Endophytic Fungi Isolated from Ageratina adenophora Exhibits Potential Antimicrobial Activity against Multidrug-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Results
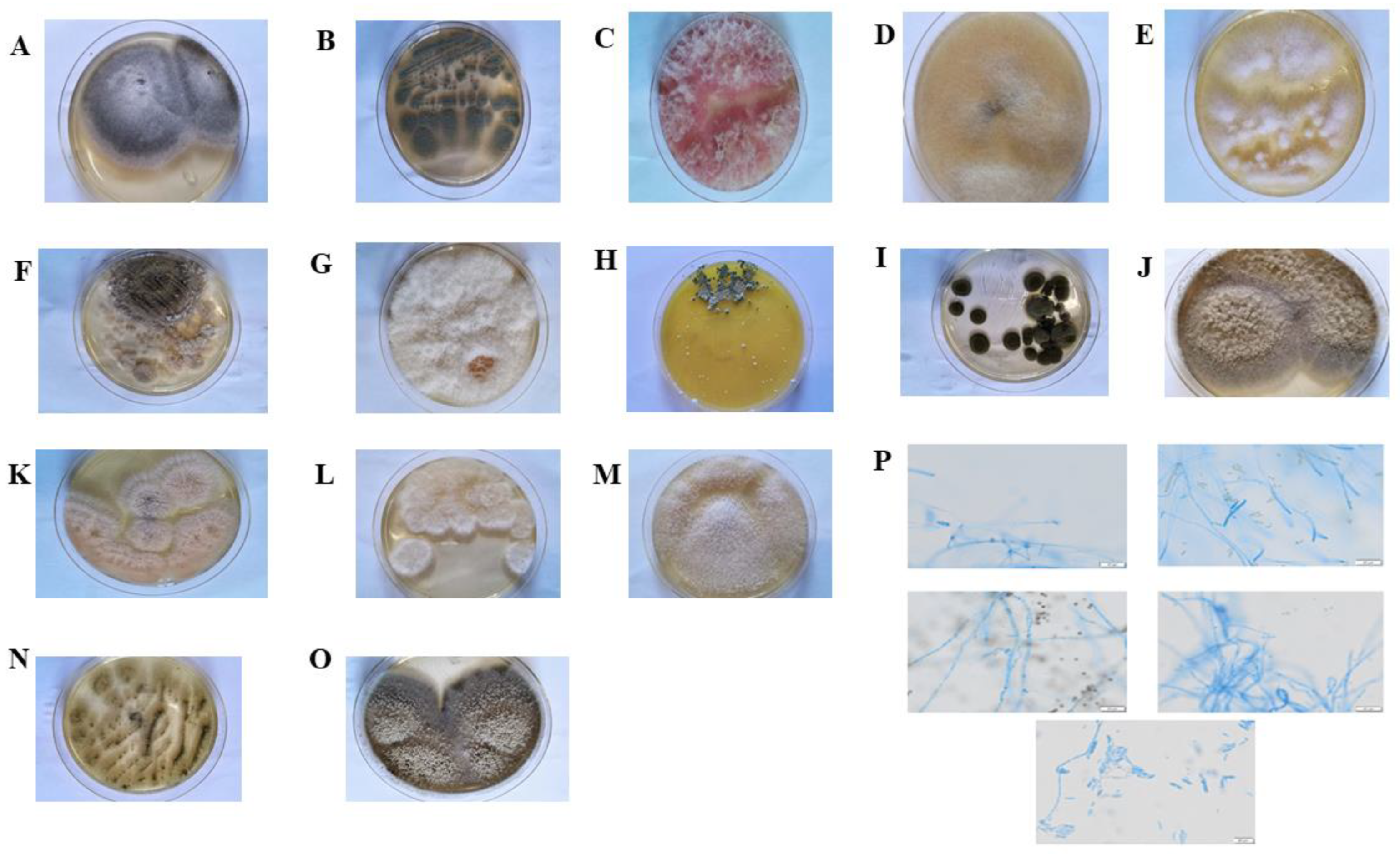
2.1. Isolation, Identification, and Diversity of Culturable Endophytic Fungi in A. adenophora
2.2. Antibacterial Activity
2.3. Liquid Chromatography—Tandem Mass Spectrometry
3. Discussion
4. Materials and Methods
4.1. Study Site and Collection of Plant Materials
4.2. Isolation of Endophytic Fungi
4.3. Identification of Endophytic Fungi
4.4. Antibacterial Activity Screening
4.4.1. Agar Well Diffusion
4.4.2. Preparation of EtOAc Extracts
4.5. Measurement of MIC and Minimum Bactericidal Concentration
4.6. Fluorescence Microscopy
4.7. Liquid Chromatography–Mass Spectrometry Analysis of the Chemical Composition of Ethyl Acetate Extracts
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BLAST | Basic Local Alignment Search Tool |
| NCBI | North Carolina Banking Institute |
| ITS | Internal transcribed spacer |
| DNA | Deoxyribonucleic Acid |
| PCR | Polymerase chain reaction |
| SSA | Similarity threshold |
| N | The number of isolates |
| IF | Isolation frequency |
References
- Potshangbam, M.; Devi, S.I.; Sahoo, D.; Strobel, G.A. Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front. Micro-Biol. 2017, 8, 325. [Google Scholar] [CrossRef]
- Li, J.L.; Sun, X.; Zheng, Y.; Lü, P.P.; Guo, L.D.; Wang, Y.-L. Diversity and community of culturable endophytic fungi from stems and roots of desert halophytes in northwest China. MycoKeys. 2020, 62, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef] [PubMed]
- Manganyi, M.C.; Ateba, C.N. Untapped potentials of endophytic fungi: A review of novel bioactive compounds with biological applications. Microorganisms 2020, 8, 1934. [Google Scholar] [CrossRef] [PubMed]
- Shweta, S.; Gurumurthy, B.R.; Ravikanth, G.; Ramanan, U.S.; Shivanna, M.B. Endophytic fungi from Miquelia dentata Bedd., produce the anti-cancer alkaloid, camptothecine. Phytomedicine 2013, 20, 337–342. [Google Scholar] [CrossRef]
- Puri, S.C.; Nazir, A.; Chawla, R.; Arora, R.; Riyaz-ul-Hasan, S.; Amna, T.; Ahmed, B.; Verma, V.; Singh, S.; Sagar, R.; et al. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryl tetralin lignans. J. Biotechnol. 2006, 122, 494–510. [Google Scholar] [CrossRef]
- Fazilath, U.; Mohan, C.D.; Abeer, H.; Konappa, N.M.; Shobith, R.; Kamath, P.V.; Singh, B.P.; Mudili, V.; Gupta, V.; Siddaiah, C.N.; et al. Endophytic fungi—Alternative sources of cytotoxic compounds: A review. Front. Pharmacol. 2018, 9, 309. [Google Scholar] [CrossRef]
- Martinez-Klimova, E.; Rodríguez-Peña, K.; Sánchez, S. Endophytes as sources of antibiotics. Biochem Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef]
- Ma, J.; Wu, Y.; Xia, L.; Zhang, Q.; Ma, Y.; Yang, Q. Elevational diversity of small mammals in Luoji Mt. Nature Reserve, Sichuan Province. Acta Theriol. Sin. 2010, 30, 400–410. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.L.; Feng, Q.S.; Jin, C.Y.; Cao, A.C.; He, L. A New Strategy for the prevention and control of Eupatorium adenophorum under climate change in China. Sustainability 2017, 9, 2037. [Google Scholar] [CrossRef]
- Yunnan Institute of Materia Medica. List of Medicinal Plants in Yunnan; Yunnan People’s Publishing House: Kunming, China, 1975. [Google Scholar]
- Tripathi, Y.C.; Saini, N.; Anjum, N.A. Review of ethnomedicinal, phytochemical, pharmacoilogical and toxicological aspects of Eupatorium adenophorum Spreng. Asian J. Biomed. Pharmaceut. Sci. 2018, 8, 25–35. [Google Scholar] [CrossRef]
