Influence of Foliar Application of Hydrogen Peroxide on Gas Exchange, Photochemical Efficiency, and Growth of Soursop under Salt Stress
Abstract
1. Introduction
2. Results
2.1. Leaf Gas Exchange
2.2. Quantum Yield
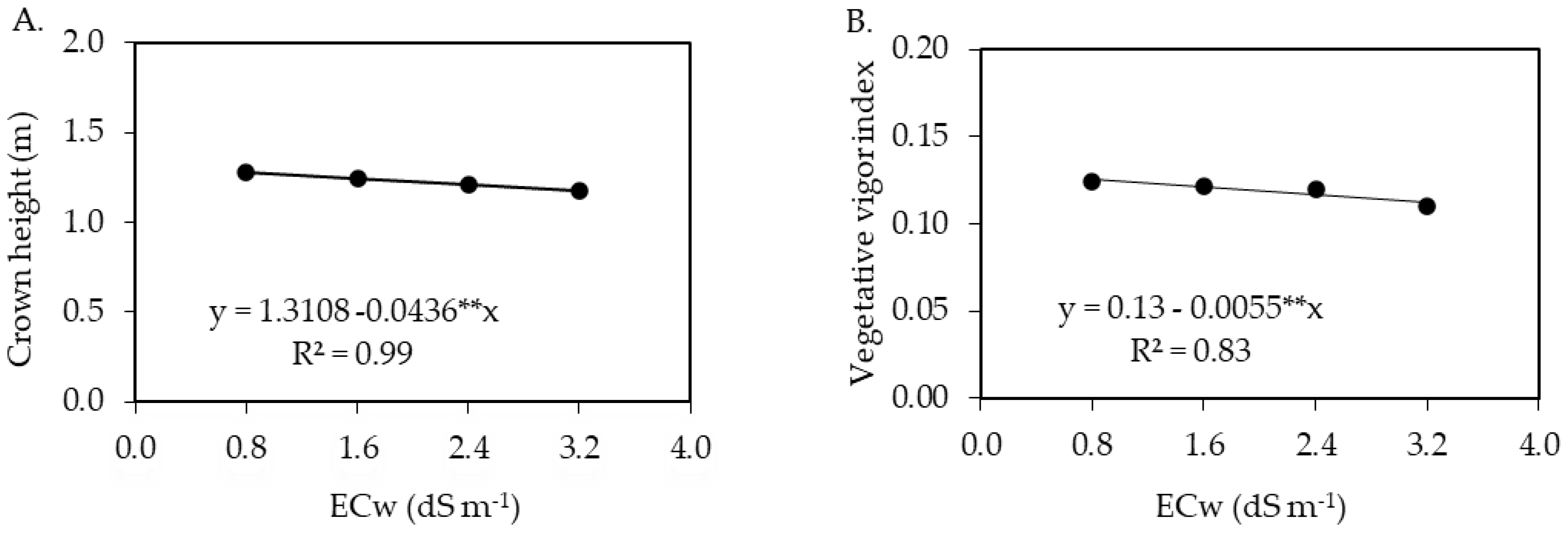
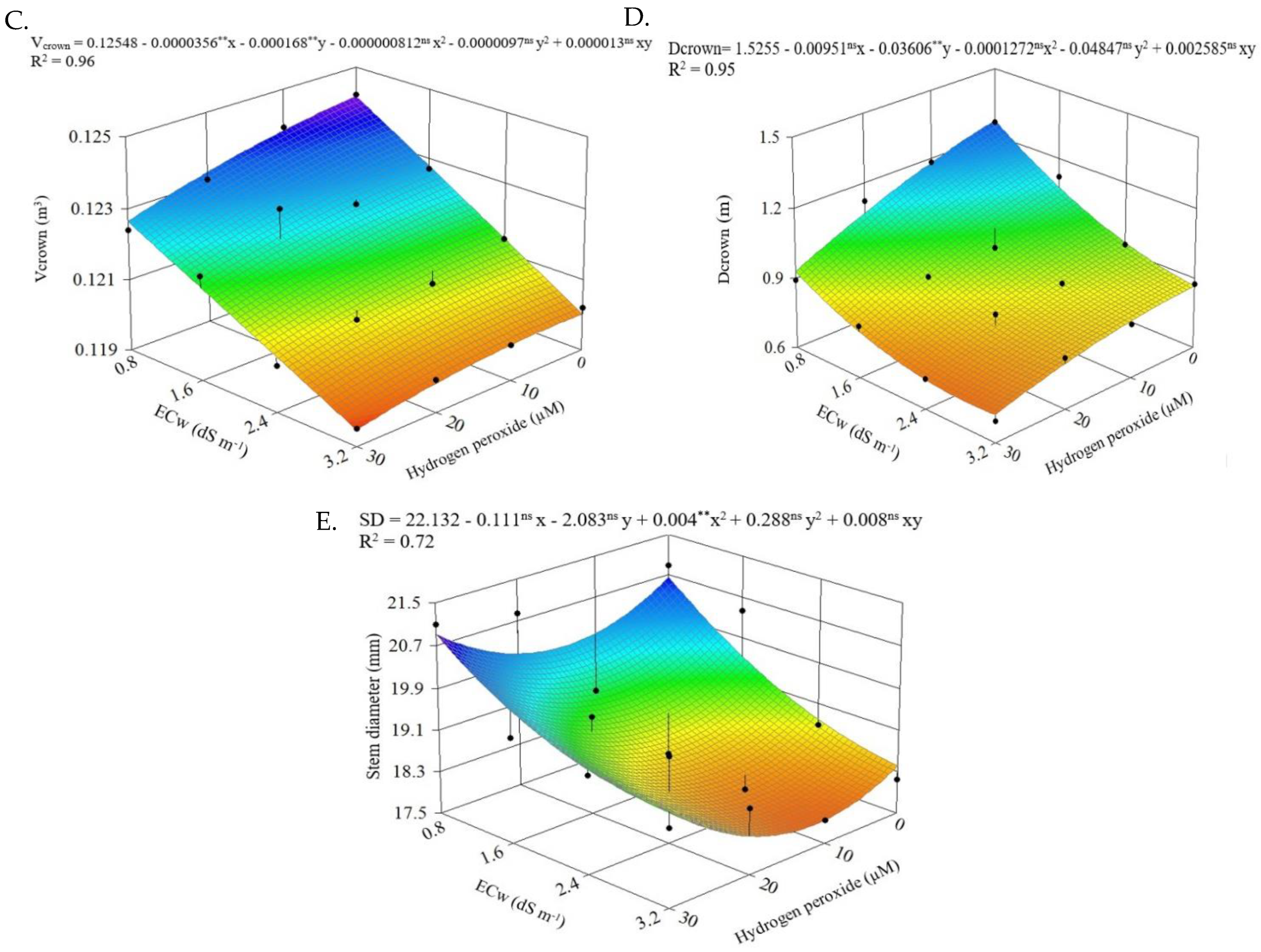
2.3. Morphological Parameters
3. Discussion
4. Materials and Methods
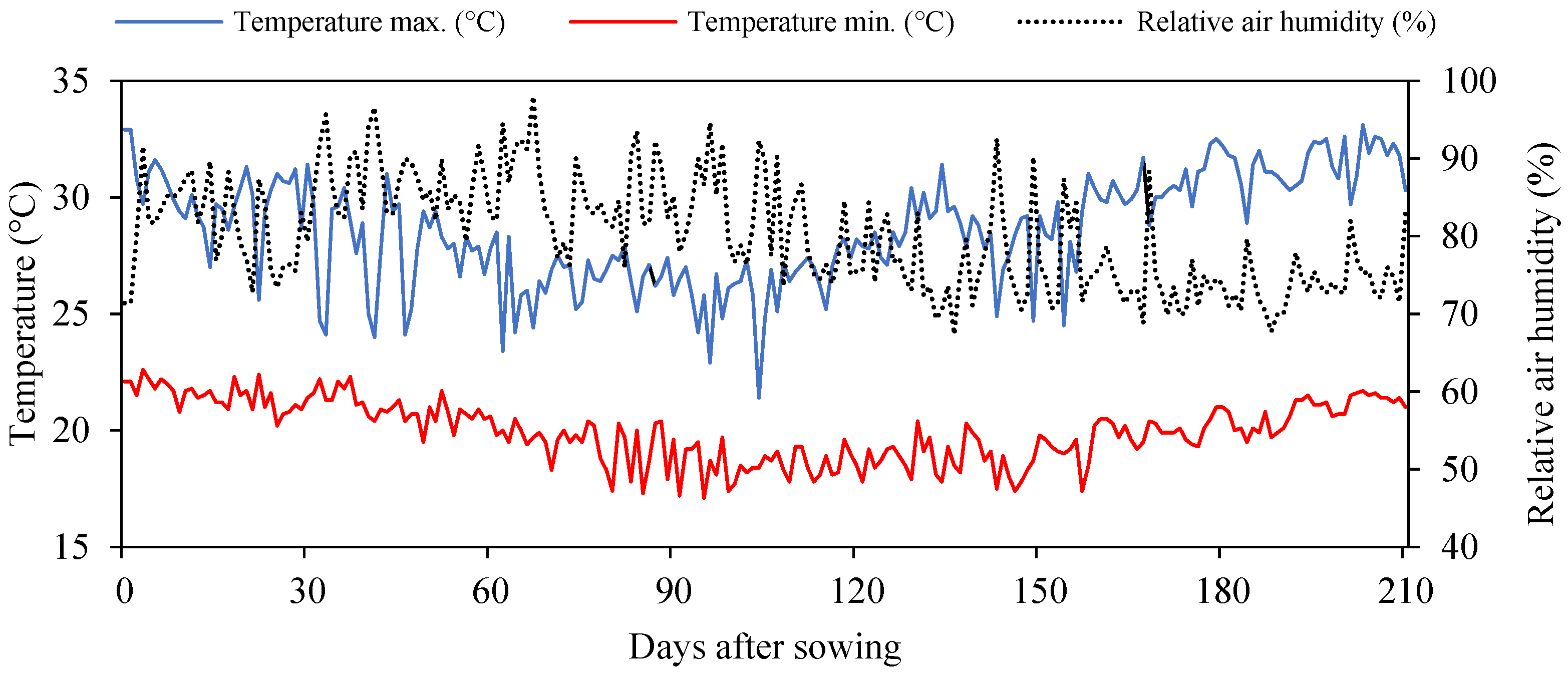
4.1. Location of the Experiment
4.2. Treatments and Experimental Design
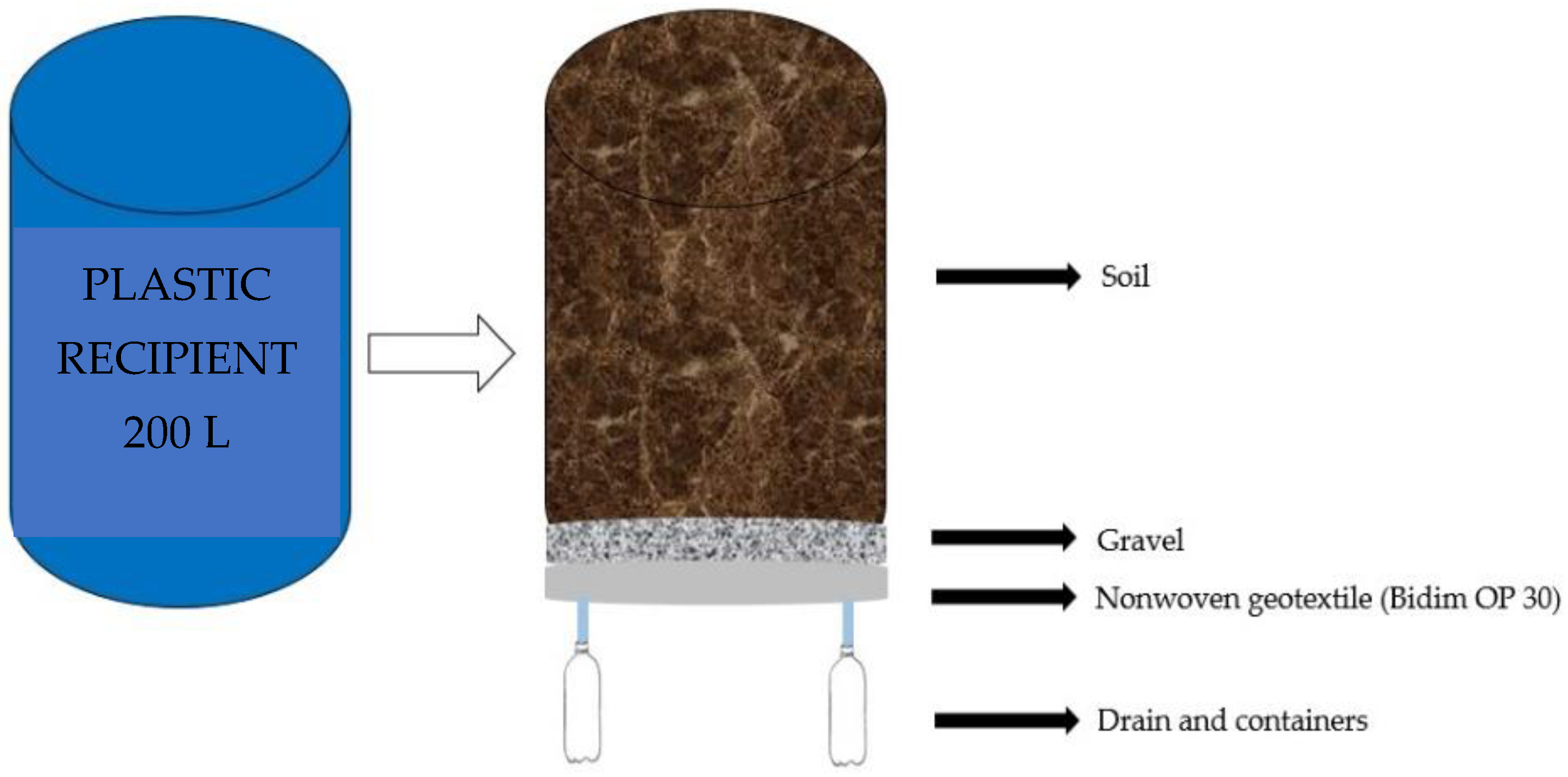
4.3. Description of the Experiment
4.4. Variables Analyzed
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavalcante, L.F.; Carvalho, S.D.; Lima, E.D.; Feitosa Filho, J.C.; Silva, D.A. Produção e qualidade da graviola sob irrigação e cobertura do solo com resíduo de sisal. Magistra 2016, 28, 91–101. [Google Scholar]
- Bento, E.B.; Monteiro, Á.B.; Lemos, I.C.S.; Brito Junior, F.E.; Oliveira, D.R.; Menezes, I.R.A.; Kerntopf, M.R. Estudio etnofarmacológico comparativo en la región del Araripe de la Annona muricata L. (Graviola). Rev. Cubana Plant. Med. 2016, 21, 9–19. [Google Scholar]
- Da Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; Veloso, L.L.D.S.A.; Souza, L.D.P.; de Fátima, R.T.; Silva, F.D.A.D.; Gheyi, H.R. Exogenous application of salicylic acid on the mitigation of salt stress in Capsicum annuum L. Ciênc. Rural. 2023, 53, e20210447. [Google Scholar] [CrossRef]
- Silva, A.A.R.; Veloso, L.L.S.A.; Lima, G.S.; Azevedo, C.A.V.; Gheyi, H.R.; Fernandes, P.D. Hydrogen peroxide in the acclimation of yellow passion fruit seedlings to salt stress. Rev. Bras. Eng. Agríc. Ambient. 2021, 25, 116–123. [Google Scholar] [CrossRef]
- De Lima, G.S.; Pinheiro, F.W.A.; Gheyi, H.R.; Soares, L.A.D.A.; Sousa, P.F.D.N.; Fernandes, P.D. Saline water irrigation strategies and potassium fertilization on physiology and fruit production of yellow passion fruit. Rev. Bras. Eng. Agric. Ambient. 2022, 26, 180–189. [Google Scholar] [CrossRef]
- De Lima, G.S.; dos Santos, J.B.; Soares, L.A.D.A.; Gheyi, H.R.; Nobre, R.G.; Pereira, R.F. Irrigação com águas salinas e aplicação de prolina foliar em cultivo de pimentão ‘All Big’. Comun. Sci. 2016, 7, 513–522. [Google Scholar] [CrossRef]
- Pinheiro, F.W.A.; de Lima, G.S.; Gheyi, H.R.; Soares, L.A.D.A.; de Oliveira, S.G.; da Silva, F.A. Gas exchange and yellow passion fruit production under irrigation strategies using brackish water and potassium. Rev. Cien. Agron. 2022, 53, e20217816. [Google Scholar] [CrossRef]
