Abstract
Background: Catasetum Rich. ex Kunth is a genus of Neotropical orchids distributed in Central and South American regions. In the Brazilian Amazon, there are more than 60 species of Catasetum. The floral aromas of orchids are little known, particularly of Catasetum species. This work aimed to analyze the chemical constituents of the volatile concentrates of eight Catasetum specimens from the Amazon: C. alatum (1), C. albovirens (2), C. barbatum (1), C. ciliatum (2), C. galeritum (1), and C. gnomus (1). Methods: Gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) analyzed and identified the constituents of the volatile concentrates, and principal component analysis (PCA) and hierarchical cluster analysis (HCA) were used in the multivariate statistical analysis. Results: The Catasetum main constituents in descending order and above 10% were trans-geranylgeraniol, 1,4-dimethoxybenzene, linalool, 2-phenylethyl acetate, geraniol, 7-epi-1,2-dehydro-sesquicineole, 1,8-cineole, benzyl acetate, limonene, methyl salicylate, (E)-β-farnesene, anisyl butyrate, cis-carvone oxide, cadin-4-en-10-ol, indole, α-pinene, and δ-cadinene. Conclusions: Multivariate statistical analysis of Catasetum species showed that C. barbatum, C. albovirens, and C. gnomus are distinct from the other studied species, while C. alatum, C. ciliatum, and C. galeritum presented the same primary classes of compounds. These results contribute to a better understanding of the genus Catasetum chemotaxonomy.
1. Introduction
Flowering plants and their volatile compounds attract birds, insects, and other animals, including mammals, as pollinators for their reproduction. More than 1700 flower scent compounds, covering 990 taxa, have already been identified [1,2,3]. The flower perianth is primarily responsible for the scent emission, though all floral organs might contribute to the emission of scents. Floral scents are stored in the oily glands, such as the trichomes before it is released into the air as volatile compounds, and in addition to flowers, volatile compounds emitted by other plant organs have involved in its defense mechanisms. Therefore, floral volatiles play a significant role in the plant’s reproductive process by attracting pollinators and acting as repellents and physiological protectors against abiotic stress [2,4]. Floral volatiles are expected to be used in the composition of perfumes, cosmetics, flavors, and therapeutic applications. However, the volatiles emitted by flowers are also the main signals captured by insects to select gratifying flower species associated with the respective flower colors [3,5]. Floral scents are composed of volatile compounds, usually lipophilic and low molecular weight. Based on their origin, function, and biosynthesis, floral scents are grouped into three main classes of compounds: Terpenoids, phenylpropanoids/benzenoids, and fatty acid and derivatives [3,6].
Orchidaceae is the most prominent flowering plant family, with 736 genera and ca 28,000 species, which shows a wide diversity of epiphytic and terrestrial specimens, colonizing almost every earth’s habitat and renowned for their abundance of morphological types, with an unending number of beautiful variations and very well represented in the monocotyledons’ floral evolution [7,8]. The orchids distributed in the natural environment have small sizes and have a limited production of flowers. These restrictions are circumvented by producing visual and olfactory signals to attract pollinators. Developing highly specialized pollination mechanisms to attract effective pollinators is a common strategy in orchids. Specializing these mechanisms leads to the formation of syndromes, in which the set of floral characteristics, including fragrances, is associated with attracting a particular group of pollinators. Euglossini syndrome is widely known among orchids and is characterized by the absence of food resources, with only the production of specialized floral fragrances collected by male Euglossini bees. Floral fragrances not only act as signaling but also represent a reward for bees because some components of these mixtures will compose their pheromones, which is the essential prerequisite for sexual recognition and selection during mating [9,10,11,12].
Catasetum Rich. ex Kunth is one of the genera of Neotropical orchids pollinated by male Euglossini bees. The genus has about 130 species distributed mainly in Central and South American tropical regions. Floral fragrances play a vital role in the diversification of Catasetum. Hybridizations that result in changes in the composition of fragrances also generate differences in pollinators, restricting gene flow and contributing to the origin of new strains. Inflorescences of Catasetum are usually unisexual with distinct morphology and sexual determination according to each individual’s environmental and nutritional characteristics. Catasetum usually shows male or female flowers, but in some situations, may form nonfunctional hermaphroditic flowers and/or flowers of both sexes. Sex expression is controlled by plant size and light intensity. Large plants under strong sunlight usually develop female flowers, whereas younger and smaller plants under moderate light develop male flowers. They are sympatric species that use floral fragrances as a fundamental part of the reproductive isolation mechanism. The composition of these aromas is represented by a mixture of constituents with attractive and repellent action of different proportions, detected in small amounts by male Euglossini bees. Therefore, orchids attract particular species of bees that, in synergy with other floral filters (e.g., morphological characteristics), act as their effective pollinators. [13,14,15,16].
The present work aimed to extract the volatile concentrates and identify the chemical constituents of the flowers of eight specimens from six species of Catasetum: C. alatum (1), C. albovirens (2), C. barbatum (1), C. ciliatum (2), C. galeritum (1), and C. gnomus (1), with occurrence in the Brazilian Amazon. In addition, submit the chemical composition data of these specimens to multivariate analysis, targeting their association with other species/specimens of Catasetum taxonomically close or previously analyzed.
