Effects of Organophosphate-Degrading Bacteria on the Plant Biomass, Active Medicinal Components, and Soil Phosphorus Levels of Paris polyphylla var. yunnanensis
Abstract
1. Introduction
2. Results
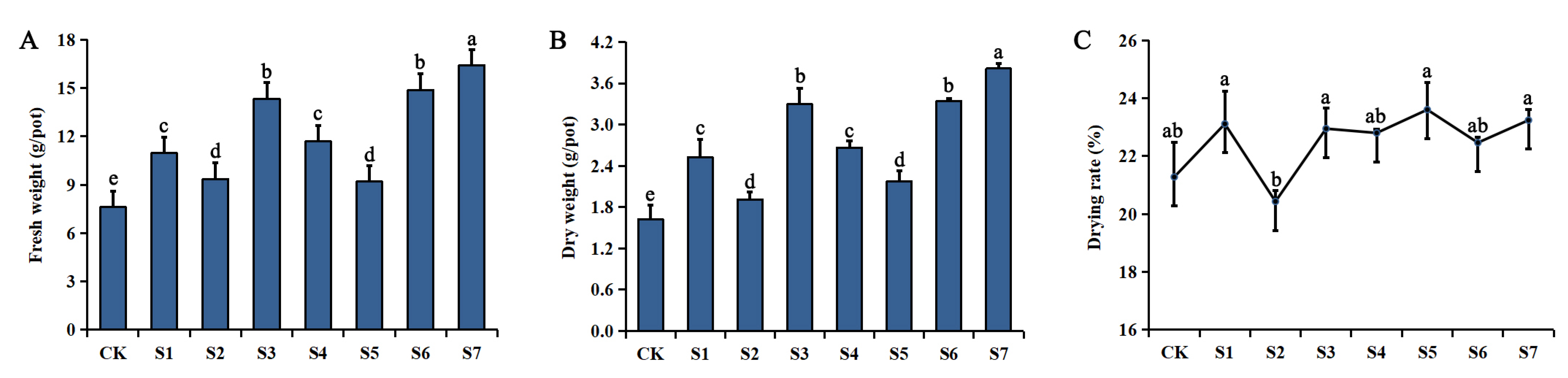
2.1. Changes in Rhizome Biomass
2.2. Changes in Steroidal Saponin Content
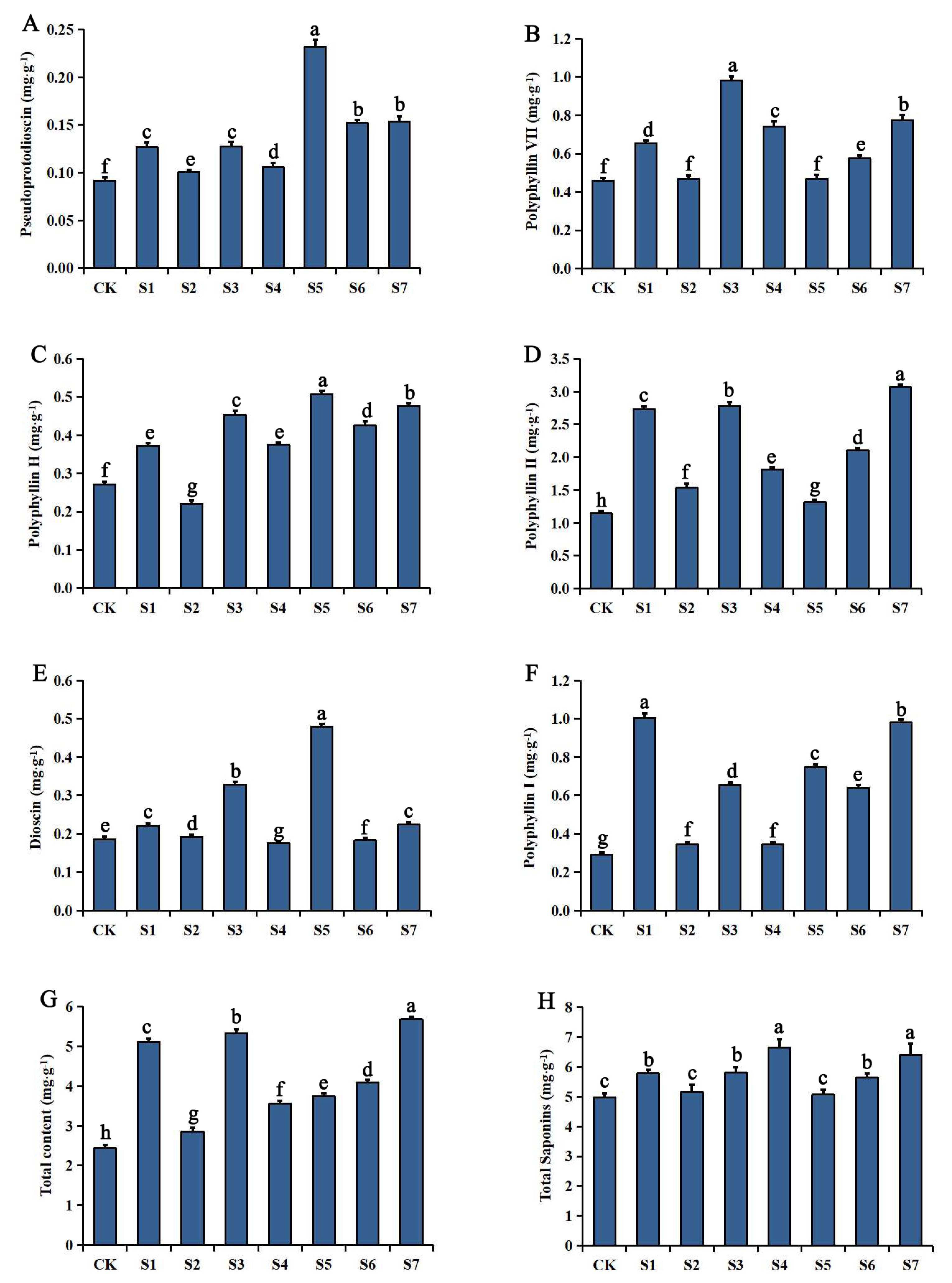
2.2.1. Changes in Steroidal Saponin Concentrations in the Rhizome
2.2.2. Principal Component Analysis of the Six Steroidal Saponins
2.3. Changes in Concentrations of Different Soil P Forms
2.4. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Bacterial Materials
4.2. Plant Materials
4.3. Determination of Rhizome Biomass
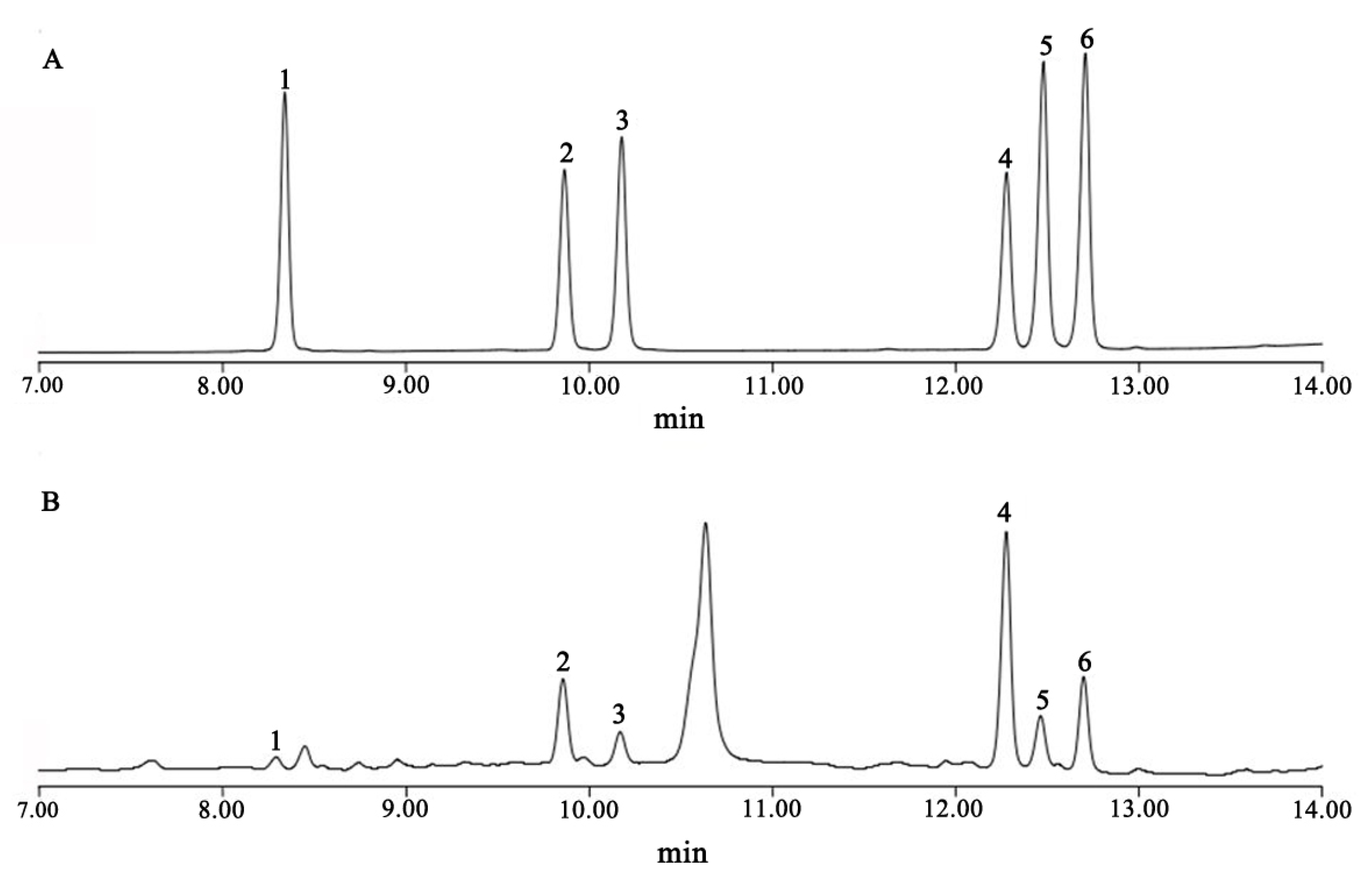
4.4. Determination of Steroidal Saponin Concentration in Rhizomes
4.5. Methodological Validation
4.6. Principal Component Analysis
4.7. Determinations of Concentrations of Different P Forms in Rhizospheric Soil
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, K.; Ren, X.; Mu, R.-F.; Zhou, T.-T.; Li, D.; Hu, H.; Liu, Y.; Li, S.-H. Ecdysteroids and spirosterane steroids from the traditional Chinese medicine Paris polyphylla var. yunnanensis. Phytochem. Lett. 2021, 45, 117–120. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China (Part 1); China Medical Science Press: Beijing, China, 2020; p. 261. [Google Scholar]
- Li, H.-L.; Xu, L.-F.; Li, Z.-W.; Zhao, S.-X.; Guo, D.-Q.; Rui, L.; Zhou, N. Mycorrhizas effect polyphyllin accumulation of Paris polyphylla var. yunnanensis through promoting PpSE expression. Phyton 2021, 90, 1535–1547. [Google Scholar]
- Zhao, J.-L.; Mou, Y.; Shan, T.-J.; Li, Y.; Zhou, L.-G.; Wang, M.-A.; Wang, J.-G. Antimicrobial metabolites from the endophytic fungus Pichia guilliermondii isolated from Paris polyphylla var. yunnanensis. Molecules 2010, 15, 7961–7970. [Google Scholar] [CrossRef]
