Horticultural Practices in Early Spring to Mitigate the Adverse Effect of Low Temperature on Fruit Set in ‘Lapins’ Sweet Cherry
Abstract
1. Introduction
2. Results
2.1. Cold Injury on Floral Organs at SuRDC1
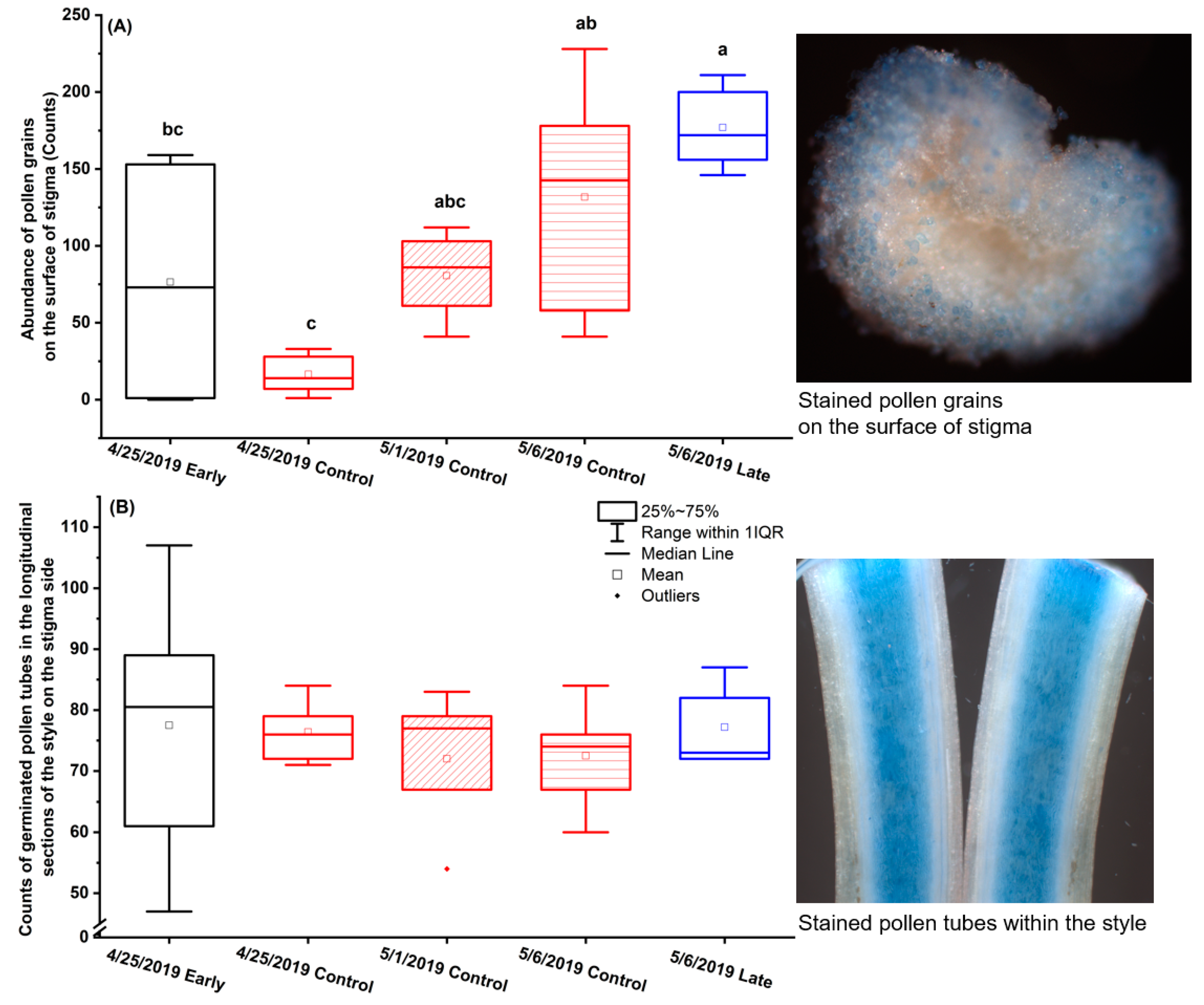
2.2. Effects of Elevated Temperature inside Polyethylene Sleeves
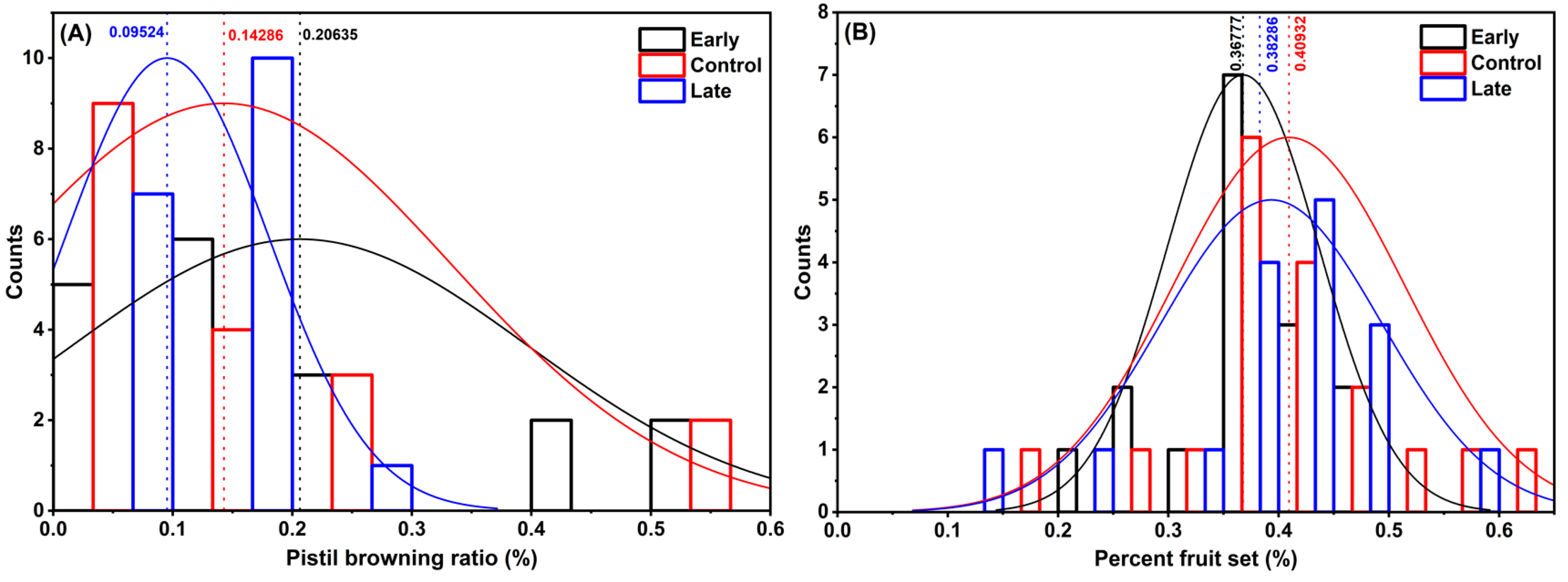
2.3. Effects of FAME Spray
2.4. Boric Acid Spray
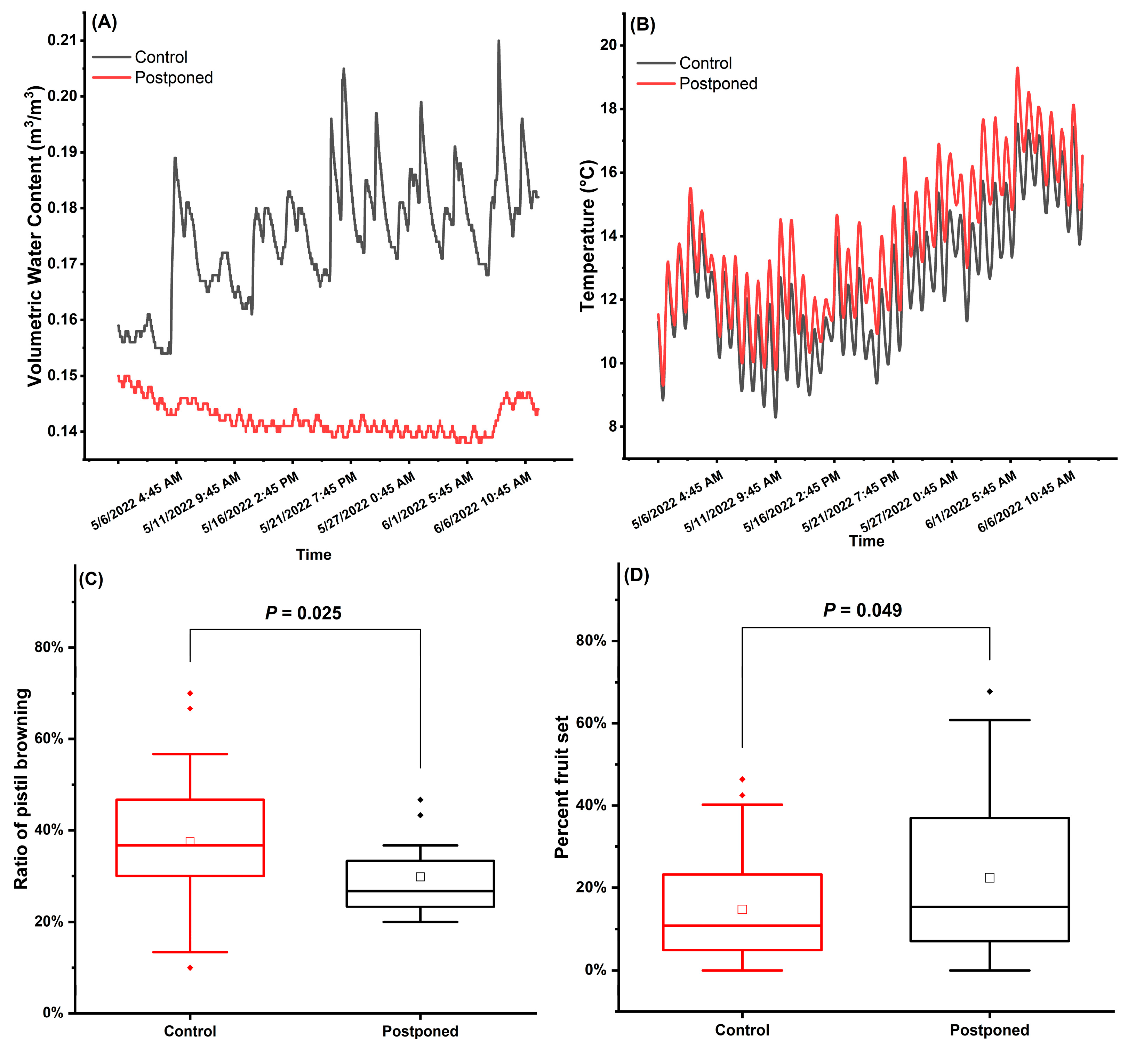
2.5. Effects of Reduction in Spring Irrigation
3. Discussion
3.1. Vulnerability of Sweet Cherry Floral Organs under Variable Spring Conditions
3.2. The Effectiveness of Horticultural Mitigations and Future Perspectives
4. Materials and Methods
4.1. Site Description and Fruit Set Monitoring
4.2. Spring Horticultural Mitigations at SuRDC1
4.2.1. Installation of Polyethylene Sleeves to Increase Floral Bud Tsurface
4.2.2. FAME Spray to Regulate Bud Break
4.2.3. Boric Acid Spray
4.2.4. Postponed Irrigation and Monitoring of Soil Moisture and Temperature
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmood, K.; Carew, J.G.; Hadley, P.; Battey, N.H. The effect of chilling and post-chilling temperatures on growth and flowering of sweet cherry (Prunus avium L.). J. Hortic. Sci. Biotechnol. 2000, 75, 598–601. [Google Scholar] [CrossRef]
- Beppu, K.; Suehara, T.; Kataoka, I. High temperature and drought stress suppress the photosynthesis and carbohydrate accumulation in ‘Satohnishiki’sweet cherry. Acta Hortic. 2002, 618, 371–377. [Google Scholar]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. The effect of temperature on stigmatic receptivity in sweet cherry (Prunus avium L.). Plant Cell Environ. 2003, 26, 1673–1680. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Effect of temperature on pollen tube kinetics and dynamics in sweet cherry, Prunus avium (Rosaceae). Am. J. Bot. 2004, 91, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Montiel, F.G.; Serrano, M.; Martinez-Romero, D.; Alburquerque, N. Factors influencing fruit set and quality in different sweet cherry cultivars. Span. J. Agric. Res. 2010, 8, 1118–1128. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. Warm temperatures at bloom reduce fruit set in sweet cherry. J. Appl. Bot. Food Qual. 2007, 81, 158–164. [Google Scholar]