2. Results and Discussion
2.1. Catasetum Rich. ex Kunth
2.1.1. Catasetum alatum M.F.F.Silva & A.T.Oliveira
Botanical description: Epiphyte. Pseudobulbs aggregated, multi-ringed, fusiform, erect; apex abruptly acuminate. Leaves membranous, lanceolate, slightly wavy margins, five to eight leaves per pseudobulb, and three thin veins. Inflorescence 10 to 20 flowers (Figure 1), basal and pendant. Flowers staminate, resupinate jade green, erect, and distribute in the rachis’s middle third. Dorsal sepal lanceolate, erect, slightly concave; lateral sepals erect, linear-lanceolate, green, arched backward, acuminate. Petals oblong-lanceolate, greenish, convex, erect, margins slightly serrated. Fleshy lip, jade-green, 90° angle with the spine; frontal opening or elliptical ostium, internally light green, white spot at base, sack form at middle portion; edges of lateral lobes strongly winged, semi-curled, slightly serrated, asymmetrical, raised; triangular terminal lobe, apiculate, facing downwards, edges smooth [17]. Flowering in December and January.

Figure 1.
Catasetum alatum. (Source Luiz Otavio Adão).
Geographic distribution: Endemic in Brazil. Occurrence for the North region of Brazil, State of Rondônia, in riparian or gallery forest areas [17].
The specimen of C. alatum in this study was sampled initially in the locality of Vila Nova California, Rondônia, Brazil. Table 1 lists the constituents of its volatile concentrate.

Table 1.
Constituents identified in the volatile concentrate of C. alatum flowers.
Oxygenated monoterpenes (57.0%) predominated in the volatile concentrate of C. alatum, followed by monoterpene hydrocarbons (36.9%). The main constituents were geraniol (29.7%), limonene (18.2%), α-pinene (11.8%), and linalool (8.8%) (see Figure 2). The floral scents of C. alatum are being described for the first time.
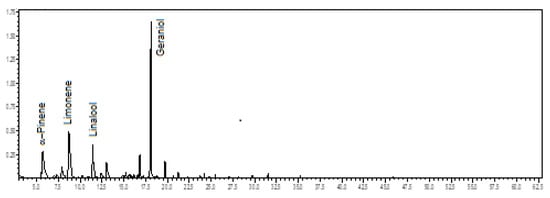
Figure 2.
Ion-chromatogram of the Catasetum alatum volatile concentrate.
2.1.2. Catasetum Albovirens Barb. Rodr.
Botanical description: Epiphyte. Fusiform pseudobulbs. Leaves with longitudinal veins, elliptical-lanceolate, sharp at the base, attenuated in canaliculate pseudo petiole. Inflorescence about 25.0 cm, erect and at middle curved in a basal arch, multiflora. Flowers (Figure 3) not resupinate, white-green to pink-green, drooping. Pedicel patent and twisted. Oval or oval-oblong sepals, obtuse or very sharp; erect dorsal sepal with five prominent veins; oblique lateral sepals. Petals lanceolate and acuminate with three very distinct veins. Lip nearly globular, distinctly 3-lobed, crass-fleshy, generally greener than other segments; lateral lobes rounded, entire, curved; terminal lobe patent, reflex, more or less 3-lobed or truncated, glabrous and smooth on the inside. Oval column, with rostellum, acuminate and straight. Antennae about 6.0 mm, extending to the front [17]. Flowering in February to May.

Figure 3.
Catasetum albovirens. (Source Luiz Otavio Adão).
Synonimy:Catasetum lamosii Rolfe [17].
Geographic distribution: Endemic in Brazil, occurring in the states of Amazonas, Pará, Maranhão, Mato Grosso, and Tocantins, in anthropic areas such as rupestrian fields, high and flooded forests, seasonal deciduous forests, and savannas [17].
Two specimens of C. albovirens were analyzed. Specimen Calb-1 was sampled initially in the municipality of Muaná, Ilha do Marajó, and specimen Calb-2 was collected initially in the municipality of Tucuruí, Pará state, Brazil. Table 2 lists the constituents of their volatile concentrates.

Table 2.
Constituents identified in the volatile concentrates of two specimens of C. albovirens flowers.
The two analyzed specimens of C. albovirens were rich in oxygenated monoterpenes (52.4% and 36.7%), oxygenated sesquiterpenes (30.4% and 20.8%), and benzenoids (7.5% to 15.4%). There was a predominance of linalool (10.0% to 39.5%), 7-epi-1,2-dehydro-sesquicineole (19.3% to 28.3%), anisyl butyrate (7.5% to 15.4%), 1,8-cineol (1.1% to 11.7%), and (E,E)-α-farnesene (1.0% to 5.2%), analyzing both specimens (see Figure 4).
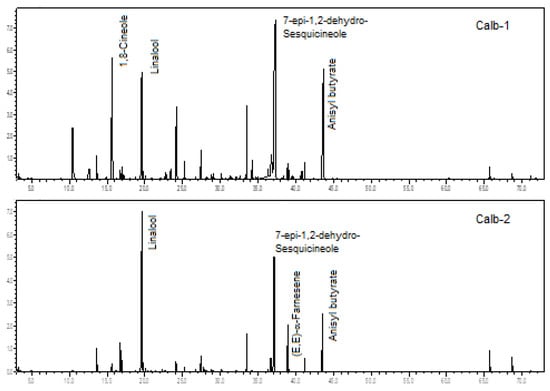
Figure 4.
Ion-chromatogram of the Catasetum albovirens volatile concentrates.
