The Content of Phenolic Compounds in Stevia rebaudiana (Bertoni) Plants Derived from Melatonin and NaCl Treated Seeds
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Acids and Flavonoids Identified in Stevia Leaves
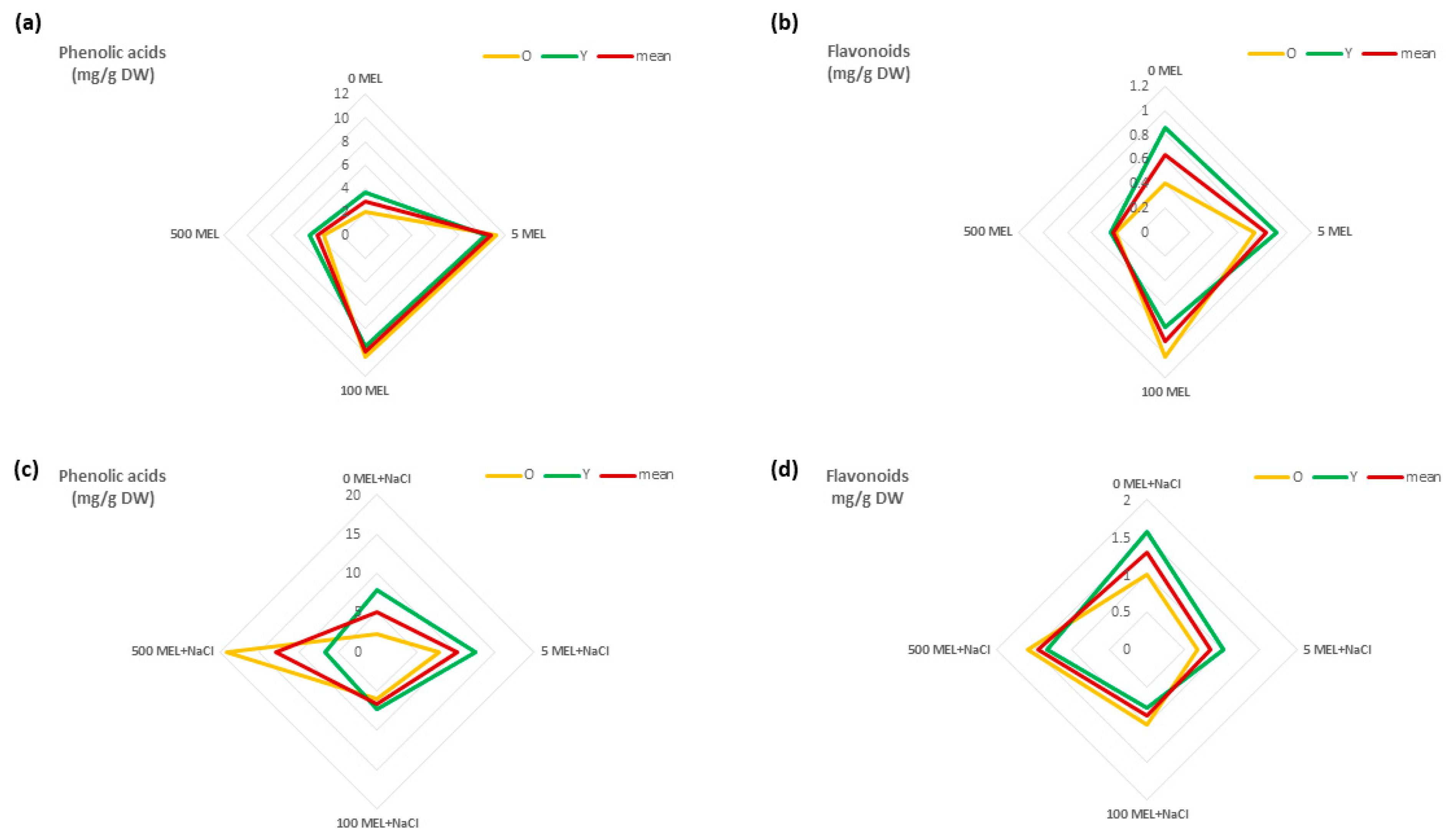
2.2. The Effect of Melatonin on Phenolic Acid and Flavonoid Content
2.3. The Effect of Melatonin on Phenolic Acid and Flavonoid Content under NaCl
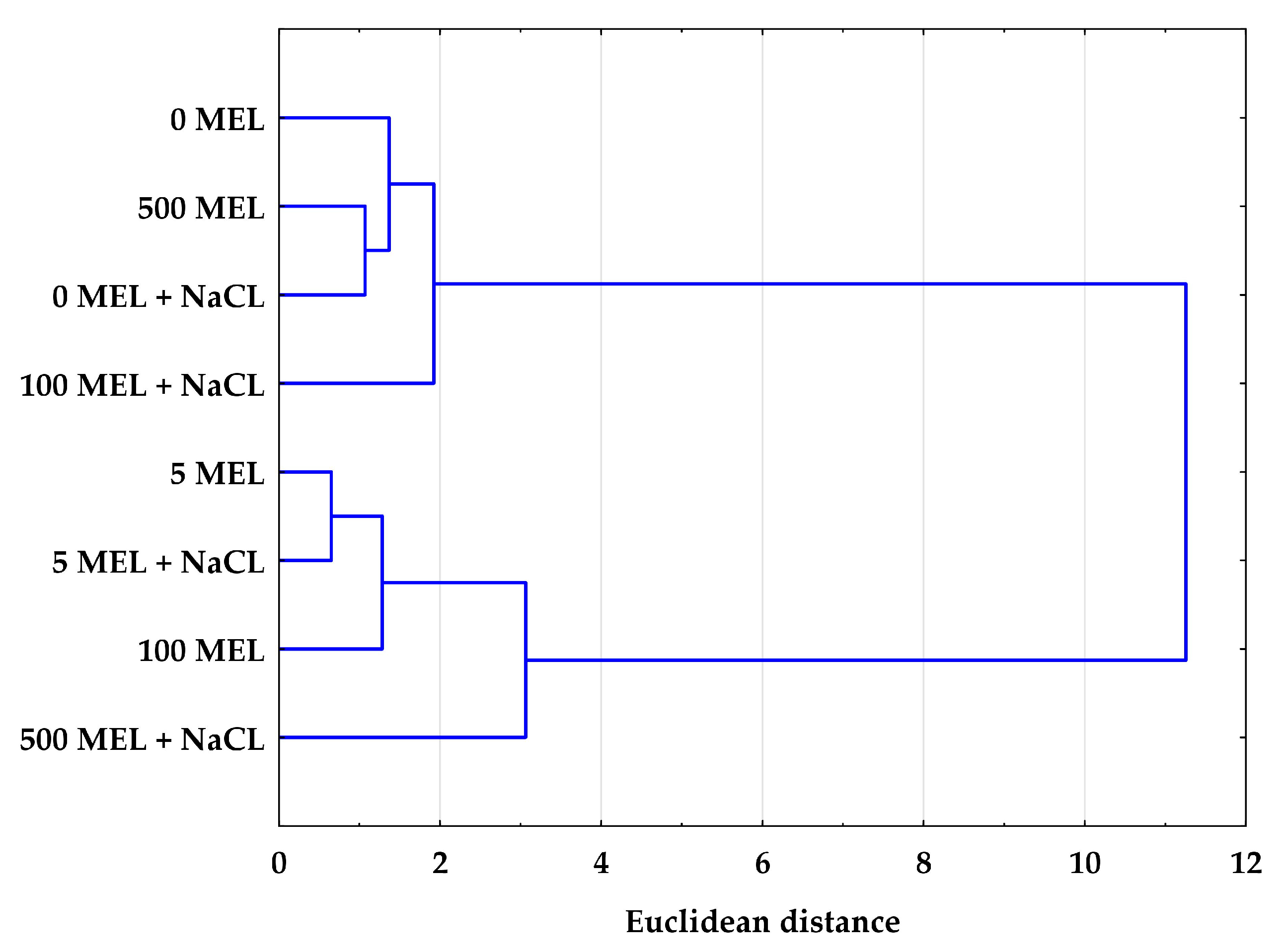
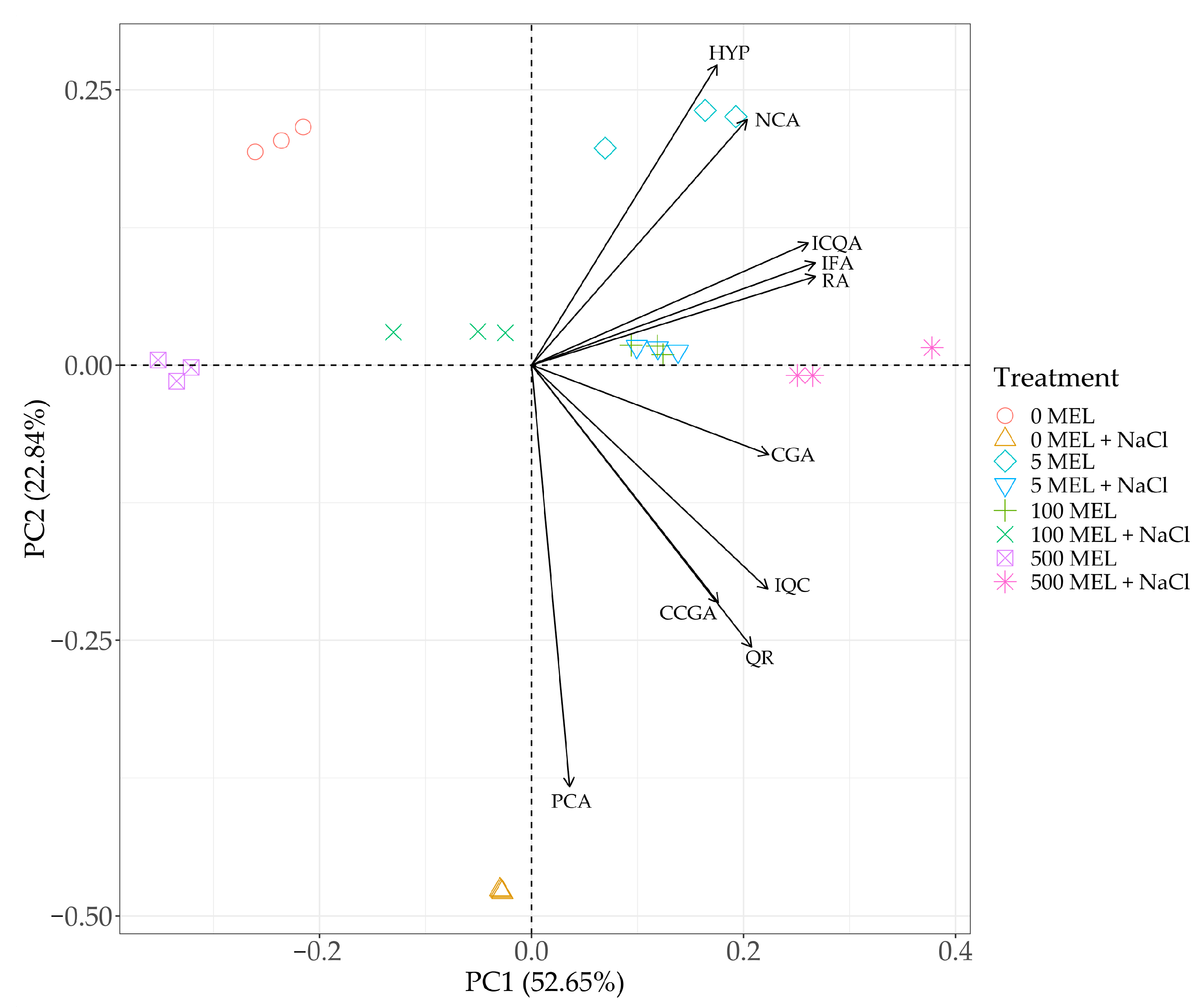
2.4. The Effect of Melatonin and NaCl on the Accumulation of Phenolic Compounds
3. Materials and Methods
3.1. Plant Material
3.2. RP-HPLC Analysis
3.3. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soejarto, D.D. Botany of Stevia and Stevia rebaudiana. In Stevia: The Genus Stevia; Kinghorn, A.D., Ed.; Taylor and Francis: London, UK, 2001; Volume 2, pp. 18–39. [Google Scholar]
- Brandle, J.E.; Telmer, P.G. Steviol glycoside biosynthesis. Phytochemistry 2007, 68, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Sasa, S.; Inoue, A.; Tamai, T.; Fujita, I.; Morita, K.; Matsuura, F. Characterization of novel steviol glycosides from leaves of Stevia rebaudiana Morita. J. Appl. Glycosci. 2010, 57, 199–209. [Google Scholar]
- Chaturvedula, V.S.; Prakash, I. Structures of the novel diterpene glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 346, 1057–1060. [Google Scholar]
- Chaturvedula, V.S.; Prakash, I. Additional minor diterpene glycosides from Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 1059–1062. [Google Scholar]
- Chaturvedula, V.S.; Rhea, J.; Milanowski, D.; Mocek, U.; Prakash, I. Two minor diterpene glycosides from the leaves of Stevia rebaudiana. Nat. Prod. Commun. 2011, 6, 175–178. [Google Scholar] [PubMed]
- Ceunen, S.; Geuns, J.M.C. Steviol glycosides: Chemical diversity, metabolism, and function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar]
- Angelini, L.G.; Martini, A.; Passera, B.; Tavarini, S. Cultivation of Stevia rebaudiana Bertoni and associated challenges. In Sweeteners. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–52. [Google Scholar]
- Atteh, J.; Onagbesan, O.; Tona, K.; Buyse, J.; Decuypere, E.; Geuns, J. Potential use of Stevia rebaudiana in animal feeds. Arch. Zootec. 2011, 60, 133–136. [Google Scholar] [CrossRef]
- Goyal, S.K.; Samsher; Goyal, R.K. Stevia (Stevia rebaudiana) a bio-sweetener: A review. Int. J. Food Sci. Nutr. 2010, 61, 1–10. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Revised exposure assessment for steviol glycosides for the proposed uses as a food additive. EFSA J. 2011, 9, 1972. [Google Scholar]
- Jeong, Y.; Lee, H.J.; Jin, G.H.; Park, Y.D.; Choi, D.S.; Kang, M.A. Anti-inflammatory activity of Stevia rebaudiana in LPS-induced RAW 264.7 cells. J. Food Sci. Nutr. 2010, 15, 14–18. [Google Scholar] [CrossRef]
- Preethi, D.; Sridhar, T.M.; Josthna, P.; Naidu, C.V. Studies on antibacterial activity, phytochemical analysis of Stevia rebaudiana (Bert.). An important calorie free biosweetner. J. Ecobiotech. 2011, 3, 5–10. [Google Scholar]
- Arya, A.; Kumar, S.; Kasana, M.S. Anti-inflammatory activity of in vitro regenerated calli and in vivo plant of Stevia rebaudiana (Bert.) Bertoni. J. Sci. Ind. Res. 2012, 2, 435–439. [Google Scholar]
