The Phenolic Profile of Sweet Cherry Fruits Influenced by Cultivar/Rootstock Combination
Abstract
1. Introduction
2. Results
2.1. The Phenolic Profile Detected in the Sweet Cherry
2.2. Content of Individual and Total Anthocyanins
2.3. Content of Individual and Total Flavanols
2.4. Content of Individual and Total Flavonols
2.5. Content of Individual and Total Flavanones
2.6. Content of Hydroxycinnamic Derivatives and Total Hydroxycinnamic Acids
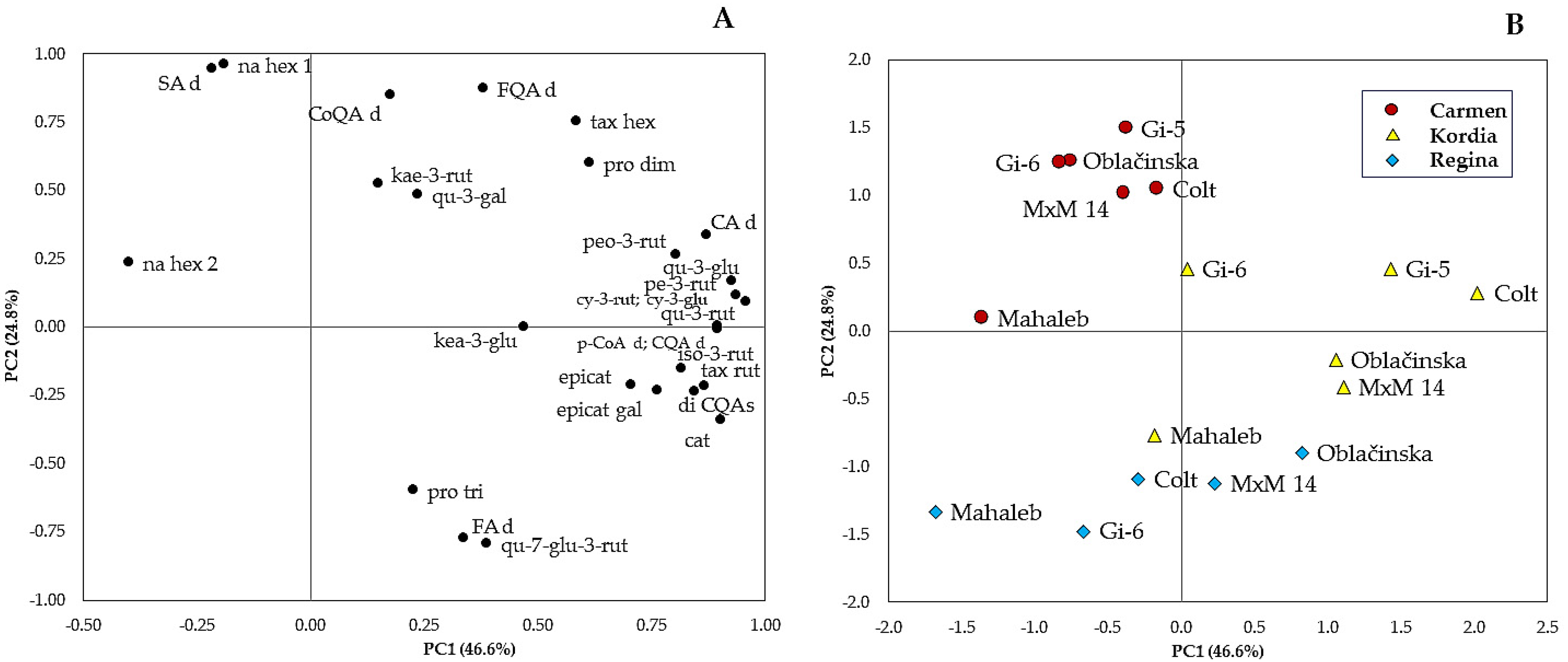
2.7. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Analysis of Phenolic Compounds
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lang, G.A. Sweet cherry orchard management: From shifting paradigms to computer modeling. Acta Hortic. 2008, 795, 597–604. [Google Scholar] [CrossRef]
- Bujdosó, G.; Hrotkó, K. Cherry production. In Cherries: Botany, Production and Uses; Quero-Garcia, J., Lezzoni, A., Pulawska, J., Lang, G., Eds.; CABI: Wallingford UK, 2017; pp. 1–13. [Google Scholar]
- Food and Agricultural Organization of the United Nation. FAOSTAT Statistical Database. Available online: http://www.fao.org (accessed on 15 October 2022).
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Butz, P.; Hofmann, C.; Tauscher, B. Recent developments in noninvasive techniques for fresh fruit and vegetable internal quality analysis. J. Food Sci. 2006, 70, 131–141. [Google Scholar] [CrossRef]
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Factors affecting quality and health promoting compounds during growth and postharvest life of sweet cherry (Prunus avium L.). Front. Plant Sci. 2017, 8, 2166. [Google Scholar] [CrossRef]
- Faienza, M.F.; Corbo, F.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Grano, M.; Wang, D.Q.-H.; D’Amato, G.; Muraglia, M.; Franchini, C.; et al. Novel insights in health-promoting properties of sweet cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef]
- Fonseca, L.R.S.; Silva, G.R.; Luís, Â.; Cardoso, H.J.; Correia, S.; Vaz, C.V.; Duarte, A.P.; Socorro, S. Sweet cherries as anti-cancer agents: From bioactive compounds to function. Molecules 2021, 26, 2941. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Goyal, A.; Algın Yapar, E.; Cavalu, S. Bioactive compounds and nanodelivery perspectives for treatment of cardiovascular diseases. Appl. Sci. 2021, 11, 11031. [Google Scholar] [CrossRef]
- De Leo, M.; Iannuzzi, A.M.; Germanò, M.P.; D’Angelo, V.; Camangi, F.; Sevi, F.; Diretto, G.; De Tommasi, N.; Braca, A. Comparative chemical analysis of six ancient Italian sweet cherry (Prunus avium L.) varieties showing antiangiogenic activity. Food Chem. 2021, 360, 129999. [Google Scholar] [CrossRef]
- Mozetič, B.; Trebše, P.; Hribar, J. Determination and quantitation of anthocyanins and hydroxycinnamic acids in different cultivars of sweet cherries (Prunus avium L.) from Nova Gorica region (Slovenia). Food Technol. Biotechnol. 2002, 40, 207–212. [Google Scholar]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Serradilla, M.J.; Lozano, M.; Bernalte, M.J.; Ayuso, M.C.; López-Corrales, M.; González-Gómez, D. Physicochemical and bioactive properties evolution during ripening of ‘Ambrunés’ sweet cherry cultivar. Food Sci. Technol. 2011, 44, 199–205. [Google Scholar] [CrossRef]
- Usenik, V.; Fajt, N.; Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. Sweet cherry pomological and biochemical characteristics influenced by rootstock. J. Agric. Food Chem. 2010, 58, 4928–4933. [Google Scholar] [CrossRef] [PubMed]
- Balducci, F.; Capriotti, L.; Mazzoni, L.; Medori, I.; Albanesi, A.; Borraccini, G.; Giampieri, F.; Mezzetti, B.; Capocasa, F. The rootstock effects on vigor, production and fruit quality in sweet cherry (Prunus avium L.). J. Berry Res. 2019, 9, 249–265. [Google Scholar] [CrossRef]
- Moreno, M.A.; Adrada, R.; Aparicio, J.; Betrán, S. Performance of ‘Sunburst’ sweet cherry grafted on different rootstocks. J. Hortic. Sci. Biotechnol. 2001, 76, 167–173. [Google Scholar] [CrossRef]
- López-Ortega, G.; García-Montiel, F.; Bayo-Canha, A.; Frutos-Ruiz, C.; Frutos-Tomás, D. Rootstock effects on the growth, yield and fruit quality of sweet cherry cv. ‘Newstar’ in the growing conditions of the region of Murcia. Sci. Hortic. 2016, 198, 326–335. [Google Scholar] [CrossRef]
