Variation in Shoot, Peduncle and Fruit Growth of Lagenaria siceraria Landraces
Abstract
:1. Introduction
2. Results
2.1. Shoot Traits of Lagenaria siceraria Landraces
2.2. Dynamics in the Development of Peduncles, Intact and Harvested Fruits of Lagenaria siceraria Landraces
2.3. Correlations between L. siceraria Shoot, Fruit and Peduncle Traits at Different Growth Stages
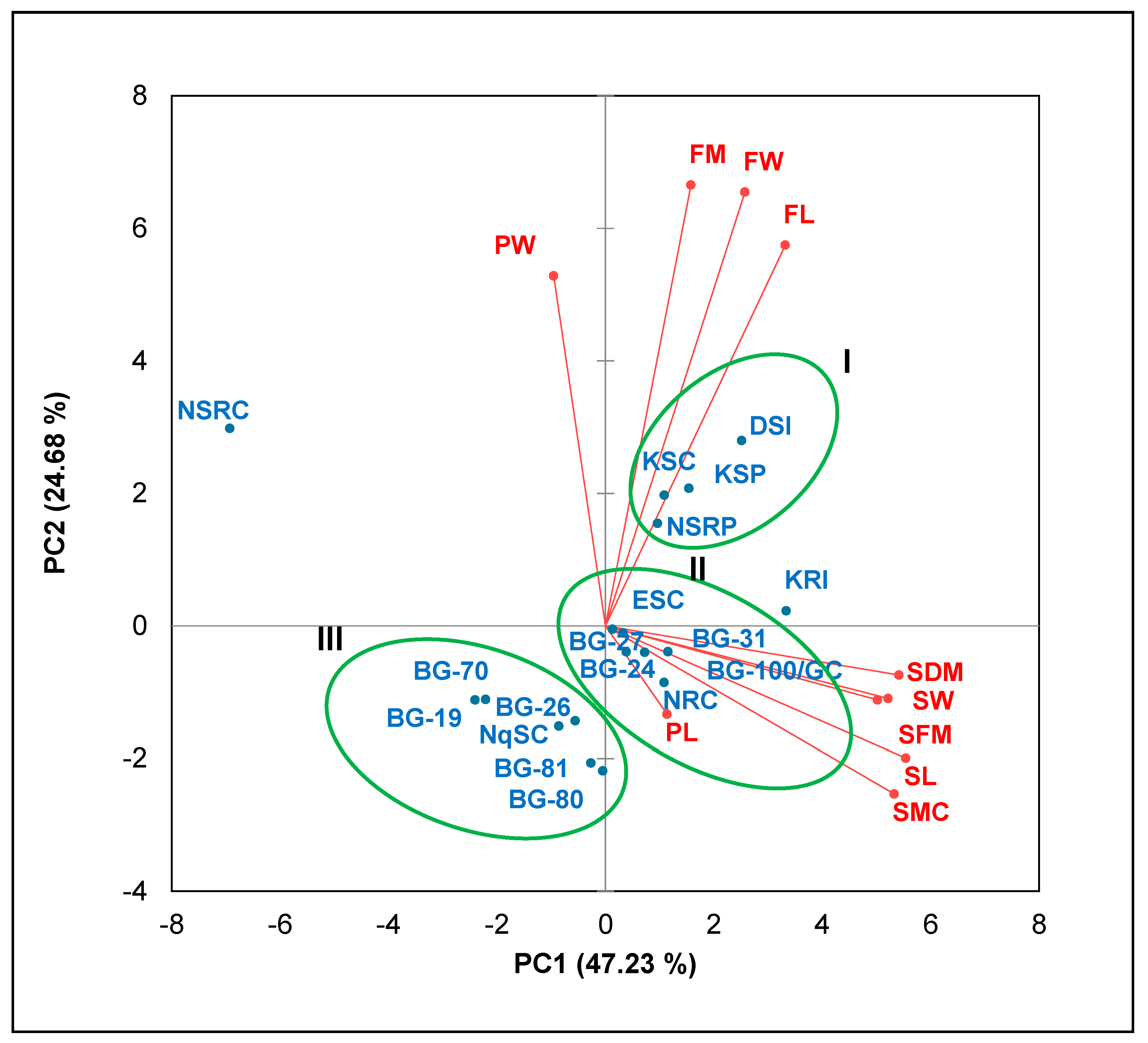
2.4. Principal Component and Cluster Analyses
Genetic Parameters
3. Discussion
3.1. Shoot Growth of Lagenaria siceraria Landraces
3.2. Peduncle and Fruit Sizes of Lagenaria siceraria Landraces at Different Growth Stages
3.3. Correlation
3.4. Principal Component Analysis
3.5. Cluster Analysis
3.6. Genetics Parameters
4. Materials and Methods
4.1. Germplasm Sourcing
4.2. Shoot Traits
4.3. Peduncle and Fruit Traits
4.4. Data Analysis
Estimation of Variance Components
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soni, N.; Raj, K. Isolation and evaluation of fruit and seed mycoflora of bottle gourd (Lagenaria siceraria (Molina) Standl.). Indian Phytopathol. 2022, 75, 83–91. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, V.; Kumar, A.; Prakash, S.; Singh, R. Studies on genetic variability, heritability and genetic advance among quantitative and qualitative characters in bottle gourd (Lagenaria siceraria Standl.). Int. J. Curr. Microbiol. Appl. Sci. 2020, 10, 617–621. [Google Scholar] [CrossRef]
- Mashilo, J.; Shimelis, H.; Ngwepe, R.M. Genetic resources of bottle gourd (Lagenaria siceraria (Molina) Standl.] and citron watermelon (Citrullus lanatus var. citroides (L.H. Bailey) Mansf. ex Greb.): Implications for genetic improvement, product development and commercialization: A review. S. Afr. J. Bot. 2021, 145, 28–47. [Google Scholar] [CrossRef]
- Mkhize, P.; Mashilo, J.; Shimelis, H. Progress on genetic improvement and analysis of bottle gourd [Lagenaria siceraria (Molina) Standl.] for agronomic traits, nutrient composition and stress tolerance: A Review. Front. Sustain. Food Syst. 2021, 5, 1–13. [Google Scholar] [CrossRef]
- Zahoor, M.; Ikram, M.; Nazir, N.; Naz, S.; Batiha, G.E.; Kamran, A.W.; Tomczyk, M.; Kabrah, A. A comprehensive review on the medicinal importance; biological and therapeutic efficacy of Lagenaria siceraria (Mol.) (bottle gourd) standley fruit. Curr. Top. Med. Chem. 2021, 21, 1788–1803. [Google Scholar] [CrossRef]
- Sithole, N.J.; Modi, A.T.; Pillay, K. An assessment of minerals and protein content in selected South African bottle gourd landraces [Lagenaria siceraria (Mol. Standl.)]. J. Hum. Ecol. 2015, 51, 279–286. [Google Scholar] [CrossRef]
- Oh, M.; Honey, S.H.; Tax, F.E. The control of cell expansion, cell division, and vascular development by brassinosteroids: A historical perspective. Int. J. Mol. Sci. 2020, 21, 1743. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Chen, C.; Wang, M.; Shen, Y.; Yang, Y.; Wang, A.; Dai, H.; Zhang, Z.; Miao, M. Comparative analysis of assimilate synthesis, translocation and partitioning between two Cucurbita maxima cultivars “Atlantic giant” and “Hubbard”. Sci. Hortic. 2021, 289, 1–9. [Google Scholar] [CrossRef]