- Da Silva, E.M.; De Lima, G.S.; Gheyi, H.R.; Nobre, R.G.; Sá, F.V.D.S.; Souza, L.D.P. Growth and gas exchanges in soursop under irrigation with saline water and nitrogen sources. Rev. Bras. Eng. Agríc. Ambient. 2018, 22, 776–781. [Google Scholar] [CrossRef]
- Veloso, L.L.D.S.A.; da Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; Gheyi, H.R.; Moreira, R.C.L. Growth and gas exchange of soursop under salt stress and hydrogen peroxide application. Rev. Bras. Eng. Agríc. Ambient. 2022, 26, 119–125. [Google Scholar] [CrossRef]
- Veloso, L.L.D.S.A.; Nobre, R.G.; Souza, L.D.P.; Gheyi, H.R.; Cavalcante, I.T.S.; Araujo, E.B.G.; Da Silva, W.L. Formation of soursop seedlings irrigated using waters with different salinity levels and nitrogen fertilization. Biosci. J. 2018, 34, 151–160. [Google Scholar] [CrossRef]
- Dos Santos, A.N.; Silva, E.F.S.; Silva, G.S.; Bezerra, R.R.; Pedrosa, M.R.P. Antioxidant response of cowpea co-inoculated with plant growth-promoting bacteria under salt stress. Braz. J. Micro. 2018, 49, 513–521. [Google Scholar] [CrossRef]
- Veloso, L.L.D.S.A.; de Azevedo, C.A.V.; Nobre, R.G.; de Lima, G.S.; Capitulino, J.D.; Silva, F.D.A.D. H2O2 alleviates salt stress effects on photochemical efficiency and photosynthetic pigments of cotton genotypes. Rev. Bras. Eng. Agríc. Ambient. 2023, 27, 34–41. [Google Scholar] [CrossRef]
- Andrade, E.M.G.; De Lima, G.S.; De Lima, V.L.A.; Da Silva, S.S.; Gheyi, H.R.; Da Silva, A.A.R. Gas exchanges and growth of passion fruit under saline water irrigation and H2O2 application. Rev. Bras. Eng. Agríc. Ambient. 2019, 23, 945–951. [Google Scholar] [CrossRef]
- Bagheri, M.; Gholami, M.; Baninasab, B. Hydrogen peroxide-induced salt tolerance in relation to antioxidant systems in pistachio seedlings. Sci. Hortic. 2019, 243, 207–213. [Google Scholar] [CrossRef]
- Da Silva, A.A.R.; De Lima, G.S.; De Azevedo, C.A.V.; Veloso, L.L.D.S.A.; Capitulino, J.D.; Gheyi, H.R. Induction of tolerance to salt stress in soursop seedlings using hydrogen peroxide. Comun. Sci. 2019, 10, 484–490. [Google Scholar] [CrossRef]
- Liu, L.; Huang, L.; Lin, X.; Sun, C.O. Hydrogen peroxide alleviates salinity-induced damage by increasing proline buildup in wheat seedlings. Plant Cell Rep. 2020, 39, 567–575. [Google Scholar] [CrossRef]