- Jiménez, S.; Pinochet, J.; Gogorcena, Y.; Betrán, J.A.; Moreno, M.A. Influence of different vigour cherry rootstocks on leaves and shoots mineral composition. Sci. Hortic. 2007, 112, 73–79. [Google Scholar] [CrossRef]
- Vosnjak, M.; Mrzlic, D.; Hudina, M.; Usenik, V. The effect of water supply on sweet cherry phytochemicals in bud, leaf and fruit. Plants 2021, 10, 1131. [Google Scholar] [CrossRef]
- Radović, M.; Milatović, D.; Tešić, Ž.; Tosti, T.; Gašić, U.; Dojčinović, B.; Zagorac-Dabić, D. Influence of rootstocks on the chemical composition of the fruits of plum cultivars. J. Food Compos. Anal. 2020, 92, 103480. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Abukauskas, V.; Janulis, V.; Kviklys, D. Phenolic content and antioxidant activity in apples of the ‘Galaval’ cultivar grown on 17 different rootstocks. Antioxidants 2022, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Šeruga, M.; Voća, S.; Šindrak, Z.; Dobričević, N. Flavonol and phenolic acid composition of sweet cherries (cv. Lapins) produced on six different vegetative rootstocks. Sci. Hortic. 2009, 123, 23–28. [Google Scholar] [CrossRef]
- Milinović, B.; Dragović-Uzelac, V.; Halapija Kazija, D.; Jelačić, T.; Vujević, P.; Čiček, D.; Biško, A.; Čmelik, Z. Influence of four different dwarfing rootstocks on phenolic acids and anthocyanin composition of sweet cherry (Prunus avium L.) cvs ‘Kordia’ and ‘Regina’. J. Appl. Bot. Food Qual. 2016, 89, 2937. [Google Scholar] [CrossRef]
- Karakaya, O.; Ozturk, B.; Aglar, E.; Balik, H.I. The influence of the rootstocks on biochemical and bioactive compound content of ‘0900 Ziraat’ sweet cherry fruit. Erwerbs-Obstbau 2021, 63, 247–253. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Novak, I.; Medvidovic-Kosanovic, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensm.-Rundsch. 2007, 103, 58–64. [Google Scholar]
- Giménez, M.J.; Valverde, J.M.; Valero, D.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Castillo, S. Quality and antioxidant properties on sweet cherries as affected by preharvest salicylic and acetylsalicylic acids treatments. Food Chem. 2014, 160, 226–232. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Campos, G.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and phytochemical composition of 23 Portuguese sweet cherries as conditioned by variety (or genotype). Food Chem. 2021, 335, 127637. [Google Scholar] [CrossRef]
- Di Matteo, A.; Russo, R.; Graziani, G.; Ritieni, A.; Di Vaio, C. Characterization of autochthonous sweet cherry cultivars (Prunus avium L.) of Southern Italy for fruit quality, bioactive compounds and antioxidant activity. J. Sci. Food Agric. 2017, 97, 2782–2794. [Google Scholar] [CrossRef]
- Antognoni, F.; Potente, G.; Mandrioli, R.; Angeloni, C.; Freschi, M.; Malaguti, M.; Hrelia, S.; Lugli, S.; Gennari, F.; Muzzi, E.; et al. Fruit quality characterization of new sweet cherry cultivars as a good source of bioactive phenolic compounds with antioxidant and neuroprotective potential. Antioxidants 2020, 9, 677. [Google Scholar] [CrossRef]
- Kim, D.-O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef]
- Usenik, V.; Zadravec, P.; Štampar, F. Influence of rain protective tree covering on sweet cherry fruit quality. Europ. J. Hortic. Sci. 2009, 74, 49–53. [Google Scholar]
- Hayaloglu, A.A.; Demir, N. Phenolic compounds, volatiles, and sensory characteristics of twelve sweet cherry (Prunus avium L.) cultivars grown in Turkey: Phenolics and volatiles in sweet cherry. J. Food Sci. 2016, 81, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Muñoz Mingarro, D. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Mozetič, B.; Trebše, P.; Simčič, M.; Hribar, J. Changes of anthocyanins and hydroxycinnamic acids affecting the skin colour during maturation of sweet cherries (Prunus avium L.). Food Sci. Technol. 2004, 37, 123–128. [Google Scholar] [CrossRef]
- Gonçalves, B.; Landbo, A.-K.; Knudsen, D.; Silva, A.P.; Moutinho-Pereira, J.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the phenolic profiles of cherries (Prunus avium L.). J. Agric. Food Chem. 2004, 52, 523–530. [Google Scholar] [CrossRef]
- Bartolini, S.; Leccese, A.; Iacona, C.; Andreini, L.; Viti, R. Influence of rootstock on fruit entity, quality and antioxidant properties of fresh apricots (cv. ‘Pisana’). N. Z. J. Crop Hortic. Sci. 2014, 42, 265–274. [Google Scholar] [CrossRef]