- Pramanik, D.; Spaans, M.; Kranenburg, T.; Bogarin, D.; Heijungs, R.; Lens, F.; Smets, E.; Gravendeel, B. Inflorescence lignification of natural species and horticultural hybrids of Phalaenopsis orchids. Sci. Hortic. 2022, 295, 1–16. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Edelmann, D.; Mori, T.F.; Szekely, G.J. On relationships between the pearson and the distance correlation coefficients. Stat. Probab. Lett. 2021, 169, 1–6. [Google Scholar] [CrossRef]
- Gogtay, N.J.; Thatte, U.M. Principles of Correlation Analysis. J. Assoc. Physicians India 2017, 65, 78–81. [Google Scholar] [PubMed]
- Shen, C.; Shia, X.; Xiea, C.; Lia, Y.; Yanga, H.; Meia, X.; Xua, Y.; Donga, C. The change in microstructure of petioles and peduncles and transporter gene expression by potassium influences the distribution of nutrients and sugars in pear leaves and fruit. J. Plant Physiol. 2019, 232, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D. Statistics Corner: Questions and answers about language testing statistics: What is an eigenvalue? Test. Eval. SIG Newsletter. 2001, 5, 15–19. [Google Scholar]
- Aghaee, M.; Mohammadi, R.; Nabovati, S. Agro-morphological characterization of durum wheat accessions using pattern analysis. Aust. J. Crop Sci. 2010, 4, 505–514. [Google Scholar]
- Earle, D.; Hurley, C.B. Advances in dendrogram seriation for application to visualization. J. Comput. Graph. Stat. 2015, 24, 1–25. [Google Scholar] [CrossRef]
- Bartaula, S.; Panthi, U.; Timilsena, K.; Acharya, S.S.; Shrestha, J. Variability, heritability and genetic advance of maize (Zea mays L.) genotypes. Res. Agric. Livest. Fish. 2019, 6, 163–169. [Google Scholar] [CrossRef]
- Kesumawati, E.; Sabaruddin; Hayati, E.; Hadisah, N.; Hayati, R.; Haidar, Y.; Pohan, N.S.; Jannah, R.; Ardika, A.; Khalil, M.; et al. Genetic variance and heritability estimation of hybridized pepper plants (Capsicum annuum L.) F2 progeny for begomovirus resistance in growth stage. Environ. Earth Sci. 2022, 951, 1–8. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M.; Sharma, V.R.; Singh, M.K.; Singh, B.; Chand, P. Genetic variability, heritability and genetic advance studies in genotypes of okra [(Abelmoschus esculentus (L.) Moench]. J. Pharmacogn. Phytochem. 2019, 8, 1285–1290. [Google Scholar]
- Jain, A.; Singh, S.P.; Pandey, V.P. Character association among the yield and yield attributes in bottle gourd [Lagenaria siceraria (Molina) Standley] genotypes. Plant Arch. 2017, 17, 711–714. [Google Scholar]
- Rani, K.U.; Reddy, E.N. Variability and correlation studies in bottle gourd. Int. J. Pure Appl. Biosci. 2017, 5, 723–731. [Google Scholar] [CrossRef]