- Okyere, S.K.; Wen, J.; Cui, Y.; Xie, L.; Gao, P.; Wang, J.; Wang, S.; Hu, Y. Toxic mechanisms and pharmacological properties of euptox A, a toxic monomer from A. adenophora. Fitoterapia. 2021, 155, 105032. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Khadayat, K.; Poudel, S.; Shrestha, S.; Marasini, B.P. Phytochemical analysis of medicinal plants of Nepal and their antibacterial and antibiofilm activities against uropathogenic Escherichia coli. BMC Complement. Med. Ther. 2021, 21, 116. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, B.; Yang, J.; Ma, X.H.; Deng, S.H.; Huang, Y.; Wen, Y.; Yuan, J.; Yang, X. Essential oil derived from Eupatorium adenophorum Spreng. mediates anticancer fffect by inhibiting STAT3 and AKT activation to induce apoptosis in hepatocellular carcinoma. Front. Pharmacol. 2018, 9, 483. [Google Scholar] [CrossRef]
- Maheo, A.R.; Vithiya, B.S.M.; Prasad, T.A.A. Biosynthesis and characterization of Eupatorium adenophorum and chitosan mediated copper oxide nanoparticles and their antibacterial activity. Results Surf. Interfaces 2022, 6, 100048. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Chimnoi, N.; Reuk-Ngam, N.; Khlaychan, P.; Makarasen, A.; Wetprasit, N.; Dechtrirat, D.; Supaphol , P.; Techasakul, S. Development of gelatin hydrogel pads incorporated with Eupatorium adenophorum essential oil as antibacterial wound dressing. Polymer Bulletin. 2018, 76, 701–724. [Google Scholar] [CrossRef]
- Liu, S.X.; Wei, H.P.; Cheng, J.; Yang, J.Q. Studies on antibacterial mechanism of the volatile oils from Eupatorium adenophorum Spreng on Staphylococcus aureus. Chin. Hosp. Pharm. J. 2012, 32, 1743–1745. [Google Scholar] [CrossRef]
- Sharma, H.; Rai, A.K.; Dahiya, D.; Chettri, R.; Nigam, P.S. Exploring endophytes for in vitro synthesis of bioactive compounds similar to metabolites produced in vivo by host plants. AIMS Microbiol. 2021, 7, 175–199. [Google Scholar] [CrossRef]
- Wen, J.; Okyere, S.K.; Wang, S.; Wang, J.C.; Xie, L.; Ran, Y.; Hu, Y. Endophytic fungi: An effective alternative source of plant-derived bioactive compounds for pharmacological studies. J. Fungi. 2022, 8, 205. [Google Scholar] [CrossRef]
- Elmoslemany, A.; Elsohaby, I.; Alorabi, M.; Alkafafy, M.; Al-Marri, T. Diversity and risk factors associated with multidrug and methicillin-resistant Staphylococci isolated from cats admitted to a veterinary clinic in Eastern Province, Saudi Arabia. Antibiotics 2021, 10, 367. [Google Scholar] [CrossRef]
- Conn, G.L.; Bavro, V.N.; Davies, C. Editorial: Bacterial mechanisms of antibiotic resistance: A structural perspective. Front. Mol. Biosci. 2019, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Macías-Rubalcava, M.L.; Garrido-Santos, M.Y. Phytotoxic compounds from endophytic fungi. Appl. Microbiol. Biotechnol. 2022, 106, 931–950. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.S.; Song, Y.P.; Meng, L.H.; Yang, S.Q.; Li, X.M. Isolation and characterization of antibacterial carotane sesquiterpenes from Artemisia argyi associated endophytic Trichoderma virens QA-8. Antibiotics 2021, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Shi, Y.T.; Zhou, Z.X.; Yang, C.; Chen, Y.J.; Chen, L.M.; Yang, M.-Z.; Zhang, H.-B. Leaf chemistry and co-occurring species interactions affecting the endophytic fungal composition of Eupatorium adenophorum. Ann. Microbiol. 2011, 61, 655–662. [Google Scholar] [CrossRef]
- Zhong, F.; Fan, X.; Ji, W.; Hai, Z.; Hu, N.; Li, X.; Liu, G.; Yu, C.; Chen, Y.; Lian, B.; et al. Soil fungal community composition and diversity of culturable endophytic fungi from plant roots in the reclaimed area of the Eastern Coast of China. J. Fungi. 2022, 8, 124. [Google Scholar] [CrossRef]
- Gupta, S.; Chaturvedi, P.; Kulkarni, M.G.; Van Staden, J.A. Critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotechnol. Adv. 2020, 9, 107462. [Google Scholar] [CrossRef]
