Effects of Arbuscular Mycorrhizal Fungi on Alleviating Cadmium Stress in Medicago truncatula Gaertn
Abstract
1. Introduction
2. Results
2.1. Effect of Inoculation with AMF on the Growth of M. truncatula
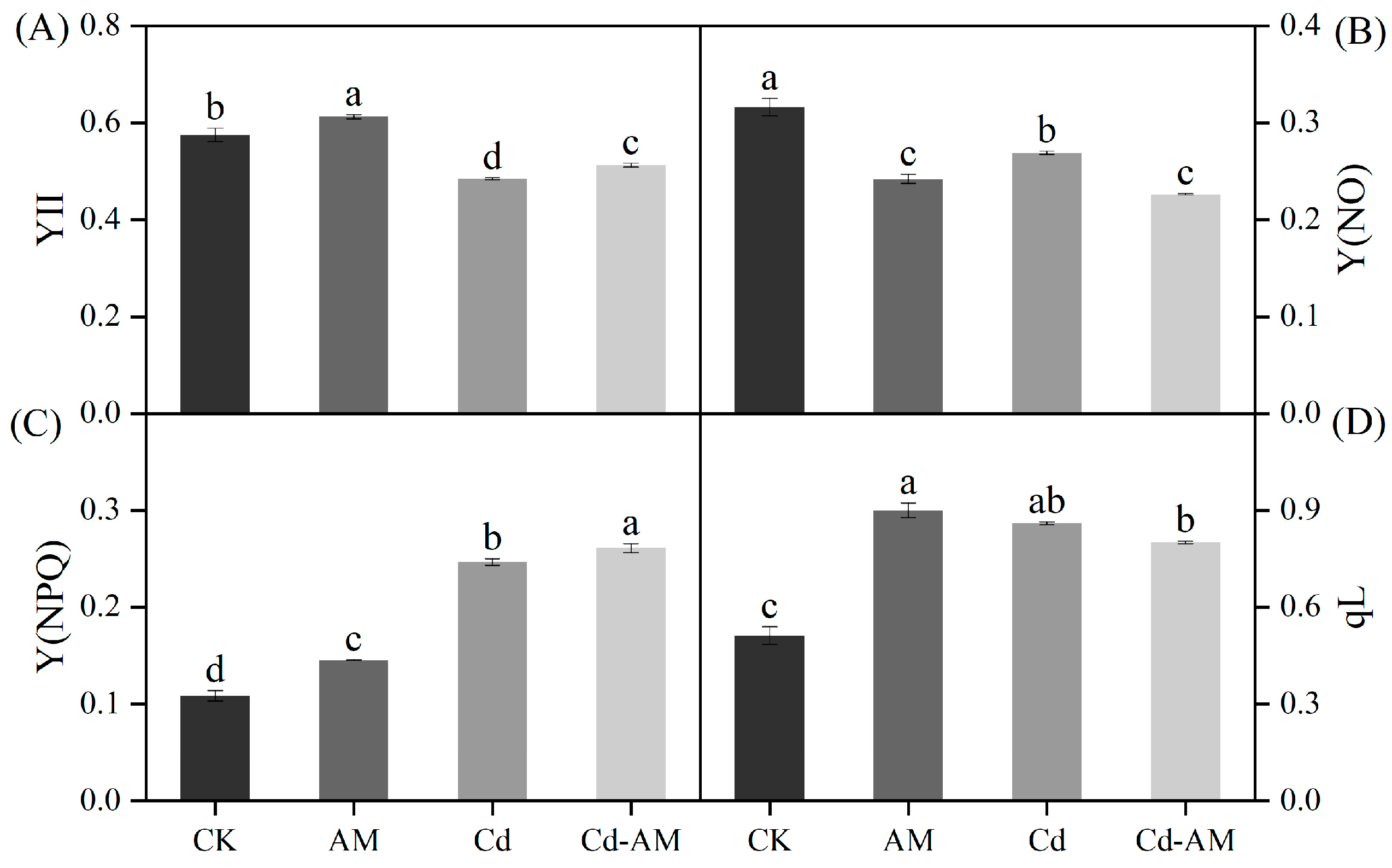
2.2. Effect of Inoculation with AMF on M. truncatula Chlorophyll Fluorescence
2.3. Effect of AMF Inoculation on the Cd2+ Distribution and Content of M. truncatula
2.4. Effect of AMF Inoculation on M. truncatula MDA Content and Antioxidant Enzyme Activity
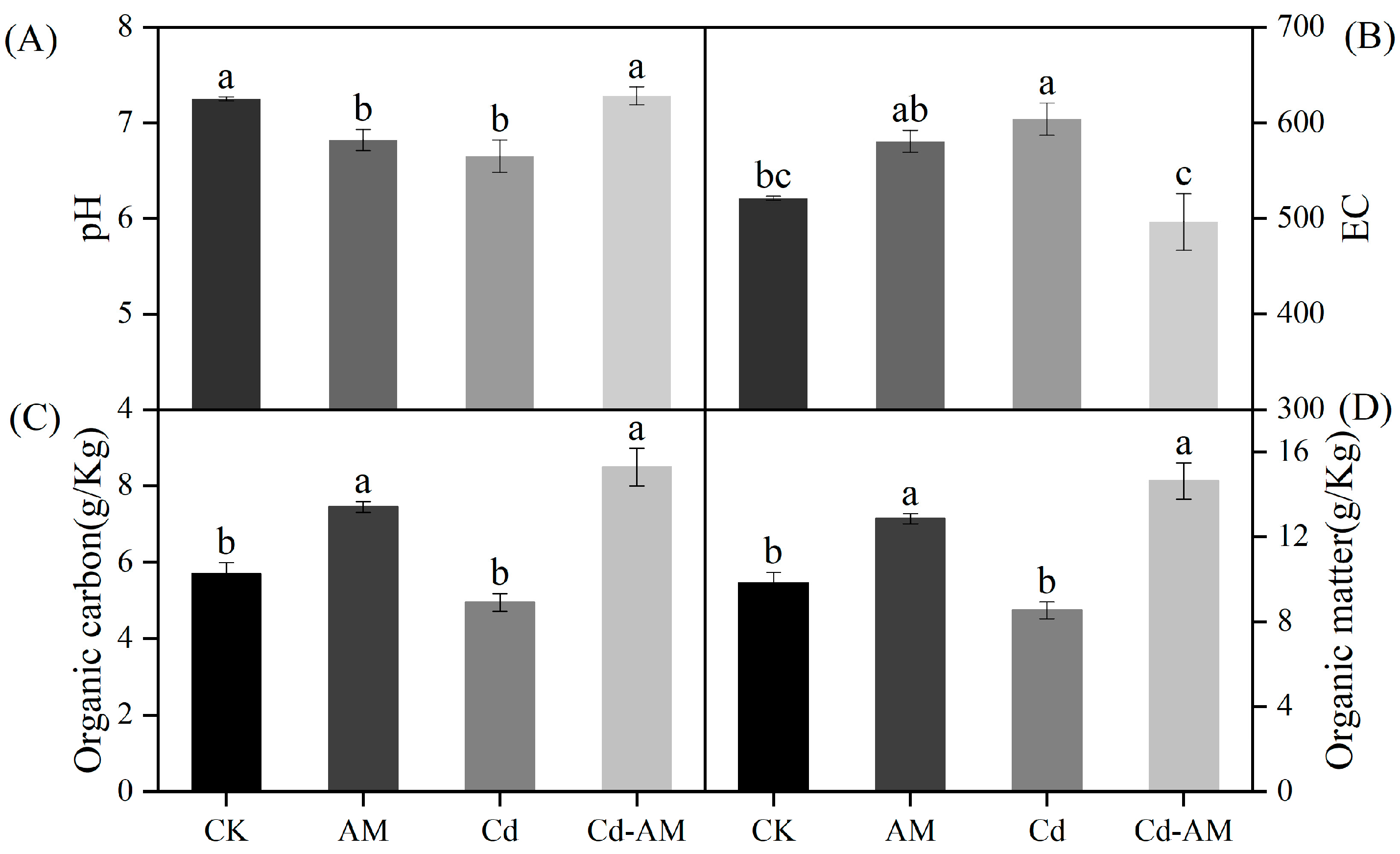
2.5. Effect of AMF Inoculation on Inter-Root Soil Properties
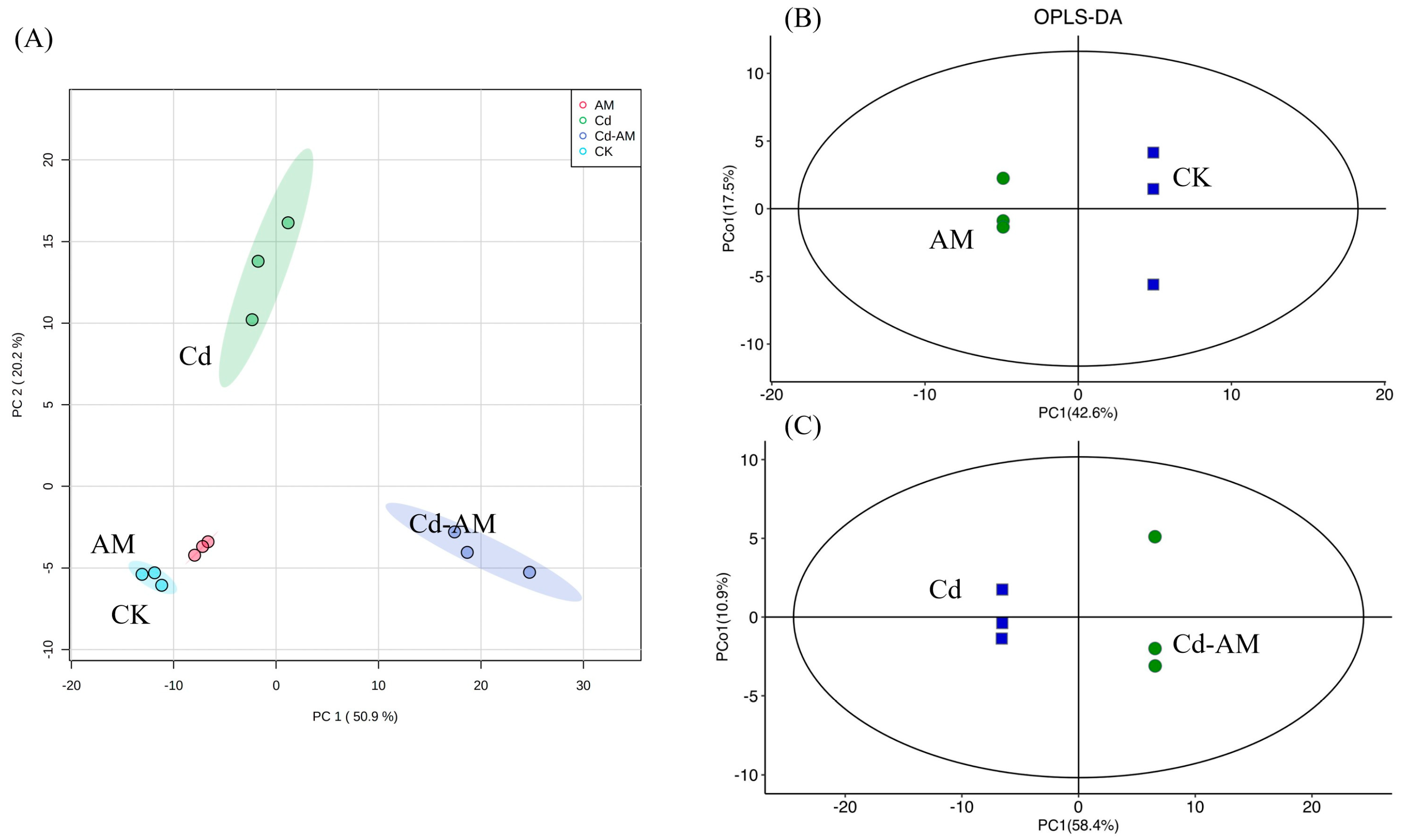
2.6. Effect of AMF Inoculation on the Inter-Root Metabolites of M. truncatula
2.6.1. Multivariate Statistical Analysis of Metabolites
2.6.2. Differential Metabolite and Enrichment Pathway Analysis
2.6.3. Visual Network Analysis of Inter-Root Amino Acid Metabolism
3. Discussion
4. Materials and Methods
4.1. Cultivation and Processing of Test Materials
4.2. Measurement of Chlorophyll Fluorescence Parameters
4.3. Measurement of Antioxidant Enzyme Activity and MDA Content
4.4. Measurement of Cd Content in Samples and Physical and Chemical Properties of Soil
4.5. Root Metabolite Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Jinadasa, N.; Collins, D.; Holford, P.; Milham, P.J.; Conroy, J.P. Reactions to cadmium stress in a cadmium-tolerant variety of cabbage (Brassica oleracea L.): Is cadmium tolerance necessarily desirable in food crops? Environ. Sci. Pollut. Res. Int. 2016, 23, 5296–5306. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.L.; Plant, J.A.; Voulvoulis, N.; Oates, C.J.; Ihlenfeld, C. Cadmium levels in Europe: Implications for human health. Environ. Geochem. Health 2010, 32, 1–12. [Google Scholar] [CrossRef]
- Liu, L.W.; Li, W.; Song, W.P.; Guo, M.X. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Ibrahim, M.; Tsang, D.C.W.; Zia-Ur-Rehman, M.; Zahir, Z.A.; Rinklebe, J.; Tack, F.M.G.; Ok, Y.S. A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 2017, 182, 90–105. [Google Scholar] [CrossRef]
- Lata, S.; Mishra, T. Cadmium Bioremediation: A Review. Int. J. Pharm. Sci. Res. 2019, 14, 4120–4128. [Google Scholar] [CrossRef]
- Hembrom, S.; Singh, B.; Gupta, S.K.; Nema, A.K. A Comprehensive Evaluation of Heavy Metal Contamination in Foodstuff and Associated Human Health Risk: A Global Perspective. In Contemporary Environmental Issues and Challenges in Era of Climate Change; Singh, P., Singh, R.P., Srivastava, V., Eds.; Springer: Singapore, 2020; pp. 33–63. [Google Scholar]