- Gamboa, F.; Chaves, M. Antimicrobial potential of ex-tracts from Stevia rebaudiana leaves against bacteria of importance in dental caries. Acta Odontol. Latinoam. 2012, 25, 171–175. [Google Scholar]
- Kedik, S.A.; Yartsev, E.I.; Stanishevskaya, I.E. Antiviral activity of dried extract of Stevia. Pharm. Chem. J. 2009, 43, 198–199. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Kozuka, M.; Tokuda, H.; Takayasu, J.; Nishino, H.; Miyakoshi, M.; Mizutani, K.; Lee, K.-H. Cancer preventive agents. Part 8: Chemopreventive effects of stevioside and related compounds. Bioorg. Med. Chem. 2009, 17, 600–605. [Google Scholar] [PubMed]
- Chen, J.; Xia, Y.; Sui, X.; Peng, Q.; Zhang, T.; Li, J.; Zhang, J. Steviol, a natural product inhibits proliferation of the gastrointestinal cancer cells intensively. Oncotarget 2018, 9, 26299–26308. [Google Scholar] [CrossRef]
- Ghanta, S.; Banerjee, A.; Poddar, A.; Chattopadhyay, S. Oxidative DNA damage preventive activity and anti-oxidant potential of Stevia rebaudiana (Bertoni) Bertoni, a natural sweetener. J. Agric. Food Chem. 2007, 55, 10962–10967. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A.; Mehta, P.; Bajpai, V.K. Antioxidant ability and total phenolic content of aqueous leaf extract of Stevia rebaudiana Bert. Exp. Toxicol. Pathol. 2012, 64, 807–811. [Google Scholar] [CrossRef]
- Muandam, F.; Soulimani, R.; Diop, B.; Dicko, A. Study on chemical composition and biological activities of essential oil and extracts from Stevia rebaudiana Bertoni leaves. LWT-Food Sci. Technol. 2011, 44, 1865–1872. [Google Scholar] [CrossRef]
- Karaköse, H.; Jaiswal, R.; Kuhnert, N. Characterization and quantification of hydroxycinnamate derivatives in Stevia rebaudiana leaves by LC-MSn. J. Agric. Food Chem. 2011, 59, 10143–10150. [Google Scholar] [CrossRef]
- Kim, I.S.; Yang, M.; Lee, O.H.; Kang, S.N. The antioxidant activity and the bioactive compound content of Stevia rebaudiana water extracts LWT. Food Sci. Technol. 2011, 44, 1328–1332. [Google Scholar]
- Lemus-Mondaca, R.; Vega-Galvez, A.; Rojas, P.; Stucken, K.; Delporte, C.; Valenzuela-Barra, G.; Jagus, J.J.; Agüero, M.V.; Pasten, A. Antioxidant, antimicrobial and anti-inflammatory potential of Stevia rebaudiana leaves: Effect of different drying methods. J. Appl. Res. Med. Aromat. Plants 2018, 11, 37–46. [Google Scholar] [CrossRef]
- Madan, S.; Ahmad, S.; Singh, G.N.; Kohli, K.; Kumar, Y.; Singh, R.; Garg, M. Stevia rebaudiana (Bert.) Bertoni—A review. Indian J. Nat. Prod. Resour. 2010, 1, 267–286. [Google Scholar]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Dal Poggetto, F.; Catauro, M. New insights into phenol and polyphenol composition of Stevia rebaudiana leaves. J. Pharm. Biomed. Anal. 2019, 163, 45–57. [Google Scholar] [PubMed]
- Wolwer-Rieck, U. The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: A review. J. Agric. Food Chem. 2012, 60, 886–895. [Google Scholar] [PubMed]
- Yu, H.; Yang, G.Q.; Sato, M.; Yamaguchi, T.; Nakano, T.; Xi, Y.C. Antioxidant activities of aqueous extract from Stevia rebaudiana stem waste to inhibit fish oil oxidation and identification of its phenolic compounds. Food Chem. 2017, 232, 379–386. [Google Scholar]