- Mashilo, J.; Shimelis, H.; Odindo, A. Genetic diversity of bottle gourd (Lagenaria siceraria (Molina) Standl.) landraces of South Africa assessed by morphological traits and simple sequence repeat markers. S. Afr. J. Plant Soil. 2016, 33, 113–124. [Google Scholar] [CrossRef]
- Mashilo, J.; Odindo, A.; Shimelis, H.; Musenge, P.; Tesfay, S.Z.; Magwaza, L.S. Drought tolerance of selected bottle gourd [Lagenaria siceraria (Molina) Standl.] landraces assessed by leaf gas exchange and photosynthetic efficiency. Plant Physiol. Biochem. 2017, 120, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Mashilo, J.; Shimelis, H.; Odindo, A. Phenotypic and genotypic characterization of bottle gourd [Lagenaria siceraria (Molina) Standley] and implications for breeding: A Review. Sci. Hortic. 2017, 222, 136–144. [Google Scholar] [CrossRef]
- Fichtner, F.; Barbier, F.F.; Mueller-Roeber, B.; Stitt, M.; Annunziata, M.G.; Feil, R.; Beveridge, C.A.; Lunn, J.E. Regulation of shoot branching in Arabidopsis by trehalose 6-phosphate. New Phytol. 2020, 229, 2135–2151. [Google Scholar] [CrossRef]
- Anwar, N.A.M.; Gad, A.A.; Bardisi, A.; Zyada, H.G. Effect of plant spacing and apical shoot pinching on growth and productivity of watermelon plants under sandy soil conditions. J. Agric. Res. 2019, 46, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Wang, R. Determining the relationship between floral initiation and source–sink dynamics of tomato seedlings affected by changes in shading and nutrients. HortScience 2020, 55, 457–464. [Google Scholar] [CrossRef]
- Barbier, F.F.; Dun, E.A.; Kerr, S.C.; Chabikwa, T.G.; Beveridge, C.A. An update on the signals controlling shoot branching. Trends Plant Sci. 2019, 24, 221–236. [Google Scholar] [CrossRef]
- Dong, S.; Beckles, D.M. Dynamic changes in the starch-sugar interconversion within plant source and sink tissues promote a better abiotic stress response. J. Plant Physiol. 2019, 234, 80–93. [Google Scholar] [CrossRef]
- Naafe, M.; Nabi, G.; Irshad, M.; Khan, M.N.; Ali, S.; Hayat, R. Influence of pinching on growth and yield of bottle gourd (Lagenaria siceraria). Pure Appl. Biol. 2022, 11, 891–901. [Google Scholar] [CrossRef]
- Sithole, N.; Modi, A.T. Responses of selected bottle gourd [Lagenaria siceraria (Molina Standly)] landraces to water stress. Acta Agric. Scand. B Soil Plant Sci. 2015, 65, 350–356. [Google Scholar] [CrossRef]
- Small, C.C.; Degenhardt, D. Plant growth regulators for enhancing revegetation success in reclamation: A review. Ecol. Eng. 2018, 118, 43–51. [Google Scholar] [CrossRef]
- Rogers, H.J. Programmed cell death in floral organs: How and why do flowers die? Ann. Bot. 2006, 97, 309–315. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Tuteja, N. Integrated signaling in flower senescence. Plant Signal. Behav. 2007, 2, 437–445. [Google Scholar] [CrossRef]