- Hasan, S.; Ali, B.; Hayat, S.; Ahmad, A. Cadmium-induced changes in the growth and carbonic anhydrase activity of chickpea. Turk. J. Biol. 2007, 31, 137–140. [Google Scholar]
- Xu, Z.M.; Li, Q.S.; Yang, P.; Ye, H.J.; Chen, Z.S.; Guo, S.H.; Wang, L.L.; He, B.Y.; Zeng, E.Y. Impact of osmoregulation on the differences in Cd accumulation between two contrasting edible amaranth cultivars grown on Cd-polluted saline soils. Environ. Pollut. 2017, 224, 89–97. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Jali, P.; Pradhan, C.; Das, A.B. Effects of Cadmium Toxicity in Plants: A Review Article. Sch. Acad. J. Biosci. 2017, 4, 1074–1081. [Google Scholar]
- Mahajan, P.; Kaushal, J. Role of Phytoremediation in Reducing Cadmium Toxicity in Soil and Water. J. Toxicol. 2018, 2018, 4864365. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, P.; Zhao, F.J. Dietary cadmium exposure, risks to human health and mitigation strategies. Crit. Rev. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Parthipan, P.; Preetham, E.; Machuca, L.L.; Rahman, P.K.S.M.; Murugan, K.; Rajasekar, A. Biosurfactant and Degradative Enzymes Mediated Crude Oil Degradation by Bacterium Bacillus subtilis A1. Front. Microbiol. 2017, 8, 193. [Google Scholar] [CrossRef]
- Chellaiah, E.R. Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: A minireview. Appl. Water Sci. 2018, 8, 154. [Google Scholar] [CrossRef]
- Varma, A.; Sherameti, I.; Tripathi, S.; Prasad, R.; Das, A.; Sharma, M.; Bakshi, M.; Johnson, J.M.; Bhardwaj, S.; Arora, M.; et al. 13 The Symbiotic Fungus Piriformospora indica: Review. In Fungal Associations; Hock, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 231–254. [Google Scholar]
- Pirdashti, H.; Yaghoubian, Y.; Goltapeh, E.; Hosseini, S. Effect of mycorrhiza-like endophyte (Sebacina vermifera) on growth, yield and nutrition of rice (Oryza sativa L.) under salt stress. J. Agric. Sci. Technol. 2012, 8, 1651–1661. [Google Scholar]