- Yu, K.; Fan, Q.-L.; Wang, Y.; Wei, J.-R.; Ma, Q.; Yu, D.; Li, J.-R. Function of leafy sepals in Paris polyphylla: Photosynthate allocation and partitioning to the fruit and rhizome. Funct. Plant Biol. 2013, 40, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.-F.; Wu, L.-H.; Zhang, Q.-Z.; Wang, Y.-Z. Geographical traceability of cultivated Paris polyphylla var. yunnanensis using ATR-FTMIR spectroscopy with three mathematical algorithms. Anal. Methods 2018, 11, 113–122. [Google Scholar] [CrossRef]
- Chan, C.; Liao, Y.-Y.; Chiou, T.-J. The impact of phosphorus on plant immunity. Plant Cell Physiol. 2021, 62, 582–589. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Ji, L.-L.; Chen, S.-C.; Wu, Z.-H.; Chang, J.; Tao, R.-P. Effects of planted grasses on soil nutrients and soil enzyme activities in Carya cathayensis forest. Non-Wood For. Res. 2022, 40, 19–25. [Google Scholar]
- Sattari, S.-Z.; Vanittersum, M.-K.; Giller, K.-E.; Zhang, F.; Bouwman, A.-F. Key role of China and its agriculture in global sustainable phosphorus management. Environ. Res. Lett. 2014, 9, 054003. [Google Scholar] [CrossRef]
- Carpenter, S.-R. Eutrophication of aquatic ecosystems: Bistability and soil phosphorus. Proc. Natl. Acad. Sci. USA 2005, 102, 10002–10005. [Google Scholar] [CrossRef]
- Qin, L.-J.; Yang, Y.-Z.; Yang, X.-Y. Advances in mechanisms of soil P solubilization and dissolution by phosphate solubilizing microorganisms. Life Sci. Res. 2019, 23, 59–64. [Google Scholar]
- Hameeda, B.; Harini, G.; Rupela, O.-P.; Wani, S.-P.; Reddy, G. Growth promotion of maize by phosphate-solubilizing bacteria isolated from composts and macrofauna. Microbiol. Res. 2008, 163, 234–242. [Google Scholar] [CrossRef]
- Raj, J.; Bagyaraj, D.-J.; Manjunath, A. Infouence of soil inoculation with vesicular-arbuscular mycorrhiza and a phosphate dissolving bacterium on plant growth and 32p-up take. Soil Biol. Biochem. 1981, 13, 105–108. [Google Scholar]
- Bashan, Y.; Kamnev, A.-A.; De-bashan, L.-E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biol. Fertil. Soils 2013, 49, 465–479. [Google Scholar] [CrossRef]