- Abdelghafar, A.; Burrell, R.; Reighard, G.; Gasic, K. Antioxidant capacity and bioactive compounds accumulation in peach breeding germplasm. J. Am. Pom. Soc. 2018, 72, 40–69. [Google Scholar]
- Martínez-Esplá, A.; Zapata, P.J.; Valero, D.; García-Viguera, C.; Castillo, S.; Serrano, M. Preharvest application of oxalic acid increased fruit size, bioactive compounds, and antioxidant capacity in sweet cherry cultivars (Prunus avium L.). J. Agric. Food Chem. 2014, 62, 3432–3437. [Google Scholar] [CrossRef]
- Martins, V.; Silva, V.; Pereira, S.; Afonso, S.; Oliveira, I.; Santos, M.; Ribeiro, C.; Vilela, A.; Bacelar, E.; Silva, A.P.; et al. Rootstock affects the fruit quality of ‘Early Bigi’ sweet cherries. Foods 2021, 10, 2317. [Google Scholar] [CrossRef]
- Trendafilova, A.; Ivanova, V.; Trusheva, B.; Kamenova-Nacheva, M.; Tabakov, S.; Simova, S. Chemical composition and antioxidant capacity of the fruits of European plum cultivar “Čačanska Lepotica” influenced by different rootstocks. Foods 2022, 11, 2844. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Evaluation of chemical constituents and antioxidant activity of sweet cherry (Prunus avium L.) cultivars: Chemical constituents of sweet cherry. Int. J. Food Sci. Technol. 2011, 46, 2530–2537. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus fruit species as a rich source of bioactive compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (sweet cherry) by-products: A source of phenolic compounds with antioxidant and anti-hyperglycemic properties—A review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhong, F.; Tian, R.; Zhang, K.; Zhang, X.; Li, T. Comparative study of phenolic compounds and antioxidant activity in different species of cherries. J. Food Sci. 2011, 76, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Remorini, D.; Tavarini, S.; Degl’Innocenti, E.; Loreti, F.; Massai, R.; Guidi, L. Effect of rootstocks and harvesting time on the nutritional quality of peel and flesh of peach fruits. Food Chem. 2008, 110, 361–367. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Mladenović, J. Combining fruit quality and main antioxidant attributes in the sour cherry: The role of new clonal rootstock. Sci. Hortic. 2020, 265, 109236. [Google Scholar] [CrossRef]
- Gonçalves, B.; Moutinho-Pereira, J.; Santos, A.; Silva, A.P.; Bacelar, E.; Correia, C.; Rosa, E. Scion-rootstock interaction affects the physiology and fruit quality of sweet cherry. Tree Physiol. 2006, 26, 93–104. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Koron, D.; Zorenc, Z.; Veberic, R. Do optimally ripe blackberries contain the highest levels of metabolites? Food Chem. 2017, 215, 41–49. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Koron, D.; Rusjan, D. The impact of food processing on the phenolic content in products made from juneberry (Amelanchier lamarckii) fruits. J. Food Sci. 2020, 85, 386–393. [Google Scholar] [CrossRef]
| Anthocyanins | Flavanones | ||
|---|---|---|---|
| Cyanidin-3-rutinoside | cy-3-rut | Naringenin hexoside 1 | na hex 1 |
| Cyanidin-3-glucoside | cy-3-glu | Naringenin hexoside 2 | na hex 2 |
| Pelargonidin-3-rutinoside | pe-3-rut | taxifolin rutinoside | tax rut |
| Peonidin-3-rutinoside | peo-3-rut | taxifolin hexoside | tax hex |
| Flavonols | Hydroxycinnamic acids | ||
| Quercetin-7-glucoside-3-rutinoside) | qu-7-glu-3-rut | Caffeoylquinic acid derivatives | CQA d |
| Quercetin-3-rutinoside | qu-3-rut | Coumaroylquinic acid derivatives | CoQA d |
| Quercetin-3-galactoside | qu-3-gal | Caffeic acid derivatives | CAd |
| Quercetin-3-glucoside | qu-3-glu | Dicaffeoylquinic acids | di CQAs |
| Kaempferol-3-rutinoside | kae-3-rut | Feruloylquinic acid derivatives | FQA d |
| Isorhamnetin-3-rutinoside | iso-3-rut | Sinapic acid derivatives | SA d |
| Kaempferol-3-glucoside | kae-3-glu | Ferulic acid derivatives | FA d |
| Flavanols | p-coumaric acid derivatives | p-CoA d | |
| Procyanidin dimers | pro dim | ||
| Catechin | cat | ||
| Epicatechin | epicat | ||
| Procyanidin trimers | pro tri | ||
| Epicatechin gallate | epicat gal | ||
| Combination Cultivar/Rootstock | Cyanidin-3- rutinoside | Cyanidin-3- glucoside | Pelargonidin-3- glucoside | Peonidin-3- glucoside | Total Anthocyanins | |