- Baba, A.I.; Mir, M.Y.; Riyazuddin, R.; Fehér, A. Plants in microgravity: Molecular and technological perspectives. Int. J. Mol. Sci. 2022, 23, 10548. [Google Scholar] [CrossRef]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Involvement of auxin in growth and stress response of cucumber. Veg. Sci. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Ma, N.; Ma, C.; Liu, Y.; Shahid, M.O.; Wang, C.; Gao, J. Petal senescence: A hormone view. J. Exp. Bot. 2018, 69, 719–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, J.B. Anatomy of axis contraction in seedlings from a fire prone habitat. Am. J. Bot. 2008, 95, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Gril, J.; Jullien, D.; Bardet, S.; Yamamoto, H. Tree growth stress and related problems. J. Wood Sci. 2017, 63, 411–432. [Google Scholar] [CrossRef]
- Eng, R.C.; Sampathkumar, A. Plant biology: Bending of plant organs. Curr. Biol. 2020, 30, R402–R405. [Google Scholar] [CrossRef]
- Pereira, L.A.R.; Pina, G.O.; Silveira, C.E.S.; Gomes, S.M.; Toledo, J.L.; Borghetti, F. Effects of Eugenia dysenterica L. extracts on roots and gravitropism of Sesamum indicum L. and Raphanus sativus L. Allelopath. J. 2017, 42, 03–20. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Molecular regulation of plant cell wall extensibility. Gravit. Space Biol. Bull. 1998, 11, 61–70. [Google Scholar] [PubMed]
- Swamy, G.N.; Meghana, D.; Kowsalya, K.B.; Sudeshna, K.; Nair, K.A.K. History: Mechanism and functions of plant growth regulators in vegetable crops. J. Pharm. Innov. 2021, 10, 556–567. [Google Scholar]
- Yap, Y.; Loh, C.; Ong, B. Regulation of flower development in Dendrobium crumenatum by changes in carbohydrate contents, water status and cell wall metabolism. Sci. Hortic. 2008, 119, 59–66. [Google Scholar] [CrossRef]
- Liu, X.; Pan, Y.; Liu, C.; Ding, Y.; Wang, X.; Cheng, Z.; Meng, H. Cucumber fruit size and shape variations explored from the aspects of morphology, histology, and endogenous hormones. Plants 2020, 9, 772. [Google Scholar] [CrossRef]
- Gosai, S.; Adhikari, S.; Khanal, S.; Poudel, P.B. Effects of plant growth regulators on growth, flowering, fruiting and fruit yield of cucumber (Cucumis sativus L.): A review. Arch. Agric. Environ. Sci. 2020, 5, 268–274. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; McGregor, C.; Liu, S.; Luan, F.; Gao, M.; Weng, Y. Genetic architecture of fruit size and shape variation in cucurbits: A comparative perspective. Theor. Appl. Genet. 2020, 133, 1–21. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Yang, Y.; Guo, X.; Feng, Q.; Dong, X.; Chen, S. Genetic analysis and QTL mapping of fruit length and diameter in a cucumber (Cucumber sativus L.) recombinant inbred line (RIL) population. Sci. Hortic. 2019, 250, 214–222. [Google Scholar] [CrossRef]
- Serrani, J.C.; Sanjuán, R.; Ruiz-Rivero, O.; Fos, M.; García-Martínez, J.L. Gibberellin regulation of fruit set and growth in tomato. Plant Physiol. 2022, 145, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Kayani, M.Z.; Mukhtar, T.; Hussain, M.A. Effects of southern root knot nematode population densities and plant age on growth and yield parameters of cucumber. Crop Prot. 2021, 92, 207–212. [Google Scholar] [CrossRef]