- Zhou, J.; Miao, Y.F.; Fang, K.; Chen, L.; Yang, Z.P.; Dong, X.F.; Zhang, H. Diversity of the endophytic and rhizosphere soil fungi of Ageratina adenophora. Ecol. Sci. 2019, 38, 1–7. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Purahong, W.; Wubet, T.; Hyde, K.D.; Zhang, W. Direct comparison of culture-dependent and culture-independent molecular approaches reveal the diversity of fungal endophytic communities in stems of grapevine (Vitis vinifera). Fungal Divers. 2018, 90, 85–107. [Google Scholar] [CrossRef]
- Rajamani, T.; Suryanarayanan, T.S.; Murali, T.S.; Thirunavukkarasu, N. Distribution and diversity of foliar endophytic fungi in the mangroves of Andaman Islands, India. Fungal Ecol. 2018, 36, 109–116. [Google Scholar] [CrossRef]
- Zheng, R.H.; Li, S.J.; Zhang, X.; Zhao, C.Q. Biological activities of some new secondary metabolites isolated from endophytic fungi: A review study. Int. J. Mol. Sci. 2021, 22, 959. [Google Scholar] [CrossRef]
- Fang, K.; Miao, Y.F.; Chen, L.; Zhou, J.; Yang, Z.P.; Dong, X.F.; Zhang, H.-B. Tissue-specific and geographical variation in endophytic fungi of Ageratina adenophora and fungal associations with the environment. Front. Microbiol. 2019, 10, 2919. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Wang, L.P.; Zhu, G.L.; Zuo, M.X.; Gong, Q.Y. New phenylpyridone derivatives from the Penicillium sumatrense GZWMJZ-313, a fungal endophyte of Garcinia multiflora. Chin. Chem. Lett. 2019, 30, 431–434. [Google Scholar] [CrossRef]
- Shi, Y.B.; Bai, Y.; Pan, H.Q.; Hu, J.C. Isolation and identification of secondary metabolites from plant endophytic fungus F4a and their hypoglycemic and antioxidant activities. J. Microbiol. 2022, 42, 26–31. [Google Scholar] [CrossRef]
- Ning, Y.W.; Fu, Y.N.; Hou, L.I.; Ma, M.G.; Wang, Z.X.; Li, X.F.; Jia, Y. iTRAQ-based quantitative proteomic analysis of synergistic antibacterial mechanism of phenyllactic acid and lactic acid against Bacillus cereus. Food Res. Int. 2021, 139, 109562. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, A.; Beyhan, S.; Choi, Y.; Morales, P.; Chan, A.P.; Espinoza, J.L.; Dupont, C.L.; Meyer, K.J.; Spoering, A.; Lewis, K.; et al. Mechanism-of-action classification of antibiotics by global transcriptome profiling. Antimicrob. Agents Chemother. 2020, 64, e01207–e01219. [Google Scholar] [CrossRef]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef]
- Costea, T.; Vlad, O.C.; Miclea, L.C.; Ganea, C. Alleviation of multidrug resistance by flavonoid and non-flavonoid compounds in breast, lung, colorectal and prostate cancer. Int. J. Mol. Sci. 2020, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ding, Y.; Zhao, P.H.; Li, W.; Li, M.; Zhu, J.B.; Ye, S. Systems pharmacology-based drug discovery and active mechanism of natural products for coronavirus pneumonia (COVID-19): An example using flavonoids. Comput. Biol. Med. 2022, 143, 105241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, Z.G.; Wang, J.H.; Wang, A. Advances in antimicrobial molecular mechanism of organic acids. Acta Vet. Et Zootech. Sin. 2011, 42, 323–328. [Google Scholar]
- Oluduro, O.A.; Aderiye, B.I.; Connolly, J.D.; Akintayo, E.T.; Famurewa, O. Characterization and antimicrobial activity of 4-(β-D-glucopyranosyl-1→4-α-L-rhamnopyranosyloxy)-benzyl thiocarboxamide; a novel bioactive compound from Moringa oleifera seed extract. Folia Microbiol. Praha. 2010, 55, 422–426. [Google Scholar] [CrossRef]
- Huang, X.S.; Zhou, D.X.; Liang, Y.; Liu, X.B.; Cao, F.; Qin, Y.Y.; Mo, T.X.; Xu, Z.L.; Li, J.; Yang, R.Y. Cytochalasins from endophytic Diaporthe sp. GDG-118. Nat. Prod. Res. 2021, 35, 3396–3403. [Google Scholar] [CrossRef]
- Lei, H.; Lin, X.P.; Han, L.; Ma, J.; Ma, Q.J. New metabolites and bioactive chlorinated benzophenone derivatives produced by a marine-derived fungus Pestalotiopsis heterocornis. Mar. Drugs. 2017, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Sun, X.; He, C.; Pulak, M.; Li, X.C.; Guo, L.D. Phyllosphere epiphytic and endophytic fungal community and network structures differ in a tropical mangrove ecosystem. Microbiome 2019, 7, 57. [Google Scholar] [CrossRef]