|---|---|---|---|---|---|---|
| Carmen/Mahaleb | 208.8 ± 32.6 g–h | 14.7 ± 2.3 e–h | 2.2 ± 0.2 d–e | 4.3 ± 0.9 de | 229.2 ± 38.6 f–g | |
| Carmen/Colt | 358.1 ± 36.9 b–g | 25.2 ± 2.6 b–g | 4.8 ± 0.6 b–d | 8.8 ± 1.0 b–d | 396.9 ± 31.7 b–e | |
| Carmen/Oblacinska | 261.4 ± 23.4 g–h | 18.4 ± 1.5 d–h | 3.1 ± 0.8 c–e | 6.6 ± 1.3 c–e | 289.5 ± 43.1 c–g | |
| Carmen/M × M 14 | 345.9 ± 38.3 b–g | 24.3 ± 2.7 b–g | 3.7 ± 0.7 c–e | 10.8 ± 1.7 a–c | 384.7 ± 68.9 b–e | |
| Carmen/Gisela 5 | 303.3 ± 36.7 c–h | 21.3 ± 2.7 c–h | 3.9 ± 1.0 c–e | 7.7 ± 2.5 c–e | 336.2 ± 58.7 b–f | |
| Carmen/Gisela 6 | 202.8 ± 28.6 f–h | 14.3 ± 2.0 f–h | 2.5 ± 0.5 d–e | 3.6 ± 1.1 de | 223.2 ± 28.3 g–i | |
| Kordia/Mahaleb | 315.9 ± 21.9 c–h | 22.2 ± 1.5 c–h | 3.5 ± 0.5 c–e | 7.1 ± 1.1 c–e | 342.1 ± 46.6 e–g | |
| Kordia/Colt | 597.1 ± 51.2 a | 42.0 ± 2.6 a | 8.6 ± 1.1 a | 15.9 ± 3.0 a | 663.6 ± 52.8 a | |
| Kordia/Oblacinska | 400.4 ± 42.4 a–f | 28.2 ± 2.1 a–f | 5.7 ± 0.8 a–c | 8.3 ± 2.5 cd | 442.6 ± 39.7 b–f | |
| Kordia/M × M 14 | 537.3 ± 64.5 ab | 37.8 ± 2.5 ab | 5.7 ± 0.9 a–c | 14.2 ± 2.2 ab | 595.0 ± 51.8 ab | |
| Kordia/Gisela 5 | 406.1 ± 52.9 a–e | 28.6 ± 3.5 a–f | 7.6 ± 1.2 ab | 15.5 ± 3.1 a | 457.8 ± 56.2 b–e | |
| Kordia/Gisela 6 | 253.6 ± 37.2 g–h | 17.8 ± 2.6 e–h | 2.6 ± 0.5 c–e | 3.7 ± 1.1 de | 266.5 ± 32.6 e–g | |
| Regina/Mahaleb | 128.4 ± 14.3 h | 9.0 ± 1.0 h | 1.5 ± 0.2 e | 2.4 ± 0.3 e | 141.3 ± 23.7 i | |
| Regina/Colt | 302.3 ± 38.6 c–h | 21.3 ± 2.1 c–h | 3.8 ± 0.6 c–e | 5.4 ± 0.9 c–e | 332.8 ± 49.4 d–g | |
| Regina/Oblacinska | 468.2 ± 38.4 a–c | 32.9 ± 2.5 a–d | 4.8 ± 0.3 b–d | 6.5 ± 0.4 c–e | 512.4 ± 53.6 a–c | |
| Regina/M × M 14 | 461.6 ± 32.0 a–d | 32.5 ± 3.3 a–d | 4.2 ± 0.9 c–e | 6.6 ± 2.4 c–e | 504.9 ± 51.1 a–d | |
| Regina/Gisela 6 | 161.5 ± 22.3 gh | 11.2 ± 1.3 gh | 2.4 ± 0.3 d–e | 3.4 ± 0.5 de | 178.5 ± 22.8 hi | |
| Cultivar | Carmen | 280.1 ± 20.5 b | 19.7 ± 1.4 b | 3.4 ± 0.3 b | 7.0 ± 0.7 bc | 303.2 ± 23.8 c |
| Kordia | 418.4 ± 31.7 a | 29.4 ± 2.6 a | 5.6 ± 0.8 a | 10.8 ± 1.4 a | 464.2 ± 32.3 a | |
| Regina | 304.4 ± 40.8 b | 21.4 ± 2.9 b | 3.4 ± 0.3 b | 4.9 ± 0.6 c | 334.1 ± 33.7 b | |
| Rootstock | Mahaleb | 217.7 ± 22.8 c | 15.3 ± 1.6 cd | 2.4 ± 0.3 b | 4.6 ± 0.7 cd | 240.1 ± 20.9 c |
| Colt | 419.1 ± 60.6 a | 29.5 ± 3.3 a | 5.7 ± 1.1 a | 10.0 ± 1.9 a | 464.3 ± 46.7 a | |
| Oblacinska | 376.7 ± 44.1 ab | 26.5 ± 3.1 ab | 4.6 ± 0.7 a | 7.2 ± 0.9 bc | 416.5 ± 39.0 b | |
| M × M 14 | 448.3 ± 51.2 a | 31.5 ± 3.6 a | 4.5 ± 0.5 a | 10.6 ± 1.4 a | 494.9 ± 42.3 a | |
| Gisela 5 | 304.1 ± 42.0 bc | 21.4 ± 3.0 bc | 4.6 ± 0.9 a | 9.0 ± 2.4 b | 339.1 ± 36.3 b | |
| Gisela 6 | 207.6 ± 22.9 c | 14.5 ± 1.7 d | 2.5 ± 0.3 b | 3.6 ± 0.6 d | 228.2 ± 22.6 c | |
| Year | 2020 | 393.74 ± 38.69 a | 27.68 ± 2.72 a | 4.65 ± 0.59 a | 8.47 ± 1.04 a | 434.5 ± 44.01 a |
| 2021 | 273.39 ± 14.81 b | 19.24 ± 1.04 b | 3.25 ± 0.2 b | 5.92 ± 0.58 b | 301.8 ± 18.24 b | |
| Statistical significance | ||||||
| Cultivar | *** | *** | *** | *** | *** | |
| Rootstock | *** | *** | *** | *** | *** | |
| Year | *** | *** | *** | *** | *** | |
| Cultivar × Rootstock | *** | *** | * | ** | *** | |
| Combination Cultivar/Rootstock | Catechin | Epicatechin | Epicatechin Gallate | Procyanidin Dimers | Procyanidin Trimers | Total Flavanols | |
|---|---|---|---|---|---|---|---|
| Carmen/Mahaleb | 2.13 ± 0.28 c–f | 7.87 ± 0.84 b–d | 0.7 5± 0.21 f | 10.6 ± 1.2 f | 8.1 ± 0.9 f | 29.4 ± 6.3 e | |
| Carmen/Colt | 2.67 ± 0.12 a–e | 10.65 ± 0.94 a–c | 0.90 ± 0.23 f | 13.1 ± 0.9 c–f | 7.1 ± 0.3 f | 34.4 ± 6.5 de | |
| Carmen/Oblacinska | 3.14 ± 0.16 a–c | 11.83 ± 0.71 ab | 2.28 ± 0.33 b–d | 12.9 ± 1.2 c–f | 12.5 ± 1.7 de | 40.2 ± 3.9 b–e | |
| Carmen/M × M 14 | 2.49 ± 0.12 a–f | 11.34 ± 0.72 ab | 0.67 ± 0.14 f | 13.7 ± 0.8 c–f | 8.3 ± 0.5 f | 36.5 ± 7.9 c–e | |
| Carmen/Gisela 5 | 3.04 ± 0.29 a–d | 10.19 ± 1.24 a–c | 1.30 ± 0.28 d–f | 14.7 ± 2.0 b–f | 9.9 ± 0.9 ef | 39.1 ± 3.4 b–e | |
| Carmen/Gisela 6 | 2.58 ± 0.24 a–f | 8.90 ± 0.48 a–d | 1.15 ± 0.20 ef | 11.2 ± 0.7 ef | 9.6 ± 1.3 ef | 33.4 ± 7.4 de | |
| Kordia/Mahaleb | 1.93 ± 0.07 d–f | 8.10 ± 1.04 b–d | 1.68 ± 0.31 c–f | 14.9 ± 1.3 b–f | 14.5 ± 0.8 b–d | 41.1 ± 4.3 b–d | |