- Kazan, K.; Lyons, R. The link between flowering time and stress tolerance. J. Exp. Bot. 2016, 67, 47–60. [Google Scholar] [CrossRef]
- Postweiler, K.; Stösser, R.; Anvari, S.F. The effect of different temperatures on the viability of ovules in cherries. Sci. Hortic. 1985, 25, 235–239. [Google Scholar] [CrossRef]
- Predieri, S.; Dris, R.; Sekse, L.; Rapparini, F. Influence of environmental factors and orchard management on yield and quality of sweet cherry. Food Agric. Environ. 2003, 1, 263–266. [Google Scholar]
- Bosch, J.; Kemp, W.P. Effect of wintering duration and temperature on survival and emergence time in males of the orchard pollinator Osmia lignaria (Hymenoptera: Megachilidae). Environ. Entomol. 2003, 32, 711–716. [Google Scholar] [CrossRef]
- Vicens, N.; Bosch, J. Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environ. Entomol. 2000, 29, 413–420. [Google Scholar] [CrossRef]
- Holzschuh, A.; Dudenhöffer, J.H.; Tscharntke, T. Landscapes with wild bee habitats enhance pollination, fruit set and yield of sweet cherry. Biol. Conserv. 2012, 153, 101–107. [Google Scholar] [CrossRef]
- Forrest, J.R. Plant–pollinator interactions and phenological change: What can we learn about climate impacts from experiments and observations? Oikos 2015, 124, 4–13. [Google Scholar] [CrossRef]
- Kaufmann, M.R. Water Deficits and Reproductive Growth. In Water Deficit and Plant Growth. Volume III Plant Responses and Control of Water Balance; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA; London, UK, 1972; pp. 91–124. [Google Scholar]
- Pallardy, S.G. Physiology of Woody Plants; Academic Press: New York, NY, USA, 2010; Volume 231–233, pp. 244–252. [Google Scholar]
- Bradford, K.J.; Yang, S.F. Physiological responses of plants to waterlogging. HortScience 1981, 16, 25–30. [Google Scholar] [CrossRef]
- Gradziel, T.M.; Weinbaum, S.A. High relative humidity reduces anther dehiscence in apricot, peach, and almond. HortScience 1999, 34, 322–325. [Google Scholar] [CrossRef]