- Zhao, L.X.; Xu, L.H.; Jiang, C.L. Methods for the Study of Endophytic microorganisms from Traditional Chinese Medicine plants. Methods Enzymol. 2012, 517, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Adhikary, S.K.; Ahmed, M. Morphological characterization of Colletotrichum gloeosporioiedes identified from Anthracnose of Mangifera indica L. Asian J. Plant Pathol. 2017, 11, 102–117. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Sutton, D.A.; Martin-Vicente, A.; Cano-Lira, J.F.; Wiederhold, N.; Guarro, J.; Gené, J. Cladosporium Species Recovered from Clinical Samples in the United States. J. Clin. Microbiol. 2015, 53, 2990–3000. [Google Scholar] [CrossRef] [PubMed]
- El-Dawy, E.G.A.E.M.; Gherbawy, Y.A.; Hussein, M.A. Morphological, molecular characterization, plant pathogenicity and biocontrol of Cladosporium complex groups associated with faba beans. Sci. Rep. 2021, 11, 14183. [Google Scholar] [CrossRef]
- Jiang, N.; Voglmayr, H.; Xue, H.; Piao, C.G.; Li, Y. Morphology and Phylogeny of Pestalotiopsis (Sporocadaceae, Amphisphaeriales) from Fagaceae Leaves in China. Microbiol. Spectr. 2022, 10, e0327222. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Duong, T.T.; Lee, H.B. Characterization of Two New Records of Mucoralean Species Isolated from Gut of Soldier Fly Larva in Korea. Mycobiology 2016, 44, 310–313. [Google Scholar] [CrossRef]
- Hassan, O.; Jeon, J.Y.; Chang, T.; Shin, J.S.; Oh, N.K.; Lee, Y.S. Molecular and Morphological Characterization of Colletotrichum Species in the Colletotrichum gloeosporioides Complex Associated with Persimmon Anthracnose in South Korea. Plant Dis. 2018, 102, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Cambaza, E. Comprehensive Description of Fusarium graminearum Pigments and Related Compounds. Foods 2018, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Aung, S.L.L.; Liu, H.F.; Pei, D.F.; Lu, B.B.; Oo, M.M.; Deng, J.X. Morphology and Molecular Characterization of a Fungus from the Alternaria alternata Species Complex Causing Black Spots on Pyrus sinkiangensis (Koerle pear). Mycobiology 2020, 48, 233–239. [Google Scholar] [CrossRef]
- Mao, Z.L.; Zhang, W.H.; Wu, C.Y.; Feng, H.; Peng, Y.H.; Shahid, H.; Cui, Z.; Ding, P.; Shan, T. Diversity and antibacterial activity of fungal endophytes from eucalyptus exserta. BMC Microbiol. 2021, 21, 155. [Google Scholar] [CrossRef]
- Win, P.M.; Matsumura, E.; Fukuda, K. Effects of pesticides on the diversity of endophytic fungi in tea plants. Microb. Ecol. 2021, 82, 62–72. [Google Scholar] [CrossRef]
- Molla, Y.; Nedi, T.; Tadesse, G.; Alemayehu, H.; Shibeshi, W. Evaluation of the in vitro antibacterial activity of the solvent fractions of the leaves of Rhamnus prinoides L’herit (rhamnaceae) against pathogenic bacteria. BMC Complement. Altern. Med. 2016, 16, 287. [Google Scholar] [CrossRef]
- An, C.; Ma, S.J.; Shi, X.W.; Xue, W.J.; Chen, L. Diversity and antimicrobial activity of endophytic fungi isolated from Chloranthus japonicus Sieb in Qinling Mountains, China. Int. J. Mol. Sci. 2021, 21, 5958. [Google Scholar] [CrossRef]
- Tang, Z.; Qin, Y.; Chen, W.; Zhao, Z.; Lin, W.; Xiao, Y.; Chen, H.; Liu, Y.; Chen, H.; Bu, T.; et al. Diversity, chemical constituents, and biological activities of endophytic fungi isolated from Ligusticum chuanxiong Hort. Front. Microbiol. 2021, 12, 771000. [Google Scholar] [CrossRef]
- Santos, I.P.; Silva, L.C.N.; Silva, M.V.; Araújo, J.M.; Cavalcanti, M.S.; Lima, V.L.M. Antibacterial activity of endophytic fungi from leaves of Indigofera suffruticosa Miller (Fabaceae). Front. Microbiol. 2015, 6, 350. [Google Scholar] [CrossRef]
