Growth, Flowering, and Fruit Production of Strawberry ‘Albion’ in Response to Photoperiod and Photosynthetic Photon Flux Density of Sole-Source Lighting
Abstract
1. Introduction
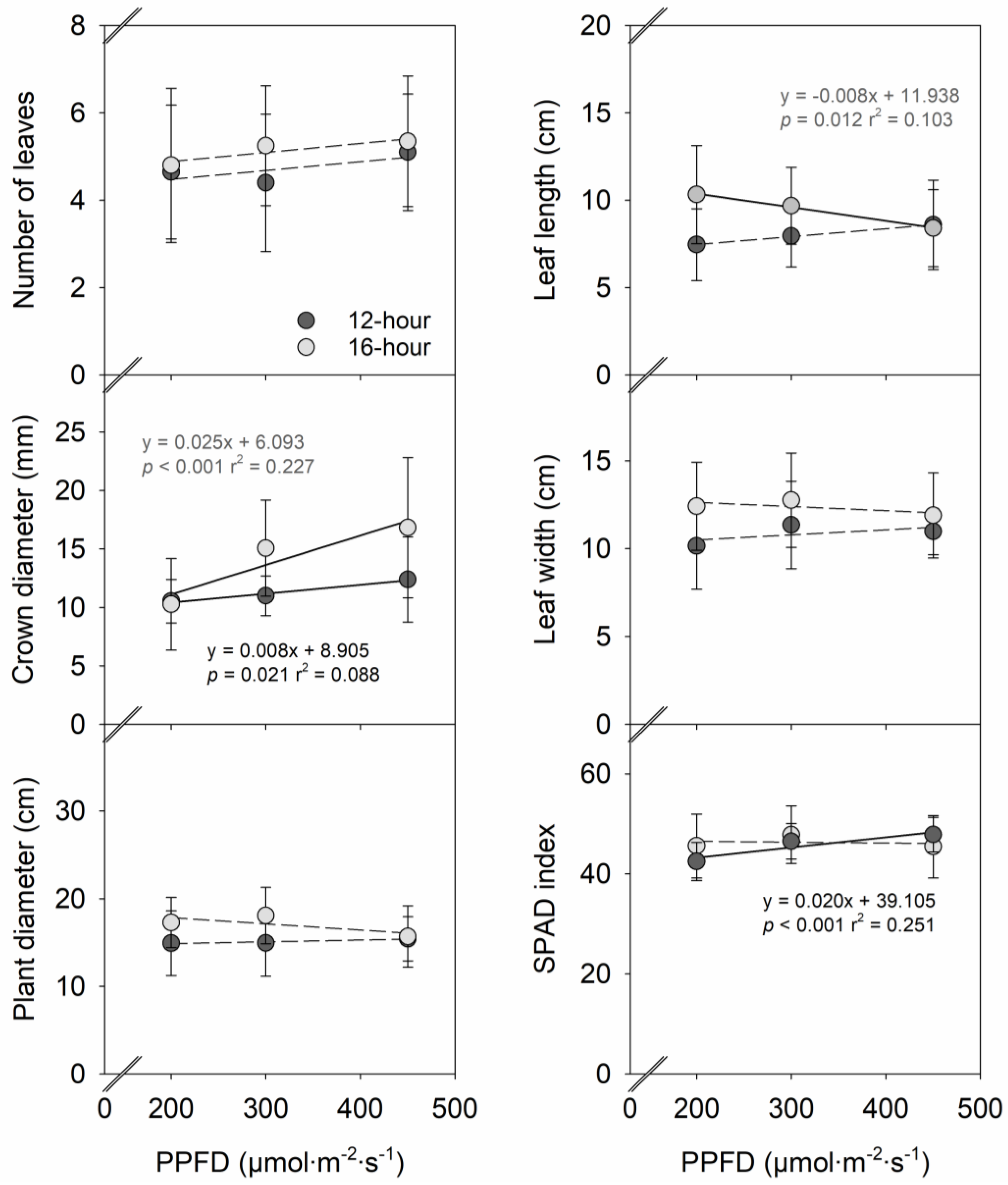
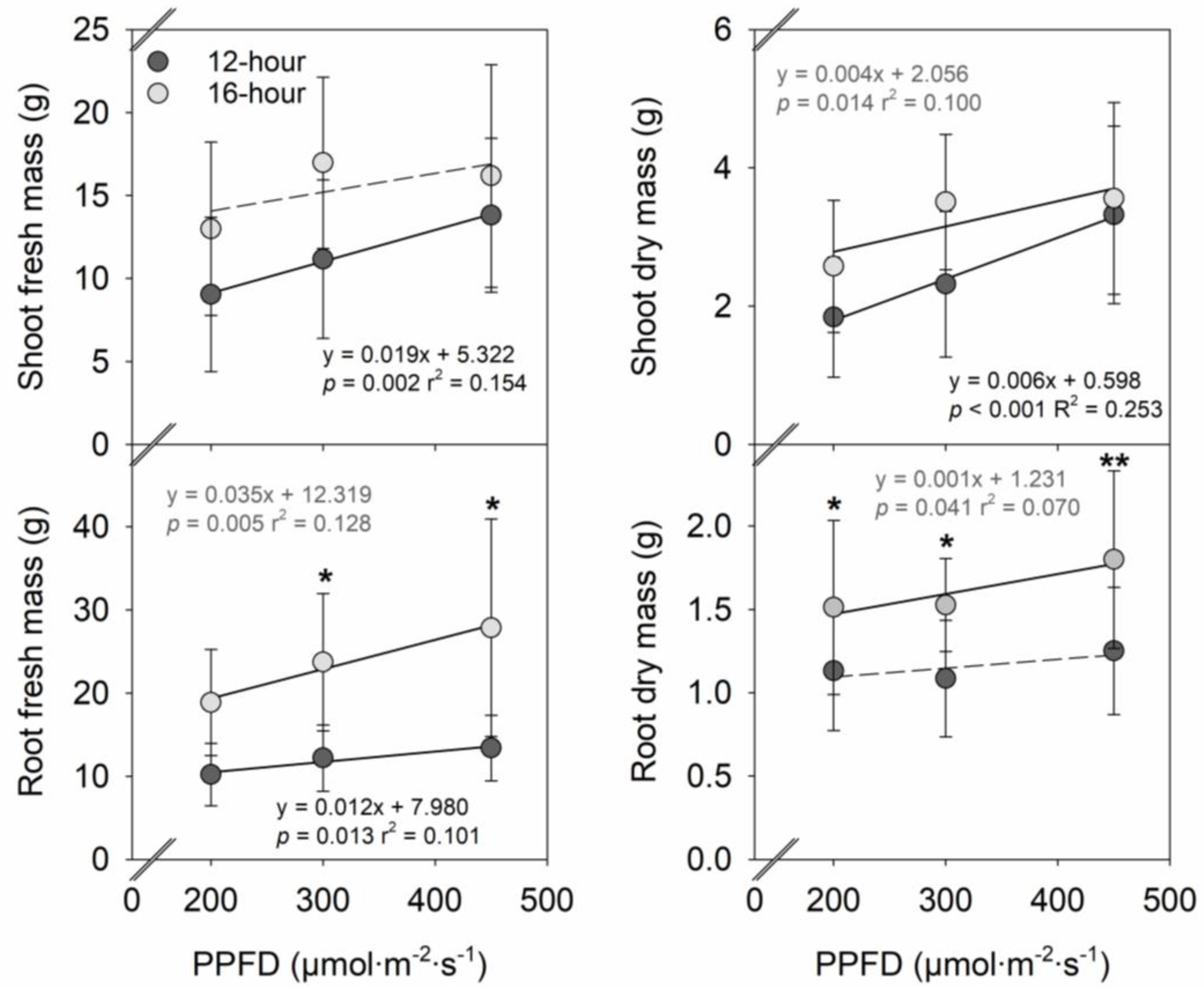
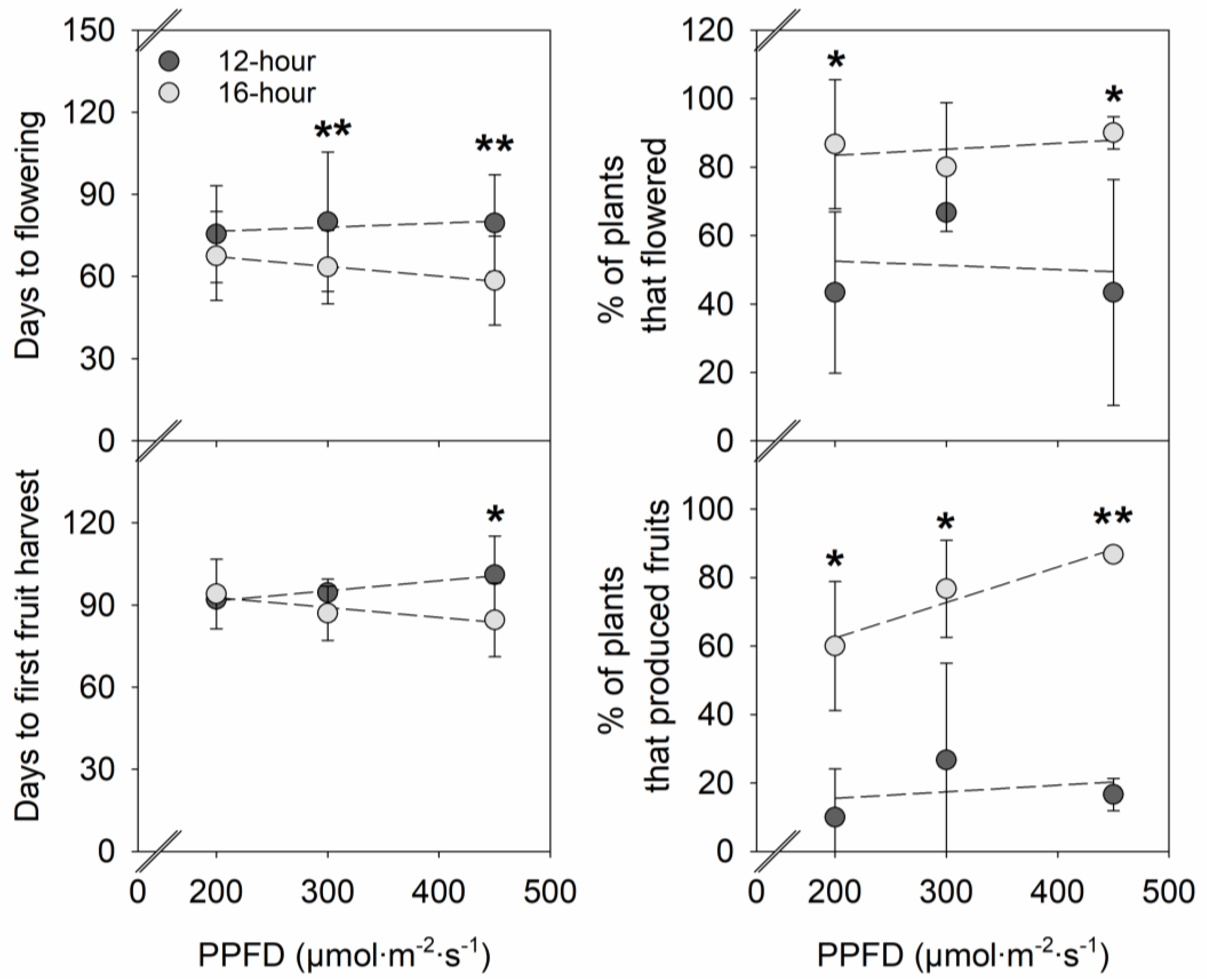
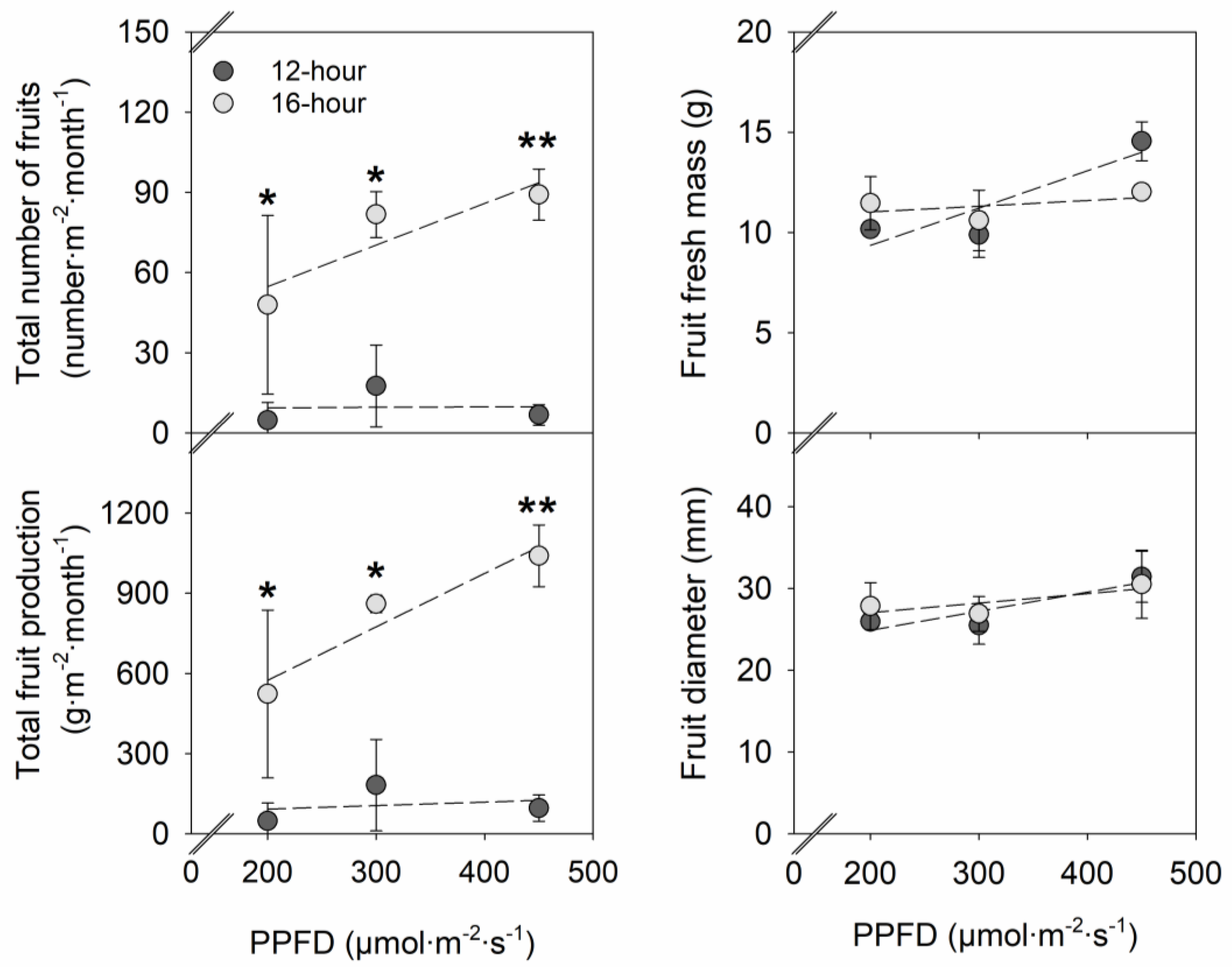
2. Results
2.1. Plant Growth
2.2. Flowering and Fruit Production
