Histochemical Analysis and Ultrastructure of Trichomes and Laticifers of Croton gratissimus Burch. var. gratissimus (Euphorbiaceae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection and Sampling
2.2. Stereomicroscopy
2.3. Scanning Electron Microscopy (SEM)
2.3.1. Chemical Fixation
2.3.2. Freeze-Drying
2.4. Sample Preparation for Light and Transmission Electron Microscopy (TEM)
2.5. Fluorescence Microscopy
2.5.1. Acridine Orange
2.5.2. Auto-Fluorescence
2.6. Histochemistry
2.6.1. Alkaloids
2.6.2. Lipids
Sudan III
Nile blue A
2.6.3. Phenolic Compounds
2.6.4. Lignin
2.6.5. Polysaccharides (Pectin and Mucilage)
2.6.6. Carboxylated Polysaccharides, Polyuronides, Macromolecules with Free Phosphate Groups, and Polyphenols
3. Results and Discussion
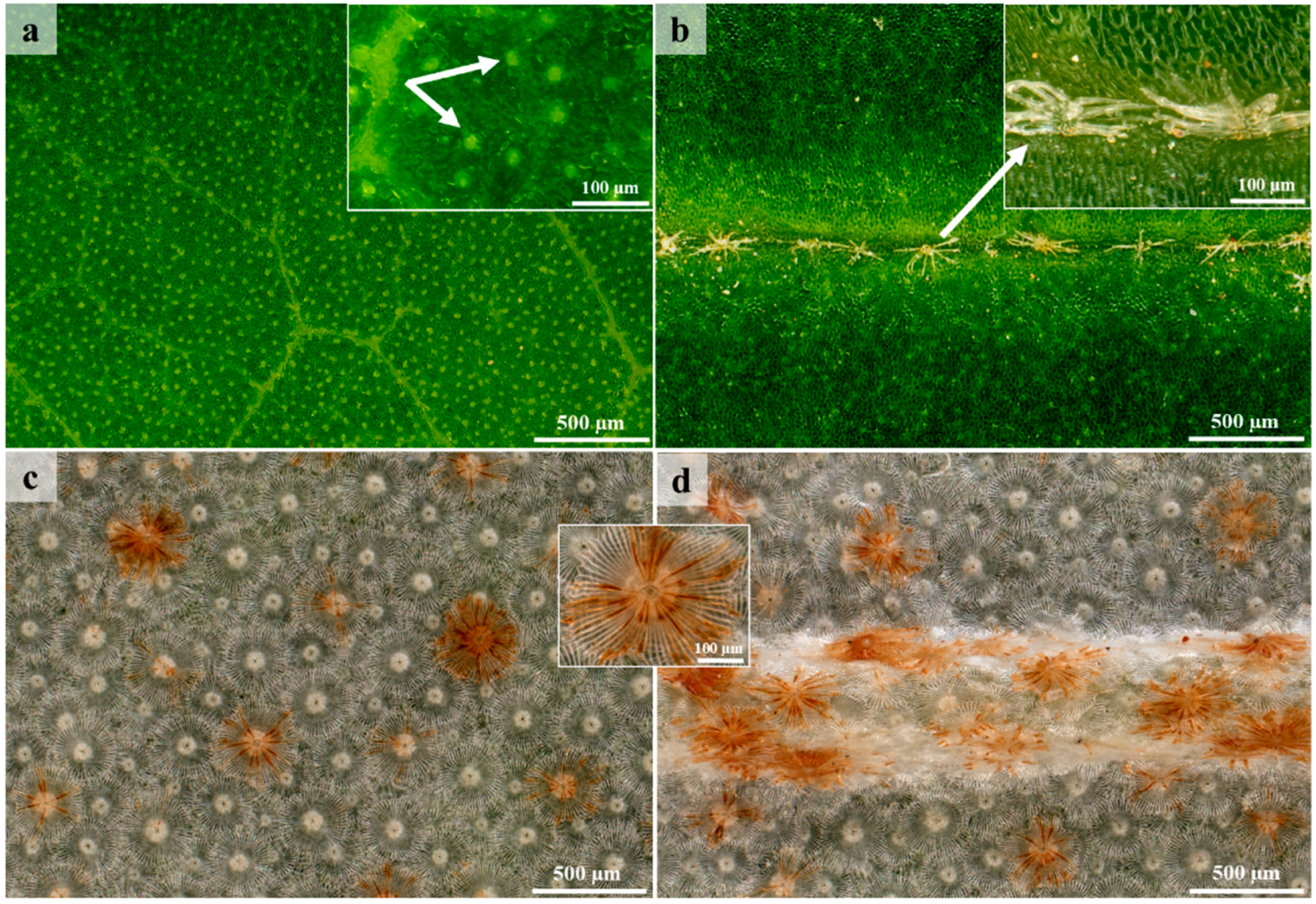
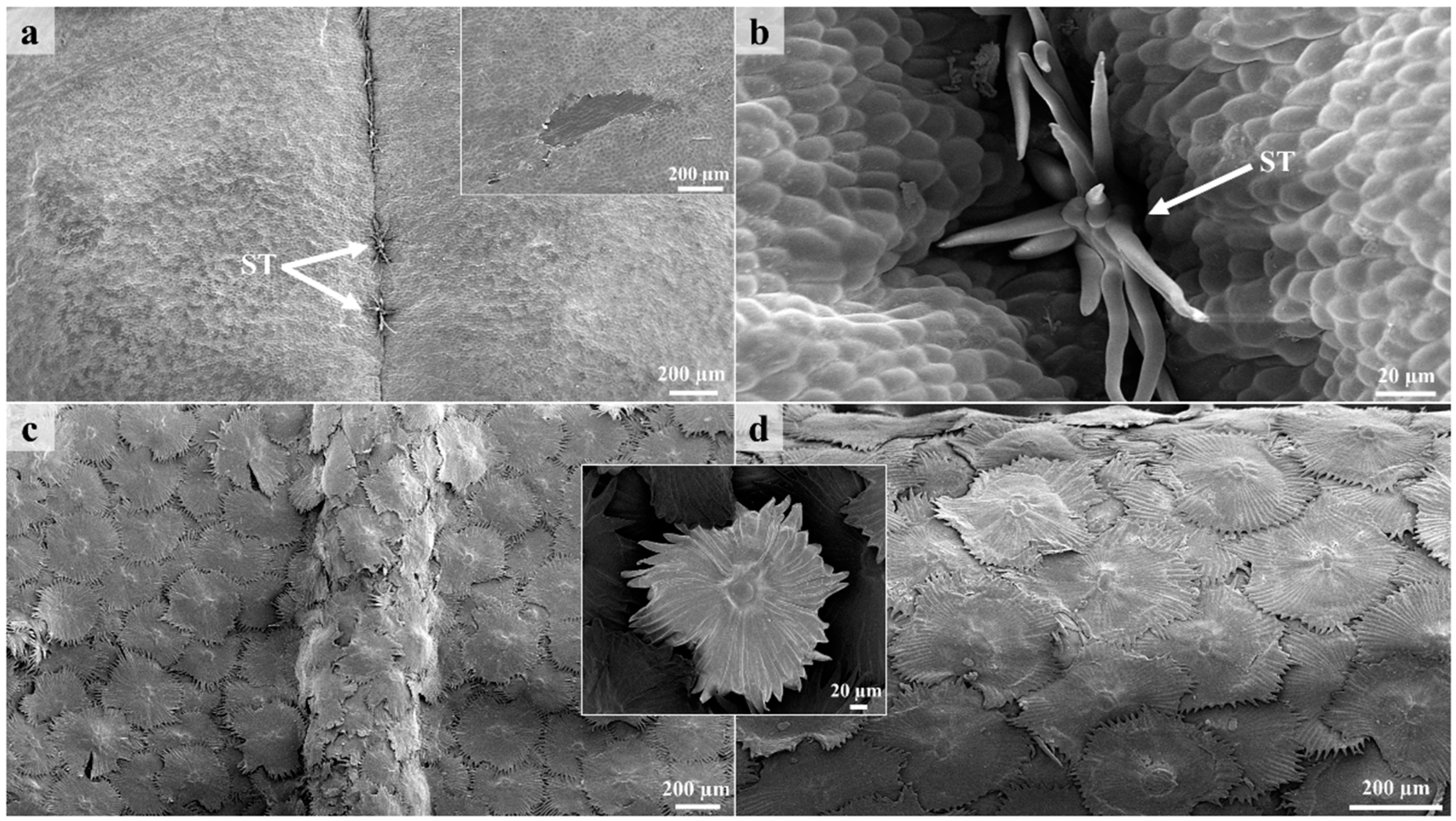
3.1. Surface Overview
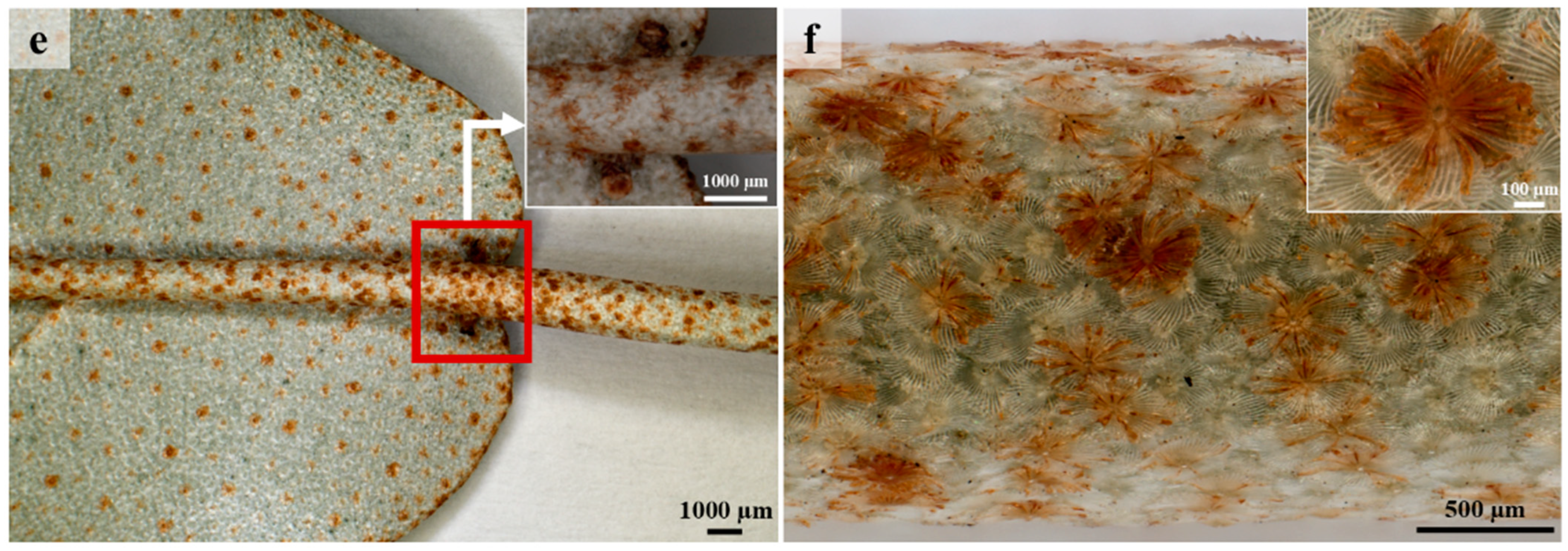
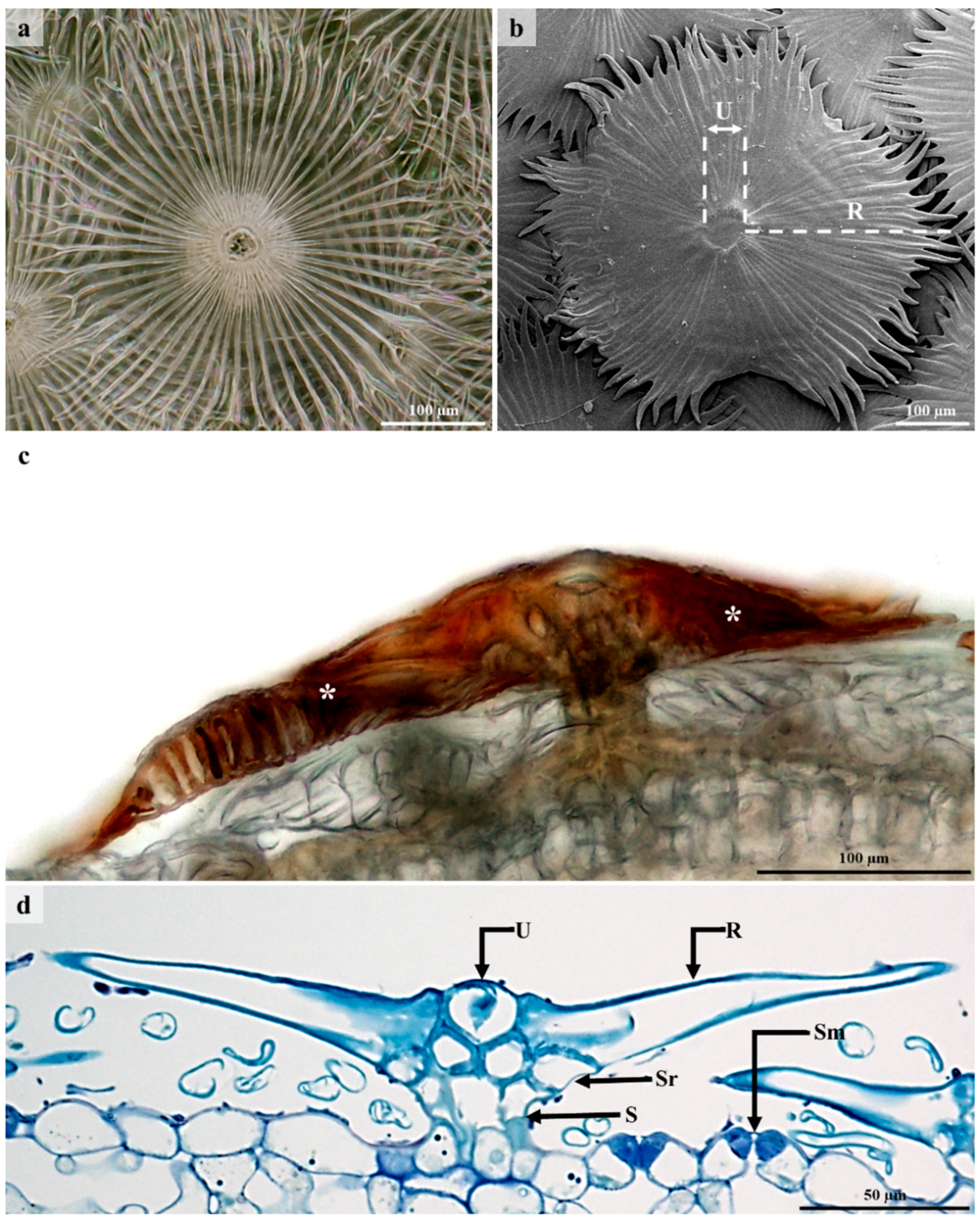
3.2. Lepidote Trichomes
3.3. Glandular Trichomes
3.4. Laticifers
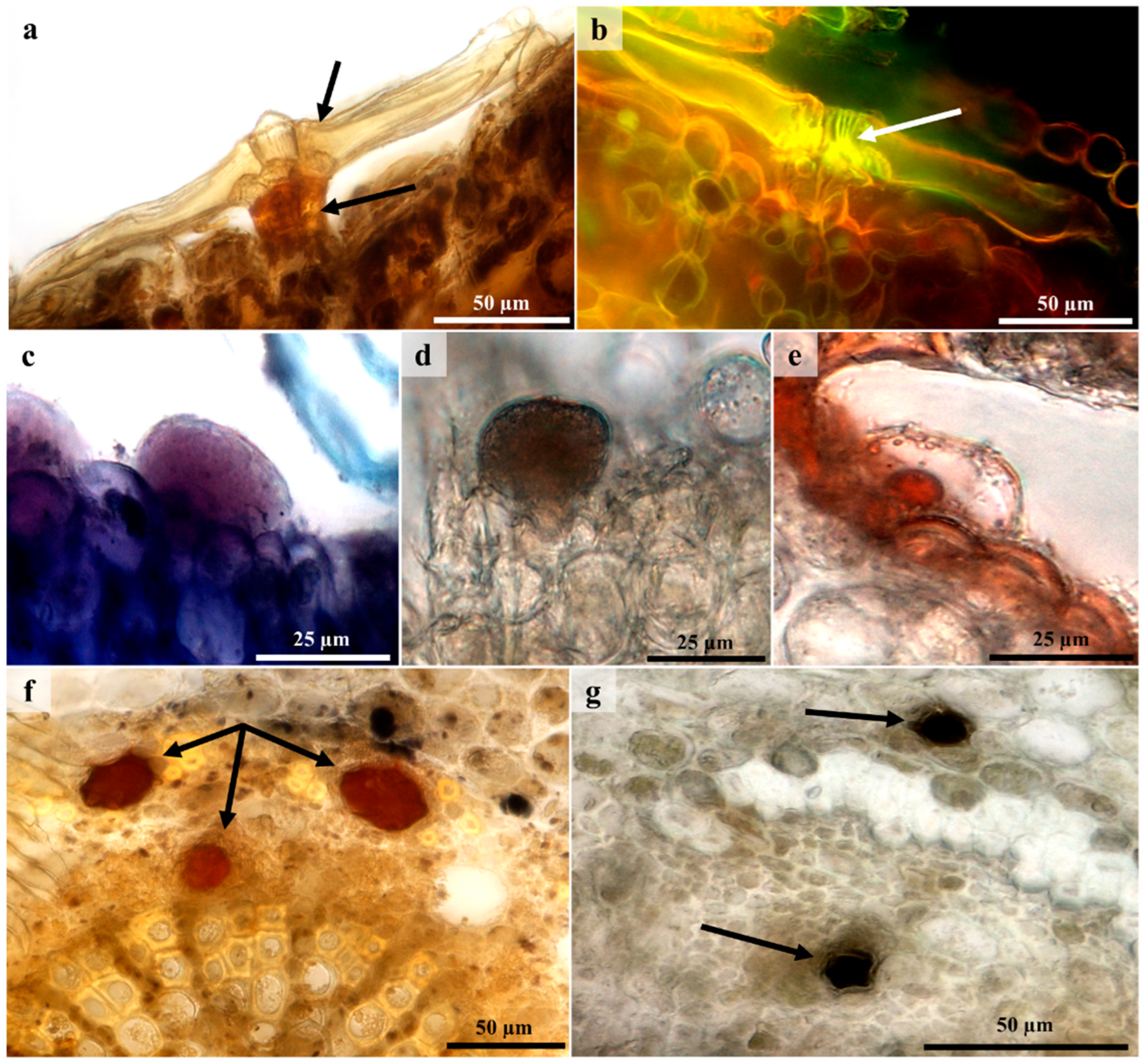
3.5. Histochemistry and Fluorescence Microscopy