- Yaghoubian, Y.; Siadat, S.A.; Moradi Telavat, M.R.; Pirdashti, H.; Yaghoubian, I. Bio-removal of cadmium from aqueous solutions by filamentous fungi: Trichoderma spp. and Piriformospora indica. Environ. Sci. Pollut. Res. Int. 2019, 26, 7863–7872. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, Y.; Yang, Y.C.; Yang, L.X.; Yang, N.; Zhang, D.P. Long-term effects of mixed planting on arbuscular mycorrhizal fungal communities in the roots and soils of Juglans mandshurica plantations. BMC Microbiol. 2020, 20, 304. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I.; et al. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Li, H.; Gao, M.Y.; Mo, C.H.; Wong, M.H.; Chen, X.W.; Wang, J.J. Potential use of arbuscular mycorrhizal fungi for simultaneous mitigation of arsenic and cadmium accumulation in rice. J. Exp. Bot. 2022, 73, 50–67. [Google Scholar] [CrossRef]
- Cui, X.; Jia, B.B.; Diao, F.W.; Li, X.; Xu, J.; Zhang, Z.C.; Li, F.Y.; Guo, W. Transcriptomic analysis reveals the molecular mechanisms of arbuscular mycorrhizal fungi and nitrilotriacetic acid on Suaeda salsa tolerance to combined stress of cadmium and salt. Process Saf. Environ. Prot. 2022, 160, 210–220. [Google Scholar] [CrossRef]
- Adeyemi, N.O.; Atayese, M.O.; Sakariyawo, O.S.; Azeez, J.O.; Sobowale, S.P.A.; Olubode, A.; Mudathir, R.; Adebayo, R.; Adeoye, S. Alleviation of heavy metal stress by arbuscular mycorrhizal symbiosis in Glycine max (L.) grown in copper, lead and zinc contaminated soils. Rhizosphere 2021, 18, 100325. [Google Scholar] [CrossRef]
- Bahmani-Babanari, L.; Mirzahosseini, Z.; Shabani, L.; Sabzalian, M.R. Effect of arbuscular mycorrhizal fungus, Funneliformis fasciculatum, on detoxification of Nickel and expression of TIP genes in Lolium perenne L. Biologia 2021, 76, 1675–1683. [Google Scholar] [CrossRef]
- Yang, Y.R.; Huang, B.T.; Xu, J.Z.; Li, Z.X.; Tang, Z.H.; Wu, X.F. Heavy metal domestication enhances beneficial effects of arbuscular mycorrhizal fungi on lead (Pb) phytoremediation efficiency of Bidens parviflora through improving plant growth and root Pb accumulation. Environ. Sci. Pollut. Res. 2022, 29, 32988–33001. [Google Scholar] [CrossRef]
- Cui, G.J.; Ai, S.Y.; Chen, K.; Wang, X.R. Arbuscular mycorrhiza augments cadmium tolerance in soybean by altering accumulation and partitioning of nutrient elements, and related gene expression. Ecotoxicol. Environ. Saf. 2019, 171, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Diao, F.W.; Wang, Q.F.; Pan, L.; Dang, Z.H.; Guo, W. Effects of arbuscular mycorrhizal symbiosis on growth, nutrient and metal uptake by maize seedlings (Zea mays L.) grown in soils spiked with Lanthanum and Cadmium. Environ. Pollut. 2018, 241, 607–615. [Google Scholar] [CrossRef]