3. Discussion
3.1. Effects of PPFD on Vegetative Growth, Flowering, and Fruit Yield in Strawberries
3.2. Effects of Photoperiod on Vegetative Growth, Flowering, and Fruit Yield in Strawberries
4. Materials and Methods
4.1. Plant Materials
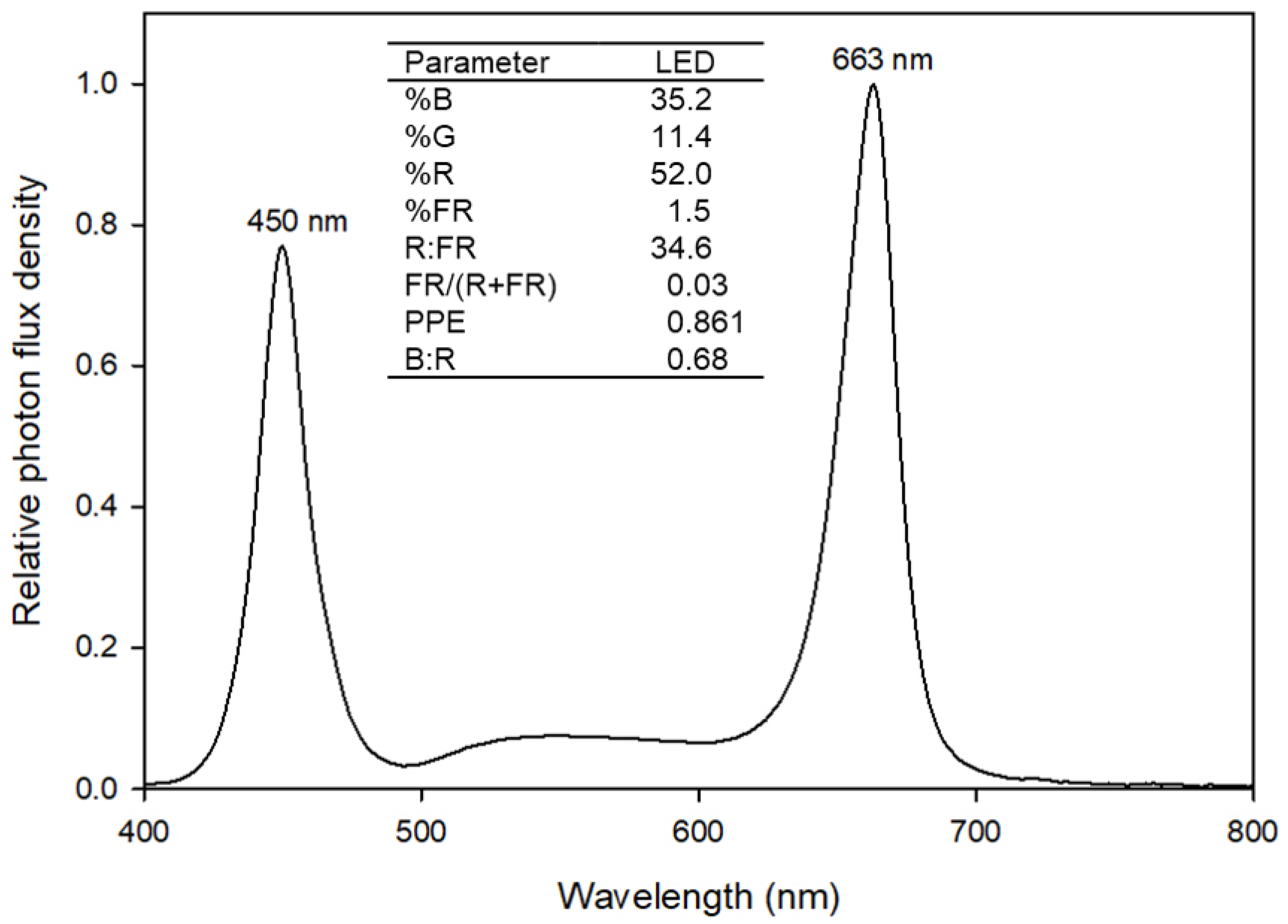
4.2. Growth Conditions and Sole-Source Lighting Treatments
4.3. Data Collection and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food Dive. Available online: https://www.fooddive.com/news/vertical-farming-startup-oishii-raises-50m-to-grow-strawberries/596515/ (accessed on 3 October 2021).