3.6. Ultrastructure of Lepidote Trichomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, S.; Chakraborty, R. Revival, modernization and integration of Indian traditional herbal medicine in clinical practice: Importance, challenges and future. J. Tradit. Complement. Med. 2017, 7, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Chikezie, P.C.; Ibegbulem, C.O.; Mbagwu, F.N. Bioactive principles from medicinal plants. Research J. Phytochem. 2015, 9, 88–115. [Google Scholar] [CrossRef]
- Lee, Y.L.; Ding, P. Production of essential oil in plants: Ontogeny, secretory structures and seasonal variations. Pertanika J. Sch. Res. Rev. 2016, 2, 1–10. [Google Scholar]
- Demarco, D. Histochemical analysis of plant secretory structures. In Histochemistry of Single Molecules: Methods and Protocols, Methods in Molecular Biology; Pellicciari, C., Biggiogera, M., Eds.; Humana Press: New York, NY, USA, 2017; pp. 313–330. [Google Scholar]
- Tissier, A. Plant secretory structures: More than just reaction bags. Curr. Opin. Biotechnol. 2018, 49, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Esau, K. Plant Anatomy, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1965. [Google Scholar]
- Dickison, W.C. Integrative Plant Anatomy; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Mwine, J.T.; Van Damme, P. Why do Euphorbiaceae tick as medicinal plants? A review of Euphorbiaceae family and its medicinal features. J. Med. Plant Res. 2011, 5, 652–662. [Google Scholar]
- Schilmiller, A.L.; Last, R.L.; Pichersky, E. Harnessing plant trichome biochemistry for the production of useful compounds. Plant J. 2008, 54, 702–711. [Google Scholar] [CrossRef]
- Wagner, G.J.; Wang, E.; Shepherd, R.W. New approaches for studying and exploiting an old protuberance, the plant trichome. Ann. Bot. 2004, 93, 3–11. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, E.S. Structural features of glandular and non-glandular trichomes in three species of Mentha. Appl. Microsc. 2013, 43, 47–53. [Google Scholar] [CrossRef]
- Huchelmann, A.; Boutry, M.; Hachez, C. Plant glandular trichomes: Natural cell factories of high biotechnological interest. Plant Physiol. 2017, 175, 6–22. [Google Scholar] [CrossRef]