- Kobus, J. The distribute on of microorganisms mobilizing P in different soils. Acta Microbiologia Plolonica 1962, 11, 255–264. [Google Scholar]
- Yadav, B.K.; Tarafdar, J.C. Penicillium purpurogenum, unique P mobilizers in arid agro-ecosystems. Arid Soil Res. Rehabil. 2011, 25, 87–99. [Google Scholar] [CrossRef]
- Bai, W.-J.; Hu, R.-R.; Zhang, J.-E.; Feng, L.-F.; Xu, H.-Q. Effects of phosphate-solubilizing bacteria on growth and P uptake of corn seedling. Ecol. Sci. 2014, 33, 401–407. [Google Scholar]
- Jyoti, S.; Minaxi; Anamika, J. Impact of a phosphate solubilizing bacterium and an arbuscular mycorrhizal fungus (glomus etunicatum) on growth, yield and P concentration in wheat plants. CLEAN-Soil Air Water 2014, 42, 1248–1252. [Google Scholar]
- Shen, M.-C.; Li, J.-G.; Dong, Y.-H.; Liu, H.; Peng, J.-W.; Hu, Y.; Sun, Y. Profiling of plant growth-promoting metabolites by phosphate-solubilizing bacteria in maize rhizosphere. Plants 2021, 10, 1071. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Peng, S.; Hua, Q.-Q.; Qiu, C.-W.; Wu, P.; Liu, X.-L.; Lin, X.-G. The long-term effects of using phosphate-solubilizing bacteria and photosynthetic bacteria as biofertilizers on peanut yield and soil bacteria community. Front. Microbiol. 2021, 12, 693535. [Google Scholar] [CrossRef]
- El, M.-S.; Elabed, A.; Alaoui, T.-Z.-E.; Meddich, A.; Filali, M.-A.; Douira, A.; Ibnsouda, K.-S.; Amir, S.; El, M.-C. Effect of arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria consortia associated with phospho-compost on phosphorus solubilization and growth of tomato seedlings (Solanum lycopersicum L.). Commun. Soil Sci. Plant Anal. 2020, 51, 622–634. [Google Scholar]
- Sirinapa, C.; Chaisit, T.; Mominul, I.; Sabina, Y. Efficiency of phosphate-solubilizing bacteria to address phosphorus fixation in Takhli soil series: A case of sugarcane cultivation, Thailand. Plant Soil 2021, 73, 1–11. [Google Scholar]