A previous comparative biology study of C. albovirens showed β-myrcene, eucalyptol (1,8-cineole), (E)-β-ocimene, linalool, 2,4-dimethylacetophenone, indole, (E)-8-hydroxy-linalool, methyl anthranilate, geranyl acetate, and (E,E)-farnesene, as primary constituents [18]. Therefore, presenting some chemical similarities to the two samples currently analyzed.
2.1.3. Catasetum barbatum Lindl.
Botanical description: Epiphytic, occasionally terrestrial. Fusiform pseudobulbs, about 15.0 to 5.0 × 3.0 to 5.0 cm long. Narrow, plicate leaves with a midrib and two lateral ones. Inflorescence suberect or arched with up to 20 flowers. Flowers (Figure 5) male resupinate, greenish with brown spots. Sepals are lanceolate to dorsal erect, the lateral ones reflexed on the pedicel. Petals lanceolate and erect, margins finely serrated, somewhat revolute. The lips include hairs that may be white or greenish; the basal callus may be simple or bifurcated, usually surrounded by pilosity. Column light green or brownish. Cream anther. Yellow pollinia are hard and compressed on a white laminar stipe and a white viscid disc. Flowering in April and May [17].

Figure 5.
Catasetum barbatum. (Source Luiz Otavio Adão).
Synonimy:Catasetum barbatum var. spinosum Rolfe, C. brachybulbon Schltr., C. buchtienii Kraenzl., C. comosum Cogn., C. crinitum Linden, C. polydactylon Schltr., C. proboscideum Lindl., C. rionegrense Campacci & G.F. Carr, C. spinosum (Hook.) Lindl., C. variabile Barb. Rodr., Myanthus barbatus Lindl., M. barbatus var. immaculatus Knowles and Westc., M. spinosus Hook. [17].
Geographic distribution: Not endemic in Brazil, but found in upland and floodplain forests, broadleaf forests, mangrove and palm groves, and vegetation on rocky outcrops in the states of Amazonas, Pará, Roraima, Tocantins, Alagoas, Bahia, Ceará, Maranhão, Paraíba, Pernambuco, Piauí, Distrito Federal, Goiás, Mato Grosso do Sul, Mato Grosso, and Minas Gerais [17].
The specimen of C. barbatum in this study was sampled initially in the municipality of Ourilândia do Norte, Pará, Brazil. Table 3 lists the constituents of its volatile concentrate.

Table 3.
Constituents identified in the volatile concentrate of C. barbatum flowers.
In this volatile concentrate of C. barbatum flowers, the major constituents were the trans-geranylgeraniol (61.2%), an oxygenated diterpene, the (E)-β-farnesene (16.4%), a sesquiterpene hydrocarbon, and the indole (11.3%), a heterocyclic aromatic compound (see Figure 6).
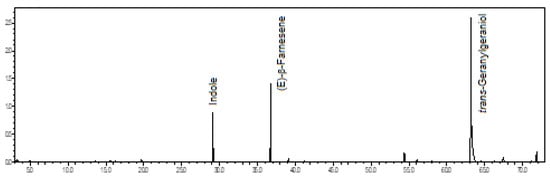
Figure 6.
Ion-chromatogram of the Catasetum barbatum volatile concentrate.
The floral scents of C. barbatum were previously analyzed. Two morphological variants originated from Brazil and Ecuador has differed in fragrance. The Brazilian form showed as primary constituents 1,8-cineole (16.1%), a-pinene (7.9%), and an unidentified main oxygenated monoterpene (46.1%), while the Ecuadorian form displayed ocimene (67.7%) as the main compound [19]. Also, another paper on C. barbatum floral scent presented germacrane-type compounds as the primary constituents, including germacra-1(10)-5-dien-4-ol (60.0%), germacrene A (9.0%), germacrene D (7.0%), and bicyclogermacrene (2.0%) [16]. A doctoral thesis in the comparative biology of Catasetum presented (Z)-α-bergamotene, neryl acetate, and (E)-β-farnesene as the main components of C. barbatum sampled in the Amazonas state, Brazil [18]. This last sample of C. barbatum is more associated with the one analyzed in the present work due to the presence of (E)-β-farnesene, except for the significant content of trans-geranylgeraniol in the sample worked by us.
2.1.4. Catasetum ciliatum Barb. Rodr.
Botanical description: Epiphytic, erect, caespitose, ca. 38.0 cm long. Short rhizome, less than 1.0 cm between pseudobulbs. Caulomas thickened into pseudobulbs, aggregated, fusiform covered by leaf sheaths. Elliptical, semi-leathery leaves, five prominent veins. Inflorescence in a raceme, lateral; green peduncle, partially covered by tubular sheaths, ca. six flowers. Flowers (Figure 7) resupinate, greenish; sepals free from each other, dorsal elliptical; apex acuminate, elliptical-falcated laterals, apex acuminate; slightly oval petals. Lip green, elm-shaped, narrow base, adnate at the foot of the gynostemium, apex narrowly curved. Flowering in April and May [20].

Figure 7.
Catasetum ciliatum. (Source Luiz Otavio Adão).
Geographic distribution: Not endemic In Brazil but found in areas of campinarana, rock fields, floodplain forests, sandbanks, and savannas, in the states of Amazonas, Amapá, Pará, Rondônia, Roraima, and Maranhão.