- HortiBiz. Available online: https://www.hortibiz.com/newsitem/news/10-new-trends-in-cea-in-2021-and-beyond/ (accessed on 3 October 2021).
- Fruit Growers News. Available online: https://fruitgrowersnews.com/article/driscolls-plenty-research-vertical-farming-for-strawberries/ (accessed on 3 October 2021).
- Mitchell, C.; Stutte, G. Sole-source lighting for controlled environment agriculture. In Light Management in Controlled Environments; Lopez, R., Runkle, E.S., Eds.; Meister Media Worldwide: Willoughby, OH, USA, 2017; pp. 119–134. [Google Scholar]
- Runkle, E.S. Sole-source lighting of plants. Greenhouse Product News 2017, 27, 58. Available online: https://gpnmag.com/article/sole-source-lighting-of-plants/ (accessed on 3 October 2021).
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an in-creasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Palmer, S.; Van Iersel, M.W. Increasing growth of lettuce and mizuna under sole-source LED lighting using longer photoperiods with the same daily light integral. Agronomy 2020, 10, 1659. [Google Scholar] [CrossRef]
- Xu, X.; Hernández, R. The effect of light intensity on vegetative propagation efficacy, growth, and morphology of “Albion” strawberry plants in a precision indoor propagation system. Appl. Sci. 2020, 10, 1044. [Google Scholar] [CrossRef]
- Zheng, J.; Ji, F.; He, D.; Niu, G. Effect of light intensity on rooting and growth of hydroponic strawberry runner plants in a LED plant factory. Agronomy 2019, 9, 875. [Google Scholar] [CrossRef]
- Tsuruyama, J.; Shibuya, T. Growth and flowering responses of seed-propagated strawberry seedlings to different photoperiods in controlled environment chambers. HortTechnology 2018, 28, 453–458. [Google Scholar] [CrossRef]
- Mattson, N.S. How many light fixtures do I need? e-Gro Edible Alert 2017, 2, 5. Available online: https://www.e-gro.org/pdf/E205a.pdf (accessed on 3 October 2021).
- Runkle, E.S. DLI ‘Requirements’. Greenhouse Product News 2019, 29, 50. Available online: https://gpnmag.com/article/dli-requirements/ (accessed on 3 October 2021).