- Uddin, A.F.M.J.; Tahidul, M.I.; Chowdhury, M.H.N.; Shiam, I.M.; Mehraj, H. Evaluation of bottle gourd (Lagenaria siceraria) to growth and yield. Int. J. Biosci. 2014, 5, 7–11. [Google Scholar] [CrossRef]
- Chanishvili, S.S.; Badridze, G.S.; Barblishvili, T.F.; Dolidze, M.D. Defoliation photosynthetic rates and assimilate transport in grapevine plants. Russ. J. Plant Physiol. 2005, 52, 448–453. [Google Scholar] [CrossRef]
- Shubha, K.; Srivastava, R.; Gangopadhyaya, K.K.; Rana, J.C. Diversity analysis of bottle gourd (Lagenaria siceraria (Molina) Standl.) germplasm by multivariate analysis. Veg. Sci. 2019, 46, 50–55. [Google Scholar]
- Contreras-Soto, R.I.; Rafael, D.Z.; Moiana, L.D.; Maldonado, C.; Mora-Poblete, F. Variation in root-related traits is associated with water uptake in Lagenaria siceraria genotypes under water-deficit conditions. Front. Plant Sci. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Chukwudi, U.P.; Agbo, C.U. Effect of trellis height and cutting frequency on leaf and fruit yield of fluted pumpkin (Telfairia occidentalis Hook F.). J. Anim. Plant Sci. 2014, 24, 1190–1197. [Google Scholar]
- Ntuli, N.R.; Zobolo, A.M. Effect of water stress on growth of colchicine induced polyploidy Coccinia palmata and Lagenaria sphaerica plants. Afr. J. Biotechnol. 2008, 7, 3548–3652. [Google Scholar]
- Nelson, L.E.; Khama, O.R.; Cedric, K. Improvement on grass establishment at a quarry rehabilitation site influenced by poultry manure in the subtropical South Africa. Afr. Crop Sci. J. 2014, 2, 98–107. [Google Scholar]
- Naidoo, S.I.M.; Laurie, S.M.; Odeny, D.A.; Vorster, B.J.; Mphela, W.M.; Greyling, M.M.; Crampton, B.G. Genetic analysis of yield and flesh colour in sweet potato. Afr. Crop Sci. J. 2016, 24, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Abishkar, K.; Pragya, A. Effect of various organic fertilizer on seedling health and vigour of different varieties of cucumber in rautahat condition. Malays. J. Sustain. Agric. 2020, 4, 81–85. [Google Scholar]
- Reeb, J.; Milota, M. Moisture Content by the Oven-Dry Method for Industrial Testing; Oregon State University: Corvallis, OR, USA, 1999; pp. 66–74. [Google Scholar]
- Burton, G.W.; Devane, E.H. Estimating the heritability in tall fescue (Festuca arundinancea) from replicated clonal material. J. Agron. 1953, 45, 478–481. [Google Scholar] [CrossRef]
- Allard, R.W. Principles of Plant Breeding; John Wiley & Sons: London, UK, 1960; pp. 83–88. [Google Scholar]
- Meena, B.L.; Das, S.P.; Meena, S.K.; Kumari, R.; Devi, A.G.; Devi, H.L. Assessment of GCV, PCV, heritability and genetic advance for yield and its components in field pea (Pisum sativum L.). Int. J. Curr. Microbiol. Appl. Sci. 2015, 6, 1025–1033. [Google Scholar] [CrossRef]