- Da Silva, A.A.R.; Sousa, P.F.D.N.; de Lima, G.S.; Soares, L.A.D.A.; Gheyi, H.R.; de Azevedo, C.A.V. Hydrogen peroxide reduces the effect of salt stress on growth and postharvest quality of hydroponic mini watermelon. Water Air Soil Pollut. 2022, 233, 198. [Google Scholar] [CrossRef]
- Tanou, G.; Job, C.; Rajjou, L.; Arc, E.; Belghazi, M.; Diamantidis, G.; Molassiotis, A.; Job, D. Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J. 2009, 60, 795–804. [Google Scholar] [CrossRef]
- Hajivar, B.; Zare-Bavani, M.R. Alleviation of salinity stress by hydrogen peroxide and nitric oxide in tomato plants. Adv. Hortic. Sci. 2019, 33, 409–416. [Google Scholar] [CrossRef]
- Gondim, F.A.; Miranda, R.S.; Gomes-Filho, E.; Prisco, J.T. Enhanced salt tolerance in maize plants induced by H2O2 leaf spraying is associated with improved gas exchange rather than with non-enzymatic antioxidant system. Theor. Exp. Plant Physiol. 2013, 25, 251–260. [Google Scholar] [CrossRef]
- Wang, X.; Hou, C.; Liu, J.; He, W.; Nan, W.; Gong, H.; Bi, Y. Hydrogen peroxide is involved in the regulation of rice (Oryza sativa L.) tolerance to salt stress. Acta Physiol. Plant. 2013, 35, 891–900. [Google Scholar] [CrossRef]
- Mendonça, A.J.T.; da Silva, A.A.R.; de Lima, G.S.; Soares, L.A.D.A.; Oliveira, V.K.N.; Gheyi, H.R.; de Lacerda, C.F.; de Azevedo, C.A.V.; de Lima, V.L.A.; Fernandes, P.D. Salicylic acid modulates okra tolerance to salt stress in hydroponic system. Agriculture 2022, 12, 1687. [Google Scholar] [CrossRef]
- De Lima, G.S.; De Andrade, J.N.F.; De Medeiros, M.N.V.; Soares, L.A.D.A.; Gheyi, H.R.; Nobre, R.G.; Fernandes, P.D.; De Lacerda, C.N. Gas exchange, growth, and quality of passion fruit seedlings cultivated with saline water. Semin. Ciências Agrárias 2021, 42, 137–154. [Google Scholar] [CrossRef]
- Dias, A.S.; De Lima, G.S.; Sá, F.V.D.S.; Gheyi, H.R.; Soares, L.A.D.A.; Fernandes, P.D. Gas exchanges and photochemical efficiency of West Indian cherry cultivated with saline water and potassium fertilization. Rev. Bras. Eng. Agric. Ambient. 2018, 22, 628–633. [Google Scholar] [CrossRef]
- Abrar, M.M.; Sohail, M.; Saqib, M.; Akhtar, J.; Abbas, G.; Wahab, H.A.; Xu, M. Interactive salinity and water stress severely reduced the growth, stress tolerance, and physiological responses of guava (Psidium guajava L.). Sci. Rep. 2022, 12, 18952. [Google Scholar] [CrossRef]
- Sá, F.V.D.S.; Gheyi, H.R.; de Lima, G.S.; Pinheiro, F.W.A.; de Paiva, E.P.; Moreira, R.C.L.; Silva, L.D.A.; Fernandes, P.D. The right combination of NPK fertilization may mitigate salt stress in custard apple (Annona squamosa L.). Acta Physiol. Plant. 2021, 43, 59. [Google Scholar] [CrossRef]