- Herrero, M.; Rodrigo, J.; Wünsch, A. Flowering, fruit set and development. In Cherries—Botany, Production and Uses; Quero-Garcia, J., Iezzoni, A., Puławska, J., Lang, G., Eds.; CABI: Oxfordshire, UK, 2017; pp. 23–29. [Google Scholar]
- Beyhan, N.; Karakaş, B. Investigation of the fertilization biology of some sweet cherry cultivars grown in the Central Northern Anatolian Region of Turkey. Sci. Hortic. 2009, 121, 320–326. [Google Scholar] [CrossRef]
- Mateos-Fierro, Z.; Garratt, M.P.; Fountain, M.T.; Ashbrook, K.; Westbury, D.B. Wild bees are less abundant but show better pollination behaviour for sweet cherry than managed pollinators. J. Appl. Entomol. 2022, 146, 361–371. [Google Scholar] [CrossRef]
- Bound, S.A.; Close, D.C.; Jones, J.E.; Whiting, M.D. Improving fruit set of ‘Kordia’ and ‘Regina’ sweet cherry with AVG. Acta Hortic. 2014, 1042, 285–292. [Google Scholar] [CrossRef]
- Bound, S.A.; Miller, P. Manipulating time of bud break, flowering and crop development of sweet cherry with the dormancy breaker Waiken®. Acta Hortic. 2016, 1130, 285–292. [Google Scholar] [CrossRef]
- Muengkaew, R.; Chaiprasart, P.; Wongsawad, P. Calcium-boron addition promotes pollen germination and fruit set of mango. Int. J. Fruit Sci. 2017, 17, 147–158. [Google Scholar] [CrossRef]
- Ureta Ovalle, A.; Atenas, C.; Larraín, P. Application of an Ecklonia maxima seaweed product at two different timings can improve the fruit set and yield in ‘Bing’ sweet cherry trees. Acta Hortic. 2019, 1235, 319−326. [Google Scholar] [CrossRef]
- Alhamid, J.O.; Mo, C.; Zhang, X.; Wang, P.; Whiting, M.D.; Zhang, Q. Cellulose nanocrystals reduce cold damage to reproductive buds in fruit crops. Biosyst. Eng. 2018, 172, 124–133. [Google Scholar] [CrossRef]
- Feldmane, D. Effects of irrigation and woodchip mulch on growth and habit of sour cherries. In Proceedings of the Annual 15th International Scientific Conference Research for Rural Development, Aguascalientes, Mexico, 18–21 June 2009; pp. 64–70. [Google Scholar]
- Feldmane, D. Precocity of sour cherry cultivars influenced by using woodchip mulch and drip irrigation. In Proceedings of the Research for Rural Development Annual 16th International Scientific Conference Proceedings, Jelgava, Latvia, 19–21 May 2010; pp. 48–54. [Google Scholar]