| Prov | LR | Area | Fruit Color | Fruit Texture | Fruit Shape | Seed Type | Seed Color | Seed Texture | Seed Size | Seed Line | Seed Shape |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KZN | KSP | Khangelani | Pale green | Smooth | Pear | Asiatica | Brown | Leathery | Large | Present | Slightly oblong to rectangular |
| KZN | KSC | Khangelani | Pale green | Smooth | Curvilinear | Asiatica | Brown | Leathery | Large | Present | Slightly oblong to rectangular |
| KZN | KRI | Khangelani | Green | Rough | Isodiametric | Siceraria | Dark brown | Leathery | Large | Present | Slightly oblong to rectangular |
| KZN | NRC | Ndumo | Dark green | Rough | Cylindrical | Siceraria | Creamy brown | Smooth | Small | Absent | Oblong |
| KZN | NSRC | Nquthu | Green | Semi-rough | Curvilinear | Intermediate | Brown | Leathery | Medium | Present | Slightly oblong |
| KZN | NSRP | Nquthu | Pale green | Semi-rough | Pear | Intermediate | Brown | Leathery | Medium | Present | Slightly oblong |
| KZN | NqSC | Nquthu | Pale green | Smooth | Semi-curvilinear | Asiatica | Light brown | Leathery | Medium | Present | Slightly oblong |
| KZN | DSI | Dundee | Dark green | Smooth | Isodiametric | Siceraria | Dark brown | Smooth | Large | Present | Oblong |
| KZN | ESC | Emkhandlwini | Pale green | Smooth | Curvilinear | Asiatica | Light brown | Leathery | Medium | Present | Slightly oblong |
| LP | BG-19 | Kgohloane | Dark green | Smooth | Isodiametric | Siceraria | Brown | Leathery | Large | Present | Slightly oblong to rectangular |
| LP | BG-24 | Go-Phasa | Pale green | Corrugate | Cavate | Siceraria | Dark brown | Smooth | Small | Absent | Oblong |
| LP | BG-26 | Kgohloane | Dark green | Smooth | Cavate | Intermediate | Brown | Leathery | Large | Present | Slightly oblong to rectangular |
| LP | BG-27 | Kgohloane | Pale and dark green | Semi-rough | Cavate | Siceraria | Brown | Leathery | Small | Present | Oblong |
| LP | BG-31 | Kgohloane | Dark green | Smooth | Cavate | Intermediate | Brown | Leathery | Large | Present | Slightly oblong to rectangular |
| LP | BG-70 | Go-Phasa | Pale green | Smooth | Pyriform | Asiatica | Light brown | Leathery | Large | Present | Slightly oblong to rectangular |
| LP | BG-80 | Moletjie-Mabokelele | Pale green | Corrugate | Cavate | Asiatica | Dark brown | Smooth | Medium | Absent | Oblong |
| LP | BG-81 | Kgohloane | Pale and dark green | Corrugate | Cavate | Asiatica | Brown | Leathery | Large | Present | Oblong |
| LP | BG-100/GC | Kgohloane | Pale green | Semi-rough | Cylindrical | Asiatica | Light brown | Leathery | Medium | Present | Slightly oblong |
| Harvest Period (Days after Sowing) | |||||
|---|---|---|---|---|---|
| Traits | Landraces | 42 DAS | 49 DAS | 56 DAS | 63 DAS |
| SL | BG-19 | NA | 232.0 lmn | 299.0 d–n | 300.0 d–n |
| BG-24 | 263.5 h–n | 307.0 d–n | 347.5 b–m | 349.0 b–l | |
| BG-26 | 252.0 j–n | 337.5 b–n | 347.0 b–m | 377.0 a–h | |
| BG-27 | 236.8 k–n | 286.4 d–n | 376.1 a–h | 333.0 b–n | |
