availability, nitrogen fertilizer rate and zinc fertilizer application method on zinc biofortification of rice. J. Agric. Sci. 2016, 154, 584–597. [Google Scholar] [CrossRef]
- Bouis, H.E.; Welch, R.M. Biofortification—A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010, 50, S20–S32. [Google Scholar] [CrossRef]
- Zaman, Q.U.; Aslam, Z.; Yaseen, M.; Ihsan, M.Z.; Khaliq, A.; Fahad, S.; Bashir, S.; Ramzani, P.M.A.; Naeem, M. Zinc biofortification in rice: Leveraging agriculture to moderate hidden hunger in developing countries. Arch. Agron. Soil Sci. 2018, 64, 147–161. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Seneweera, S. Effects of elevated CO2 on plant growth and nutrient partitioning of rice (Oryza sativa L.) at rapid tillering and physiological maturity. J. Plant Interactions 2011, 6, 35–42. [Google Scholar] [CrossRef]
- Nishiyama, R.; Kato, M.; Nagata, S.; Yanagisawa, S.; Yoneyama, T. Identification of Zn–nicotianamine and Fe–2′-deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L.). Plant Cell Physiol. 2012, 53, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Kitagishi, K.; Obata, H. Effects of zinc deficiency on the nitrogen metabolism of meristematic tissues of rice plants with reference to protein synthesis. Soil Sci. Plant Nutr. 1986, 32, 397–405. [Google Scholar] [CrossRef]
- Mae, T.; Ohira, K. The remobilization of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.). Plant Cell Physiol. 1981, 22, 1067–1074. [Google Scholar]
- Impa, S.M.; Morete, M.J.; Ismail, A.M.; Schulin, R.; Johnson-Beebout, S.E. Zn uptake, translocation and grain Zn loading in rice (Oryza sativa L.) genotypes selected for Zn deficiency tolerance and high grain Zn. J. Exp. Bot. 2013, 64, 2739–2751. [Google Scholar] [CrossRef]
- OECD. The OECD Environmental Outlook to 2050. The Consequences of Inaction. In Key Findings on Health and the Environment; Organisation for Economic Co-Operation and Development: Paris, France, 2012. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakandalage, N.; Milham, P.J.; Holford, P.; Seneweera, S. Luxury Zinc Supply Prevents the Depression of Grain Nitrogen Concentrations in Rice (Oryza sativa L.) Typically Induced by Elevated CO2. Plants 2023, 12, 839. https://doi.org/10.3390/plants12040839
Nakandalage N, Milham PJ, Holford P, Seneweera S. Luxury Zinc Supply Prevents the Depression of Grain Nitrogen Concentrations in Rice (Oryza sativa L.) Typically Induced by Elevated CO2. Plants. 2023; 12(4):839. https://doi.org/10.3390/plants12040839
Chicago/Turabian StyleNakandalage, Niluka, Paul James Milham, Paul Holford, and Saman Seneweera. 2023. "Luxury Zinc Supply Prevents the Depression of Grain Nitrogen Concentrations in Rice (Oryza sativa L.) Typically Induced by Elevated CO2" Plants 12, no. 4: 839. https://doi.org/10.3390/plants12040839
APA StyleNakandalage, N., Milham, P. J., Holford, P., & Seneweera, S. (2023). Luxury Zinc Supply Prevents the Depression of Grain Nitrogen Concentrations in Rice (Oryza sativa L.) Typically Induced by Elevated CO2. Plants, 12(4), 839. https://doi.org/10.3390/plants12040839