- Zhang, Q.N.; Yang, H.; Li, Y.N.; Liu, H.B.; Jia, X.D. Toxicological evaluation of ethanolic extract from Stevia rebaudiana Bertoni leaves: Genotoxicity and subchronic oral toxicity. Regul. Toxicol. Pharmacol. 2017, 86, 253–259. [Google Scholar]
- Amriteswori, R.; Margaret, F.R. The flavonoids of Stevia rebaudiana. J. Nat. Prod. 1983, 46, 194–195. [Google Scholar]
- Myint, K.Z.; Wu, K.; Xia, Y.; Fan, Y.; Shen, J.; Zhang, P.; Gu, J. Polyphenols from Stevia rebaudiana (Bertoni) leaves and their functional properties. J. Food Sci. 2020, 85, 240–248. [Google Scholar]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Posmyk, M.; Bałabusta, M.; Wieczorek, M.; Śliwińska, E.; Janas, K.M. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Tiryaki, I.; Keles, H. Reversal of the inhibitory effect of light and high temperature on germination of Phacelia tanacetifolia seeds by melatonin. J. Pineal Res. 2012, 52, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin: A growth-stimulating compound present in lupin tissues. Planta 2004, 220, 140–144. [Google Scholar]
- Hernandez-Ruiz, J.; Cano, A.; Arnao, M.B. Melatonin acts as a growth-stimulating compound in some monocot species. J. Pineal Res. 2005, 39, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ruiz, J.; Arnao, M.B. Melatonin stimulates the expansion of etiolated lupin cotyledons. Plant Growth Regul. 2008, 55, 29–34. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.-J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Park, S.; Back, K. Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. J. Pineal Res. 2013, 53, 385–389. [Google Scholar] [CrossRef]
- Sarrou, E.; Therios, I.; Dimassi-Theriou, K. Melatonin and other factors that promote rooting and sprouting of shoot cuttings in Punica granatum cv. Wonderful. Turk. J. Botany 2014, 38, 293–301. [Google Scholar]
- Chen, Q.; Qi, W.B.; Reiter, R.J.; Wei, W.; Wang, B.M. Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J. Plant Physiol. 2009, 166, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Simlat, M.; Ptak, A.; Skrzypek, E.; Warchoł, M.; Morańska, E.; Piórkowska, E. Melatonin significantly influences seed germination and seedling growth of Stevia rebaudiana Bertoni. PeerJ 2018, 6, e5009. [Google Scholar] [CrossRef] [PubMed]
- Simlat, M.; Szewczyk, A.; Ptak, A. Melatonin promotes seed germination under salinity and enhances the biosynthesis of steviol glycosides in Stevia rebaudiana Bertoni leaves. PLoS ONE 2020, 15, e0230755. [Google Scholar]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar]
- Bałabusta, M.; Szafrańska, K.; Posmyk, M.M. Exogenous melatonin improves antioxidant defense in cucumber seeds (Cucumis sativus L.) germinated under chilling stress. Front. Plant Sci. 2016, 7, 575. [Google Scholar]
- Wang, L.Y.; Liu, J.L.; Wang, W.X.; Sun, Y. Exogenous melatonin improves growth and photosynthetic capacity of cucumber under salinity-induced stress. Photosynthetica 2016, 54, 19–27. [Google Scholar] [CrossRef]
- Wang, Y.; Reiter, R.J.; Chan, Z. Phytomelatonin: A universal abiotic stress regulator. J. Exp. Bot. 2018, 69, 963–974. [Google Scholar] [CrossRef]