- Du, H.-H.; Zhu, F.-R.; Yang, M.; Guo, D.-Q.; Zhao, S.-X.; Li, Q.-T.; Zhou, N. Isolation and identification of phosphatolytic bacteria in Paris polyphylla var. yunnanensis. China J. Chin. Mater. Med. 2021, 46, 915–922. [Google Scholar]
- You, Y.-L.; Zhao, H.-M.; Li, Y.; Wu, R.-X.; Liu, G.-B.; Zhou, J.-D.; Chen, J.-F. Dynamic changes in biomass accumulation and nutritional quality of triticeae forages. Acta Prataculturae Sin. 2022, 31, 189–201. [Google Scholar]
- Zhang, D.-L.; Liu, S.; Liu, Z.-Y.; Ma, C.-C.; Jiang, Y.-H.; Sun, C.; Li, K.-L.; Cao, G.-S.; Lin, Z.-M.; Wang, P. Polyphyllin I induces cell cycle arrest in prostate cancer cells via the upregulation of IL6 and P21 expression. Medicine 2019, 98, e17743. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Huang, P.; Liu, X.-W.; Xiang, Y.-C.; Zhang, T.; Wu, Y.-Z.; Xu, J.-X.; Sun, Z.-T.; Zhen, W.-G.; Zhang, L. Polyphyllin I inhibits growth and invasion of cisplatin-resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis. J. Pharmacol. Sci. 2018, 137, 305–312. [Google Scholar] [CrossRef]
- Niu, W.-P.; Xu, L.; Li, J.-W.; Zhai, Y.; Sun, Z.-H.; Shi, W.; Jiang, Y.-H.; Ma, C.-C.; Lin, H.-Q.; Guo, Y.-X. Polyphyllin II inhibits human bladder cancer migration and invasion by regulating EMT-associated factors and MMPs. Oncol. Lett. 2020, 20, 2928–2936. [Google Scholar] [CrossRef]
- Pang, D.; Yang, C.; Li, C.; Zou, Y.; Feng, B.; Huang, C. Polyphyllin II inhibits liver cancer cell proliferation, migration and invasion through downregulated cofifilin activity and the AKT/NF-κB pathway. Biol. Open 2020, 9, bio046854. [Google Scholar]
- Zhou, L.; Song, H.-Y.; Zhang, Y.-Q.; Ren, Z.-Z.; Li, M.-H.; Fu, Q. Polyphyllin VII attenuated RANKL-induced osteoclast differentiation via inhibiting of TRAF6/c-Src/PI3K pathway and ROS production. BMC Musculoskelet. Disord. 2020, 21, 136. [Google Scholar] [CrossRef]
- Zhang, C.; Li, C.; Jia, X.; Wang, K.; Tu, Y.; Wang, R.-C.; Liu, K.-C.; Lu, T.; He, C.-W. In vitro and in vivo anti-inflflammatory effects of polyphyllin VII through downregulating MAPK and NF-κB pathways. Molecules 2019, 24, 875. [Google Scholar]