Two specimens of C. ciliatum were analyzed. Specimen Ccil-1 was sampled initially in the municipality of Prainha, and specimen Ccil-2 was collected initially in the locality of Lago Preto, municipality of Santarém, Pará state Brazil. Table 4 lists the constituents of their volatile concentrates.

Table 4.
Constituents identified in the volatile concentrates of two specimens of C. ciliatum flowers.
The two analyzed specimens of C. ciliatum were rich in oxygenated monoterpenes (67.5% and 24.6%), benzenoids/phenylpropanoids (15.0% and 53.5%), and monoterpene hydrocarbons (14.8% and 8.4%). There was a predominance of 2-phenylethyl acetate (3.2% to 30.5%), 1,8-cineole (3.7% to 23.7%), benzyl acetate (11.8% to 22.8%), geraniol (5.0% to 16.6%), cis-carvone oxide (10.1% to 14.3%), α-pinene (4.8% to 10.5%), and carvone (4.4% to 7.8%), analyzing both specimens (see Figure 8). The floral scents of C. ciliatum are being described for the first time.
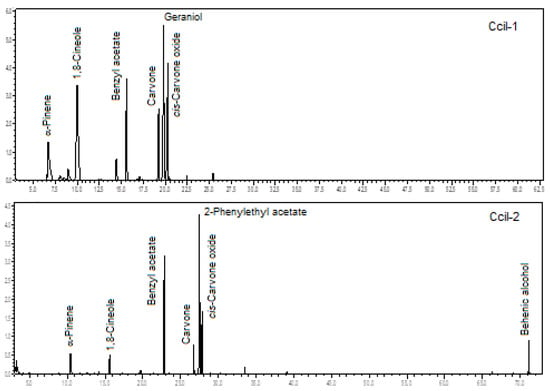
Figure 8.
Ion-chromatogram of the Catasetum ciliatum volatile concentrates.
2.1.5. Catasetum galeritum Rchb.f.
Botanical description: Epiphyte. Conical-fusiform pseudobulbs, compressed on the sides, ringed and furrowed. Leaves lanceolate-spatulate, acuminate towards the base, attenuated in canaliculate pseudo petiole, with five to seven longitudinal veins. Inflorescence from 20.0 to 25.0 cm, racemose, basal, pendant, and robust. Pedicel patent, arched, plump with pseudo-ovary. Flowers (Figure 9) are not resupinate and patent. Petals oblong-ligulate, sharp, erect, conniving with the dorsal sepal and embraced by its margins. Sepals are slightly convex and attenuated at the base. Very patent lip, somewhat reflexive, inferior, thickly fleshy, rigid, longer than the lateral sepals, with an oblong sack form protuberance, laterally compressed, entire, the sides erect over the sac projected forward. Fleshy column, elongated, at apex short conical rostrum, slightly curved. Antennae about 18.0 mm long, parallel, converging, with two yellow pollinia. The flowers of C. galeritum release an intense and sweet perfume, easily detectable by the human nose up to 2.0 m away [21]. Flowering in April.

Figure 9.
Catasetum galeritum. (Source Luiz Otavio Adão).
Synonimy:Catasetum galeritum var. pachyglossum Rchb.f. [17].
Geographic distribution: Endemic in Brazil, occurs in riparian or gallery forests, Upland and floodplain forests, and broadleaf forests in the states of Amazonas, Pará, Tocantins, Maranhão, and Mato Grosso [17].
This C. galeritum specimen was sampled initially in the municipality of São Félix do Xingu, Pará state, Brazil, and the constituents of their volatile concentrate are listed in Table 5.

Table 5.
Constituents identified in the volatile concentrate of C. galeritum flowers.
Benzenoids/phenylpropanoids (59.3%) predominated in the volatile concentrate of C. galeritum, followed by oxygenated monoterpenes (37.6%). The main constituents were 1,4-dimethoxybenzene (54.1%), linalool (34.9%), and indole (5.2%) (see Figure 10). A previous floral scent of another C. galeritum specimen, also occurring in the municipality of São Felix do Xingu, Pará state, Brazil, and visited only by male bees, was similarly composed of 1,4-dimethoxybenzene (85-94%), followed by minor amounts of linalool, indole, β-elemene, and (E)-caryophyllene [21].
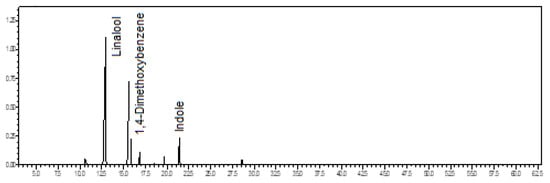
Figure 10.
Ion-chromatogram of the Catasetum galeritum volatile concentrate.
2.1.6. Catasetum gnomus L. Linden & Rchb.f.
Botanical description: Epiphyte. Pseudobulbs fusiform, erect, slightly compressed on the sides, long attenuated and acuminate, aggregated. Leaves oblong-lanceolate, 7 to 9 per pseudobulb, 3-veined, erect-patent. Inflorescence male basal, racemose, arched to pending. Bracts amplexicaul lanceolate, acuminate. Flowers (Figure 11) 5 to 15 non-resupinate, alternate on the rachis, somewhat inclined or drooping. Triangular floral bracts run to the pedicels. Pedicel cylindrical, sinuous. Dorsal sepal oboval-lanceolate, attenuated base, acuminate and acute apex; the sides oboval-lanceolate, slightly united at the base, apex very acuminate. Petals linear-lanceolate, erect, partially covered by the dorsal sepal and covering the column, oblique. Fleshy lip, with sack form protuberance (25.0 mm bristle), undulating and crenulated margins, sometimes serrated, internally under the column with a carina and around the thickened ostium, the anterior side always with the margins reclined downwards. Fleshy column, erect, triangular in cross-section, apex rostriform, threadlike, and long. Crossed antennas. Two yellow pollinia. Anther yellowish [17]. Flowering in February.