- Carvalho, F.E.L.; Lobo, A.K.M.; Bonifacio, A.; Martins, M.O.; Lima Neto, M.C.; Silveira, J.A.G. Aclimatação ao estresse salino em plantas de arroz induzida pelo pré-tratamento com H2O2. Rev. Bras. Eng. Agric. Ambient. 2011, 15, 416–423. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. EROS as key players in plant stress signalling. JXB 2014, 65, 1229–1240. [Google Scholar] [CrossRef]
- Simões, W.L.; Coelho, D.S.; Mesquita, A.C.; Calgaro, M.; da Silva, J.S. Physiological and biochemical responses of sugarcane varieties to salt stress. Rev. Caatinga 2020, 32, 1069–1076. [Google Scholar] [CrossRef]
- Dias, A.S.; De Lima, G.S.; Pinheiro, F.W.A.; Gheyi, H.R.; Soares, L.A.D.A. Gas exchanges, quantum yield and photosynthetic pigments of West Indian cherry under salt stress and potassium fertilization. Rev. Caatinga 2019, 32, 429–439. [Google Scholar] [CrossRef]
- Silveira, A.G.; Silva, S.L.F.; Silva, E.V.; Viégas, R.V. Mecanismos biomoleculares envolvidos com a resistência ao estresse salino em plantas. In Manejo da Salinidade na Agricultura: Estudos Básicos e Aplicados, 2nd ed.; Gheyi, H.R., Dias, N.d.S., de Lacerda, C.F., Gomes Filho, É., Eds.; INCTSal: Fortaleza, Brazil, 2016; Chapter 13; pp. 181–197. [Google Scholar]
- Hnilicková, H.; Hnilička, F.; Martinkova, J.; Kraus, K. Effects of salt stress on water status, photosynthesis and chlorophyll fluorescence of rocket. Plant Soil Environ. 2017, 63, 362–367. [Google Scholar] [CrossRef]
- Turan, M.; Ekinci, M.; Kul, R.; Boynueyri, F.G.; Yildirim, E. Mitigation of salinity stress in cucumber seedlings by exogenous hydrogen sulfide. J. Plant Res. 2022, 135, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, A.; Soundararajan, P.; Muneer, S.; Ko, C.H.; Jeong, B.R. Silicon mitigates salinity stress by regulating the physiology, antioxidant enzyme activities, and protein expression in Capsicum annuum ‘Bugwang’. BioMed Res. Int. 2016, 2016, 3076357. [Google Scholar] [CrossRef]
- Da Silva, A.A.R.; De Lima, G.S.; De Azevedo, C.A.V.; Gheyi, H.R.; Souza, L.D.P.; Veloso, L.L.D.S.A. Gas exchanges and growth of passion fruit seedlings under salt stress and hydrogen peroxide. Pesq. Agropecu. Trop. 2019, 49, e55671. [Google Scholar] [CrossRef]