- Glas, J.J.; Schimmel, B.C.; Alba, J.M.; Escobar-Bravo, R.; Schuurink, R.C.; Kant, M.R. Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef] [PubMed]
- Tozin, L.R.D.S.; de Melo Silva, S.C.; Rodrigues, T.M. Non-glandular trichomes in Lamiaceae and Verbenaceae species: Morphological and histochemical features indicate more than physical protection. N. Z. J. Bot. 2016, 54, 446–457. [Google Scholar] [CrossRef]
- Fahn, A. Secretory Tissues in Plants; Academic Press: London, UK, 1979. [Google Scholar]
- Pickard, W.F. Laticifers and secretory ducts: Two other tube systems in plants. New Phytol. 2008, 177, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Castelblanque, L.; Balaguer, B.; Martí, C.; Rodríguez, J.J.; Orozco, M.; Vera, P. Novel insights into the organization of laticifer cells: A cell comprising a unified whole system. Plant Physiol. 2016, 172, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Hagel, J.M.; Yeung, E.C.; Facchini, P.J. Got milk? The secret life of laticifers. Trends Plant Sci. 2008, 13, 631–639. [Google Scholar] [CrossRef]
- Lange, B.M. The evolution of plant secretory structures and emergence of terpenoid chemical diversity. Annu. Rev. Plant Biol. 2015, 66, 139–159. [Google Scholar] [CrossRef]
- Castro, M.D.; Demarco, D. Phenolic compounds produced by secretory structures in plants: A brief review. Nat. Prod. Commun. 2008, 3, 1273–1284. [Google Scholar]
- Salatino, A.; Salatino, M.L.F.; Negri, G. Traditional uses, chemistry and pharmacology of Croton species (Euphorbiaceae). J. Braz. Chem. Soc. 2007, 18, 11–33. [Google Scholar] [CrossRef]
- Block, S.; Baccelli, C.; Tinant, B.; Van Meervelt, L.; Rozenberg, R.; Jiwan, J.L.H.; Llabres, G.; De Pauw-Gillet, M.C.; Quetin-Leclercq, J. Diterpenes from the leaves of Croton zambesicus. Phytochemistry 2004, 65, 1165–1171. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Langat, M.K.; Crouch, N.R.; Coley, H.M.; Mutambi, E.M.; Nuzillard, J.M. Cembranolides from the stem bark of the southern African medicinal plant, Croton gratissimus (Euphorbiaceae). Phytochemistry 2010, 71, 1381–1386. [Google Scholar] [CrossRef]
- PlantZAfrica. Croton gratissimus Burch. Available online: http://pza.sanbi.org/croton-gratissimus (accessed on 5 February 2018).