- Yang, Y.R.; Song, Y.Y.; Scheller, H.V.; Ghosh, A.; Ban, Y.H.; Chen, H.; Tang, M. Community structure of arbuscular mycorrhizal fungi associated with Robinia pseudoacacia in uncontaminated and heavy metal contaminated soils. Soil Biol. Biochem. 2015, 86, 146–158. [Google Scholar] [CrossRef]
- Zhao, X.F.; Lei, M.; Gu, R.Y. Knowledge Mapping of the Phytoremediation of Cadmium-Contaminated Soil: A Bibliometric Analysis from 1994 to 2021. Int. J. Environ. Res. Public Health 2022, 19, 6987. [Google Scholar] [CrossRef] [PubMed]
- Pozgajova, M.; Navratilova, A.; Kovar, M. Curative Potential of Substances with Bioactive Properties to Alleviate Cd Toxicity: A Review. Int. J. Environ. Res. Public Health 2022, 19, 12380. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Wang, Z.S. The Epitranscriptomic Mechanism of Metal Toxicity and Carcinogenesis. Int. J. Mol. Sci. 2022, 23, 11830. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Xiong, J.; Li, M.; Wang, M.X.; Tan, W.F. Interactive Effects of Cd and Pb on the Photosynthesis Efficiency and Antioxidant Defense System of Capsicum annuum L. Bull. Environ. Contam. Toxicol. 2022, 108, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.L.; Wang, H.X.; Wang, Y.; Hu, X.Q.; Wen, X.L. Effects of Cd2+ and Pb2+ on Growth and Photosynthesis of Two Freshwater Algae Species. Pol. J. Environ. Stud. 2022, 31, 2059–2068. [Google Scholar] [CrossRef]
- Basyal, B.; Emery, S.M. An arbuscular mycorrhizal fungus alters switchgrass growth, root architecture, and cell wall chemistry across a soil moisture gradient. Mycorrhiza 2021, 31, 251–258. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, N.; Li, X.; Long, J.; Sui, X.; Wu, Y.; Li, J.; Wang, J.; Zhong, H.; Sun, G.Y. Arbuscular Mycorrhizal Fungi (Glomus mosseae) Improves Growth, Photosynthesis and Protects Photosystem II in Leaves of Lolium perenne L. in Cadmium Contaminated Soil. Front. Plant Sci. 2018, 9, 1156. [Google Scholar] [CrossRef]
- Wu, N.; Li, Z.; Liu, H.G.; Tang, M. Influence of arbuscular mycorrhiza on photosynthesis and water status of Populus cathayana Rehder males and females under salt stress. Acta Physiol. Plant. 2015, 37, 183. [Google Scholar] [CrossRef]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Wirth, S.; Egamberdieva, D. Arbuscular mycorrhizal fungi alleviate salt stress in lupine (Lupinus termis Forsik) through modulation of antioxidant defense systems and physiological traits. Legume Res. 2016, 39, 198–207. [Google Scholar] [CrossRef]