- Cattivelli, L.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Maré, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crops Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Monteiro, D.R.; De Melo, H.F.; Lins, C.M.T.; Dourado, P.R.M.; Santos, H.R.B.; De Souza, E.R. Chlorophyll a fluorescence in saccharine sorghum irrigated with saline water. Rev. Bras. Eng. Agríc. Ambient. 2018, 22, 673–678. [Google Scholar] [CrossRef]
- Da Silva, A.A.R.; de Lima, G.S.; de Azevedo, C.A.V.; Gheyi, H.R.; Soares, L.A.D.A.; Veloso, L.L.D.S.A. Salicylic acid improves physiological indicators of soursop irrigated with saline water. Rev. Bras. Eng. Agríc. Ambient. 2022, 26, 412–419. [Google Scholar] [CrossRef]
- Reis, F.O.; Campostrini, E. Microaspersão de água sobre a copa: Um estudo relacionado às trocas gasosas e à eficiência fotoquímica em plantas de mamoeiro. Rev. Bras. Agroc. 2011, 17, 284–295. [Google Scholar] [CrossRef]
- Veloso, L.L.D.S.A.; da Silva, A.A.R.; Capitulino, J.D.; de Lima, G.S.; de Azevedo, C.A.V.; Gheyi, H.R.; Nobre, R.G.; Fernandes, P.D. Photochemical efficiency and growth of soursop rootstocks subjected to salt stress and hydrogen peroxide. AIMS Agric. Food 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Yadav, S.P.; Bharadwaj, R.B.; Nayak, H.; Mahto, R.; Singh, R.K.; Prasad, S.K. Impact of salt stress on growth, productivity and physicochemical properties of plants: A Review. Int. J. Chem. Stud. 2019, 7, 1793–1798. [Google Scholar]
- Veloso, L.L.D.S.A.; De Lima, G.S.; De Azevedo, C.A.V.; Nobre, R.G.; Da Silva, A.A.R.; Capitulino, J.D.; Gheyi, H.R.; Bonifácio, B.F. Physiological changes and growth of soursop plants under irrigation with saline water and H2O2 in post-grafting phase. Semin. Ciênc. Agrár. 2020, 41, 3023–3038. [Google Scholar] [CrossRef]
- Shobha, S.; Ashwani, K.; Sehrawat, N.; Kumar, K.; Kumar, N.; Lata, C.; Mann, A. Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi J. Biol. Sci. 2021, 28, 2510–2517. [Google Scholar] [CrossRef]
- Najar, R.; Aydi, S.; Sassi-Aydi, S.; Zarai, A.; Abdelly, C. Effect of salt stress on photosynthesis and chlorophyll fluorescence in Medicago truncatula. Plant Biosyst. 2019, 153, 88–97. [Google Scholar] [CrossRef]
- Kilic, S.; Kahraman, A. The mitigation effects of exogenous hydrogen peroxide when alleviating seed germination and seedling growth inhibition on salinity-induced stress in barley. Pol. J. Environ. Stud. 2016, 25, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.A.R.; De Lima, G.S.; De Azevedo, C.A.V.; Veloso, L.L.D.S.A.; Gheyi, H.R.; Soares, L.A.D.A. Salt stress and exogenous application of hydrogen peroxide on photosynthetic parameters of soursop. Rev. Bras. Eng. Agríc. Ambient. 2019, 23, 257–263. [Google Scholar] [CrossRef]