- Guan, X.; Li, R.-S.; Duan, B.-Z.; Wang, Y.; Fan, M.; Wang, S.; Zhang, H.-Z.; Xia, C.-L. Advances in research on chemical constituents and pharmacological effects of Paris genus and prediction and analysis of quality markers. Chin. Herb. Med. 2019, 50, 4838–4852. [Google Scholar]
- Zhang, Z.; Zhao, X.-R.; Gao, M.; Xu, L.-N.; Qi, Y.; Wang, J.-H.; Yin, L.-H. Dioscin alleviates myocardial infarction injury via regulating BMP4/NOX1-mediated oxidative stress and inflammation. Phytomedicine 2022, 103, 154222. [Google Scholar] [CrossRef]
- Mao, Z.; Gao, M.; Zhao, X.-R.; Li, L.-L.; Peng, J.-Y. Neuroprotective effect of dioscin against parkinson’s disease via adjusting dual-specificity phosphatase 6 (DUSP6)-mediated oxidative stress. Molecules 2022, 27, 3151. [Google Scholar] [CrossRef]
- Bao, R.-X.; Wang, W.; Chen, B.-B.; Pan, J.-J.; Chen, Q.; Liu, M.-Y.; Wang, D.; Wu, Y.-Z.; Yu, H.-Y.; Han, L.-F. Dioscin ameliorates hyperuricemia-induced atherosclerosis by modulating of cholesterol metabolism through FXR-Signaling pathway. Nutrients 2022, 14, 1983. [Google Scholar] [CrossRef]
- Gai, Y.N.; Li, Y.-S.; Xu, Z.-L.; Chen, J. Pseudoprotodioscin inhibits SREBPs and microRNA 33a/b levels and reduces the gene expression regarding the synthesis of cholesterol and triglycerides. Fitoterapia 2019, 139, 104393. [Google Scholar] [CrossRef]
- Gao, Y.-T.; Yang, L.-R.; Yang, Y.-L.; Wang, X.-M. Study on scavenging reactive oxygen species and antioxidant effects of Paris poiyphylla extract in vitro. Chin. Tradit. Pat. Med. 2007, 30, 195–198. [Google Scholar]
- Gu, W.-C.; Guo, D.-Q.; Yang, M.; Huang, X.-L.; Li, H.-L.; Zhou, N. Determination of 9 steroidal saponins in rhizome and fibrous roots of wild and cultivated Paris polyphylla var. yunnanensis by UPLC. Tradit. Chin. Drug Res. Clin. Pharmacol. 2020, 31, 838–847. [Google Scholar]