Figure 11.
Catasetum gnomus. (Source Luiz Otavio Adão).
Synonimy:Catasetum gnomus var. phasma (Rchb.f.) Cogn., Catasetum heteranthum Barb. Rodr., Catasetum huebneri Mansf., Catasetum mocuranum Schltr., Catasetum negrense Schltr., Catasetum phasma Rchb.f.
Geographic distribution: Endemic in Brazil occurs in riparian or gallery forests, Upland and floodplain forests, and broadleaf forests in the states of Amazonas, Pará, and Rondônia.
This C. gnomus specimen was sampled initially in Manaus, Amazonas state, Brazil, and the constituents of its volatile concentrate are listed in Table 6.

Table 6.
Constituents identified in the volatile concentrate of C. gnomus flowers.
Sesquiterpene hydrocarbons (29.0%) predominated in the volatile concentrate of C. gnomus, followed by oxygenated sesquiterpenes (26.3%), benzenoids/phenylpropanoids (21.4%), monoterpene hydrocarbons (13.2%), and oxygenated monoterpenes (8.5%). The main constituents were methyl salicylate (17.0%), cadin-4-en-10-ol (12.1%), δ-cadinene (10.3%), limonene (7.5%), and epi-α-muurolol (6.9%) (see Figure 12). The floral scents of two other C. gnomus specimens grown in a greenhouse at the University of Miami, Florida, USA, were previously reported, showing methyl salicylate, α-pinene, 1,8-cineole, and methyl benzoate as their main constituents [16,19,22].
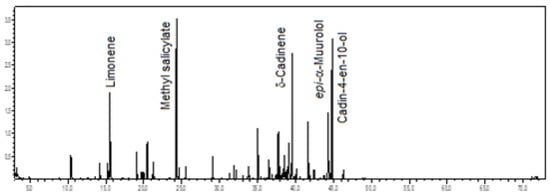
Figure 12.
Ion-chromatogram of the Catasetum gnomus volatile concentrate.
2.2. The Catasetum Floral Scent Chemistry
The primary constituents found in the Catasetum flowers analyzed in this work were the monoterpenes linalool, 1,8-cineole, α-pinene, limonene, geraniol, carvone, and cis-carvone oxide; the sesquiterpenes 7-epi-1,2-dehydro-sesquicineole, (E,E)-farnesene, (E)-β-farnesene, δ-cadinene, epi-α-muurolol, and cadin-4-en-10-ol; the benzenoids anisyl butyrate, indole, benzyl acetate, 2-phenylethyl acetate, methyl salicylate, and 1,4-dimethoxybenzene; and the diterpene trans-geranylgeraniol. By analogy, consulting the literature, it was found that the floral aromas of 30 species of Catasetum (around 17% of existing) have already been chemically characterized. In these studies, 124 volatile compounds were reported, belonging to the following classes of compounds: monoterpenes (44), sesquiterpenes (26), irregular terpenes (1), aliphatics (14), aromatics (38), and N-bearing compounds (1). Individually, 1,8-cineole and α-pinene were the most reported, followed by β-pinene, (E)-dihydrocarvone, (E)-carvone epoxide, carvone, and p-cymene (10) [2,16].
The complexity of floral scents in the species of Catasetum investigated so far varies considerably. In the present work, more than 93% of the scent profile has been characterized, while the number of identified constituents varied from 1 in C. micranthum to 74 in C. uncatum [23,24]. This variation in scent floral complexity across Catasetum species certainly reflects an inherent characteristic for each species. As expected in angiosperms, floral scents of Catasetum are species-specific, although dominated by some significant constituents usually shared by several species [25]. Most Catasetum species have 2 or 3 main constituents that account for more than 70% of the fragrances. These constituents are potent attractants to many Euglossa and Eulaema bees, but the attractiveness to individuals and species is reduced as more components compose the mixtures so that specific scents attract only a few pollinator species [11,19].
The pivotal role of floral fragrances in pollinator shifts and as a reproductive isolating mechanism in Catasetum was previously highlighted [16]. However, floral scents may not be enough to assure the effective reproductive isolation in Catasetum. Sympatric species usually produce similar fragrances, thereby attracting the same pollinator species. In these cases, different reproductive isolating mechanisms (e.g., geographical, morphological/mechanical, temporal/seasonal), acting alone or together, will be necessary to contribute to or prevent the hybridization [16,19]. Presently, considering the well-defined separation of the pollinating genera of Catasetum and the higher sensorial similarity between the closely related bee species, have been speculated that the olfactory adaptations have shaped the evolution of floral fragrances of Catasetum due to the partitioning with pollinator’s bees, particularly from the genera Euglossa and Eulaema [16,19].
Therefore, pollinator affinity with phylogeny is correlated with differences found in floral aromas. More generally, the question is why flowers produce different odors or why mixtures of odors tend to be species-specific. The answer to this question demands more complex functional analyses, attributing phylogenetic, physiological, and ecological influences to the chemical variation of floral scents [25].