- Ngadjui, B.T.; Abegaz, B.M.; Keumedjio, F.; Folefoc, G.N.; Kapche, G.W. Diterpenoids from the stem bark of Croton zambesicus. Phytochemistry 2002, 60, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, S.F.; Viljoen, A.M. In vitro evidence of phyto-synergy for plant part combinations of Croton gratissimus (Euphorbiaceae) used in African traditional healing. J. Ethnopharmacol. 2008, 119, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Langat, M.K.; Crouch, N.R.; Smith, P.J.; Mulholland, D.A. Cembranolides from the leaves of Croton gratissimus. J. Nat. Prod. 2011, 74, 2349–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Munien, P.; Naidoo, Y.; Naidoo, G. Micromorphology, histochemistry and ultrastructure of the foliar trichomes of Withania somnifera (L.) Dunal (Solanaceae). Planta 2015, 242, 1107–1122. [Google Scholar] [CrossRef]
- Talamond, P.; Verdeil, J.L.; Conéjéro, G. Secondary metabolite localization by autofluorescence in living plant cells. Molecules 2015, 20, 5024–5037. [Google Scholar] [CrossRef]
- Furr, M.; Mahlberg, P.G. Histochemical analyses of laticifers and glandular trichomes in Cannabis sativa. J. Nat. Prod. 1981, 44, 153–159. [Google Scholar] [CrossRef]
- Pearse, A.G.E. Histochemistry: Theoretical and Applied, 4th ed.; Longman: Harlow, UK, 1985; Volume II. [Google Scholar]
- Guo, J.; Yuan, Y.; Liu, Z.; Zhu, J. Development and structure of internal glands and external glandular trichomes in Pogostemon cablin. PLoS One 2013, 8, 1–13. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill: London, UK, 1940. [Google Scholar]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Zander, R.H. A new progressive polychrome protocol for staining bryophytes. Phytoneuron 2016, 2, 1–12. [Google Scholar]
- De Andrade, E.A.; Folquitto, D.G.; Luz, L.E.C.; Paludo, K.S.; Farago, P.V.; Budel, J.M. Anatomy and histochemistry of leaves and stems of Sapium glandulosum. Rev. Bras. Farmacogn. 2017, 27, 282–289. [Google Scholar] [CrossRef]
- Leandri, J. Contribution a l’etude des Croton malgaches a grandes feuilles argentees. Adansonia 1972, 12, 403–408. [Google Scholar]
- Berry, P.E.; van Ee, B.W.; Kainulainen, K.; Achtemeier, L. Croton cupreolepis (Euphorbiaceae), a new coppery-lepidote tree species from eastern Madagascar. Syst. Bot. 2016, 41, 977–982. [Google Scholar] [CrossRef]
- Vitarelli, N.C.; Riina, R.; Caruzo, M.B.R.; Cordeiro, I.; Fuertes-Aguilar, J.; Meira, R.M. Foliar secretory structures in Crotoneae (Euphorbiaceae): Diversity, anatomy, and evolutionary significance. Am. J. Bot. 2015, 102, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Werker, E. Trichome diversity and development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar]
- Webster, G.L.; Del-Arco-Aguilar, M.J.; Smith, B.A. Systematic distribution of foliar trichome types in Croton (Euphorbiaceae). Bot. J. Linn. Soc. 1996, 121, 41–57. [Google Scholar] [CrossRef]
- Vitarelli, N.C.; Riina, R.; Cassino, M.F.; Meira, R.M.S.A. Trichome-like emergences in Croton of Brazilian highland rock outcrops: Evidences for atmospheric water uptake. Perspect. Plant Ecol. Evol. Syst. 2016, 22, 23–35. [Google Scholar] [CrossRef]
- Kalicharan, B.; Naidoo, Y.; Heneidak, S.; Bhatt, A. Distribution, morphological and histochemical characteristics of foliar trichomes of Plectranthus zuluensis (Lamiaceae). Rev. Bras. Bot. 2015, 38, 961–971. [Google Scholar] [CrossRef]
- Thakur, H.A.; Patil, D.A. Foliar epidermal studies of plants in Euphorbiaceae. Taiwania 2014, 59, 59–70. [Google Scholar]
- De Sá-Haiad, B.; Serpa-Ribeiro, A.C.C.; Barbosa, C.N.; Pizzini, D.; Leal, D.D.O.; de Senna-Valle, L.; de Santiago-Fernandes, L.D.R. Leaf structure of species from three closely related genera from tribe Crotoneae Dumort. (Euphorbiaceae ss, Malpighiales). Plant Syst. Evol. 2009, 283, 179–202. [Google Scholar] [CrossRef]
- Wurdack, K.J.; Hoffmann, P.; Chase, M.W. Molecular phylogenetic analysis of uniovulate Euphorbiaceae (Euphorbiaceae sensu stricto) using plastid rbcL and trnL-F DNA sequences. Am. J. Bot. 2005, 92, 1397–1420. [Google Scholar] [CrossRef] [PubMed]