- Nazir, F.; Fariduddin, Q.T.; Khan, A. Hydrogen peroxide as a signaling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 2020, 252, 126486. [Google Scholar] [CrossRef]
- Farouk, S.; Amira, M.S.A.Q. Enhancing seed quality and productivity as well as physio-anatomical responses of pea plants by folic acid and/or hydrogen peroxide application. Sci. Hortic. 2018, 240, 29–37. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, H.; Chen, T.; Pen, J.; Yu, S.; Zhao, X. Morphological and physiological responses of cotton plants (Gossypium hirsutum L.) to salinity. PLoS ONE 2014, 9, e112807. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa Solos: Brasília, Brazil, 2017; p. 573. [Google Scholar]
- Medeiros, J.F. Qualidade de Água de Irrigação e Evolução da Salinidade nas Propriedades Assistidas Pelo GAT nos Estados de RN, PB e CE. Master’s Dissertation, Universidade Federal da Paraíba, Campina Grande, Brazil, 1992; 196p. [Google Scholar]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; U.S. Department of Agriculture: Washington, DC, USA, 1954; 160p.
- Cavalcante, F.J. Recomendações de Adubação Para o Estado de Pernambuco, 2nd ed.; IPA: Recife, Brazil, 2008; Volume 3, p. 212. [Google Scholar]
- EMBRAPA. A Cultura da Gravioleira, 1st ed.; Embrapa Amazônia Ocidental: Manaus, Brazil, 1999; 19p. [Google Scholar]
- Fernandes, E.A.; Soares, L.A.D.A.; de Lima, G.S.; Neta, A.M.D.S.S.; Roque, I.A.; da Silva, F.A.; Fernandes, P.D.; de Lacerda, C.N. Cell damage, gas exchange, and growth of Annona squamosa L. under saline water irrigation and potassium fertilization. Semin. Ciências Agrárias 2021, 42, 999–1018. [Google Scholar] [CrossRef]
- Portella, C.R.; Marinho, C.S.; Amaral, B.D.; Carvalho, W.S.G.; Campos, G.S.; Silva, M.P.S.; Sousa, M.C. Desempenho de cultivares de citros enxertados sobre o trifoliateiro ‘Flying Dragon’ e limoeiro ‘Cravo’ em fase de formação do pomar. Bragantia 2016, 1, 70–75. [Google Scholar] [CrossRef]
- Ferreira, D.F. SISVAR: A computer analysis system to fixed effects split plot type designs. Rev. Bras. Biom. 2019, 37, 529–535. [Google Scholar] [CrossRef]
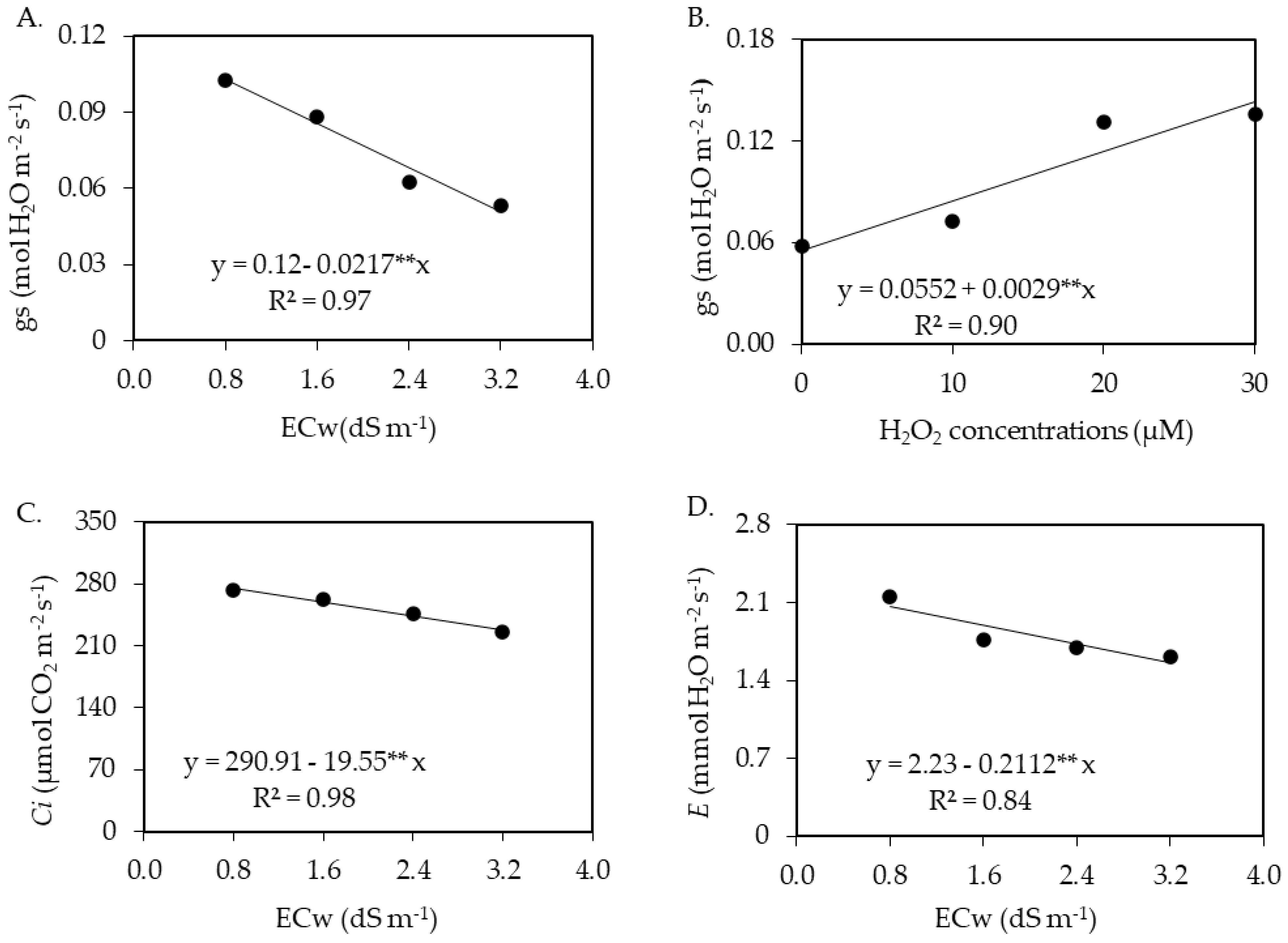
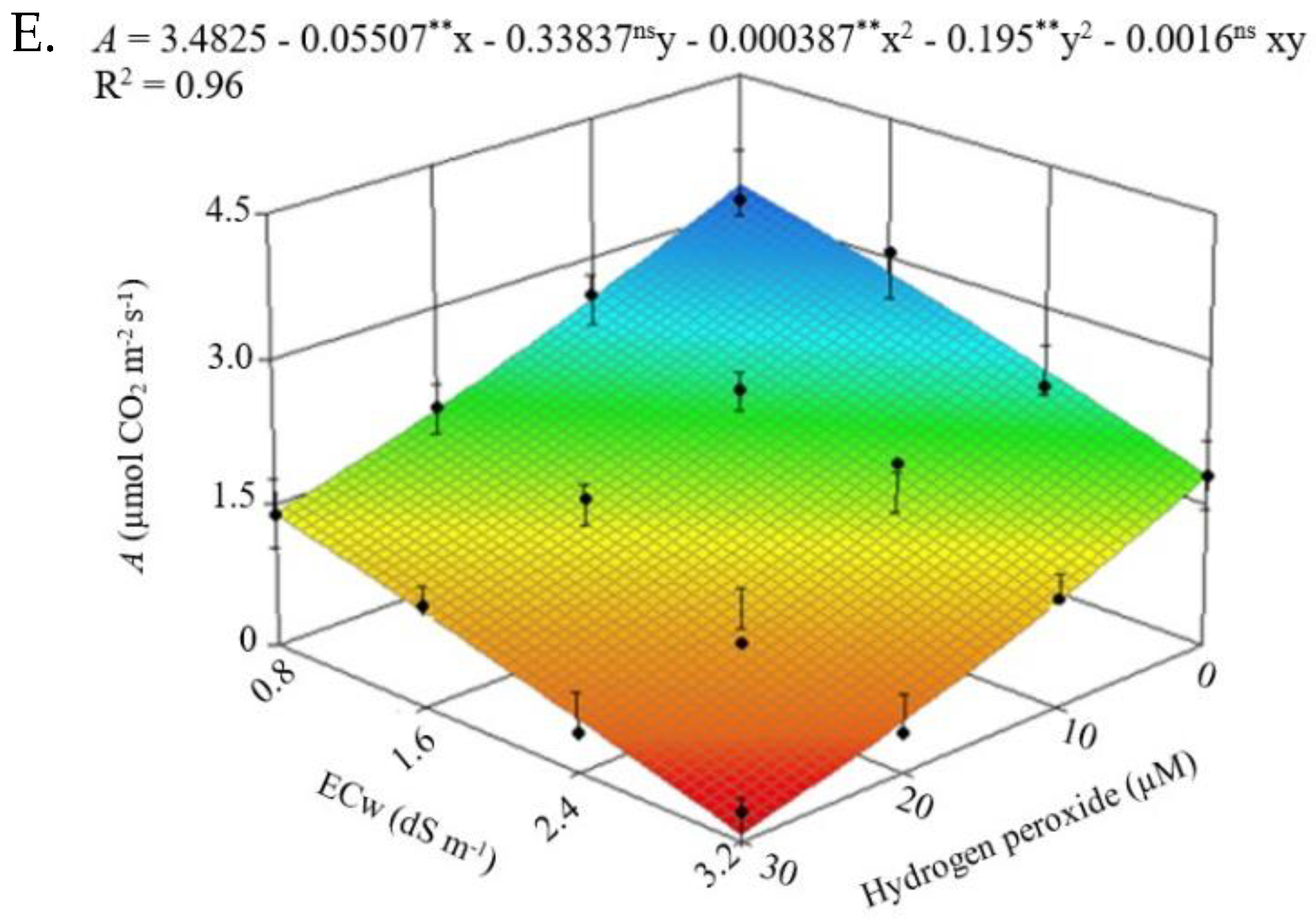
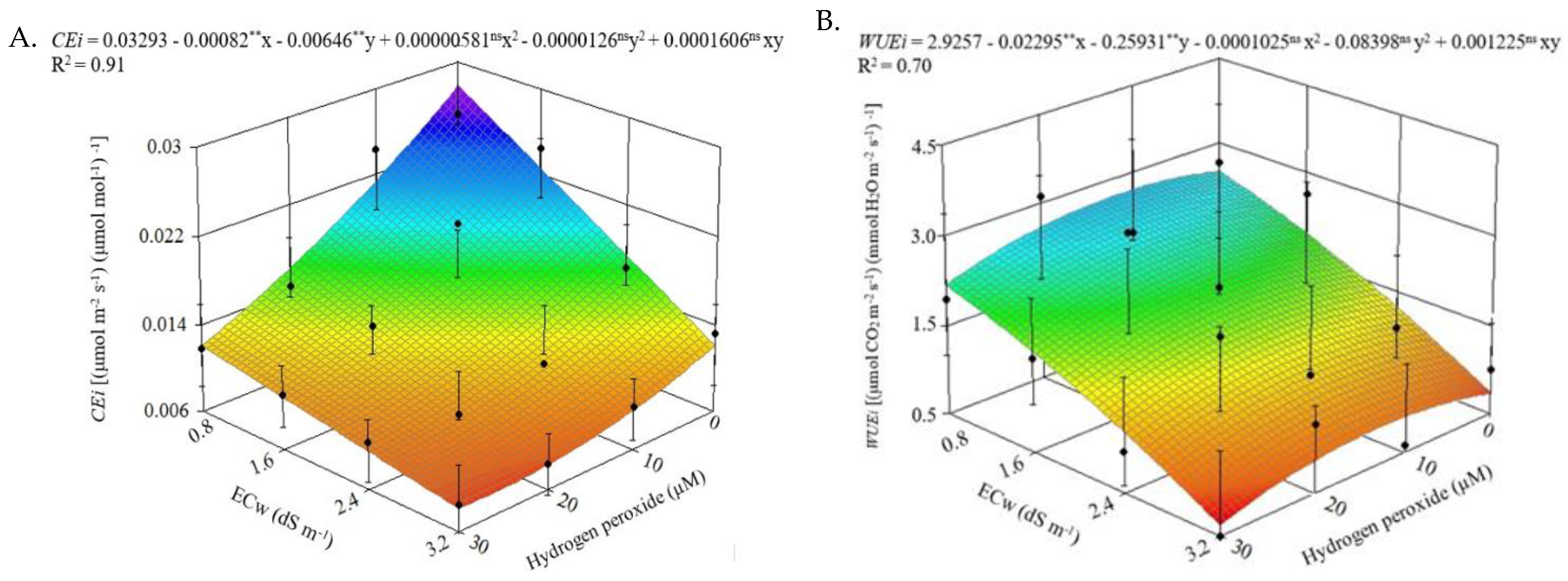
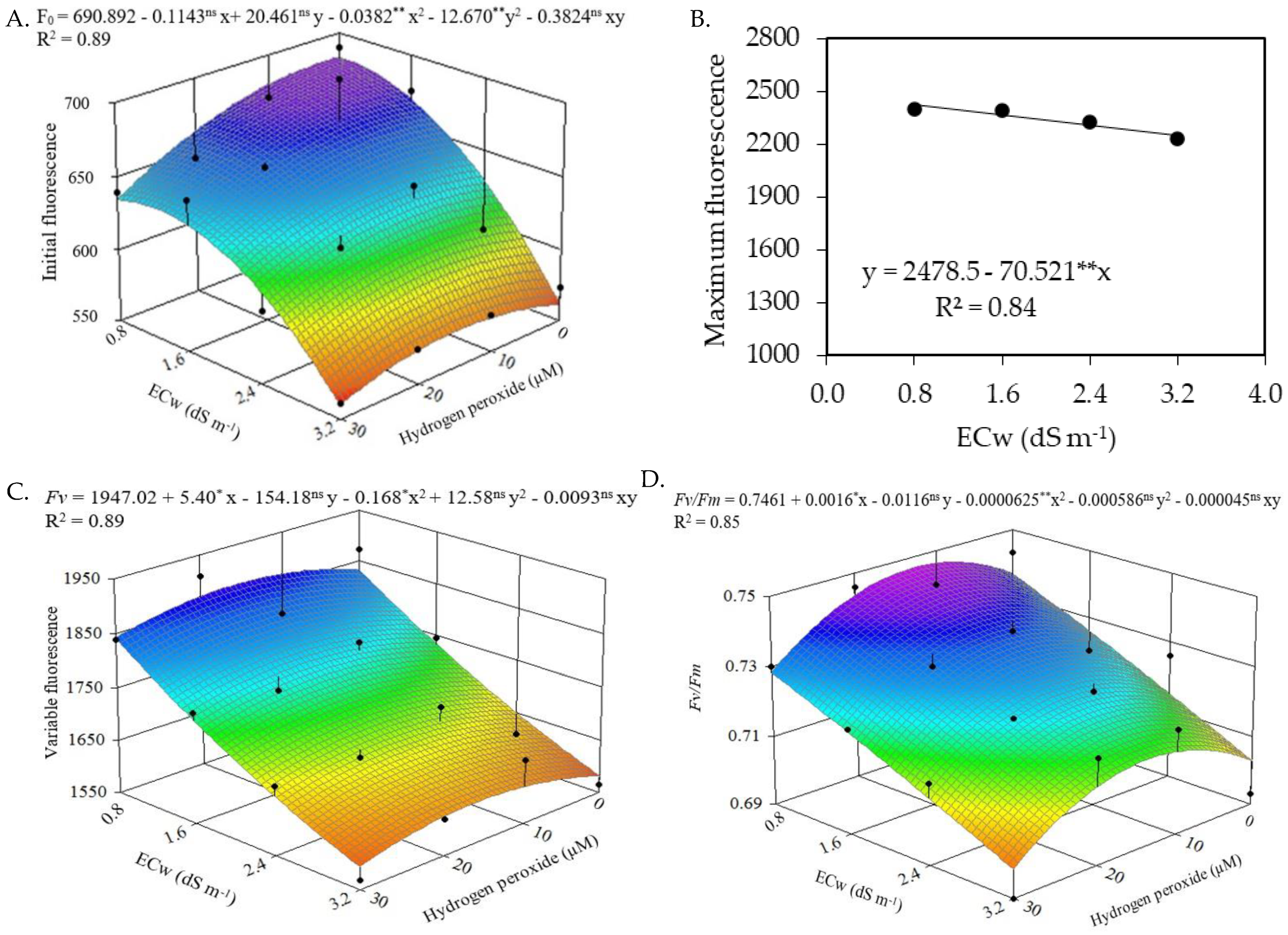