| Kordia/Colt | 3.63 ± 0.27 a | 14.04 ± 1.69 a | 1.63 ± 0.28 c–f | 21.9 ± 1.9 a | 17.9 ± 1.3 bc | 59.1 ± 4.9 a | |
| Kordia/Oblacinska | 2.99 ± 0.29 a–d | 9.13 ± 1.16 a–d | 2.33 ± 0.20 b–d | 18.1 ± 1.4 a–c | 17.9 ± 1.5 bc | 50.4 ± 5.9 ab | |
| Kordia/M × M 14 | 2.31 ± 0.38 b–f | 8.85 ± 0.79 a–d | 1.44 ± 0.21 c–f | 19.3 ± 1.5 ab | 13.3 ± 1.2 de | 45.2 ± 6.1 bc | |
| Kordia/Gisela 5 | 3.43 ± 0.17 ab | 11.88 ± 1.84 ab | 2.45 ± 0.19 a–c | 19.3 ± 1.4 ab | 22.1 ± 1.7 a | 59.2 ± 9.1 a | |
| Kordia/Gisela 6 | 3.18 ± 0.33 a–c | 10.57 ± 1.32 a–c | 2.02 ± 0.18 b–e | 15.8 ± 1.4 b–f | 18.3 ± 0.6 ab | 49.9 ± 5.8 ab | |
| Regina/Mahaleb | 0.71 ± 0.07 g | 4.55 ± 0.98 d | 1.97 ± 0.31 b–e | 12.4 ± 0.6 d–f | 10.1 ± 1.0 ef | 29.7 ± 3.5 e | |
| Regina/Colt | 1.70 ± 0.35 e–g | 9.55 ± 1.57 a–d | 2.04 ± 0.29 b–e | 16.4 ± 1.9 b–e | 13.2 ± 1.4 de | 42.9 ± 5.9 b–d | |
| Regina/Oblacinska | 2.29 ± 0.33 b–f | 11.73 ± 1.08 ab | 3.00 ± 0.27 ab | 19.4 ± 0.6 ab | 14.1 ± 1.4 cd | 50.5 ± 5.4 ab | |
| Regina/M × M 14 | 1.60 ± 0.38 e–g | 7.30 ± 1.06 b–d | 2.47 ± 0.25 a–c | 17.9 ± 1.4 a–c | 12.7 ± 1.6 de | 41.9 ± 6.7 b–d | |
| Regina/Gisela 6 | 1.44 ± 0.13 fg | 5.46 ± 0.61 cd | 3.48 ± 0.13 a | 16.9 ± 0.6 a–d | 14.3 ± 0.8 cd | 41.6 ± 4.5 b–d | |
| Cultivar | Carmen | 2.67 ± 0.11 a | 10.13 ± 0.62 ab | 1.17 ± 0.13 c | 12.7 ± 0.6 b | 13.7 ± 1.2 ab | 40.3 ± 1.7 b |
| Kordia | 2.97 ± 0.23 a | 10.43 ± 0.73 a | 1.93 ± 0.11 b | 18.2 ± 0.8 a | 10.5 ± 0.5 b | 44.3 ± 3.5 a | |
| Regina | 1.55 ± 0.15 b | 7.72 ± 0.75 b | 2.59 ± 0.17 a | 16.6 ± 0.6 ab | 15.8 ± 0.7 a | 44.1 ± 2.6 a | |
| Rootstock | Mahaleb | 1.59 ± 0.18 c | 6.84 ± 0.85 b | 1.47 ± 0.21 b | 12.6 ± 0.7 b | 10.9 ± 0.8 d | 33.4 ± 2.7 c |
| Colt | 2.67 ± 0.33 a | 11.41 ± 1.64 a | 1.52 ± 0.22 b | 17.1 ± 1.4 a | 12.7 ± 1.7 cd | 45.4 ± 5.8 ab | |
| Oblacinska | 2.81 ± 0.17 a | 10.90 ± 0.97 a | 2.54 ± 0.17 a | 16.8 ± 1.1 a | 14.9 ± 1.4 ab | 48.3 ± 3.9 a | |
| M × M 14 | 2.13 ± 0.19 bc | 9.17 ± 0.58 ab | 1.52 ± 0.21 b | 17.0 ± 0.9 a | 11.4 ± 0.8 d | 41.2 ± 4.3 bc | |
| Gisela 5 | 2.82 ± 0.18 a | 10.89 ± 1.21 a | 1.77 ± 0.19 b | 17.0 ± 1.4 a | 16.0 ± 1.6 a | 46.5 ± 6.0 ab | |
| Gisela 6 | 2.60 ± 0.20 ab | 7.15 ± 0.65 b | 2.54 ± 0.30 a | 14.6 ± 0.8 ab | 14.1 ± 1.0 bc | 41.0 ± 4.3 bc | |
| Year | 2020 | 2.46 ± 0.16 | 11.98 ± 0.69 a | 1.73 ± 0.13 b | 15.2 ± 1.9 b | 12.4 ± 1.6 b | 43.8 ± 2.5 |
| 2021 | 2.39 ± 0.14 | 7.07 ± 0.57 b | 1.98 ± 0.14 a | 16.4 ± 1.0 a | 14.0 ± 1.3 a | 41.8 ± 1.6 | |
| Statistical significance | |||||||
| Cultivar | *** | *** | *** | *** | *** | *** | |
| Rootstock | *** | *** | *** | *** | *** | *** | |
| Year | ns | *** | * | * | *** | ns | |
| Cultivar × Rootstock | * | *** | ** | * | ** | * | |
| Combination Cultivar/Rootstock | Isorhamnetin -3-rutinoside | Kaempferol -3-glucoside | Kaempferol -3-rutinoside | Quercetin-3- galactoside | Quercetin-3- glucoside | Quercetin-3- rutinoside | Quercetin-7- glucoside-3- rutinoside | Total Flavonols | |
|---|---|---|---|---|---|---|---|---|---|
| Carmen/Mahaleb | 0.03 ± 0.006 d | 0.06 ± 0.02 c | 0.07 ± 0.02 c | 0.33 ± 0.03 b–d | 0.25 ± 0.05 d | 7.66 ± 1.37 d | 0.96 ± 0.28 c | 15.1 ± 1.5 d | |
| Carmen/Colt | 0.03 ± 0.013 d | 0.13 ± 0.02 a–c | 0.26 ± 0.05 ab | 0.49 ± 0.08 a–d | 0.58 ± 0.19 a–d | 16.21 ± 1.70 a–d | 0.98 ± 0.15 c | 29.0 ± 2.8 a–c | |
| Carmen/Oblacinska | 0.02 ± 0.007 d | 0.11 ± 0.03 a–c | 0.10 ± 0.03 bc | 0.42 ± 0.05 a–d | 0.37 ± 0.09 cd | 9.84 ± 0.70 cd | 1.52 ± 0.19 c | 20.9 ± 2.3 b–d | |
| Carmen/M × M 14 | 0.03 ± 0.009 d | 0.06 ± 0.01 c | 0.11 ± 0.04 bc | 0.37 ± 0.07 b–d | 0.36 ± 0.14 cd | 11.97 ± 1.42 b–d | 1.04 ± 0.38 c | 22.9 ± 2.7 a–d | |
| Carmen/Gisela 5 | 0.03 ± 0.008 d | 0.11 ± 0.01 a–c | 0.22 ± 0.05 a–c | 0.44 ± 0.06 a–d | 0.40 ± 0.12 cd | 11.90 ± 1.06 b–d | 1.28 ± 0.28 c | 22.9 ± 2.4 a–d | |
| Carmen/Gisela 6 | 0.03 ± 0.010 d | 0.16 ± 0.02 a–c | 0.36 ± 0.03 a | 0.77 ± 0.24 a | 0.40 ± 0.08 cd | 12.94 ± 1.21 a–d | 1.13 ± 0.19 c | 20.1 ± 1.9 b–d | |
| Kordia/Mahaleb | 0.06 ± 0.009 a–d | 0.07 ± 0.01 bc | 0.10 ± 0.04 bc | 0.41 ± 0.11 a–d | 0.43 ± 0.07 b–d | 12.18 ± 1.87 b–d | 2.90 ± 0.18 b | 20.2 ± 3.3 b–d | |
| Kordia/Colt | 0.08 ± 0.013 a | 0.13 ± 0.04 a–c | 0.16 ± 0.03 bc | 0.43 ± 0.06 a–d | 0.85 ± 0.05 ab | 20.69 ± 2.49 a | 2.89 ± 0.34 b | 31.9 ± 3.8 a | |
| Kordia/Oblacinska | 0.08 ± 0.022 a | 0.21 ± 0.03 a | 0.22 ± 0.08 a–c | 0.41 ± 0.08 a–d | 0.75 ± 0.12 a–c | 17.64 ± 1.68 a–c | 2.67 ± 0.22 b | 28.1 ± 2.3 a–c | |