- Garica, K.; Kubota, C. Flowering responses of North American strawberry cultivars. Acta Hortic. 2017, 1156, 483–490. [Google Scholar] [CrossRef]
- van Iersel, M. Light, photosynthesis, and plant growth. In Light Management in Controlled Environments; Lopez, R., Runkle, E.S., Eds.; Meister Media Worldwide: Willoughby, OH, USA, 2017; pp. 119–134. [Google Scholar]
- Fan, X.; Xu, Z.; Liu, X.; Tang, C.; Wang, L.; Han, X. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Park, S.; Kwack, Y.; Chun, C. Growth of runner plants grown in a plant factory as affected by light intensity and container volume. Hortic. Sci. Technol. 2017, 35, 439–445. [Google Scholar] [CrossRef]
- Carlen, C.; Potel, A.M.; Ancay, A. Photosynthetic response of strawberry leaves to changing temperatures. Acta Hortic. 2009, 838, 73–76. [Google Scholar] [CrossRef]
- Macias-Rodriguez, L.; Quero, E.; Lopez, M.G. Carbohydrate differences in strawberry crowns and fruit (Fragaria × ananassa) during plant development. J. Agric. Food Chem. 2002, 50, 3317–3321. [Google Scholar] [CrossRef]
- Morgan, L. Hydroponic Strawberry Production; Suntec (NZ) Ltd.: Tokomaru, New Zealand, 2006. [Google Scholar]
- Torres-Quezada, E.A.; Zotarelli, L.; Whitaker, V.M.; Santos, B.M.; Hernández-Ochoa, I. Initial crown diameter of strawberry bare-root transplant affects early and total fruit yield. HortTechnology 2015, 25, 203–208. [Google Scholar] [CrossRef]
- Wu, C.C.; Hsu, S.T.; Chang, M.Y.; Fang, W. Effect of light environment on runner plant propagation of strawberry. Acta Hortic. 2011, 907, 297–302. [Google Scholar] [CrossRef]
- Fagherazzi, A.F.; Suek Zanin, D.; Soares dos Santos, M.F.; Martins de Lima, J.; Welter, P.D.; Francis Richter, A.; Regianini Nerbass, F.; Anneliese Kretzschmar, A.; Rufato, L.; Baruzzi, G. Initial crown diameter influences on the fruit yield and quality of strawberry Pircinque. Agronomy 2021, 11, 184. [Google Scholar] [CrossRef]
- Cocco, C.; Gonçalves, M.A.; Reisser Júnior, C.; Marafon, A.C.; Antunes, L.E.C. Carbohydrate content and development of strawberry transplants from Rio Grande do Sul and imported. Rev. Bras. Frutic. 2016, 38, e581. [Google Scholar] [CrossRef]
- Marcelis, L.F.M.; Broekhuijsen, A.G.M.; Meinen, E.; Nijs, L.; Raaphorst, M.G.M. Quantification of the growth response to light quantity of greenhouse grown crops. Acta Hortic. 2006, 711, 97–104. [Google Scholar] [CrossRef]
- Na, M.H.; Jeon, W.B.; Cho, Y.; Lee, H.R.; Park, Y.; Cho, W.H.; Kim, D.H. A study on environmental factors affecting strawberry yield using pattern recognition techniques. Acta Hortic. 2019, 1265, 271–280. [Google Scholar] [CrossRef]
- Hytönen, T.; Kurokura, T. Control of flowering and runnering in strawberry. Hortic. J. 2020, 89, 96–107. [Google Scholar] [CrossRef]
- Sim, H.S.; Kim, D.S.; Ahn, M.G.; Ahn, S.R.; Kim, S.K. Prediction of strawberry growth and fruit yield based on environmental and growth data in a greenhouse for soil cultivation with applied autonomous facilities. Hortic. Sci. Technol. 2020, 38, 840–849. [Google Scholar] [CrossRef]
- Ji, F.; Wei, S.; Liu, N.; Xu, L.; Yang, P. Growth of cucumber seedlings in different varieties as affected by light environment. Int. J. Agric. Biol. Eng. 2020, 13, 73–78. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Durner, E.F.; Barden, J.A.; Himelrick, D.G.; Poling, E.B. Photoperiod and temperature effects on flower and runner development in day-neutral, Junebearing, and everbearing strawberries. J. Am. Soc. Hortic. Sci. 1984, 109, 396–400. [Google Scholar] [CrossRef]