- Yin, X.; Long, L.E.; Huang, X.L.; Jaja, N.; Bai, J.; Seavert, C.F.; le Roux, J. Transitional effects of double-lateral drip irrigation and straw mulch on irrigation water consumption, mineral nutrition, yield, and storability of sweet cherry. HortTechnology 2012, 22, 484–492. [Google Scholar] [CrossRef]
- Long, L.E.; Yin, X.; Huang, X.L.; Jaja, N. Responses of sweet cherry water use and productivity and soil quality to alternate groundcover and irrigation systems. Acta Hortic. 2014, 1020, 331–338. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Kappel, F.; Forge, T. Interaction of irrigation and soil management on sweet cherry productivity and fruit quality at different crop loads that simulat those occurring by environmental extremes. HortScience 2014, 49, 215–220. [Google Scholar] [CrossRef]
- Blanco, V.; Blaya-Ros, P.J.; Torres-Sánchez, R.; Domingo, R. Influence of regulated deficit irrigation and environmental conditions on reproductive response of sweet cherry trees. Plants 2020, 9, 94. [Google Scholar] [CrossRef]
- Lang, G.A.; Sage, L.; Wilkinson, T. Ten years of studies on systems to modify sweet cherry production environments: Retractable roofs, high tunnels, and rain-shelters. Acta Hortic. 2016, 1130, 83–90. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Rahimi Devin, S.; Salazar, J.A.; López-Alcolea, J.; Rubio, M.; Martínez-García, P.J. Principles and Prospects of Prunus Cultivation in Greenhouse. Agronomy 2021, 11, 474. [Google Scholar] [CrossRef]
- Zhang, L.; Ferguson, L.; Whiting, M.D. Temperature effects on pistil viability and fruit set in sweet cherry. Sci. Hortic. 2018, 241, 8–17. [Google Scholar] [CrossRef]
- Wojcik, P.; Wojcik, M. Effect of boron fertilization on sweet cherry tree yield and fruit quality. J. Plant Nutr. 2006, 29, 1755–1766. [Google Scholar] [CrossRef]
- Botelho, R.V.; Müller, M.M.L.; Umburanas, R.C.; Laconski, J.M.O.; Terra, M.M. Boron in fruit crops: Plant physiology, deficiency, toxicity, and sources for fertilization. In Boron in Plants and Agriculture; Academic Press: New York, NY, USA, 2022; pp. 29–50. [Google Scholar]