- Xu, H.Y.; Lv, J.; Yu, C. Study on Growth Promoting of Pinus massoniana Seedlings Regulated by Rhizosphere Phosphate-solubilizing Paraburkholderia spp. Biotechnol. Bull. 2019, 31, 1–12. [Google Scholar]
- Qi, P.; Wang, X.-J.; Jiao, Y.-P.; Guo, G.-W.; Ma, J.-J.; Wu, J.; Cai, L.-Q.; Zhang, R.-Z. Effects of P application on P composition and availability of spring wheat topsoil on the Loess Plateau of Longzhong. Agric. Res. Arid Areas 2021, 39, 99–106. [Google Scholar]
- Li, R.-N.; Wang, Z.-P.; Batbayar, J.; Zhang, D.-J.; Zhang, S.-L.; Yang, X.-Y. Relationship between soil available P and inorganic P forms under equivalent organic matter condition in a tier soil. Sci. Agric. Sin. 2019, 52, 3852–3865. [Google Scholar]
- Wang, Q.-J.; Guo, D.-J.; Ma, Y. Stoichiometric characteristics of soil carbon, nitrogen and P and their relationship with soil available P under continuous application of organic fertilizer for vegetable cultivation in greenhouse. Jiangsu J. Agric. Sci. 2021, 37, 893–901. [Google Scholar]
- Zhao, S.X.; Deng, Q.S.; Jiang, C.Y. Inoculation with Potassium Solubilizing Bacteria and Its Effect on the Medicinal Characteristics of Paris polyphylla var. yunnanensis. Agriculture 2022, 13, 21. [Google Scholar] [CrossRef]
- Huang, Y.P.; Zhang, J.; Yang, M.; Ding, B.; Guo, D.Q.; Pan, X.J.; Zang, D.Q.; Zhou, N. Effects of different inoculation periods on seedling growth and steroidal saponin content of Paris polyphylla var. yunnanensis. Chin. Tradit. Herb. Drugs 2019, 50, 4438–4448. [Google Scholar]
- Liu, D.Z.; Chen, Y.Y.; Zhang, M.; Tian, Y.; Li, Z.H.; Yu, D.; Dou, X.W. Study on biotransformation of endophytic fungus to enhance saponins content and antitumour activity of Paridis Rhizoma. Chin. Tradit. Herb. Drugs 2022, 53, 4486–4492. [Google Scholar]
- Zhang, J.; Zhou, N.; Pan, X.-J.; Guo, D.-Q.; Ding, B.; Wang, L.; Yang, M.; Zhu, L.; Zhang, H. Screening of preponderant arbuscular mycorrhizal fungi species from Paris polyphylla var. yunnanensis seedlings based on biomass and active components. Chin. Herb. Med. 2018, 49, 1897–1906. [Google Scholar]