2.3. Catasetum Specimens’ Multivariate Analysis
The floral variability of samples of Catasetum volatile concentrates was evaluated using multivariate statistical analyses (PCA, principal component analysis; HCA, hierarchical cluster analysis) based on their classes of compounds. The percentage values of monoterpene hydrocarbons (MH), oxygenated monoterpenes (OM), sesquiterpene hydrocarbons (SH), oxygenated sesquiterpenes (OS), oxygenated diterpenes (OD), benzenoids/phenylpropanoids (B/P), and fatty acids and derivatives were obtained based on the GC-MS analyses of the volatile concentrate constituents. The data were used as variables (see Table 7).

Table 7.
Classes of compounds identified in Catasetum specimens used in the multivariate statistical analyses.
The HCA analysis (Figure 13) showed the formation of five groups. The first group comprised C. alatum and C. ciliatum-1 (I); the second group the two specimens of C. albovirens (II); the third group of C. gnomus (III); the fourth group of C. barbatum (IV); and fifth group by C. ciliatum-2 and C. galeritum (V).
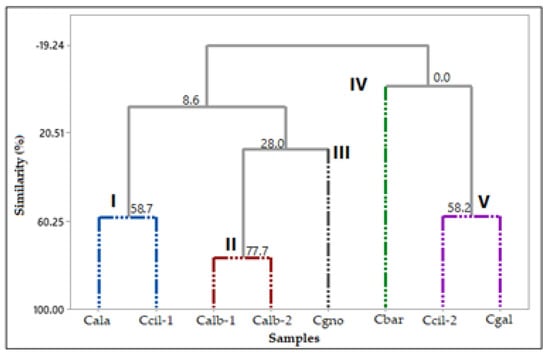
Figure 13.
Hierarchical cluster analysis (HCA) of the Catasetum volatile concentrates, based on their classes of compounds.
The PCA analysis (Figure 14) explained 69.5% of the data variability. The PC1 justified 35.32% of the data, showing negative correlations with monoterpene hydrocarbons (MH, λ = −0.52), oxygenated monoterpenes (OM, λ = −0.43), oxygenated sesquiterpenes (OS, λ = −0.12) and positive correlations with sesquiterpene hydrocarbons (SH, λ = 0.09), oxygenated diterpene (OD, λ = 0.28), benzenoids/phenylpropanoids (BZ-PP, λ = 0.46), and fatty acids and derivatives (FA, λ = 0.45). The PC2 clarified 34.3% of the data, showing a positive correlation with oxygenated sesquiterpenes (OS, λ = 0.32), oxygenated diterpene (OD, λ = 0.36), and sesquiterpene hydrocarbons (SH, λ = 0.56), and negative correlation with monoterpene hydrocarbons (MH, λ = −0.08), oxygenated monoterpenes (OM, λ = −0.42), benzenoids/phenylpropanoids (BZ-PP, λ = 0.35), and fatty acids and derivatives (FA, λ = −0.38). Similar to HCA, the PCA analysis confirmed the formation of five distinct groups.
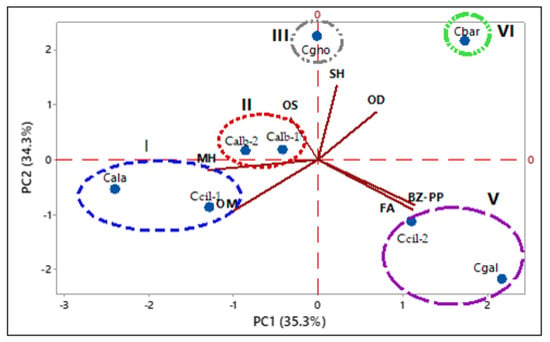
Figure 14.
Principal componente analysis (PCA) of the Catasetum volatile concentrates, Based on their classes of compounds.
Group I was characterized by oxygenated monoterpene (57.0–67.5%) and monoterpene hydrocarbons (14.8–36.9%). Group II was characterized by oxygenated monoterpenes (36.7–52.4%) and oxygenated sesquiterpenes (20.8–30.4%). Group III was characterized by sesquiterpene hydrocarbons (29.0%), oxygenated sesquiterpenes (26.3%), and benzenoids/phenylpropanoids (21.4%). Group IV was characterized by oxygenated diterpenes (62.0%). Group V was characterized by benzenoids/phenylpropanoids (53.5–59.3%) and oxygenated monoterpenes (24.6–37.6%).
3. Materials and Methods
3.1. Plant Material
The orchids Catasetum alatum, C. albovirens, C. barbatum, C. ciliatum, C. galeritum, and C. gnomus (Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6), which provided the flowers for this work, are live plants cultivated in pots containing charcoal and wood shavings, existing in the private nursery of Mr. Luiz Otávio Adão Teixeira, located in the Amazon Garden Condominium, BR-316, km 6, 67015-795 Ananindeua, PA, Brazil (coordinates: 1°22′20.96″ S/48°23′34.14′′ W). These orchid specimens were previously sampled in various localities and cities of the Brazilian Amazon, as already described for each one in the Results. Each plant exemplar (exsiccate) was deposited in the Herbarium of Emílio Goeldi Museum, Belém, Para state, Brazil. The orchid flowers were collected during the flowering period, at 6 am, to extract their volatile constituents.