- Lei, Y.-B.; Xia, H.-X.; Chen, K.; Plenković-Moraj, A.; Huang, W.; Sun, G. Photosynthetic regulation in response to fluctuating light conditions under temperature stress in three mosses with different light requirements. Plant Sci. 2021, 311, 111020. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Chen, K.; Liu, L.; Plenkovic-Moraj, A.; Sun, G.; Lei, Y. Photosynthetic regulation in fluctuating light under combined stresses of high temperature and dehydration in three contrasting mosses. Plant Sci. 2022, 323, 111379. [Google Scholar] [CrossRef]
- He, L.; Kong, J.; Li, G.; Meng, G.; Chen, K. Similar responses in morphology, growth, biomass allocation, and photosynthesis in invasive Wedelia trilobata and native congeners to CO2 enrichment. Plant Ecol. 2018, 219, 145–157. [Google Scholar] [CrossRef]
- Fu, Y.; Mason, A.S.; Zhang, Y.; Lin, B.; Xiao, M.; Fu, D.; Yu, H. MicroRNA-mRNA expression profiles and their potential role in cadmium stress response in Brassica napus. BMC Plant Biol. 2019, 19, 570. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, W.; Li, F.; Zhang, L.; Deng, X.; Liu, Y.; Yang, S. The Plastidial Glyceraldehyde-3-Phosphate Dehydrogenase Is Critical for Abiotic Stress Response in Wheat. Int. J. Mol. Sci. 2019, 20, 1104. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular Mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, R.; Jiang, L.; Chen, K.; Zhu, W. Alleviation of Cadmium Toxicity to Medicago truncatula by AMF Involves the Changes of Cd Speciation in Rhizosphere Soil and Subcellular Distribution. Phyton-Int. J. Exp. Bot. 2021, 90, 403–415. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X.; Shi, Z.; Tong, R.; Adams, C.A.; Shi, X. Arbuscular mycorrhizae alleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants—A soil microcosm experiment. Chemosphere 2016, 147, 88–97. [Google Scholar] [CrossRef]
- Choudhury, S.; Panda, P.; Sahoo, L.; Panda, S.K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal. Behav. 2013, 8, e23681. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Zou, Y.-N.; Fathi Abd-Allah, E. Chapter 15—Mycorrhizal Association and ROS in Plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 453–475. [Google Scholar]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D. Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2016, 23, 39–47. [Google Scholar] [CrossRef]