- Du, W.; Yao, Z.G.; Li, J.L.; Sun, C.L.; Xia, J.B.; Wang, B.G.; Shi, D.; Ren, L. Diversity and antimicrobial activity of endophytic fungi isolated from Securinega suffruticosa in the Yellow River Delta. PLoS ONE. 2020, 15, e0229589. [Google Scholar] [CrossRef]

| No. | Closest Species in ITS Gene Sequences Database | Strain Number (DNA Sequence Data) | Identity (%) | N | IF |
|---|---|---|---|---|---|
| 1 | Alternaria alternata isolate 1 (MH368103) | DCL02, DCL03, DCL11, DCL12, DCL13, DCL15, DCL16, DCL17, DCL18, DCL24,DCL26, DCL34, DCL37, DCL39,DCL46, DCS01, DCS04, DCS06,DCS11, DCS13, DCS15, DCS16, DCS17, DCS18, DCS28, DCS29, DCS32, DCR23 | 99.47 | 28 | 22.58 |
| 2 | Alternaria dauci strain SM19 (MZ314740) | DCS03 | 99.46 | 1 | 0.81 |
| 3 | Alternaria tenuissima voucher HGUP191067 (MZ541977) | DCL14, DCL21, DCL25,DCL32, DCS05 | 100.00 | 5 | 4.03 |
| 4 | Aspergillus flavus isolate AMS_3 (MW522551) | DCL04, DCL10 | 99.49 | 2 | 1.61 |
| 5 | Aporospora terricola strain (MW961422) | DCL01 | 100.00 | 1 | 0.81 |
| 6 | Cercospora sp. IPBCC 13.1012 (KC776152.1) | DCL22, DCR24 | 99.43 | 2 | 1.61 |
| 7 | Botrytis cinerea isolate ET 63 (MH992149) | DCL50, DCL54 | 99.62 | 2 | 1.61 |
| 8 | Diaporthe novem strain MLT18 (MH299960) | DCL27, DCL40, DCL43, DCR08, DCR35 | 99.47 | 5 | 4.03 |
| 9 | Pestalotiopsis trachycarpicola voucher HGUP194013 (MZ724924) | DCL44, DCL47, DCL48, DCR34 | 99.67 | 4 | 3.23 |
| 10 | Trametes versicolor isolate Au-I-1.1 (MF475935) | DCL31 | 99.34 | 1 | 0.81 |
| 11 | Trametes hirsuta isolate HH1 (MF377430) | DCL33 | 99.84 | 1 | 0.81 |
| 12 | Ampelomyces sp. isolate X13 (KJ958371) | DCL28 | 99.81 | 1 | 0.81 |
| 13 | Phoma sp. strain CN1 (ON025541) | DCS07 | 99.62 | 1 | 0.81 |
| 14 | Phomopsis sp. FH-2012b isolate cgyg1 (JQ954648) | DCL38, DCR04 | 98.95 | 2 | 1.61 |
| 15 | Botrytis fabae strain DH-6 (MN589851) | DCL51 | 99.43 | 1 | 0.81 |
| 16 | Cladosporium sp. WSN6 (KC178629) | DCL23, DCS14, DCS23, DCS24, DCR05,DCR21 | 100.00 | 6 | 4.84 |
| 17 | Cladosporium oxysporum ALSHB3 (KU561865) | DCR19 | 100.00 | 1 | 0.81 |
| 18 | Cladosporium perangustum isolate A743 (KU529857) | DCS21 | 99.81 | 1 | 0.81 |
| 19 | Colletotrichum sp. JT2 (KC507287.1) | DCL30 | 99.65 | 1 | 0.81 |
| 20 | Colletotrichum gloeosporioides strain GZBY01 (KM044004) | DCL36, DCL49 | 100.00 | 2 | 1.61 |
| 21 | Colletotrichum godetiae strain 435E (MZ078527.1) | DCL55 | 99.82 | 1 | 0.81 |
| 22 | Colletotrichum liriopes isolate SJM3-2 (MN589679) | DCS20 | 99.47 | 1 | 0.81 |
| 23 | Colletotrichum siamense isolate GQH57(MN296041.1) | DCL35, DCL52 | 99.65 | 2 | 1.61 |
| 24 | Fusarium oxysporum clone SF_72 (MT529348.1) | DCL07, DCL53, DCS22, DCR06, DCR29, DCR30, DCR31 | 100.00 | 7 | 5.65 |
| 25 | Fusarium acuminatum isolate Kt6.1 (MN489462) | DCR20 | 99.46 | 1 | 0.81 |
| 26 | Fusarium graminearum isolate TD2 (MT228970) | DCL19 | 99.81 | 1 | 0.81 |
| 27 | Fusarium kyushuense isolate G797 (MK247795) | DCR07 | 100.00 | 1 | 0.81 |
| 28 | Fusarium solani strain K. L. Chen L035 (KX034335) | DCR09, DCR12, DCR15, DCR16 | 99.82 | 4 | 3.23 |
| 29 | Fusarium verticillioides strain JINF002 (KX196811) | DCS10 | 99.44 | 1 | 0.81 |
| 30 | Trichoderma gamsii strain ICC080 (GQ351597) | DCR22 | 99.33 | 1 | 0.81 |
| 31 | Trichoderma longibrachiatum isolate BM10 (MK910065) | DCL05 | 99.37 | 1 | 0.81 |
| 32 | Trichothecium roseum isolate JZB57004 (MW440515) | DCS25 | 99.83 | 1 | 0.81 2.42 |
| 33 | Trichoderma tomentosum DAOM 229898 (AY605737) | DCS08,DCS09,DCS30,DCR28 | 99.67 | 4 | 3.23 |
| 34 | Trichoderma sulphureum clone SF_102 (MT529378) | DCS27, DCS31 | 100.00 | 2 | 1.61 |
| 35 | Brunneomyces brunnescens isolate NTOU5435 (MN592899) | DCL41 | 98.56 | 1 | 0.81 |
| 36 | Paecilomyces variotii isolate RS3-S2-27 (MN547409) | DCR18 | 99.65 | 1 | 0.81 |