| Kordia/M × M 14 | 0.07 ± 0.007 ab | 0.10 ± 0.01 a–c | 0.10 ± 0.01 bc | 0.37 ± 0.05 b–d | 0.73 ± 0.09 a–c | 19.47 ± 1.81 ab | 2.96 ± 0.23 b | 29.0 ± 2.2 ab | |
| Kordia/Gisela 5 | 0.07 ± 0.019 a–c | 0.19 ± 0.03 ab | 0.23 ± 0.03 a–c | 0.70 ± 0.19 ab | 0.95 ± 0.16 a | 19.78 ± 1.79 ab | 3.30 ± 0.38 b | 31.0 ± 2.7 a | |
| Kordia/Gisela 6 | 0.05 ± 0.006 a–d | 0.11 ± 0.04 a–c | 0.17 ± 0.02 bc | 0.66 ± 0.17 a–c | 0.56 ± 0.09 a–d | 14.42 ± 1.84 a–d | 3.10 ± 0.38 b | 28.8 ± 2.5 a–d | |
| Regina/Mahaleb | 0.03 ± 0.003 d | 0.08 ± 0.02 bc | 0.07 ± 0.04 c | 0.36 ± 0.05 b–d | 0.21 ± 0.07 d | 6.53 ± 0.50 d | 2.84 ± 0.36 b | 13.6 ± 1.6 d | |
| Regina/Colt | 0.03 ± 0.005 d | 0.10 ± 0.01 a–c | 0.08 ± 0.01 c | 0.32 ± 0.03 cd | 0.35 ± 0.03 cd | 12.20 ± 1.34 b–d | 3.16 ± 0.49 b | 20.1 ± 2.7 b–d | |
| Regina/Oblacinska | 0.03 ± 0.003 d | 0.12 ± 0.01 a–c | 0.14 ± 0.03 bc | 0.54 ± 0.11 a–d | 0.50 ± 0.05 b–d | 17.88 ± 1.92 a–c | 4.71 ± 0.47 a | 29.6 ± 2.9 ab | |
| Regina/M × M 14 | 0.04 ± 0.005 b–d | 0.12 ± 0.01 a–c | 0.09 ± 0.02 c | 0.42 ± 0.05 a–d | 0.49 ± 0.07 b–d | 14.80 ± 1.64 a–d | 4.37 ± 0.41 a | 25.7 ± 1.5 a–c | |
| Regina/Gisela 6 | 0.04 ± 0.005 b–d | 0.17 ± 0.04 a–c | 0.19 ± 0.02 a–c | 0.27 ± 0.02 d | 0.33 ± 0.03 cd | 10.23 ± 1.46 cd | 4.61 ± 0.28 a | 19.5 ± 2.3 cd | |
| Cultivar | Carmen | 0.03 ± 0.002 b | 0.10 ± 0.01 | 0.19 ± 0.03 a | 0.48 ± 005 | 0.40 ± 0.04 b | 11.76 ± 0.81 b | 1.16 ± 0.06 b | 14.7 ± 1.2 c |
| Kordia | 0.07 ± 0.006 a | 0.14 ± 0.01 | 0.17 ± 0.02 b | 0.50 ± 0.06 | 0.72 ± 0.07 a | 17.37 ± 1.20 a | 2.98 ± 0.10 ab | 22.8 ± 1.7 a | |
| Regina | 0.04 ± 0.002 | 0.04 ± 0.01 | 0.12 ± 0.01 c | 0.38 ± 0.03 | 0.38 ± 0.03 b | 12.33 ± 1.03 b | 3.94 ± 0.24 a | 18.2 ± 1.4 b | |
| Rootstock | Mahaleb | 0.04 ± 0.007 | 0.08 ± 0.01 c | 0.08 ± 0.02 c | 0.37 ± 0.04 b | 0.30 ± 0.04 b | 8.79 ± 1.01 c | 2.24 ± 0.23 d | 16.2 ± 2.6 b |
| Colt | 0.05 ± 0.002 | 0.13 ± 0.02 a | 0.17 ± 0.03 b | 0.42 ± 0.04 b | 0.60 ± 0.11 a | 16.37 ± 1.63 a | 2.35 ± 0.29 c | 27.0 ± 3.1 a | |
| Oblacinska | 0.05 ± 0.004 | 0.15 ± 0.03 a | 0.16 ± 0.03 b | 0.46 ± 0.05 ab | 0.54 ± 0.09 a | 15.13 ± 1.51 ab | 2.97 ± 0.40 a | 26.2 ± 2.8 a | |
| M × M 14 | 0.05 ± 0.011 | 0.09 ± 0.01 bc | 0.10 ± 0.01 bc | 0.39 ± 0.03 b | 0.53 ± 0.06 a | 15.42 ± 1.15 ab | 2.76 ± 0.37 ab | 25.9 ± 2.0 a | |
| Gisela 5 | 0.05 ± 0.006 | 0.15 ± 0.02 a | 0.28 ± 0.04 a | 0.64 ± 0.13 a | 0.59 ± 0.09 a | 14.89 ± 1.27 ab | 1.91 ± 0.25 d | 24.6 ± 2.9 a | |
| Gisela 6 | 0.05 ± 0.008 | 0.14 ± 0.02 a | 0.18 ± 0.04 b | 0.47 ± 0.10 ab | 0.45 ± 0.06 ab | 12.33 ± 1.73 bc | 3.86 ± 0.28 a | 24.2 ± 2.5 ab | |
| Year | 2020 | 0.05 ± 0.001 | 0.14 ± 0.01 a | 0.18 ± 0.02 a | 0.51 ± 0.05 | 0.62 ± 0.05 a | 15.60 ± 1.00 a | 2.45 ± 0.16 b | 20.1 ± 1.3 a |
| 2021 | 0.04 ± 0.002 | 0.11 ± 0.01 b | 0.14 ± 0.01 b | 0.42 ± 0.04 | 0.39 ± 0.03 b | 12.21 ± 0.75 b | 2.79 ± 0.22 a | 16.6 ± 1.0 b | |
| Statistical significance | |||||||||
| Cultivar | *** | *** | ns | ns | *** | *** | *** | *** | |
| Rootstock | ns | ns | *** | * | *** | *** | *** | *** | |
| Year | ns | ns | * | ns | *** | *** | *** | *** | |
| Cultivar × Rootstock | * | * | * | *** | * | * | *** | * | |
| Combination Cultivar/Rootstock | Naringenin Hexoside 1 | Naringenin Hexoside 2 | Taxifolin Hexoside | Taxifolin Rutinoside | Total Flavanones | |
|---|---|---|---|---|---|---|
| Carmen/Mahaleb | 0.08 ± 0.01 | 0.13 ± 0.04 bc | 1.89 ± 0.41 ef | 0.99 ± 0.10 c–e | 3.09 ± 0.36 de | |
| Carmen/Colt | 0.12 ± 0.02 | 0.11 ± 0.02 c | 1.99 ± 0.56 d–f | 1.27 ± 0.16 ab | 3.49 ± 0.21 b–e | |
| Carmen/Oblacinska | 0.13 ± 0.02 | 0.19 ± 0.03 a–c | 2.53 ± 0.56 b–f | 1.38 ± 0.09 a | 4.23 ± 0.44 ab | |
| Carmen/M× M 14 | 0.06 ± 0.02 | 0.11 ± 0.02 c | 2.80 ± 0.15 a–e | 0.68 ± 0.04 fg | 3.65 ± 0.30 b–d | |
| Carmen/Gisela 5 | 0.09 ± 0.03 | 0.17 ± 0.03 a–c | 3.47 ± 0.29 ab | 0.85 ± 0.03 d–q | 4.58 ± 0.33 ab | |
| Carmen/Gisela 6 | 0.09 ± 0.03 | 0.24 ± 0.06 a–c | 3.34 ± 0.11 ab | 0.98 ± 0.04 c–d | 4.65 ± 0.37 a | |
| Kordia/Mahaleb | 0.07 ± 0.02 | 0.10 ± 0.04 c | 1.61 ± 0.25 f | 0.77 ± 0.04 d–g | 2.55 ± 0.34 e | |
| Kordia’/Colt | 0.05 ± 0.01 | 0.10 ± 0.02 c | 3.61 ± 0.41 a | 0.72 ± 0.08 c–g | 4.51 ± 0.26 ab | |
| Kordia/Oblacinska | 0.11 ± 0.01 | 0.23 ± 0.02 a–c | 2.38 ± 0.48 c–f | 1.24 ± 0.13 a–c | 3.96 ± 0.33 b–d | |