3.2. Obtaining and Analyzing Volatile Concentrates
The orchid flowers were subjected to micro distillation-extraction in a Likens & Nickerson-type apparatus (3 flowers each, 15 g in total, 2 h, duplicate) to obtain their volatile concentrates, using n-pentane (99% HPLC grade, 3 mL) as the solvent [26].
The volatile concentrates of orchids were submitted to GC and GC-MS analysis. It was performed on a GCMS-QP2010 Ultra system (Shimadzu Corporation, Tokyo, Japan), equipped with an AOC-20i auto-injector and the GCMS-Solution software containing the Adams (2007), Mondello (2011), and Nist (2011) libraries [27,28,29]. A Rxi-5ms (30 m × 0.25 mm; 0.25 μm film thickness) silica capillary column (Restek Corporation, Bellefonte, PA, USA) was used. The conditions of analysis were as follows. Injector temperature: 250 °C; Oven temperature programming: 60–240 °C (3 °C min−1); Helium as the carrier gas, adjusted to a linear velocity of 36.5 cm s−1 (1.0 mL min−1); split mode injection (split ratio 1:20) of 1.0–2.0 µL of the n-pentane solution; electron ionization at 70 eV; ionization source and transfer line temperatures of 200 and 250 °C, respectively. The mass spectra were obtained by automatically scanning every 0.3 s, with mass fragments in the 35–400 m/z. The retention index was calculated for all volatile components using a homologous series of C8-C40 n-alkanes (Sigma-Aldrich, Milwaukee, WI, USA) according to the linear equation of van den Dool and Kratz (1963) [30]. Individual components were identified by comparing their retention indices and mass spectra (molecular mass and fragmentation pattern) with those existing in the GCMS-Solution system libraries [27,28,29]. The quantitative data regarding the volatile constituents were obtained using a GC2010 Series gas chromatograph, operated under similar conditions to those of the GC-MS system. The relative amounts of individual components were calculated by peak-area normalization using a flame ionization detector (GC-FID). Chromatographic analyses were performed in duplicate.
3.3. Multivariate Statistical Analysis
Principal Component Analysis (PCA) was applied to verify the interrelationship of the samples of volatile concentrates analyzed with the classes of identified compounds, monoterpene hydrocarbons (MH), oxygenated monoterpenes (OM), sesquiterpene hydrocarbons (SH), oxygenated sesquiterpenes (OS), oxygenated diterpenes (OD), benzenoids/phenylpropanoids, and fatty acids and derivatives. The data matrix was standardized for multivariate analysis by subtracting the mean and dividing it by the standard deviation. Hierarchical Cluster Analysis (HCA), considering the Euclidean distance and complete linkage, was used to verify the similarity of the samples based on the distribution of the constituents selected in the PCA analysis (Software Minitab, free version 390, Minitab Inc., State College, PA, USA) [31].
4. Conclusions
In conclusion, the present study showed that previous reports were not found in the literature concerning the chemotaxonomy of the Catasetum species. Thus, considering their classes of compounds, Catasetum albovirens, C. gnomus, and C. barbatum can be distinguished from the other studied species, while C. alatum, C. galeritum, and C. ciliatum showed the same primary compound classes. Also, there are two chemotypes of C. ciliatum, the first one rich in oxygenated monoterpene (67.5%) and the second rich in benzenoids/phenylpropanoids (53.5%). Thus, we think these findings could contribute to a better understanding of the chemical profiles of Catasetum species.
Author Contributions
Conceptualization, J.G.S.M. and E.H.A.A.; methodology, J.G.S.M. and F.M.d.V.; software, P.L.B.F.; formal analysis, F.M.d.V., E.H.A.A. and P.L.B.F.; resources, L.O.A.T. and E.H.A.A.; data curation, F.M.d.V. and L.O.A.T.; writing—original draft preparation, F.M.d.V. and J.G.S.M. writing—review and editing, F.M.d.V., E.H.A.A. and J.G.S.M.; project administration, J.G.S.M. and E.H.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are grateful to CNPq and CAPES, scientific research agencies of the Brazilian Government for their financial of fellowships support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dudareva, N.; Pichersky, E. Biochemical and molecular genetic aspects of floral scents. Plant Physiol. 2000, 122, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- de Vasconcelos, F.M.; Andrade, E.H.A.; Teixeira, L.O.A.; Maia, J.G.S. Volatile constituents of floral scents from Encyclia cordigera (Kunth) Dressler and E. randii (Barb. Rodr.) Porto & Brade (Orchidaceae). J. Braz. Chem. Soc. 2022, 33, 96–101. [Google Scholar] [CrossRef]
- Kessler, D.; Gase, K.; Baldwin, I.T. Field experiments with transformed plants reveal the sense of floral scents. Science 2008, 321, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Delle-Vedore, R.; Juillet, N.; Bessiere, J.M.; Grison, C.; Barthes, N.; Pailler, T.; Dormont, L.; Schatz, B. Colour-scent associations in a tropical orchid: Three colours but two odours. Phytochemistry 2011, 72, 735–742. [Google Scholar] [CrossRef]
- Hsiao, Y.Y.; Pan, Z.J.; Hsu, Z.Z.; Yang, Y.P.; Hsu, Y.C.; Chuang, Y.C.; Shih, H.H.; Chen, Y.H.; Tsai, W.C.; Chen, H.H. Research on Orchid Biology and Biotechnology. Plant Cell Physiol. 2011, 52, 1467–1486. [Google Scholar] [CrossRef]
- The Plant List 2014. Available online: www.theplantlist.org/1.1/browse/A/Orchidaceae (accessed on 16 November 2022).