- Coskun, Y.; Duran, R.E.; Kilic, S. Striking effects of melatonin on secondary metabolites produced by callus culture of rosemary (Rosmarinus officinalis L.). Plant Cell Tiss. Organ Cult. 2019, 138, 89–95. [Google Scholar] [CrossRef]
- Ptak, A.; Simlat, M.; Morańska, E.; Skrzypek, E.; Warchoł, M.; Tarakemeh, A.; Laurain-Mattar, D. Exogenous melatonin stimulated Amaryllidaceae alkaloid biosynthesis in in vitro cultures of Leucojum aestivum L. Ind. Crops Prod. 2019, 138, 111458. [Google Scholar] [CrossRef]
- Rajbhandari, A.; Roberts, M.F. Flavonoids of Stevia nepetifolia. J. Nat. Prod. 1984, 47, 559–560. [Google Scholar] [CrossRef]
- Marchyshyn, S.; Hudz, N.A.; Dakhym, I.; Husak, L.V.; Demydyak, O.L. HPLC analysis of phenolic compounds from Stevia rabaudiana Bertoni leaves. Pharma Innov. 2018, 7, 515–517. [Google Scholar]
- Carrera-Lanestosa, A.; Coral-Martínez, T.; Ruíz-Ciau, D.; Moguel-Ordoñez, Y.; Rubí Segura-Campos, M. Phenolic compounds and major steviol glucosides by HPLC-DAD-RP and in vitro evaluation of the biological activity of aqueous and ethanolic extracts of leaves and stems: S. rebaudiana Bertoni (creole variety INIFAP C01). Int. J. Food Prop. 2020, 23, 199–212. [Google Scholar] [CrossRef]
- Khiraoui, A.; Al Faiz, C.; Hasib, A.; Bakha, M.; Benhmimou, A.; Amchra, F.Z.; Boulli, A. Antioxidant ability, total phenolic and flavonoid contents of leaf extract of Stevia rebaudiana Bertoni cultivated in Morocco. Int. J. Sci. Eng. Res. 2018, 9, 1585–1590. [Google Scholar]
- Dos Santos Szewczyk, K.; Pietrzak, W.; Klimek, K.; Grzywa-Celińska, A.; Celiński, R.; Gogacz, M. LC-ESI-MS/MS identification of biologically active phenolics in different extracts of Alchemilla acutiloba Opiz. Molecules 2022, 27, 621. [Google Scholar]
- Yin, Y.; Tian, X.; He, X.; Yang, J.; Yang, Z.; Fang, W. Exogenous melatonin stimulated isoflavone biosynthesis in NaCl-stressed germinating soybean (Glycine max L.). Plant Physiol. Biochem. 2022, 185, 123–131. [Google Scholar]
- Xu, L.; Yue, Q.; Bian, F.; Sun, H.; Zhai, H.; Yao, Y. Melatonin enhances phenolics accumulation partially via ethylene signaling and resulted in high antioxidant capacity in grape berries. Front. Plant Sci. 2017, 18, 1426. [Google Scholar]
- Gaweł-Bęben, K.; Bujak, T.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karaś, M.; Rybczyńska, K. Stevia rebaudiana Bert. leaf extracts as a multifunctional source of natural antioxidants. Molecules 2015, 20, 468–486. [Google Scholar]
- Liu, Z.; Bruins, M.E.; de Bruijn, W.J.C.; Vincken, J.-P. A comparison of the phenolic composition of old and young tea leaves reveals a decrease in flavanols and phenolic acids and an increase in flavonols upon tea leaf maturation. J. Food Compos. Anal. 2020, 86, 103385. [Google Scholar]
- Padda, M.; Picha, D.H. Antioxidant activity and phenolic composition in ‘Beauregard’ sweetpotato are affected by root size and leaf age. J. Am. Soc. Hortic. Sci. 2007, 132, 447–451. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-term exogenous application of melatonin improves nutrient uptake fluxes in apple plants under moderate drought stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar]
- Flowers, T.J. Improving crop salt tolerance. Am. J. Bot. 2004, 55, 307–319. [Google Scholar]