| Kordia/M × M 14 | 0.07 ± 0.01 | 0.17 ± 0.03 a–c | 2.33 ± 0.25 c–f | 1.18 ± 0.07 a–c | 3.75 ± 0.18 b–d | |
| Kordia/Gisela 5 | 0.08 ± 0.01 | 0.06 ± 0.01 c | 2.72 ± 0.38 a–e | 1.18 ± 0.12 a–c | 4.04 ± 0.22 a–c | |
| Kordia/Gisela 6 | 0.07 ± 0.02 | 0.33 ± 0.04 ab | 3.22 ± 0.37 a–c | 1.02 ± 0.09 b–d | 4.16 ± 0.17 a–d | |
| Regina/Mahaleb | 0.05 ± 0.02 | 0.24 ± 0.05 a–c | 3.60 ± 0.47 a | 0.95 ± 0.06 d–f | 4.64 ± 0.36 a | |
| Regina/Colt | 0.06 ± 0.01 | 0.13 ± 0.03 bc | 3.40 ± 0.45 ab | 0.74 ± 0.04 e–g | 4.33 ± 0.13 a–c | |
| Regina/Oblacinska | 0.07 ± 0.01 | 0.13 ± 0.03 bc | 2.72 ± 0.28 a–e | 0.68 ± 0.03 fg | 3.64 ± 0.23 b–d | |
| Regina/M × M 14 | 0.11 ± 0.01 | 0.15 ± 0.03 a–c | 2.92 ± 0.14 a–d | 0.78 ± 0.06 d–g | 4.56 ± 0.36 b–d | |
| Regina/Gisela 6 | 0.14 ± 0.03 | 0.35 ± 0.02 a | 3.22 ± 0.14 a–c | 0.65 ± 0.02 g | 4.36 ± 0.17 a–d | |
| Cultivar | Carmen | 0.11 ± 0.01 a | 0.11 ± 0.01 b | 2.31 ± 0.19 b | 1.21 ± 0.07 a | 3.78 ± 0.16 b |
| Kordia | 0.07 ± 0.01 b | 0.24 ± 0.03 a | 3.31 ± 0.12 a | 0.87 ± 0.04 ab | 4.58 ± 0.18 a | |
| Regina | 0.09 ± 0.02 ab | 0.18 ± 0.03 a | 2.82 ± 0.18 ab | 0.72 ± 0.02 b | 3.85 ± 0.20 ab | |
| Rootstock | Mahaleb | 0.07 ± 0.01 | 0.14 ± 0.02 b | 2.10 ± 0.19 b | 0.81 ± 0.05 c | 3.20 ± 0.19 b |
| Colt | 0.06 ± 0.01 | 0.24 ± 0.05 a | 2.88 ± 0.25 a | 0.94 ± 0.06 b | 4.23 ± 0.29 a | |
| Oblacinska | 0.09 ± 0.01 | 0.22 ± 0.05 a | 3.03 ± 0.29 a | 0.95 ± 0.08 b | 4.39 ± 0.26 a | |
| M × M 14 | 0.08 ±0.01 | 0.12 ± 0.02 b | 3.01 ± 0.19 a | 0.90 ± 0.06 bc | 4.21 ± 0.20 a | |
| Gisela 5 | 0.11 ± 0.02 | 0.18 ± 0.04 ab | 2.93 ± 0.29 a | 1.18 ± 0.15 a | 4.60 ± 0.26 a | |
| Gisela 6 | 0.11 ± 0.03 | 0.13 ± 0.02 b | 2.94 ± 0.26 a | 0.97 ± 0.11 b | 4.26 ± 0.25 a | |
| Year | 2020 | 0.09 ± 0.01 | 0.17 ± 0.02 | 1.05 ± 0.06 a | 0.72 ± 0.02 a | 2.39 ± 0.17 b |
| 2021 | 0.08 ± 0.01 | 0.17 ± 0.02 | 0.84 ± 0.03 b | 0.17 ± 0.02 b | 3.23 ± 0.09 a | |
| Statistical significance | ||||||
| Cultivar | * | *** | *** | *** | *** | |
| Rootstock | ns | *** | *** | *** | *** | |
| Year | ns | ns | *** | *** | *** | |
| Cultivar × Rootstock | ns | ns | *** | *** | * | |
| Comination Cultivar/Rootstock | Caffeic Acid Derivatives | Caffeoyl- Quinic Acid Derivatives | Coumaroyl- Quinic Acid Derivatives | Dicaffeoyl- Quinic Acids | Ferulic Acid Derivatives | Feruloyl- Quinic Acid Derivatives | Sinapic Acid Derivatives | p-Coumaric Acid Derivatives | Total Hydroxycin- Namic Acids | |
|---|---|---|---|---|---|---|---|---|---|---|
| Carmen/Mahaleb | 29.6 ± 2.9 d–f | 43.4 ± 5.0 | 31.0 ± 3.7 d–g | 0.030 ± 0.002 | 0.32 ± 0.03 de | 1.05 ± 0.06 | 0.21 ± 0.02 b–d | 5.48 ± 0.92 e–h | 111.1 ± 14.8 fg | |
| Carmen/Colt | 52.0 ± 5.5 b–f | 59.4 ± 5.3 | 33.7 ± 2.6 d–f | 0.027 ± 0.003 | 0.39 ± 0.04 c–e | 1.27 ± 0.05 | 0.25 ± 0.02 ab | 9.66 ± 1.02 c–e | 156.7 ± 19.5 b–d | |
| Carmen/Oblacinska | 36.7 ± 4.2 d–g | 63.2 ± 7.5 | 58.6 ± 3.6 a | 0.019 ± 0.003 | 0.34 ± 0.04 de | 1.29 ± 0.07 | 0.27 ± 0.03 ab | 6.81 ± 1.71 d–h | 167.2 ± 21.7 ab | |
| Carmen/M × M 14 | 50.0 ± 5.1 b–f | 61.1 ± 7.0 | 38.6 ± 3.1 c–e | 0.021 ± 0.003 | 0.46 ± 0.06 a–e | 1.32 ± 0.06 | 0.25 ± 0.03 a–c | 9.29 ± 1.29 c–f | 161,1 ± 19.8 a–c | |
| Carmen/Gisela 5 | 42.4 ± 4.7 c–g | 87.0 ± 7.5 | 52.4 ± 4.9 ab | 0.026 ± 0.003 | 0.42 ± 0.04 a–e | 1.45 ± 0.14 | 0.29 ± 0.03 a | 7.87 ± 0.81 d–g | 190.9 ± 26.8 a | |
| Carmen/Gisela 6 | 29.0 ± 4.6 e–g | 46.3 ± 4.4 | 40.9 ± 3.9 b–d | 0.028 ± 0.002 | 0.40 ± 0.05 b–e | 1.32 ± 0.07 | 0.26 ± 0.02 ab | 5.36 ± 0.85 f–h | 123.6 ± 14.1 de | |
| Kordia/Mahaleb | 46.0 ± 3.6 c–g | 55.9 ± 1.4 | 27.6 ± 1.4 e–g | 0.029 ± 0.004 | 0.47 ± 0.05 a–e | 0.97 ± 0.03 | 0.14 ± 0.01 g | 8.54 ± 0.68 c–f | 139.6 ± 15.0 c–e | |
| Kordia/Colt | 86.1 ± 9.6 a | 61.8 ± 8.3 | 32.3 ± 3.4 d–g | 0.033 ± 0.002 | 0.60 ± 0.06 ab | 1.34 ± 0.13 | 0.20 ± 0.01 c–f | 16.02 ± 1.03 a | 198.4 ± 17.8 a | |
| Kordia/Oblacinska | 57.6 ± 5.5 a–e | 69.4 ± 4.8 | 39.4 ± 3.3 b–e | 0.036 ± 0.006 | 0.58 ± 0.05 a–c | 1.23 ± 0.07 | 0.18 ± 0.01 d–g | 10.70 ± 1.96 b–d | 179.1 ± 19.9 ab | |
| Kordia/M × M 14 | 77.0 ± 7.8 ab | 64.4 ± 6.3 | 29.9 ± 2.6 d–g | 0.031 ± 0.009 | 0.57 ± 0.05 a–c | 1.12 ± 0.07 | 0.16 ± 0.01 d–g | 14.32 ± 1.83 ab | 187.5 ±2 5.8 ab | |