| BG-31 | 238.5 k–n | 327.5 c–n | 341.2 b–n | 371.5 a–j | |
| BG-70 | 276.5 e–n | NA | 332.5 b–n | 391.0 a–f | |
| BG-80 | 278.0 e–n | 328.5 c–n | 306.5 d–n | 335.5 b–n | |
| BG-81 | 252.2 j–n | 254.3 i–n | 376.0 a–h | 396.0 a–e | |
| BG-100/GC | 228.2 mn | 264.4 g–n | 328.5 c–n | 365.0 a–j | |
| DSI | 404.2 a–d | 367.0 a–j | 313.0 c–n | 296.5 d–n | |
| ESC | 226.9 n | 319.9 c–n | 289.9 d–n | 367.5 a–j | |
| KRI | 375.0 a–h | 400.0 a–d | 450.0 ab | 476.0 a | |
| KSC | 373.9 a–i | 223.0 n | 293.0 d–n | 287.9 d–n | |
| KSP | 430.4 abc | 297.0 d–n | 278.5 e–n | 327.0 c–n | |
| NqSC | 370.5 a–j | NA | 299.0 d–n | 330.0 c–n | |
| NRC | 273.6 f–n | 382.0 a–h | 378.0 a–h | 406.0 a–d | |
| NSRP | 268.5 g–n | 356.0 b–k | 342.5 b–n | 384.0 a–g | |
| SW | BG-19 | NA | 7.0 de | 6.9 de | 5.9 e |
| BG-24 | 4.9 e | 6.2 e | 6.1 e | 7.1 de | |
| BG-26 | 7.9 cde | 6.9 de | 7.0 de | 7.1 de | |
| BG-27 | 5.5 e | 6.2 e | 6.1 e | 6.9 de | |
| BG-31 | 5.6 e | 7.0 de | 14.3 ab | 7.2 de | |
| BG-70 | 5.0 e | NA | 6.5 e | 7.0 de | |
| BG-80 | 6.6 de | 5.9 e | 7.6 de | 6.6 de | |
| BG-81 | 6.7 de | 7.0 de | 6.7 de | 7.5 de | |
| BG-100/GC | 6.8 de | 7.4 de | 8.3 cde | 8.6 cde | |
| DSI | 6.7 de | 7.0 de | 6.7 de | 6.6 de | |
| ESC | 6.5 e | 6.4 e | 6.2 e | 6.2 e | |
| KRI | 6.8 de | 13.3 abc | 11.9 bcd | 10.3 b–e | |
| KSC | 6.6 de | 6.2 e | 6.8 de | 7.8 de | |
| KSP | 5.9 e | 17.6 a | 6.8 de | 7.0 de | |
| NqSC | 4.9 e | NA | 7.0 de | 9.0 b–e | |
| NRC | 4.9 e | 5.7 e | 7.1 de | 7.3 de | |
| NSRP | 5.5 e | 7.0 de | 6.8 de | 7.1 de | |
| SFM | BG-19 | NA | 15.23 g–o | 15.63 g–o | 15.48 g–o |
| BG-24 | 16.73 f–o | 17.14 e–o | 13.51 i–o | 13.85 i–o | |
| BG-26 | 6.29 o | 12.69 k–o | 14.42 h–o | 18.98 d–m | |
| BG-27 | 7.71 no | 10.69 l–o | 24.06 a–i | 13.00 j–o | |
| BG-31 | 9.29 m–o | 18.18 d–n | 12.79 k–o | 17.44 e–n | |
| BG-70 | 9.08 m–o | NA | 12.98 j–o | 18.78 d–m | |
| BG-80 | 11.99 k–o | 14.85 g–o | 14.40 h–o | 16.84 f–o | |
| BG-81 | 10.59 l–o | 14.07 i–o | 17.58 e–n | 19.61 d–m | |
| BG-100/GC | 13.31 i–o | 16.61 f–o | 17.30 e–n | 26.82 a–f | |
| DSI | 32.68 ab | 25.33 a–h | 15.71 g–o | 17.57 e–n | |
| ESC | 10.44 l–o | 15.12 g–o | 11.41 l–o | 15.97 f–o | |
| KRI | 29.00 a–d | 25.50 a–g | 34.75 a | 30.62 abc | |
| KSC | 15.39 g–o | 13.27 i–o | 13.09 i–o | 16.73 f–o | |
| KSP | 11.43 l–o | 13.99 i–o | 12.66 k–o | 15.88 f–o | |
| NqSC | 28.05 a–e | NA | 13.79 i–o | 22.73 b–k | |
| NRC | 31.79 abc | 23.80 a–j | 21.17 c–l | 26.87 a–f | |
| NSRP | 9.81 m–o | 16.84 f–o | 13.85 i–o | 22.64 b–k | |
| SDM | BG-19 | NA | 1.87 d–n | 2.17 c–l | 1.30 j–n |
| BG-24 | 2.19 c–k | 1.99 c–m | 1.68 e–n | 1.30 j–n | |
| BG-26 | 1.54 g–n | 1.68 e–n | 1.77 d–m | 1.58 g–n | |
| BG-27 | 1.44 h–n | 1.27 k–n | 1.58 g–n | 0.98n | |
| BG-31 | 1.43 h–n | 2.02 c–m | 1.66 e–n | 1.37 j–n | |
| BG-70 | 1.68 e–n | NA | 1.59 g–n | 1.67 e–n | |
| BG-80 | 1.81 d–m | 1.95 c–m | 1.38 i–n | 1.55 g–n | |