- Mubarak, M.H.; Belal, A.H.; El-Dein, T.N.; El-Sarag, E.I. In vitro response growth Stevia rebaudiana to salinity and drought. Sinai J. Appl. Sci. 2012, 1, 13–20. [Google Scholar] [CrossRef]
- Zeng, J.; Cheng, A.; Lim, D.; Yi, B.; Wu, W. Effects of salt stress on the growth, physiological responses, and glycoside contents of Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2013, 61, 5720–5726. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Chikara, S.K. In vitro regeneration and effect of abiotic stress on physiology and biochemical content of Stevia rebaudiana ‘Bertoni’. J. Plant Sci. Res. 2014, 1, 113. [Google Scholar]
- Shahverdi, M.A.; Omidi, H.; Tabatabaei, S.J. Stevia (Stevia rebaudiana Bertoni) responses to NaCl stress: Growth, photosynthetic pigments, diterpene glycosides and ion content in root and shoot. J. Saudi Soc. Agric. Sci. 2019, 18, 355–360. [Google Scholar]
- Gerami, M.; Majidian, P.; Ghorbanpour, A.; Alipour, Z. Stevia rebaudiana Bertoni responses to salt stress and chitosan elicitor. Physiol. Mol. Biol. Plants 2020, 26, 965–974. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, W.; Tian, R.; Li, C.; Ji, Y.; Li, T.; Wei, C.; Chen, Z. Morphological, physiological, and secondary metabolic responses of Taraxacum officinale to salt stress. Plant Physiol. Biochem. 2022, 189, 71–82. [Google Scholar] [CrossRef]
- Wei, Z.; Li, C.; Gao, T.; Zhang, Z.; Liang, B.; Lv, Z.; Zou, Y.; Ma, F. Melatonin increases the performance of Malus hupehensis after UV-B exposure. Plant Physiol. Biochem. 2019, 139, 630–641. [Google Scholar]
- Bistgani, Z.E.; Hashemi, M.; DaCosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Nazir, M.; Asad Ullah, M.; Mumtaz, S.; Siddiquah, A.; Shah, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Interactive effect of melatonin and UV-C on phenylpropanoid metabolite production and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var.s purpurascens). Molecules 2020, 25, 1072. [Google Scholar] [PubMed]
- Wei, L.; Liu, C.; Wang, J.; Younas, S.; Zheng, H.; Zheng, L. Melatonin immersion affects the quality of fresh-cut broccoli (Brassica oleracea L.) during cold storage: Focus on the antioxidant system. J. Food Process. Preserv. 2020, 44, e14691. [Google Scholar]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Ghasemi-Omran, V.O.; Ghorbani, A.; Sajjadi-Otaghsara, S.A. Melatonin alleviates NaCl-induced damage by regulating ionic homeostasis, antioxidant system, redox homeostasis, and expression of steviol glycosides-related biosynthetic genes in in vitro cultured Stevia rebaudiana Bertoni. In Vitro Cell. Dev. Biol.-Plant 2021, 57, 319–331. [Google Scholar] [CrossRef]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant Physiol. 1962, 15, 473–497. [Google Scholar]
- Ellnain-Wojtaszek, M.; Zgórka, G. High-performance liquid chromatography and thin-layer chromatography of phenolic acids from Ginkgo biloba L. leaves collected within vegetative period. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 1457–1471. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team R: Vienna, Austria, 2019; Available online: https://www.R-project.org (accessed on 2 November 2022).