- Chagas, E.A.; Barbosa, W.; Saito, A.; Pio, R.; Feldberg, N.P. Temperature, pH and development period on in vitro pollen germination in Pyrus calleryana. Acta Hortic. 2008, 800, 521–526. [Google Scholar] [CrossRef]
- Feldmane, D.; Ruisa, S.; Rubauskis, E.; Kaufmane, E. Winter hardiness of sour cherries influenced by cultivar and soil moisture treatment. Acta Hortic. 2016, 1130, 111–115. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Forge, T. Environmental limiting factors for cherry production. In Cherries—Botany, Production and Uses; Quero-Garcia, J., Iezzoni, A., Puławska, J., Lang, G., Eds.; CABI: Oxfordshire, UK, 2017; pp. 205–213. [Google Scholar]
- Houghton, E.A.; Bevandick, K.; Neilsen, D.; Hannam, K.D.; Nelson, L.M. Effects of postharvest deficit irrigation on sweet cherry (Prunus avium) in five Okanagan Valley, Canada orchards: I. Tree water status, photosynthesis, and growth. Can. J. Plant Sci. 2022. Just-In. [Google Scholar] [CrossRef]
- DeMoranville, C.J.; Deubert, K.H. Effect of commercial calcium-boron and manganese-zinc formulations on fruit set of cranberries. J. Hortic. Sci. 1987, 62, 163–169. [Google Scholar] [CrossRef]
- Usenik, V.; Stampar, F. Effect of foliar application of zinc plus boron on sweet cherry fruit set and yield. Acta Hortic. 2001, 594, 245–249. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Satya, S.; Pitchai, J.G.; Indirani, R. Boron nutrition of crops in relation to yield and quality—A review. Agric. Rev. 2009, 30, 139–144. [Google Scholar]
- Hassan, G.; Mir, M.; Khachi, B.; Slathia, S. Effect of calcium and boron on reproductive performance of sweet cherry cv Bigarreau Noir Grossa. Int. J. Farm Sci. 2016, 6, 120–127. [Google Scholar]
- van Doorn, W.G.; Stead, A.D. Abscission of flowers and floral parts. J. Exp. Bot. 1997, 48, 821–837. [Google Scholar] [CrossRef]
- Greer, D.H.; Wünsche, J.N.; Norling, C.L.; Wiggins, H.N. Root-zone temperatures affect phenology of bud break, flower cluster development, shoot extension growth and gas exchange of ‘Braeburn’(Malus domestica) apple trees. Tree Physiol. 2006, 26, 105–111. [Google Scholar] [CrossRef]