- Yang, X.; Zhang, Z.-Q. Contents determination of total saponin of Paridis. Chin. Arch. Tradit. Chin. Med. 2007, 26, 2420–2422. [Google Scholar]
- Bao, S.-D. Soil Agrochemical Analysis; China Agricultural Press: Beijing, China, 2003; pp. 14–15. [Google Scholar]


| Steroidal Saponins | Principal Component 1 | Principal Component 2 |
|---|---|---|
| Pseudoprotodioscin | 0.748 | −0.638 |
| Polyphyllin VII | 0.507 | 0.714 |
| Polyphyllin H | 0.939 | −0.110 |
| Polyphyllin II | 0.630 | 0.754 |
| Dioscin | 0.639 | −0.640 |
| Polyphyllin I | 0.845 | 0.181 |
| Eigenvalue | 3.221 | 1.940 |
| Contribution rate (%) | 53.68 | 32.34 |
| Cumulative contribution rate (%) | 53.68 | 86.02 |
| Treatment Group | F1 | Sorting | F2 | Sorting | Fz | Sorting |
|---|---|---|---|---|---|---|
| CK | −2.515 | 8 | −0.514 | 7 | −1.763 | 8 |
| S1 | 0.716 | 4 | 0.940 | 3 | 0.800 | 3 |
| S2 | −2.381 | 7 | −0.256 | 6 | −1.582 | 7 |
| S3 | 1.439 | 3 | 1.183 | 1 | 1.343 | 2 |
| S4 | −1.033 | 6 | 0.601 | 4 | −0.419 | 6 |
| S5 | 1.881 | 1 | −3.048 | 8 | 0.027 | 4 |
| S6 | 0.070 | 5 | −0.049 | 5 | 0.025 | 5 |
| S7 | 1.824 | 2 | 1.143 | 2 | 1.568 | 1 |
| Indicators | Biomass | Pseudoprotodioscin | Polyphyllin VII | Polyphyllin H | Polyphyllin II | Dioscin | Polyphyllin I | Total Saponins | Organic P | Olsen-P | Total P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomass | 1.000 | ||||||||||
| Pseudoprotodioscin | 0.219 | 1.000 | |||||||||
| Polyphyllin VII | 0.732 * | −0.123 | 1.000 | ||||||||
| Polyphyllin H | 0.673 | 0.788 * | 0.461 | 1.000 | |||||||
| Polyphyllin II | 0.837 ** | 0.005 | 0.788 * | 0.465 | 1.000 | ||||||
| Dioscin | −0.044 | 0.821 * | 0.015 | 0.628 | −0.104 | 1.000 | |||||
| Polyphyllin I | 0.584 | 0.539 | 0.331 | 0.672 | 0.742 * | 0.324 | 1.000 | ||||
| Total Saponins | 0.702 | −0.142 | 0.709 * | 0.361 | 0.649 | −0.319 | 0.279 | 1.000 | |||
| Organic P | 0.437 | 0.487 | −0.018 | 0.345 | 0.100 | 0.218 | 0.141 | 0.008 | 1.000 | ||