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van Den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Acherman, J.D. Specificity and mutual dependency of the orchid-euglossine bee interaction. Biol. J. Linn. Soc. 1983, 20, 301–313. [Google Scholar] [CrossRef]
- Dressler, R.L. Phylogeny and Classification of the Orchid Family; Dioscorides Press: Portland, OR, USA, 1993. [Google Scholar]
- Dobson, H.E.M. Relationship between floral fragrance composition and type of pollinator. In Biology of Floral Scent; Dudareva, N., Pichersky, E., Eds.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Zimmermann, Y.; Ramírez, S.R.; Eltz, T. Chemical niche differentiation among sympatric species of orchid bees. Ecology 2009, 90, 2994–3008. [Google Scholar] [CrossRef]
- Gerlach, G. The true sexual life of Catasetum and Cycnoches. Caesiana 2007, 28, 57–62. [Google Scholar]
- Romero, G.A.; Carnevali, G. Catasetum. In Genera Orchidacearum, Epidendroideae—Part II; Pridgeon, A.M., Cribb, P.J., Chase, M.W., Rasmussen, F.N., Eds.; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Schiestl, F.P.; Schluter, P.M. Floral isolation, specialized pollination, and pollinator behavior in orchids. Annu. Rev. Entomol. 2009, 54, 425–446. [Google Scholar] [CrossRef]
- Millet-Pinheiro, P.; Gerlach, G. Biology of the neotropical orchid genus Catasetum: A historical review on floral scent chemistry and pollinators. Perspect. Plant Ecol. Evol. Syst. 2017, 27, 23–34. [Google Scholar] [CrossRef]
- Barros, F.; Vinhos, F.; Rodrigues, V.T.; Barberena, F.F.V.A.; Fraga, C.N.; Pessoa, E.M.; Foster, W.; Menini Neto, L.; Furtado, S.G.; Nardy, C.; et al. Orchidaceae. In Lista de Espécies da Flora do Brasil; Jardim Botânico do Rio de Janeiro: Rio de Janeiro, Brasil, 2015. Available online: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB179 (accessed on 16 November 2022).
- Franken, E.P. Biologia Floral Comparada do Gênero Catasetum Rich. ex Kunth (Orchidaceae, Catasetinae) Baseado em Estudos Filogenéticos. D.Sc. Thesis, Programa de Pós-Graduação em Biologia Comparada, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil, 2017. [Google Scholar]
- Hills, H.G.; Williams, N.H.; Dodson, C.H. Floral fragrances and isolating mechanisms in the genus Catasetum (Orchidaceae). Biotropica 1972, 4, 61–76. [Google Scholar] [CrossRef]
- Afonso, E.A.L.; Kock, A.K.; Costa, J.M. Flora preliminar de Orchidaceae no município de Abaetetuba, Pará, Brasil. Biota Amazônia 2016, 6, 107–118. [Google Scholar] [CrossRef]
- Millet-Pinheiro, P.; Silva, J.B.F.; Navarro, D.M.A.F.; Machado, I.C.S.; Gerlach, G. Notes on pollination ecology and floral scent chemistry of the rare neotropical orchid Catasetum galeritum Rchb.f. Plant Species Biol. 2018, 33, 158–163. [Google Scholar] [CrossRef]
- Hills, H.G.; Williams, N.H.; Dodson, C.H. Identification of some orchid fragrance components. Orchid Soc. Bull. 1968, 37, 967–971. [Google Scholar]
- Hills, H.G.; Williams, N.H.; Whitten, W.M. Fragrance of Catasetum. In The World of Catasetums; Holst, A.W., Ed.; Timber Press Inc.: Portland, OR, USA, 1999; pp. 263–272. [Google Scholar]
- Millet-Pinheiro, P.; Navarro, D.M.A.F.; Dötterl, S.; Carvalho, A.T.; Pinto, C.E.; Ayasse, M.; Schlindwein, C. Pollination biology in the dioecious orchid Catasetum uncatum: How does floral scent influence the behavior of pollinators? Phytochemistry 2015, 116, 149–161. [Google Scholar] [CrossRef]
- Raguso, R.A. Flowers as sensory billboards: Progress toward and integrate understanding of floral advertisement. Curr. Opin. Plant Biol. 2004, 7, 434–440. [Google Scholar] [CrossRef]
- Likens, S.T.; Nickerson, G.B. Detection of certain hop oil constituents in brewing products. Am. Soc. Brew. Chem. Proc. 1964, 22, 5–13. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mondello, L. FFNSC 2—Flavors and Fragrances of Natural and Synthetic Compounds, Mass Spectral Database; John Wiley and Sons Inc.: New York, NY, USA, 2011. [Google Scholar]
- NIST—National Institute of Standards and Technology. Mass Spectral Library (NIST/EPA/NIH, v.2.0d); The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2011.
- Van de Doll, H.; Kratz, P. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- da Costa, J.S.; Andrade, W.M.S.; de Figueiredo, R.O.; Santos, P.V.L.; Freitas, J.J.S.; Setzer, W.S.; da Silva, J.K.R.; Maia, J.G.S.; Figueiredo, P.L.B. Chemical composition and variability of the volatile components of Myrciaria species growing in the Amazon Region. Molecules 2022, 27, 2234. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).