- Hammond, M.W.; Seeley, S.D. Spring bud development of Malus and Prunus species in relation to soil temperature1. J. Am. Soc. Hortic. Sci. 1978, 103, 655–657. [Google Scholar] [CrossRef]
- Friedman, S.P.; Naftaliev, B. A survey of the aeration status of drip-irrigated orchards. Agric. Water Manag. 2012, 115, 132–147. [Google Scholar] [CrossRef]
- Rodrigo, J. Spring frosts in deciduous fruit trees—Morphological damage and flower hardiness. Sci. Hortic. 2000, 85, 155–173. [Google Scholar] [CrossRef]
- Kappel, F. Sweet cherry cultivars vary in their susceptibility to spring frosts. HortScience 2010, 45, 176–177. [Google Scholar] [CrossRef]
- Long, L.E.; Lang, G.A.; Whiting, M.D.; Musacchi, S. Cherry Training Systems; PNW 667. A Pacific Northwest Extension Publication; Oregon State University: Corvallis, OR, USA; Washington State University: Pullman, WA, USA; University of Idaho: Perimeter, MO, USA; Michigan State University: East Lansing, MI, USA, 2015; pp. 43–56. [Google Scholar]
- Fadón, E.; Herrero, M.; Rodrigo, J. Flower development in sweet cherry framed in the BBCH scale. Sci. Hortic. 2015, 192, 141–147. [Google Scholar] [CrossRef]
- Lu, Y. Arabidopsis pollen tube aniline blue staining. Bio-Protocol 2011, 1, e88. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]

| Sites | Elevation, Frost Risk | Rootstock, Canopy Structure | Replications for % Fruit Set | 2019 | 2022 | Year Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Bloom–Petal Fall | Tmin (°C) | PP (mm) | % Fruit Set | First Bloom–Petal Fall | Tmin (°C) | PP (mm) | % Fruit Set | |||||
| SuRDC1 | 487 m, High risk | Krymsk 5, TSA | 6 plots, 3 trees/plot, 1–2 branches/tree | 27 April –7 May | −4.10 | 3 | 40.9 ± 2.5 c | 21 April –12 May | −4.12 | 12 | 23.0 ± 2.5 c * | F(1,53) = 20.06 p < 0.001 |
| SuRDC2 | 416 m, Low risk | Krymsk 5, SSA | 4 plots, 4 trees/plot, 1 trunk section/tree | 24 April –3 May | −0.16 | 3 | 46.0 ± 1.9 bc | 17 April –9 May | −1.72 | 12 | 35.8 ± 1.8 b * | F(1,31) = 8.90 p = 0.004 |