- Molina, A.S.; Lugo, M.A.; Perez Chaca, M.V.; Vargas-Gil, S.; Zirulnik, F.; Leporati, J.; Ferrol, N.; Azcon-Aguilar, C. Effect of Arbuscular Mycorrhizal Colonization on Cadmium-Mediated Oxidative Stress in Glycine max (L.) Merr. Plants 2020, 9, 108. [Google Scholar] [CrossRef]
- Lakmali, D.; Karunarathna, S.C.; Dauner, L.A.P.; Yapa, N. Potential of Vetiver (Chrysopogon zizanioides L.), Inoculated With Arbuscular Mycorrhizal Fungi, To Improve Soil Quality in Degraded Soil. Chiang Mai J. Sci. 2021, 48, 1247–1258. [Google Scholar]
- Yun, D.-Y.; Kang, Y.-G.; Kim, M.; Kim, D.; Kim, E.-H.; Hong, Y.-S. Metabolomic understanding of pod removal effect in soybean plants and potential association with their health benefit. Food Res. Int. 2020, 138, 109797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, H.; White, J.C.; Chen, X.; Li, H.; Qu, X.; Ji, R. Metabolomics reveals that engineered nanomaterial exposure in soil alters both soil rhizosphere metabolite profiles and maize metabolic pathways. Environ. Sci. Nano 2019, 6, 1716–1727. [Google Scholar] [CrossRef]
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics, and Ionomics. Front. Plant Sci. 2015, 6, 1143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhang, H.J.; Zhang, Y.X.; Liu, Y.Q.; Zhang, H.Q.; Tang, M. Arbuscular mycorrhizal fungi alter carbohydrate distribution and amino acid accumulation in Medicago truncatula under lead stress. Environ. Exp. Bot. 2020, 171, 103950. [Google Scholar] [CrossRef]
- Garg, N.; Kaur, H. Response of Antioxidant Enzymes, Phytochelatins and Glutathione Production Towards Cd and Zn Stresses in Cajanus cajan (L.) Millsp. Genotypes Colonized by Arbuscular Mycorrhizal Fungi. J. Agron. Crop Sci. 2013, 199, 118–133. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Role of arbuscular mycorrhiza in arresting reactive oxygen species (ROS) and strengthening antioxidant defense in Cajanus cajan (L.) Millsp. nodules under salinity (NaCl) and cadmium (Cd) stress. Plant Growth Regul. 2015, 75, 521–534. [Google Scholar] [CrossRef]
- Liu, X.; Bush, D.R. Expression and transcriptional regulation of amino acid transporters in plants. Amino Acids 2006, 30, 113–120. [Google Scholar] [CrossRef]