- Demarco, D.; de Moraes Castro, M.; Ascensão, L. Two laticifer systems in Sapium haematospermum—New records for Euphorbiaceae. Botany 2013, 91, 545–554. [Google Scholar] [CrossRef]
- Da Cunha, M.; Costa, C.G.; Machado, R.D.; Miguens, F.C. Distribution and differentiation of the laticifer system in Chamaesyce thymifolia (L.) Millsp. (Euphorbiaceae). Acta Bot. Neerl. 1998, 47, 209–218. [Google Scholar]
- Rudall, P. Laticifers in vascular cambium and wood of Croton spp. (Euphorbiaceae). Inter. Assoc. Wood Anatom. J. 1989, 10, 379–383. [Google Scholar] [CrossRef]
- Prado, E.; Demarco, D. Laticifers and Secretory Ducts: Similarities and Differences. In Ecosystem Services and Global Ecology; IntechOpen: London, UK, 2018; pp. 103–123. [Google Scholar]
- Southorn, W.A. Physiology of Hevea (latex flow). J. Rubber Res. J. 1969, 21, 494–512. [Google Scholar]
- Demarco, D. Micromorphology and histochemistry of the laticifers from vegetative organs of asclepiadoideae species (Apocynaceae). Acta Biol. Colomb. 2015, 20, 57–65. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Horner, H.T. Calcium oxalate crystals in plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- Anitha, R.; Sandhiya, T. Occurrence of calcium oxalate Crystals in the leaves of medicinal plants. Int. J. Pharmacogn. 2014, 1, 389–393. [Google Scholar]
- Pennisi, S.V.; McConnell, D.B.; Gower, L.B.; Kane, M.E.; Lucansky, T. Intracellular calcium oxalate crystal structure in Dracaena sanderiana. New Phytol. 2001, 150, 111–120. [Google Scholar] [CrossRef]
- Konyar, S.T.; Öztürk, N.; Dane, F. Occurrence, types and distribution of calcium oxalate crystals in leaves and stems of some species of poisonous plants. Bot. Stud. 2014, 55, 1–9. [Google Scholar]
- Solereder, H. Systematic Anatomy of the Dicotyledons; Ajay Book Service: New Delhi, India, 1908; Volume II. [Google Scholar]
- Nakata, P.A. Calcium oxalate crystal morphology. Trends Plant Sci. 2002, 7, 324. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, J.A.; Gangadhara, M. Studies on the trichomes of some Euphorbiaceae. Feddes Repert. 1977, 88, 103–111. [Google Scholar] [CrossRef]
- Liu, H.F.; Deng, Y.F.; Liao, J.P. Foliar trichomes of Croton L. (Euphorbiaceae: Crotonoideae) from China and its taxonomic implications. Bangladesh J. Plant Taxon. 2013, 20, 85–94. [Google Scholar] [CrossRef]
- Feio, A.C.; Meira, R.M.; Riina, R. Leaf anatomical features and their implications for the systematics of dragon’s blood, Croton section Cyclostigma (Euphorbiaceae). Bot. J. Linn. Soc. 2018, 187, 614–632. [Google Scholar] [CrossRef]
- Voragen, A.G.; Coenen, G.J.; Verhoef, R.P.; Schols, H.A. Pectin, a versatile polysaccharide present in plant cell walls. Struct. Chem. 2009, 20, 263–275. [Google Scholar] [CrossRef]
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Roy, A. A review on the alkaloids an important therapeutic compound from plants. Inter. J. Plant Biotechnol. 2017, 1, 1–9. [Google Scholar]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Bribi, N. Pharmacological activity of Alkaloids: A Review. Asian J. Bot. 2018, 1, 1–6. [Google Scholar]
- Huang, W.Y.; Cai, Y.Z.; Zhang, Y. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr Cancer. 2009, 62, 1–20. [Google Scholar] [CrossRef]
- Maslennikov, P.V.; Chupakhina, G.N.; Skrypnik, L.N. The content of phenolic compounds in medicinal plants of a botanical garden (Kaliningrad oblast). Biol. Bull. 2014, 41, 133–138. [Google Scholar] [CrossRef]
- Valkama, E.; Salminen, J.P.; Koricheva, J.; Pihlaja, K. Comparative analysis of leaf trichome structure and composition of epicuticular flavonoids in Finnish birch species. Ann. Bot. 2003, 91, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Feio, A.C.; Riina, R.; Meira, R.M.S.A. Secretory structures in leaves and flowers of two Dragon’s blood Croton (Euphorbiaceae): New evidence and interpretations. Int. J. Plant Sci. 2016, 177, 511–522. [Google Scholar] [CrossRef]
- Machado, S.R.; Gregório, E.A.; Guimarães, E. Ovary peltate trichomes of Zeyheria montana (Bignoniaceae): Developmental ultrastructure and secretion in relation to function. Ann. Bot. 2005, 97, 357–369. [Google Scholar] [CrossRef]