- Shaw, D.V.; Larson, K.D. Strawberry Plant Named ‘Albion’. U.S. Patent No. US PP16,228 P3, 31 January 2006. [Google Scholar]
- Shaw, D.V.; Larson, K.D. Strawberry Plant Named ‘Monterey’. U.S. Patent No. US PP19,767 P2, 24 February 2009. [Google Scholar]
- Shaw, D.V.; Larson, K.D. Strawberry Plant Named ‘Portola’. U.S. Patent No. US 2009/0144866 P1, 4 June 2009. [Google Scholar]
- Shaw, D.V.; Larson, K.D. Strawberry Plant Named ‘San Andreas’. U.S. Patent No. PP19,975 P2, 12 May 2009. [Google Scholar]
- Sonsteby, A.; Heide, O.M. Long-day control of flowering in everbearing strawberries. J. Hortic. Sci. Biotechnol. 2007, 82, 875–884. [Google Scholar] [CrossRef]
- Durner, E.F. Photoperiod affects floral ontogeny in strawberry (Fragaria × ananassa Duch.) plug plants. Sci. Hortic. 2015, 194, 154–159. [Google Scholar] [CrossRef]
- Eshghi, S.; Tafazoli, E.; Dokhani, S.; Rahemi, M.; Enam, Y. Changes in carbohydrate contents in shoot tips, leaves and roots of strawberry (Fragaria × ananassa Duch.) during flower-bud differentiation. Sci. Hortic. 2007, 113, 255–260. [Google Scholar] [CrossRef]
- Ito, H.; Saito, T. Studies on the flower formation in the strawberry plant. I. Effects of temperature and photoperiod on the flower formation. Tohoku J. Agric. Res. 1962, 13, 191–203. Available online: http://hdl.handle.net/10097/29388 (accessed on 4 January 2022).
- Controlled Environment Berry Production Information. Available online: https://u.osu.edu/indoorberry/photosynthetic-lighting/ (accessed on 13 July 2022).
- Samad, S.; Butare, D.; Marttila, S.; Sønsteby, A.; Khalil, S. Effects of temperature and photoperiod on the flower potential in everbearing strawberry as evaluated by meristem dissection. Horticulturae 2021, 7, 484. [Google Scholar] [CrossRef]
- Kusuma, P.; Bugbee, B. Far-red fraction: An improved metric for characterizing phytochrome effects on morphology. J. Am. Soc. Hortic. Sci. 2021, 146, 3–13. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. Strawberries Grades and Standards. 2006. Available online: https://www.ams.usda.gov/grades-standards/strawberries-grades-and-standards (accessed on 1 September 2020).

| Sole-Source Lighting Treatments | Measured PPFD | Calculated DLI | |
| Photoperiod | PPFD | ||
| 12 | 200 | 207.4 ± 3.9 | 8.6 |
| 300 | 310.5 ± 7.5 | 13.0 | |
| 450 | 441.3 ± 6.0 | 19.4 | |
| 16 | 200 | 200.1 ± 8.7 | 11.5 |
| 300 | 312.3 ± 10.2 | 17.3 | |
| 450 | 456.7 ± 4.8 | 25.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Sethi, R.; Temnyk, S. Growth, Flowering, and Fruit Production of Strawberry ‘Albion’ in Response to Photoperiod and Photosynthetic Photon Flux Density of Sole-Source Lighting. Plants 2023, 12, 731. https://doi.org/10.3390/plants12040731
Park Y, Sethi R, Temnyk S. Growth, Flowering, and Fruit Production of Strawberry ‘Albion’ in Response to Photoperiod and Photosynthetic Photon Flux Density of Sole-Source Lighting. Plants. 2023; 12(4):731. https://doi.org/10.3390/plants12040731
Chicago/Turabian StylePark, Yujin, Rashmi Sethi, and Stephanie Temnyk. 2023. "Growth, Flowering, and Fruit Production of Strawberry ‘Albion’ in Response to Photoperiod and Photosynthetic Photon Flux Density of Sole-Source Lighting" Plants 12, no. 4: 731. https://doi.org/10.3390/plants12040731
APA StylePark, Y., Sethi, R., & Temnyk, S. (2023). Growth, Flowering, and Fruit Production of Strawberry ‘Albion’ in Response to Photoperiod and Photosynthetic Photon Flux Density of Sole-Source Lighting. Plants, 12(4), 731. https://doi.org/10.3390/plants12040731