- Hannah, M.A.; Caldana, C.; Steinhauser, D.; Balbo, I.; Fernie, A.R.; Willmitzer, L. Combined Transcript and Metabolite Profiling of Arabidopsis Grown under Widely Variant Growth Conditions Facilitates the Identification of Novel Metabolite-Mediated Regulation of Gene Expression. Plant Physiol. 2010, 152, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yan, Q.; Yang, G.; Wang, Y. Impact of the Arbuscular Mycorrhizal Fungus Funneliformis mosseae on the Physiological and Defence Responses of Canna indica to Copper Oxide Nanoparticles Stress. J. Fungi 2022, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Xie, X.M. Cadmium release in contaminated soils due to organic acids. Pedosphere 2004, 14, 223–228. [Google Scholar]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Ahmad, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef] [PubMed]
- Miras-Moreno, B.; Senizza, B.; Regni, L.; Tolisano, C.; Proietti, P.; Trevisan, M.; Lucini, L.; Rouphael, Y.; Del Buono, D. Biochemical Insights into the Ability of Lemna minor L. Extract to Counteract Copper Toxicity in Maize. Plants 2022, 11, 2613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Miras-Moreno, B.; Yildiztugay, E.; Ozfidan-Konakci, C.; Arikan, B.; Elbasan, F.; Ak, G.; Rouphael, Y.; Zengin, G.; Lucini, L. Metabolomics and Physiological Insights into the Ability of Exogenously Applied Chlorogenic Acid and Hesperidin to Modulate Salt Stress in Lettuce Distinctively. Molecules 2021, 26, 6291. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2020, 10, 37. [Google Scholar] [CrossRef]
- Xu, X.B.; Tian, S.P. Salicylic acid alleviated pathogen-induced oxidative stress in harvested sweet cherry fruit. Postharvest. Biol. Technol. 2008, 49, 379–385. [Google Scholar] [CrossRef]
- Ahmad, P.; Nabi, G.; Ashraf, M. Cadmium-induced oxidative damage in mustard Brassica juncea (L.) Czern. & Coss. plants can be alleviated by salicylic acid. S. Afr. J. Bot. 2011, 77, 36–44. [Google Scholar] [CrossRef]
- Yin, M.; Pan, L.; Liu, J.; Yang, X.; Tang, H.; Zhou, Y.; Huang, S.; Pan, G. Proanthocyanidins Alleviate Cadmium Stress in Industrial Hemp (Cannabis sativa L.). Plants 2022, 11, 2364. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, K.; Jiang, H.; Yu, L.; Duan, B. Contrasting responses in the growth and energy utilization properties of sympatric Populus and Salix to different altitudes: Implications for sexual dimorphism in Salicaceae. Physiol. Plant 2017, 159, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Zai, X.; Zhu, S.; Qin, P.; Wang, X.; Che, L.; Luo, F. Effect of Glomus mosseae on chlorophyll content, chlorophyll fluorescence parameters, and chloroplast ultrastructure of beach plum (Prunus maritima) under NaCl stress. Photosynthetica 2012, 50, 323–328. [Google Scholar] [CrossRef]