| Source of Variation | DF | Mean Squares | |||||
|---|---|---|---|---|---|---|---|
| gs | E | A | Ci | CEi | WUEi | ||
| Salinity levels (SL) | 4 | 0.000547 ** | 0.1781 ** | 0.880 ns | 87.729 ** | 0.00009 ns | 0.3614 ns |
| Linear regression | 1 | 0.000240 * | 0.111 ** | - | 122.90 ** | - | - |
| Quadratic regression | 1 | 0.00010 ns | 0.1220 ns | - | 124.32 ns | - | - |
| Hydrogen peroxide (H2O2) | 4 | 0.001836 ** | 0.0206 ns | 2.650 * | 3594.42 ns | 0.000083 ** | 3.1369 ** |
| Linear regression | 1 | 0.004160 ** | - | 0.0811 ns | - | 0.00009 ns | 5.693 ** |
| Quadratic regression | 1 | 0.000300 ns | - | 4.656 ** | - | 0.00030 ** | 0.0965 ns |
| Interaction (SL × H2O2) | 16 | 0.001775 ns | 0.3500 ns | 9.450 ** | 2442.85 ns | 0.000186 ** | 1.074 ** |
| Blocks | 3 | 0.00015 ns | 0.0687 ns | 0.1692 ns | 23.003 ns | 0.000053 ns | 0.2020 ns |
| Residual | 30 | 0.000149 | 0.0347 | 0.334 | 105.27 | 0.000060 | 0.112 |
| CV (%) | 15.65 | 10.33 | 12.39 | 3.97 | 10.26 | 13.91 | |
| Source of Variation | DF | Mean Squares | |||
|---|---|---|---|---|---|
| F0 | Fm | Fv | Fv/Fm | ||
| Salinity levels (SL) | 4 | 7842.51 ** | 75277.36 ** | 139489.6 ** | 0.002239 ** |
| Linear regression | 1 | 19634.8 ** | 190976.0 ** | 415168.01 ** | 0.006202 ** |
| Quadratic regression | 1 | 2257.7 ns | 32870.5 ns | 3088.02 ns | 0.000033 ns |
| Hydrogen peroxide (H2O2) | 4 | 658.99 ** | 1771.91 ns | 4881.95 * | 0.000928 ** |
| Linear regression | 1 | 436.32 * | - | 608.01 ns | 0.000375 ns |
| Quadratic regression | 1 | 21.60 ns | - | 13736.3 ** | 0.002408 ** |
| Interaction (SL × H2O2) | 16 | 362.87 ** | 7207.74 ns | 5538.60 ** | 0.000163 * |
| Blocks | 3 | 140.15 ns | 16949.8 ns | 1220.47 ns | 0.000065 ns |
| Residual | 30 | 82.83 | 3186.54 | 1279.87 | 0.000049 |
| CV (%) | 1.36 | 2.42 | 2.08 | 0.97 | |
| Source of Variation | DF | Mean Squares | ||||
|---|---|---|---|---|---|---|
| SD | Hcrown | Dcrown | Vcrown | VVI | ||
| Salinity level (SL) | 4 | 0.00002 ns | 0.0250 * | 0.0748 ns | 0.00262 * | 0.0007 * |
| Linear regression | 1 | - | 0.0297 * | - | 0.00662 ** | 0.00001 * |
| Quadratic regression | 1 | - | 0.0285 ns | - | 0.00063 ns | 0.00002 ns |
| Hydrogen peroxide (H2O2) | 4 | 0.000021 ns | 0.0129 ns | 0.0344 ns | 0.01284 ** | 0.00006 ns |
| Linear regression | 1 | - | - | - | 0.01460 ** | - |
| Quadratic regression | 1 | - | - | - | 0.0107 ns | - |
| Interaction (SL × H2O2) | 16 | 0.000021 ** | 0.0109 ns | 0.1119 ** | 0.0067 ns | 0.00005 ns |