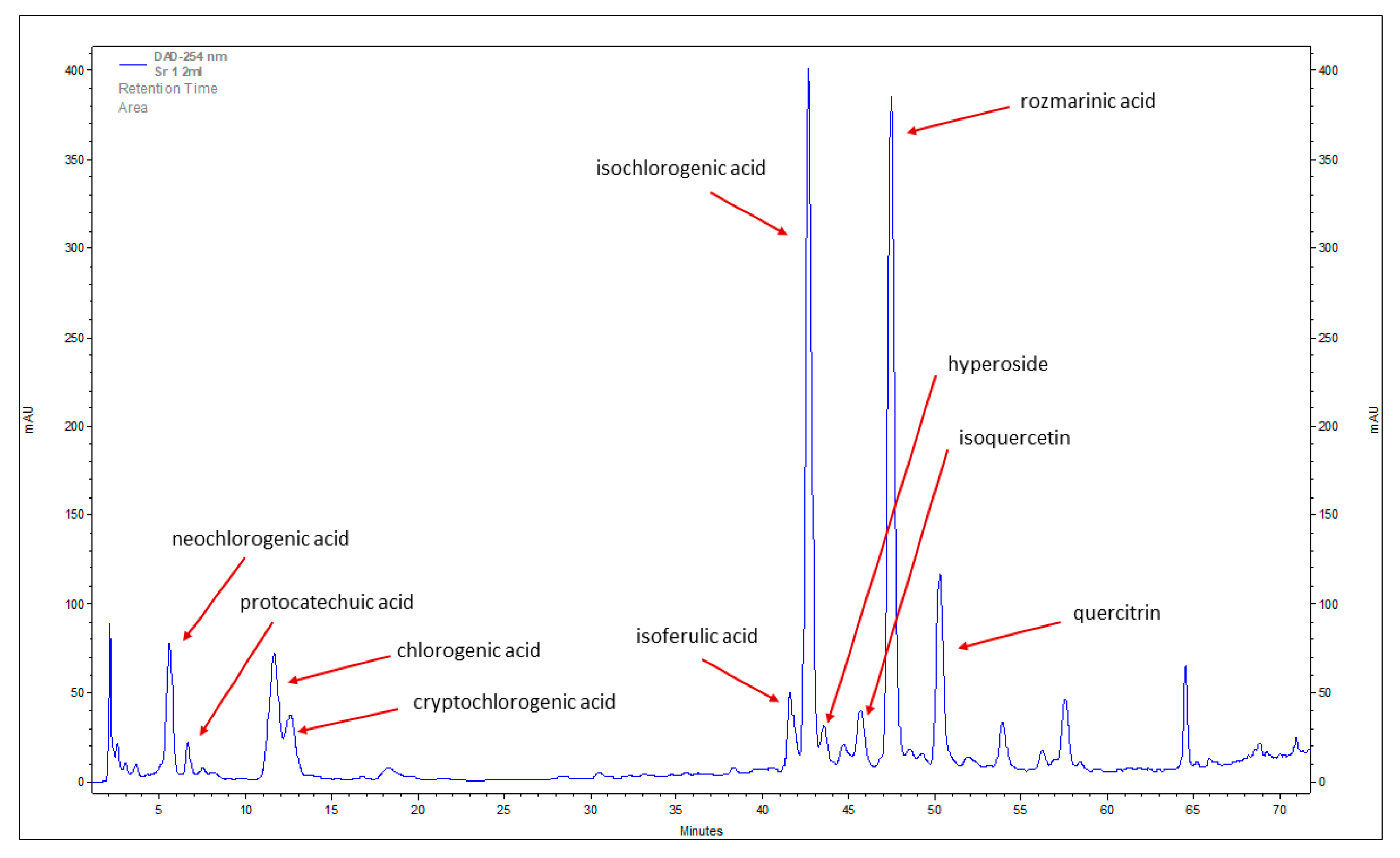
| Compound | Retention Time (tr min) | Formula | Molecular Mass (Rr) |
|---|---|---|---|
| neochlorogenic acid | 5.88 | C16H18O9 | 354.31 |
| protocatechuic acid | 6.98 | C7H6O4 | 154.12 |
| chlorogenic acid | 11.12 | C16H18O9 | 354.31 |
| cryptochlorogenic acid | 12.63 | C16H18O9 | 354.31 |
| isoferulic acid | 39.32 | C10H10O4 | 194.18 |
| isochlorogenic acid | 42.33 | C16H18O9 | 354.31 |
| hyperoside | 43.94 | C21H20O12 | 464.38 |
| isoquercetin | 45.43 | C21H20O12 | 464.38 |
| rosmarinic acid | 47.16 | C18H16O8 | 360.31 |
| quercitrin | 49.9 | C21H20O11 | 448.38 |
| Compound | Source | df | MS | F–Value | MS | F–Value |
|---|---|---|---|---|---|---|
| –NaCL | +NaCl | |||||
| neochlorogenic acid | MEL concentration (M) | 3 | 0.277576 | 223.510 *** | 0.040497 | 45.423 *** |
| Type of leaves (T) | 1 | 0.015395 | 12.397 ** | 0.017980 | 20.167 *** | |
| M × T | 3 | 0.009742 | 7.844 ** | 0.101441 | 113.781 *** | |
| Error | 16 | 0.001242 | 0.000892 | |||
| protocatechuic acid | M | 3 | 0.001284 | 162.482 *** | 0.006266 | 1140.207 *** |
| T | 1 | 0.000442 | 55.952 *** | 0.000432 | 78.547 *** | |
| M × T | 3 | 0.000066 | 8.398 ** | 0.000087 | 15.741 *** | |
| Error | 16 | 0.000008 | 0.000005 | |||
| chlorogenic acid | MEL concentration (M) | 3 | 0.583775 | 181.817 *** | 0.20679 | 33.541 *** |
| Type of leaves (T) | 1 | 0.779998 | 242.931 *** | 4.09826 | 664.724 *** | |
| M × T | 3 | 0.049906 | 15.543 *** | 0.54243 | 87.981 *** | |
| Error | 16 | 0.003211 | 0.00617 | |||
| cryptochlorogenic acid | MEL concentration (M) | 3 | 0.112743 | 364.286 * | 0.040865 | 77.858 *** |
| Type of leaves (T) | 1 | 0.000318 | 1.029 ns | 0.016176 | 30.819 *** | |
| M × T | 3 | 0.000863 | 2.789 ns | 0.051125 | 97.408 *** | |
| Error | 16 | 0.000309 | 0.000525 | |||
| isoferulic acid | MEL concentration (M) | 3 | 18.7137 | 323.605 *** | 0.079112 | 116.3412 *** |
| Type of leaves (T) | 1 | 4.9781 | 86.083 *** | 0.087028 | 127.9835 *** | |
| M × T | 3 | 0.1069 | 1.849 *** | 0.092553 | 136.1074 *** | |
| Error | 16 | 0.0578 | 0.000680 | |||
| isochlorogenic acid | MEL concentration (M) | 3 | 0.060263 | 216.828 *** | 15.7731 | 113.472 *** |
| Type of leaves (T) | 1 | 0.033797 | 121.603 *** | 3.4197 | 24.601 *** | |
| M × T | 3 | 0.009105 | 32.762 ns | 19.6256 | 141.186 *** | |
| Error | 16 | 0.000278 | 0.1390 | |||
| rosmarinic acid | MEL concentration (M) | 3 | 15.7940 | 292.521 *** | 20.4823 | 104.381 *** |
| Type of leaves (T) | 1 | 5.5199 | 102.234 *** | 18.9816 | 96.732 *** | |
| M × T | 3 | 1.6759 | 31.040 *** | 21.1283 | 107.672 *** | |
| Error | 16 | 0.0540 | 0.1962 | |||
| hyperoside | MEL concentration (M) | 3 | 0.002441 | 77.082 *** | 0.001401 | 31.794 *** |
| Type of leaves (T) | 1 | 0.000001 | 0.025 ns | 0.002616 | 59.368 *** | |
| M × T | 3 | 0.000842 | 26.589 *** | 0.002523 | 57.248 *** | |