- Xia, H.; Cheng, X.; Zheng, L.; Ren, H.; Li, W.; Lei, Y.; Plenković-Moraj, A.; Chen, K. Sex-Specific Physiological Responses of Populus cathayana to Uranium Stress. Forests 2022, 13, 1123. [Google Scholar] [CrossRef]
- Roessner-Tunali, U.; Hegemann, B.r.; Lytovchenko, A.; Carrari, F.; Bruedigam, C.; Granot, D.; Fernie, A.R. Metabolic profiling of transgenic tomato plants overexpressing hexokinase reveals that the influence of hexose phosphorylation diminishes during fruit development. Plant Physiol. 2003, 133, 84–99. [Google Scholar] [CrossRef] [PubMed]




| Shoot Length (cm) | Shoot DW (g plant−1) | Shoot Water Content (%) | Root Length (cm) | Root DW (g plant−1) | Root Water Content (%) | |
|---|---|---|---|---|---|---|
| CK | 52.761 ± 3.631 a | 0.041 ± 0.003 b | 73.18 c | 109.341 ± 8.548 b | 0.017 ± 0.006 a | 53.21 a |
| AM | 64.263 ± 5.582 a | 0.074 ± 0.016 a | 76.67 b | 172.272 ± 10.016 a | 0.020 ± 0.002 a | 54.48 a |
| Cd | 56.942 ± 7.514 a | 0.019 ± 0.002 b | 72.99 c | 90.799 ± 6.582 b | 0.010 ± 0.004 a | 52.31 a |
| Cd-AM | 58.353 ± 5.930 a | 0.028 ± 0.006 b | 81.17 a | 111.648 ± 2.141 b | 0.012 ± 0.001 a | 63.37 a |
| Cd2+ Content in Shoots (mg/g FW) | Cd2+ Content in Roots (mg/g FW) | Shoot-Root Translation Factors | |
|---|---|---|---|
| Cd | 0.0321 ± 0.0003 a | 0.1396 ± 0.0047 a | 0.2297 ± 0.0086 b |
| Cd-AM | 0.0293 ± 0.0006 b | 0.0683 ± 0.0004 b | 0.4297 ± 0.0117 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Chen, K.; Li, Q.; Tang, Y.; Jiang, Y.; Su, Y. Effects of Arbuscular Mycorrhizal Fungi on Alleviating Cadmium Stress in Medicago truncatula Gaertn. Plants 2023, 12, 547. https://doi.org/10.3390/plants12030547
Li W, Chen K, Li Q, Tang Y, Jiang Y, Su Y. Effects of Arbuscular Mycorrhizal Fungi on Alleviating Cadmium Stress in Medicago truncatula Gaertn. Plants. 2023; 12(3):547. https://doi.org/10.3390/plants12030547
Chicago/Turabian StyleLi, Wanting, Ke Chen, Qiong Li, Yunlai Tang, Yuying Jiang, and Yu Su. 2023. "Effects of Arbuscular Mycorrhizal Fungi on Alleviating Cadmium Stress in Medicago truncatula Gaertn" Plants 12, no. 3: 547. https://doi.org/10.3390/plants12030547
APA StyleLi, W., Chen, K., Li, Q., Tang, Y., Jiang, Y., & Su, Y. (2023). Effects of Arbuscular Mycorrhizal Fungi on Alleviating Cadmium Stress in Medicago truncatula Gaertn. Plants, 12(3), 547. https://doi.org/10.3390/plants12030547