| Blocks | 3 | 0.000030 ns | 0.0520 ns | 0.0230 ns | 0.00129 ns | 0.000075 ns |
| Residual | 30 | 0.000023 | 0.078 | 0.0201 | 0.0045 | 0.00004 |
| CV (%) | 7.29 | 7.28 | 15.81 | 15.39 | 1.63 | |
| Chemical characteristics | ||||||||
| pHH2O | OM | P | K+ | Na+ | Ca2+ | Mg2+ | Al3+ | H+ |
| 1:2.5 | g dm−3 | mg dm−3 | cmolc kg−1 | |||||
| 6.5 | 8.1 | 79 | 0.24 | 0.51 | 14.9 | 5.4 | 0 | 0.9 |
| Chemical characteristics | Physical characteristics | |||||||
| ECse | CEC | SARse | ESP | Particle-size fraction (g kg−1) | Moisture (dag kg−1) | |||
| dS m−1 | cmolc kg−1 | (mmol L−1)0.5 | % | Sand | Silt | Clay | 33.42 kPa 1 | 1519.5 kPa 2 |
| 2.15 | 16.54 | 0.16 | 3.08 | 572.7 | 100.7 | 326.6 | 25.91 | 12.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capitulino, J.D.; Lima, G.S.d.; Azevedo, C.A.V.d.; Silva, A.A.R.d.; Arruda, T.F.d.L.; Soares, L.A.d.A.; Gheyi, H.R.; Dantas Fernandes, P.; Sobral de Farias, M.S.; Silva, F.d.A.d.; et al. Influence of Foliar Application of Hydrogen Peroxide on Gas Exchange, Photochemical Efficiency, and Growth of Soursop under Salt Stress. Plants 2023, 12, 599. https://doi.org/10.3390/plants12030599
Capitulino JD, Lima GSd, Azevedo CAVd, Silva AARd, Arruda TFdL, Soares LAdA, Gheyi HR, Dantas Fernandes P, Sobral de Farias MS, Silva FdAd, et al. Influence of Foliar Application of Hydrogen Peroxide on Gas Exchange, Photochemical Efficiency, and Growth of Soursop under Salt Stress. Plants. 2023; 12(3):599. https://doi.org/10.3390/plants12030599
Chicago/Turabian StyleCapitulino, Jessica Dayanne, Geovani Soares de Lima, Carlos Alberto Vieira de Azevedo, André Alisson Rodrigues da Silva, Thiago Filipe de Lima Arruda, Lauriane Almeida dos Anjos Soares, Hans Raj Gheyi, Pedro Dantas Fernandes, Maria Sallydelândia Sobral de Farias, Francisco de Assis da Silva, and et al. 2023. "Influence of Foliar Application of Hydrogen Peroxide on Gas Exchange, Photochemical Efficiency, and Growth of Soursop under Salt Stress" Plants 12, no. 3: 599. https://doi.org/10.3390/plants12030599
APA StyleCapitulino, J. D., Lima, G. S. d., Azevedo, C. A. V. d., Silva, A. A. R. d., Arruda, T. F. d. L., Soares, L. A. d. A., Gheyi, H. R., Dantas Fernandes, P., Sobral de Farias, M. S., Silva, F. d. A. d., & Dias, M. d. S. (2023). Influence of Foliar Application of Hydrogen Peroxide on Gas Exchange, Photochemical Efficiency, and Growth of Soursop under Salt Stress. Plants, 12(3), 599. https://doi.org/10.3390/plants12030599