| 37 | Didymella sp. SS14 (KC507292.1) | DCL08, DCR02, DCR11 DCR02 DCR11 | 100.00 | 3 | 2.42 |
| 38 | Didymella sinensis isolate BY42 (MH257405) | DCL42 | 99.05 | 1 | 0.81 |
| 39 | Diaporthe ambigua isolate UT15JD (MF319487) | DCL56, DCR27, DCR36 | 99.47 | 3 | 2.42 |
| 40 | Diaporthe actinidiae strain JL2 (KT163360) | DCL29 | 100.00 | 1 | 0.81 |
| 41 | Diaporthe kochmanii voucher HGUP193007 (MZ724751) | DCL09 | 99.82 | 1 | 0.81 |
| 42 | Gregarithecium curvisporum HHUF 30134 (NR154049) | DCR17 | 92.40 | 1 | 0.81 |
| 43 | Mucor fragilis isolate MZC-1 (MN069560) | DCR10, DCR14 | 99.83 | 2 | 1.61 |
| 44 | Nigrospora sphaerica strain AL2 (MT466514) | DCR33 | 99.45 | 1 | 0.81 |
| 45 | Penicillium commune isolate K19(MK179259) | DCL20,DCS12, DCR01 | 99.47 | 3 | 2.42 |
| 46 | Penicillium concentricum isolate C3 (EU551180) | DCR03 | 99.47 | 1 | 0.81 |
| 47 | Penicillium ochrochloron strain KD-F1 (MK720828) | DCS26, DCR25 | 99.65 | 2 | 0.81 |
| 48 | Penicillium olsonii strain KG 08/11/15 #2(MG252481) | DCS19 | 99.47 | 1 | 0.81 |
| 49 | Penicillium sp. 14 BRO-2013 (KF367512.1) | DCR26 | 99.65 | 1 | 0.81 |
| 50 | Penicillium sclerotigenum isolate INF71(MZ227345) | DCL06 | 100.00 | 1 | 0.81 |
| 51 | Plectosphaerella sp. isolate F49 (MT771317) | DCR13 | 98.79 | 1 | 0.81 |
| 52 | Stagonosporopsis cucurbitacearum strain CAP14C (JQ936326.1) | DCL45, DCS02, DCR32 | 99.81 | 3 | 2.42 |
| Total | 124 | 100 | |||
| Species Richness (S) | 52 | ||||
| Margalef index (D/) | 7.3337 | ||||
| Shannon-Wiener index (H/) | 3.6745 | ||||
| Simpson’ s diversity index(D) | 0.9304 | ||||
| No. | Phylum | Class | Order | Family | Genus | N |
|---|---|---|---|---|---|---|
| 1 | Ascomycota | Dothideomycetes | Pleosporales | Pleosporineae | Alternaria | 34 |
| 2 | Didymellaceae | Didymella | 4 | |||
| 3 | Phoma | 1 | ||||
| 4 | Leptosphaeriaceae | Ampelomyces | 1 | |||
| 5 | Dictyosporiaceae | Gregarithecium | 1 | |||
| 6 | Mycosphaerellales | Mycosphaerellaceae | Cercospora | 2 | ||
| 7 | Stagonosporopsis | 3 | ||||
| 8 | Eurotiomycetes | Capnodiales | Cladosporiaceae | Cladosporium | 8 | |
| 9 | Eurotiales | Aspergillaceae | Aspergillus | 2 | ||
| 10 | Penicillium | 9 | ||||
| 11 | Thermoascaceae | Paecilomyces | 1 | |||
| 12 | Sordariomycetes | Diaporthales | Diaporthaceae | Diaporthe | 10 | |
| 13 | Valsaceae | Phomopsis | 2 | |||
| 14 | Hypocreales | Nectriaceae | Fusarium | 15 | ||
| 15 | Hypocreaceae | Trichoderma | 8 | |||
| 16 | Trichothecium | 1 | ||||
| 17 | Glomerellales | Plectosphaerellaceae | Brunneomyces | 1 | ||
| 18 | Plectosphaerella | 1 | ||||
| 19 | Xylariales | Sporocadaceae | Pestalotiopsis | 4 | ||
| 20 | Apiosporaceae | Nigrospora | 1 | |||
| 21 | Glomerellales | Glomerellaceae | Colletotrichum | 7 | ||
| 22 | Leotiomycetes | Helotiales | Sclerotiniaceae | Botrytis | 3 | |
| 23 | Pezizomycotina | Aporospora | 1 | |||
| 24 | Basidiomycota | Agaricomycetes | Polyporales | Polyporaceae | Trametes | 2 |
| 25 | Mucoromycota | Mucoromycetes | Mucorales | Mucoraceae | Mucor | 2 |
| Total | 3 | 7 | 12 | 19 | 25 | 124 |
| Endophytic Fungi | Pathogens Inhibitory Zone Measurement (mm) | |||||
|---|---|---|---|---|---|---|
| E. coli CICC21530 | S. enteritidis CICC24119 | S. paratyphi CICC10437 | S. agalactiae ATCC13813 | S. aureus CPCC140594 | ||
| DCL03 | Alternaria alternata | 16.19 ± 0.48 cd | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 10.88 ± 0.58 fg |
| DCL06 | Penicillium sclerotigenum | 20.62 ± 0.74 fg | 19.28 ± 0.12 ef | 17.35 ± 1.22 cd | 18.69 ± 0.44 fg | 8.89 ± 0.73 de |
| DCL09 | Diaporthe kochmanii | 19.05 ± 1.35 ef | 18.65 ± 0.34 de | 17.86 ± 0.47 cd | 19.09 ± 0.66 fg | 8.05 ± 0.57 cd |
| DCL12 | Alternaria alternata | 0.00 ± 0.00 a | 14.80 ± 0.53 b | 14.44 ± 0.97 b | 8.14 ± 0.44 b | 7.18 ± 0.78 c |