| Error | 16 | 0.000032 | 0.000044 | |||
| isoquercetin | MEL concentration (M) | 3 | 0.005569 | 286.379 *** | 0.030799 | 243.874 *** |
| Type of leaves (T) | 1 | 0.008554 | 439.882 *** | 0.020344 | 161.088 *** | |
| M × T | 3 | 0.000727 | 37.401 *** | 0.002065 | 16.355 *** | |
| Error | 16 | 0.000019 | 0.000126 | |||
| quercitrin | MEL concentration (M) | 3 | 0.175491 | 199.646 *** | 0.32960 | 81.646 *** |
| Type of leaves (T) | 1 | 0.028423 | 32.335 *** | 0.03266 | 8.089 * | |
| M × T | 3 | 0.113650 | 129.292 *** | 0.19715 | 48.838 *** | |
| Error | 16 | 0.000879 | 0.00404 | |||
| MEL Concentration | Type of Leaves | Phenolic Acids | Flavonoids | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCA | PCA | GCA | CCGA | ICQA | IFA | RA | HYP | IQC | QR | ||
| −NaCl | |||||||||||
| 0 MEL | O | 0.209 c ± 0.05 | 0.018 e ± 0.00 | 0.238 f ± 0.06 | 0.128 c ± 0.03 | 0.716 f ± 0.15 | 0.037 de ± 0.01 | 0.664 f ± 0.17 | 0.059 d ± 0.01 | 0.031 f ± 0.01 | 0.313 e ± 0.04 |
| Y | 0.367 b ± 0.01 | 0.018 e ± 0.00 | 0.640 cd ± 0.01 | 0.138 c ± 0.00 | 1.694 e ± 0.04 | 0.035 e ± 0.00 | 0.770 ef ± 0.01 | 0.094 a ± 0.00 | 0.086 c ± 0.00 | 0.685 c ± 0.03 | |
| mean | 0.288 C | 0.018 C | 0.440 B | 0.133C | 1.205 D | 0.306 B | 0.717 B | 0.076 A | 0.059 B | 0.499 BC | |
| 5 MEL | O | 0.609 a ± 0.08 | 0.043 bc ± 0.00 | 0.628 d ± 0.09 | 0.274 b ± 0.03 | 4.722 b ± 0.52 | 0.307 a ± 0.04 | 4.555 a ± 0.58 | 0.082 b ± 0.01 | 0.065 d ± 0.01 | 0.588 d ± 0.04 |
| Y | 0.581 a ± 0.04 | 0.033 d ± 0.00 | 1.229 a ± 0.11 | 0.247 b ± 0.02 | 5.542 a ± 0.34 | 0.139 c ± 0.01 | 2.481 c ± 0.02 | 0.067 cd ± 0.00 | 0.118 b ± 0.01 | 0.738 b ± 0.03 | |
| mean | 0.595 A | 0.038 B | 0.929 A | 0.261 B | 5.132 A | 0.223 A | 3.518 A | 0.075 A | 0.092 A | 0.663 AB | |
| 100 MEL | O | 0.337 b ± 0.00 | 0.055 a ± 0.00 | 0.738 c ± 0.02 | 0.368 a ± 0.01 | 3.799 c ± 0.01 | 0.271 b ± 0.01 | 4.819 a ± 0.03 | 0.071 c ± 0.00 | 0.092 c ± 0.00 | 0.868 c ± 0.02 |
| Y | 0.397 b ± 0.01 | 0.045 b ± 0.00 | 0.939 b ± 0.02 | 0.396 a ± 0.01 | 4.404 b ± 0.10 | 0.160 c ± 0.01 | 3.188 b ± 0.10 | 0.068 cd ± 0.00 | 0.129 a ± 0.00 | 0.578 a± 0.01 | |
| mean | 0.367 B | 0.050 A | 0.838 A | 0.382A | 4.101 B | 0.215 A | 4.003 A | 0.070 A | 0.110 A | 0.723 A | |
| 500 MEL | O | 0.068 d ± 0.01 | 0.055 a ± 0.01 | 0.160 f ± 0.02 | 0.066 d ± 0.00 | 1.668 e ± 0.18 | 0.066 d ± 0.01 | 1.402 d ± 0.10 | 0.041 e ± 0.00 | 0.040 e ± 0.01 | 0.322 e ± 0.03 |
| Y | 0.081d ± 0.00 | 0.040 c ± 0.00 | 0.398 e ± 0.03 | 0.083 d ± 0.00 | 2.907 d ± 0.08 | 0.047 de ± 0.00 | 1.165 de ± 0.06 | 0.026 f ± 0.00 | 0.047 e ± 0.00 | 0.365 e± 0.01 | |
| mean | 0.074 D | 0.048 A | 0.279 B | 0.075 D | 2.287 C | 0.057 B | 1.284 B | 0.034 B | 0.044 B | 0.343 C | |
| +NaCl (50 mM) | |||||||||||
| 0 MEL | O | 0.017 e ± 0.00 | 0.091 a ± 0.00 | 0.301 f ± 0.00 | 0.234 c ± 0.00 | 0.780 e ± 0.00 | 0.039 e ± 0.00 | 0.694 e ± 0.00 | 0.037 d ± 0.00 | 0.143 d ± 0.00 | 0.821 c ± 0.00 |