| Kordia/Gisela 5 | 58.0 ± 6.9 a–d | 72.2 ± 8.7 | 48.0 ± 4.4 a–c | 0.036 ± 0.009 | 0.56 ± 0.02 a–c | 1.31 ± 0.02 | 0.21 ± 0.01 c–e | 10.77 ± 1.41 b–d | 191.1 ± 22.4 a | |
| Kordia/Gisela 6 | 37.5 ± 5.7 d–g | 60.1 ± 5.1 | 48.3 ± 5.0 a–c | 0.026 ± 0.007 | 0.54 ± 0.06 a–c | 1.31 ± 0.20 | 0.21 ± 0.03 c–f | 6.94 ± 1.08 d–h | 154.4 ± 18.7 b–d | |
| Regina/Mahaleb | 18.4 ± 1.8 g | 42.7 ± 3.3 | 10.2 ± 1.4 h | 0.034 ± 0.004 | 0.27 ± 0.04 e | 0.81 ± 0.07 | 0.16 ± 0.01 e–g | 3.40 ± 0.33 h | 75.9 ± 13.2 g | |
| Regina/Colt | 43.7 ± 4.2 c–g | 46.1 ± 4.5 | 23.3 ± 3.1 f–h | 0.035 ± 0.005 | 0.46 ± 0.05 a–e | 0.96 ± 0.08 | 0.14 ± 0.01 g | 8.11 ± 1.72 d–g | 122.8 ± 15.9 ef | |
| Regina/Oblacinska | 66.9 ± 6.6 a–c | 47.6 ± 5.7 | 27.9 ± 5.2 d–g | 0.038 ± 0.004 | 0.61 ± 0.07 a | 1.14 ± 0.03 | 0.15 ± 0.02 fg | 12.43 ± 1.18 a–c | 156.8 ± 26.1 b–d | |
| Regina/M × M 14 | 68.0 ± 5.8 a–c | 49.4 ± 4.7 | 22.1 ± 3.7 f–h | 0.041 ± 0.008 | 0.49 ± 0.05 a–d | 1.06 ± 0.08 | 0.16 ± 0.01 e–g | 12.66 ± 1.70 ab | 153.9 ± 23.2 b–e | |
| Regina/Gisela 6 | 23.4 ± 2.9 fg | 42.6 ± 4.8 | 19.6 ± 1.8 gh | 0.038 ± 0.008 | 0.57 ± 0.05 a–c | 1.01 ± 0.09 | 0.16 ± 0.03 d–g | 4.00 ± 0.50 gh | 91.4 ± 24.4 fg | |
| Cultivar | Carmen | 55.8 ± 6.1 a | 62.0 ± 4.2 a | 36.3 ± 2.4 a | 0.034 ± 0.002 a | 0.39 ± 0.03 c | 1.27 ± 0.05 a | 0.25 ± 0.01 | 7.41 ± 0.56 b | 163.4 ± 9.9 a |
| Kordia | 52.9 ± 4.6 a | 50.8 ± 2.9 b | 32.7 ± 2.4 c | 0.028 ± 0.002 b | 0.56 ± 0.02 a | 1.09 ± 0.04 c | 0.18 ± 0.01 | 11.21 ± 1.01 a | 154.5 ± 15.0 a | |
| Regina | 34.2 ± 2.4 b | 60.9 ± 2.3 a | 33.9 ± 3.6 b | 0.032 ± 0.003 ab | 0.48 ± 0.03 b | 1.16 ± 0.05 bc | 0.16 ± 0.01 | 8.12 ± 1.14 b | 139.9 ± 13.5 b | |
| Rootstock | Mahaleb | 31.3 ± 3.4 c | 44.0 ± 3.4 c | 22.9 ± 2.6 e | 0.031 ± 0.002 | 0.35 ± 0.03 b | 0.95 ± 0.04 c | 0.17 ± 0.01 c | 5.81 ± 0.63 c | 105.5 ± 17.4 c |
| Colt | 60.6 ± 6.8 ab | 62.4 ± 6.5 ab | 29.8 ± 2.0 de | 0.032 ± 0.003 | 0.48 ± 0.04 a | 1.19 ± 0.06 b | 0.20 ± 0.01 b | 11.26 ± 1.63 ab | 155.9 ± 24.2 b | |
| Oblacinska | 53.7 ± 6.5 ab | 60.1 ± 4.2 ab | 41.9 ± 3.8 b | 0.031 ± 0.004 | 0.51 ± 0.05 a | 1.22 ± 0.03 b | 0.20 ± 0.02 b | 9.98 ± 1.21 ab | 163.64 ± 17.2 ab | |
| M × M 14 | 65.0 ± 5.6 a | 63.3 ± 4.5 a | 30.2 ± 2.4 cd | 0.031 ± 0.004 | 0.51 ± 0.03 a | 1.17 ± 0.05 b | 0.19 ± 0.01 bc | 12.09 ± 1.42 a | 172.49 ± 21.8 b | |
| Gisela 5 | 50.2 ± 6.0 b | 69.1 ± 5.5 a | 50.2 ± 4.4 a | 0.031 ± 0.004 | 0.49 ± 0.05 a | 1.38 ± 0.07 a | 0.25 ± 0.03 a | 9.32 ± 1.50 b | 180.97 ± 22.0 a | |
| Gisela 6 | 30.0 ± 2.8 c | 51.3 ± 3.0 bc | 36.3 ± 4.7 bc | 0.03 1± 0.003 | 0.50 ± 0.05 a | 1.21 ± 0.08 b | 0.21 ± 0.02 b | 5.43 ± 0.54 c | 124.98 ± 21.5 c | |
| Year | 2020 | 56.8 ± 5.8 a | 54.0 ± 3.7 b | 26.3 ± 2.9 b | 0.033 ± 0.002 | 0.41 ± 0.03 b | 1.18 ± 0.29 | 0.22 ± 0.01 a | 10.53 ± 0.99 a | 149.5 ± 11.1 b |
| 2021 | 40.0 ± 2.5 b | 61.5 ± 1.8 a | 42.3 ± 2.7 a | 0.029 ± 0.002 | 0.54 ± 0.02 a | 1.17 ± 0.22 | 0.18 ± 0.01 b | 7.40 ± 0.37 b | 153.1 ± 10.1 a | |
| Statistical significance | ||||||||||
| Cultivar | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| Rootstock | *** | *** | *** | ns | *** | *** | ** | *** | *** | |
| Year | *** | *** | *** | ns | *** | ns | *** | *** | *** | |
| Cultivar × Rootstock | *** | ns | *** | ns | ** | ns | * | *** | * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boskov, D.; Milatovic, D.; Rakonjac, V.; Zec, G.; Hudina, M.; Veberic, R.; Mikulic-Petkovsek, M. The Phenolic Profile of Sweet Cherry Fruits Influenced by Cultivar/Rootstock Combination. Plants 2023, 12, 103. https://doi.org/10.3390/plants12010103
Boskov D, Milatovic D, Rakonjac V, Zec G, Hudina M, Veberic R, Mikulic-Petkovsek M. The Phenolic Profile of Sweet Cherry Fruits Influenced by Cultivar/Rootstock Combination. Plants. 2023; 12(1):103. https://doi.org/10.3390/plants12010103
Chicago/Turabian StyleBoskov, Djordje, Dragan Milatovic, Vera Rakonjac, Gordan Zec, Metka Hudina, Robert Veberic, and Maja Mikulic-Petkovsek. 2023. "The Phenolic Profile of Sweet Cherry Fruits Influenced by Cultivar/Rootstock Combination" Plants 12, no. 1: 103. https://doi.org/10.3390/plants12010103
APA StyleBoskov, D., Milatovic, D., Rakonjac, V., Zec, G., Hudina, M., Veberic, R., & Mikulic-Petkovsek, M. (2023). The Phenolic Profile of Sweet Cherry Fruits Influenced by Cultivar/Rootstock Combination. Plants, 12(1), 103. https://doi.org/10.3390/plants12010103