| DCL14 | Alternaria tenuissima | 21.95 ± 1.44 g | 20.31 ± 0.19 fg | 0.00 ± 0.00 a | 19.92 ± 0.23 g | 0.00 ± 0.00 a |
| DCL16 | Alternaria alternata | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 9.98 ± 0.81 ef |
| DCL18 | Alternaria alternata | 0.00 ± 0.00 a | 21.37 ± 1.02 g | 0.00 ± 0.00 a | 17.65 ± 1.30 def | 12.12 ± 0.91 g |
| DCL24 | Alternaria alternata | 17.79 ± 1.79 cde | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 14.07 ± 0.76 c | 0.00 ± 0.00 a |
| DCL28 | Ampelomyces sp. | 17.04 ± 1.28 cde | 0.00 ± 0.00 a | 16.73 ± 1.08 cd | 16.64 ± 0.47 d | 8.93 ± 0.79 de |
| DCL31 | Trametes versicolor | 0.00 ± 0.00 a | 17.49 ± 0.36 cd | 0.00 ± 0.00 a | 17.49 ± 0.93 def | 0.00 ± 0.00 a |
| DCL36 | Colletotrichum gloeosporioides | 15.55 ± 0.60 c | 17.57 ± 1.24 cd | 0.00 ± 0.00 a | 17.50 ± 0.74 def | 10.38 ± 0.63 ef |
| DCL44 | Pestalotiopsis trachycarpicola | 18.06 ± 1.01 de | 19.95 ± 0.51 ef | 16.43 ± 1.64 c | 16.82 ± 0.97 d | 10.33 ± 0.63 ef |
| DCL48 | Pestalotiopsis trachycarpicola | 16.56 ± 0.57 cd | 16.21 ± 1.05 bc | 14.54 ± 0.71 b | 0.00 ± 0.00 a | 7.57 ± 0.32 cd |
| DCL51 | Botrytis fabae | 12.96 ± 0.82 b | 0.00 ± 0.00 a | 14.84 ± 0.42 b | 0.00 ± 0.00 a | 7.96 ± 0.55 cd |
| DCS07 | Phoma sp. | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 7.89 ± 0.24 cd |
| DCS23 | Cladosporium sp. | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 19.85 ± 1.25 g | 0.00 ± 0.00 a |
| DCS28 | Alternaria alternata | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 19.04 ± 0.48 fg | 0.00 ± 0.00 a |
| DCS30 | Trichoderma tomentosum | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 19.27 ± 0.55 c | 7.01 ± 1.06 fg |
| DCR04 | Phomopsis sp. | 0.00 ± 0.00 a | 19.73 ± 0.91 ef | 17.47 ± 1.07 cd | 19.42 ± 0.54 g | 10.94 ± 0.71 fg |
| DCR09 | Fusarium solani | 19.02 ± 0.42 ef | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 10.39 ± 0.41 ef |
| DCR12 | Fusarium solani | 17.97 ± 1.47 de | 19.54 ±0.88 ef | 16.23 ± 0.20 c | 17.62 ± 0.07 def | 5.58 ± 0.15 b |
| DCR16 | Fusarium solani | 17.76 ± 1.48 cde | 15.52 ± 0.80 b | 0.00 ± 0.00 a | 17.18 ± 1.19 de | 10.13 ± 0.81 ef |
| DCR19 | Cladosporium oxysporum | 0.00 ± 0.00 a | 19.19 ± 0.93 df | 18.21 ± 0.60 d | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| DCR25 | Penicillium ochrochloron | 11.41 ± 0.97 b | 18.38 ± 0.33 de | 16.49 ± 0.46 c | 13.36 ± 1.36 c | 7.93 ± 0.93 cd |
| DCR34 | Pestalotiopsis trachycarpicola | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 19.98 ± 0.89 g | 11.99 ± 0.30 g |
| Crude Extracts of the Fungal Endophytes | Gram-Negative Bacteria | Gram-Positive Bacteria | ||||||
|---|---|---|---|---|---|---|---|---|
| E. coli SMU1710 | Salmonella SMU3256 | MRSA SMU3194 | S.agalactiae SMU5052 | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| P. sclerotigenum | 0.5 | 2 | 2 | nd | 0.5 | 2 | 0.5 | nd |
| D. kochmanii | 2 | nd | 1 | 2 | 0.5 | 1 | 2 | nd |
| P. trachycarpicola | 1 | 2 | 0.5 | 2 | 1 | 2 | 2 | nd |
| F. solani | nd | nd | nd | nd | 2 | nd | nd | nd |
| P. ochrochloron | nd | nd | 1 | nd | nd | nd | 2 | nd |
| S/N | Name of Identified Compound | Adducts | Molecular Formula | RT (Min) | M/Z | Endophytic Fungi (µmol/g) | ||
|---|---|---|---|---|---|---|---|---|
| P. sclerotigenum | D. kochmanii | P. trachycarpicola | ||||||
| 1 | D-(+)-Mannose | [M-H]− | C6H12O6 | 1.419 | 179.056 | 2.29 | 0.36 | 1.85 |
| 2 | Sucrose | [M-H]− | C12H22O11 | 1.495 | 341.109 | 0.64 | 1.50 | 1.09 |
| 3 | D-(−)-Fructose | [M-H]− | C6H12O6 | 1.560 | 179.056 | 0.75 | 0.18 | 1.93 |
| 4 | DL-Malic acid | [M-H]− | C4 H6O5 | 1.579 | 133.014 | 4.61 | nd | 3.39 |
| 5 | trans-Aconitic acid | [M-H]− | C6H6O6 | 1.598 | 173.009 | 0.38 | nd | 0.01 |
| 6 | Pipecolic acid | [M-H]− | C6H11NO2 | 1.743 | 130.086 | 0.83 | 1.29 | 0.02 |
| 7 | D-(+)-Malic acid | [M-H]− | C4H6O5 | 1.767 | 133.014 | 1.02 | nd | 0.13 |