- Huang, S.S.; Kirchoff, B.K.; Liao, J.P. The capitate and peltate glandular trichomes of Lavandula pinnata L. (Lamiaceae): Histochemistry, ultrastructure, and secretion. J. Torrey Bot. Soc. 2008, 135, 155–167. [Google Scholar] [CrossRef]
- Werker, E.; Fahn, A. Secretory hairs of Inula viscosa (L.) Ait-development, ultrastructure, and secretion. Bot. Gazette. 1981, 142, 461–476. [Google Scholar] [CrossRef]
- Ascensão, L.; Marques, N.; Pais, M.S. Peltate glandular trichomes of Leonotis leonurus leaves: Ultrastructure and histochemical characterization of secretions. Int. J. Plant Sci. 1997, 158, 249–258. [Google Scholar] [CrossRef]
- Ascensão, L.; Pais, M.S. The leaf capitate trichomes of Leonotis leonurus: Histochemistry, ultrastructure and secretion. Ann. Bot. 1998, 81, 263–271. [Google Scholar] [CrossRef]
- Turner, G.W.; Croteau, R. Organization of monoterpene biosynthesis in Mentha. Immunocytochemical localizations of geranyl diphosphate synthase, limonene-6-hydroxylase, isopiperitenol dehydrogenase, and pulegone reductase. Plant Physiol. 2004, 136, 4215–4227. [Google Scholar] [CrossRef]
- Naidoo, Y.; Heneidak, S.; Bhatt, A.; Kasim, N.; Naidoo, G. Morphology, histochemistry, and ultrastructure of foliar mucilage-producing trichomes of Harpagophytum procumbens (Pedaliaceae). Turk. J. Bot. 2014, 38, 60–67. [Google Scholar] [CrossRef]






| Compound Class | Histochemical Test | LT | GT | L | Reaction Observed |
|---|---|---|---|---|---|
| Alkaloids | Wagner’s reagent | ++ | ++ | ++ | The stalk of lepidote trichomes and laticifer cells stained orange–brown. Contents of glandular trichomes stained dark brown. |
| Lipids | Sudan III | + | + | ++ | Stalk cells and contents of lepidote trichomes stained orange. Lipid droplet in glandular trichome stained bright orange. Laticiferous cells stained orange. |
| Nile Blue A | ++ | ++ | ++ | Subradial, radial, and central cells of lepidote trichomes, and laticifer cells stained blue. Stalk cells of lepidote trichomes and contents of glandular trichomes stained pink. | |
| Phenolic compounds | Ferric chloride | ++ | ++ | ++ | Stalk and radial cells of lepidote trichomes stained brown and dark brown, respectively. Contents of glandular trichomes stained dark brown. Laticifers stained dark brown to black. |
| Lignin | Phloroglucinol | + | NT | NT | Central cells of lepidote trichomes stained light red. |
| Mucilage and pectin | Ruthenium Red | + | NT | ++ | The subradial, central, and radial cell walls of lepidote trichomes stained pink. Laticiferous cells stained pinkish red. |
| Carboxylated polysaccharides, polyuronides, macromolecules with free phosphate groups and polyphenols | Toluidine blue O | ++ | NT | ++ | Subradial and central cells of lepidote trichomes stained bright blue. Laticifers stained dark blue. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidoo, D.; Naidoo, Y.; Naidoo, G.; Kianersi, F.; Dewir, Y.H. Histochemical Analysis and Ultrastructure of Trichomes and Laticifers of Croton gratissimus Burch. var. gratissimus (Euphorbiaceae). Plants 2023, 12, 772. https://doi.org/10.3390/plants12040772
Naidoo D, Naidoo Y, Naidoo G, Kianersi F, Dewir YH. Histochemical Analysis and Ultrastructure of Trichomes and Laticifers of Croton gratissimus Burch. var. gratissimus (Euphorbiaceae). Plants. 2023; 12(4):772. https://doi.org/10.3390/plants12040772
Chicago/Turabian StyleNaidoo, Danesha, Yougasphree Naidoo, Gonasageran Naidoo, Farzad Kianersi, and Yaser Hassan Dewir. 2023. "Histochemical Analysis and Ultrastructure of Trichomes and Laticifers of Croton gratissimus Burch. var. gratissimus (Euphorbiaceae)" Plants 12, no. 4: 772. https://doi.org/10.3390/plants12040772
APA StyleNaidoo, D., Naidoo, Y., Naidoo, G., Kianersi, F., & Dewir, Y. H. (2023). Histochemical Analysis and Ultrastructure of Trichomes and Laticifers of Croton gratissimus Burch. var. gratissimus (Euphorbiaceae). Plants, 12(4), 772. https://doi.org/10.3390/plants12040772