| Effective P | 0.737 * | 0598 | 0.388 | 0.695 | 0.600 | 0.316 | 0.666 | 0.481 | 0.721 * | 1.000 | |
| Total P | 0.655 | 0.627 | 0.314 | 0.754 * | 0.418 | 0.284 | 0.540 | 0.623 | 0.558 | 0.890 ** | 1.000 |
| Treatments | Details |
|---|---|
| S1 | Inoculation with Bacillus mycoides |
| S2 | Inoculation with B. wiedmannii |
| S3 | Inoculation with B. proteolyticus |
| S4 | Inoculation with B. mycoides and B. wiedmannii |
| S5 | Inoculation with B. mycoides and B. proteolyticus |
| S6 | Inoculation with B. wiedmannii and B. proteolyticus |
| S7 | Inoculation with B. mycoides, B. wiedmannii, and B. proteolyticus |
| CK | No inoculation with any organophosphate-degradation bacteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-W.; Wang, Y.-H.; Liu, C.; Wu, Y.-M.; Lan, G.-X.; Xue, Y.-B.; Wu, Q.-S.; Zhou, N. Effects of Organophosphate-Degrading Bacteria on the Plant Biomass, Active Medicinal Components, and Soil Phosphorus Levels of Paris polyphylla var. yunnanensis. Plants 2023, 12, 631. https://doi.org/10.3390/plants12030631
Li Z-W, Wang Y-H, Liu C, Wu Y-M, Lan G-X, Xue Y-B, Wu Q-S, Zhou N. Effects of Organophosphate-Degrading Bacteria on the Plant Biomass, Active Medicinal Components, and Soil Phosphorus Levels of Paris polyphylla var. yunnanensis. Plants. 2023; 12(3):631. https://doi.org/10.3390/plants12030631
Chicago/Turabian StyleLi, Zhuo-Wei, Yue-Heng Wang, Chang Liu, Ying-Mei Wu, Guo-Xin Lan, Yan-Bin Xue, Qiang-Sheng Wu, and Nong Zhou. 2023. "Effects of Organophosphate-Degrading Bacteria on the Plant Biomass, Active Medicinal Components, and Soil Phosphorus Levels of Paris polyphylla var. yunnanensis" Plants 12, no. 3: 631. https://doi.org/10.3390/plants12030631
APA StyleLi, Z.-W., Wang, Y.-H., Liu, C., Wu, Y.-M., Lan, G.-X., Xue, Y.-B., Wu, Q.-S., & Zhou, N. (2023). Effects of Organophosphate-Degrading Bacteria on the Plant Biomass, Active Medicinal Components, and Soil Phosphorus Levels of Paris polyphylla var. yunnanensis. Plants, 12(3), 631. https://doi.org/10.3390/plants12030631