| 8 | D-α-Hydroxyglutaric acid | [M-H]− | C5H8O5 | 1.999 | 147.030 | 1.27 | 0.01 | nd |
| 9 | Citric acid | [M-H]− | C6 H8O7 | 2.006 | 191.020 | 3.60 | 5.61 | 21.14 |
| 10 | Glutaconic acid | [M-H]− | C5H6O4 | 2.112 | 129.019 | nd | 1.91 | nd |
| 11 | Coumarin | [M-H]− | C9H6O2 | 2.357 | 147.044 | 0.01 | 0.14 | 0.39 |
| 12 | Fumaric acid | [M-H]− | C4H4O4 | 2.531 | 115.003 | nd | 0.01 | 2.51 |
| 13 | Succinic acid | [M-H]− | C4H6O4 | 2.696 | 117.019 | 0.58 | nd | 1.34 |
| 14 | 4-Hydroxybutyric acid | [M-H]− | C4H8O3 | 3.756 | 103.040 | nd | nd | 0.26 |
| 15 | 2′-Deoxyinosine | [M-H]− | C10H12N4O | 4.094 | 251.078 | 0.80 | nd | 0.24 |
| 16 | Homogentisic acid | [M-H]− | C8H8O4 | 4.846 | 167.035 | 1.71 | 1.05 | nd |
| 17 | 2-Furoic acid | [M-H]− | C5H4O3 | 4.894 | 111.008 | 0.76 | nd | nd |
| 18 | Pantothenic acid | [M-H]− | C9H17NO5 | 5.111 | 218.103 | 1.12 | nd | 2.32 |
| 19 | Caffeic acid | [M-H]− | C9H8O4 | 5.167 | 179.035 | 0.32 | 4.38 | 0.71 |
| 20 | Salicylic acid | [M-H]− | C7H6O3 | 5.413 | 137.024 | 0.32 | 0.19 | 0.34 |
| 21 | Taxifolin | [M-H]− | C15H12O7 | 5.555 | 303.051 | 1.15 | nd | nd |
| 22 | Benzoic acid | [M-H]− | C7H6O2 | 5.561 | 121.029 | 0.22 | 0.01 | 0.17 |
| 23 | 2,3-Dihydroxybenzoic acid | [M-H]− | C7H6O4 | 5.586 | 153.019 | 0.63 | nd | 0.11 |
| 24 | 3-Coumaric acid | [M-H]− | C9H8O3 | 5.649 | 163.040 | nd | 0.59 | 1.71 |
| 25 | N-Acetylvaline | [M-H]− | C7H13NO3 | 5.663 | 158.082 | 0.37 | 1.07 | 0.48 |
| 26 | Phenylacetylglycine | [M-H]− | C10H11NO3 | 5.685 | 192.067 | 3.39 | 0.92 | 0.50 |
| 27 | Suberic acid | [M-H]− | C8H14O4 | 5.719 | 173.082 | 0.03 | 0.22 | 2.61 |
| 28 | N-Acetyl-L-phenylalanine | [M-H]− | C11H13NO3 | 5.730 | 206.082 | nd | nd | 3.21 |
| 29 | 2-Hydroxycaproic acid | [M-H]− | C6 H12O3 | 5.737 | 131.071 | 1.66 | nd | nd |
| 30 | Phenyllactic acid | [M-H]− | C9H10O3 | 5.754 | 165.056 | 1.20 | 9.59 | nd |
| 31 | Glycitein | [M-H]− | C16H12O5 | 5.888 | 284.069 | 0.93 | 1.17 | 0.28 |
| 32 | Azelaic acid | [M-H]− | C9H16O4 | 5.908 | 187.098 | 0.30 | 0.81 | 1.37 |
| 33 | Genistein | [M-H]− | C15H10O5 | 6.043 | 269.045 | 2.85 | nd | 4.70 |
| 34 | Nootkatone | [M-H]− | C15H22O | 6.303 | 219.174 | 1.67 | nd | nd |
| 35 | Dulcitol | [M-H]− | C6H14O6 | 7.527 | 181.072 | 0.11 | 0.04 | 0.29 |
| 36 | 1,5-Anhydro-D-glucitol | [M-H]− | C6H12O5 | 8.058 | 163.061 | 2.66 | nd | 0.13 |
| 37 | Xylitol | [M-H]− | C5H12O5 | 8.707 | 151.061 | 1.10 | 0.14 | 0.19 |
| 38 | Isorhamnetin | [M-H]− | C16H12O7 | 10.283 | 315.051 | 6.21 | 0.12 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, J.; Okyere, S.K.; Wang, J.; Huang, R.; Wang, Y.; Liu, L.; Nong, X.; Hu, Y. Endophytic Fungi Isolated from Ageratina adenophora Exhibits Potential Antimicrobial Activity against Multidrug-Resistant Staphylococcus aureus. Plants 2023, 12, 650. https://doi.org/10.3390/plants12030650
Wen J, Okyere SK, Wang J, Huang R, Wang Y, Liu L, Nong X, Hu Y. Endophytic Fungi Isolated from Ageratina adenophora Exhibits Potential Antimicrobial Activity against Multidrug-Resistant Staphylococcus aureus. Plants. 2023; 12(3):650. https://doi.org/10.3390/plants12030650
Chicago/Turabian StyleWen, Juan, Samuel Kumi Okyere, Jianchen Wang, Ruya Huang, Ya Wang, Lin Liu, Xiang Nong, and Yanchun Hu. 2023. "Endophytic Fungi Isolated from Ageratina adenophora Exhibits Potential Antimicrobial Activity against Multidrug-Resistant Staphylococcus aureus" Plants 12, no. 3: 650. https://doi.org/10.3390/plants12030650
APA StyleWen, J., Okyere, S. K., Wang, J., Huang, R., Wang, Y., Liu, L., Nong, X., & Hu, Y. (2023). Endophytic Fungi Isolated from Ageratina adenophora Exhibits Potential Antimicrobial Activity against Multidrug-Resistant Staphylococcus aureus. Plants, 12(3), 650. https://doi.org/10.3390/plants12030650