| SuRDC3 | 424 m, Low risk | Mazzard, Open heart | 9 trees in 4 rows, 3–4 branches | 23 April –3 May | −0.98 | 3 | 59.2 ± 2.2 a | 15 April –8 May | −2.10 | 12 | 66.4 ± 2.9 a | F(1,59) = 3.74 p = 0.06 |
| Oliver | 332 m, Low risk | Mazzard, Central leader | 2 plots, 5 trees/plot, 3 branch/tree | 17 April –30 April | −1.07 | 3 | 55.2 ± 0.3 a | 11 April –4 May | −2.27 | 3 | 36.6 ± 1.8 b * | F(1,59) = 60.51 p < 0.001 |
| Kelowna | 483 m, Low risk | Mazzard, Central leader | 2 plots, 5 trees/plot, 3 branch/tree | 19 April –2 May | −1.27 | 1 | 53.1 ± 0.2 ab | 12 April –6 May | −2.46 | 2 | 57.3 ± 3.5 a | F(1,59) = 0.84 p = 0.36 |
| Sites | Weather Station Location | Tsum in March (°C) | Tsum in April (°C) | ||||
|---|---|---|---|---|---|---|---|
| Historical Average | 2019 | 2022 | Historical Average | 2019 | 2022 | ||
| Summerland EC | Latitude: 49.5700°; Longitude: −119.6769°; Elevation: 454 m | 143.62 | 108.2 | 192.4 | 269.9 | 275.5 | 215.8 |
| Oliver Central | Latitude: 49.1575°; Longitude: −119.5514°; Elevation: 349 m | 163.9 | 150.02 | 214.87 | 280.88 | 317.84 | 217.37 |
| Kelowna East | Latitude: 49.8738°; Longitude: −119.4436°; Elevation: 432 m | 148.83 | 144.93 | 182.84 | 268.54 | 278.88 | 213.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Ediger, D.; Sharifi, M. Horticultural Practices in Early Spring to Mitigate the Adverse Effect of Low Temperature on Fruit Set in ‘Lapins’ Sweet Cherry. Plants 2023, 12, 468. https://doi.org/10.3390/plants12030468
Xu H, Ediger D, Sharifi M. Horticultural Practices in Early Spring to Mitigate the Adverse Effect of Low Temperature on Fruit Set in ‘Lapins’ Sweet Cherry. Plants. 2023; 12(3):468. https://doi.org/10.3390/plants12030468
Chicago/Turabian StyleXu, Hao, Danielle Ediger, and Mehdi Sharifi. 2023. "Horticultural Practices in Early Spring to Mitigate the Adverse Effect of Low Temperature on Fruit Set in ‘Lapins’ Sweet Cherry" Plants 12, no. 3: 468. https://doi.org/10.3390/plants12030468
APA StyleXu, H., Ediger, D., & Sharifi, M. (2023). Horticultural Practices in Early Spring to Mitigate the Adverse Effect of Low Temperature on Fruit Set in ‘Lapins’ Sweet Cherry. Plants, 12(3), 468. https://doi.org/10.3390/plants12030468