| Y | 0.377 b ± 0.00 | 0.092 a ± 0.00 | 1.530 b ± 0.00 | 0.548 a ± 0.00 | 3.303 c ± 0.00 | 0.097 d ± 0.00 | 1.982 d ± 0.00 | 0.045 cd ± 0.00 | 0.215 b ± 0.00 | 1.323 a ± 0.00 | |
| mean | 0.197 B | 0.091 A | 0.915 A | 0.391 A | 2.041 C | 0.068 C | 1.338 B | 0.041 B | 0.179 B | 1.072 A | |
| 5 MEL | O | 0.331 b ± 0.02 | 0.040 c ± 0.00 | 0.619 e ± 0.04 | 0.367 b ± 0.03 | 2.932 c ± 0.21 | 0.164 b ± 0.01 | 3.394 b ± 0.25 | 0.052 c ± 0.00 | 0.094 e ± 0.01 | 0.531 d ± 0.03 |
| Y | 0.446 a ± 0.02 | 0.029 e ± 0.00 | 1.942 a ± 0.12 | 0.365 b ± 0.02 | 6.514 b ± 0.31 | 0.150 bc ± 0.01 | 3.108 b ± 0.15 | 0.069 b ± 0.00 | 0.178 c ± 0.01 | 0.777 c ± 0.03 | |
| mean | 0.389 A | 0.035 C | 1.281 A | 0.366 AB | 4.723 AB | 0.157 AB | 3.251 AB | 0.061 AB | 0.136 BC | 0.653 B | |
| 100 MEL | O | 0.265 c ± 0.05 | 0.019 f ± 0.00 | 0.580 e ± 0.13 | 0.258 c ± 0.05 | 2.034 d ± 0.37 | 0.106 cd ± 0.02 | 2.801 bc ± 0.61 | 0.069 b ± 0.01 | 0.094 e ± 0.01 | 0.824 c ± 0.11 |
| Y | 0.275 c ± 0.01 | 0.012 g ± 0.00 | 1.327 c ± 0.06 | 0.271 c ± 0.01 | 3.456 c ± 0.18 | 0.069 de ± 0.00 | 1.923 d ± 0.09 | 0.035 d ± 0.00 | 0.097 e ± 0.00 | 0.639 d ± 0.03 | |
| mean | 0.270 AB | 0.015 D | 0.953 A | 0.264 BC | 2.745 BC | 0.088 C | 2.362 B | 0.052 AB | 0.095 C | 0.732 B | |
| 500 MEL | O | 0.464 a ± 0.05 | 0.051 b ± 0.01 | 0.872 d ± 0.10 | 0.276 c ± 0.02 | 7.739 a ± 0.89 | 0.565 a ± 0.07 | 9.285 a ± 1.05 | 0.113 a ± 0.02 | 0.226 b ± 0.03 | 1.247 a ± 0.13 |
| Y | 0.197 d ± 0.00 | 0.034 d ± 0.00 | 0.880 d ± 0.00 | 0.158 d ± 0.00 | 3.231 c ± 0.00 | 0.077 de ± 0.00 | 2.046 cd ± 0.00 | 0.041 cd ± 0.00 | 0.300 a ± 0.00 | 0.981 b ± 0.00 | |
| mean | 0.330 AB | 0.043 B | 0.876 A | 0.217 C | 5.485 A | 0.321 A | 5.665 A | 0.077 A | 0.263 A | 1.114 A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simlat, M.; Ptak, A.; Wójtowicz, T.; Szewczyk, A. The Content of Phenolic Compounds in Stevia rebaudiana (Bertoni) Plants Derived from Melatonin and NaCl Treated Seeds. Plants 2023, 12, 780. https://doi.org/10.3390/plants12040780
Simlat M, Ptak A, Wójtowicz T, Szewczyk A. The Content of Phenolic Compounds in Stevia rebaudiana (Bertoni) Plants Derived from Melatonin and NaCl Treated Seeds. Plants. 2023; 12(4):780. https://doi.org/10.3390/plants12040780
Chicago/Turabian StyleSimlat, Magdalena, Agata Ptak, Tomasz Wójtowicz, and Agnieszka Szewczyk. 2023. "The Content of Phenolic Compounds in Stevia rebaudiana (Bertoni) Plants Derived from Melatonin and NaCl Treated Seeds" Plants 12, no. 4: 780. https://doi.org/10.3390/plants12040780
APA StyleSimlat, M., Ptak, A., Wójtowicz, T., & Szewczyk, A. (2023). The Content of Phenolic Compounds in Stevia rebaudiana (Bertoni) Plants Derived from Melatonin and NaCl Treated Seeds. Plants, 12(4), 780. https://doi.org/10.3390/plants12040780