| BG-81 | 1.97 c–m | 1.73 e–n | 2.02 c–m | 1.49 g–n | |
| BG-100/GC | 2.29 b–i | 2.11 c–l | 2.60 a–e | 2.13 c–l | |
| DSI | 3.23 ab | 2.54 b–f | 2.21 c–j | 1.87 d–n | |
| ESC | 1.85 d–n | 2.02 c–m | 1.25 l–n | 1.47 h–n | |
| KRI | 2.32 b–h | 2.41 b–g | 3.50a | 2.54 b–f | |
| KSC | 2.69 a–d | 1.70 e–n | 1.82 d–m | 1.67 e–n | |
| KSP | 2.56 b–f | 1.76 d–n | 2.08 c–l | 1.86 d–n | |
| NqSC | 2.85 abc | NA | 1.73 e–n | 1.89 d–n | |
| NRC | 2.31 b–i | 2.57 a–f | 2.08 c–l | 2.08 c–l | |
| NSRP | 1.48 g–n | 2.09 c–l | 1.65 f–n | 1.12mn | |
| SMC | BG-19 | NA | 87.70 d–m | 85.80 j–r | 85.94 ab |
| BG-24 | 87.00 g–p | 88.60 b–l | 87.00 g–p | 87.36 ab | |
| BG-26 | 74.20 u | 85.90 j–r | 86.90 g–p | 85.80 ab | |
| BG-27 | 80.20 st | 88.10 c–l | 89.60 a–k | 90.27 ab | |
| BG-31 | 84.30 l–s | 89.00 b–l | 85.90 j–r | 86.78 ab | |
| BG-70 | 81.60 qrs | NA | 87.30 e–n | 87.48 ab | |
| BG-80 | 85.00 k–s | 86.90 g–p | 90.30 a–j | 90.42 a | |
| BG-81 | 81.20 rst | 87.60 d–m | 87.90 c–l | 87.85 ab | |
| BG-100/GC | 82.60 n–s | 87.00 g–p | 84.40 l–s | 84.94 ab | |
| DSI | 89.40 a–k | 89.50 a–k | 86.20 i–q | 86.24 ab | |
| ESC | 82.30 p–s | 86.80 h–p | 88.40 b–l | 87.88 ab | |
| KRI | 92.10 a–e | 90.20 a–j | 89.70 a–k | 89.83 ab | |
| KSC | 82.40 o–s | 87.00 g–p | 85.80 j–r | 85.62 ab | |
| KSP | 76.60 tu | 87.40 e–n | 83.00 m–s | 83.48 b | |
| NqSC | 90.00 a–j | NA | 87.20 f–o | 87.25 ab | |
| NRC | 93.00 ab | 89.40 a–k | 90.10 a–j | 89.88 ab | |
| NSRP | 84.90 k–s | 87.80 d–m | 88.10 c–l | 88.14 ab | |
| Traits | Landraces | Days after Anthesis | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| PL | BG-19 | 36.3 o–B | 29.9 r–B | 27.8 s–B | 25.1 v–B | 20.4 z–B | 17.1 B |
| BG-24 | 85.4 bcd | 75.0 b–f | 67.5 c–k | 66.8 c–k | 63.3 d–m | 54.4 e–r | |
| BG-26 | 46.6 g–x | 35.4 o–P | 30.0 r–B | 26.4 t–B | 27.9 s–B | 24.0 w–B | |
| BG-27 | 99.8 ab | 90.1 abc | 72.9 c–g | 55.2 e–r | 38.0 m–B | 45.6 i–A | |
| BG-31 | 67.3 c–k | 52.3 f–t | 42.9 j–B | 57.8 e–p | 37.0 n–B | 25.8 u–B | |
| BG-70 | 36.8 n–B | 30.0 r–B | 27.0 s–B | 23.8 w–B | 22.5 x–B | 20.0 AB | |
| BG-80 | 57.1 e–p | 50.2 f–v | 45.1 i–A | 43.6 j–A | 39.3 l–B | 36.3 o–B | |
| BG-81 | 72.5 c–h | 54.6 f–v | 49.5 f–w | 46.6 h–y | 46.2 i–z | 42.5 j–B | |
| BG-100/GC | 111.5 a | 79.4 b–e | 65.5 c–k | 57.8 e–p | 52.7 f–s | 51.6 f–u | |
| DSI | 68.1 c–j | 51.2 f–u | 39.5 l–B | 30.9 q–B | 21.1 y–B | 20.7 y–B | |
| ESC | 72.7 c–g | 56.3 e–q | 48.4 g–x | 48.5 g–w | 43.1 j–A | 37.7 m–B | |
| KRI | 64.4 c–l | 62.3 d–n | 57.8 e–p | 34.3 o–B | 30.9 o–B | 27.3 s–B | |
| KSC | 70.8 c–i | 58.7 e–o | 54.4 e–r | 48.3 g–x | 43.5 j–A | 36.8 n–B | |
| KSP | 56.4 e–q | 41.9 k–B | 35.2 o–B | 32.1 p–B | 30.5 r–B | 30.0 r–B | |
| NqSC | NA | NA | NA | NA | NA | NA | |
| NRC | NA | NA | NA | NA | NA | NA | |
| NSRC | NA | NA | NA | NA | NA | NA | |
| NSRP | 48.2 g–x | 41.8 k–B | 36.5 n–B | 33.7 o–B | 27.8 s–B | 24.0 w–B | |
| PW | BG-19 | 3.8 r–B | 4.3 m–B | 4.6 i–A | 5.1 d–u | 5.6 c–o | 7.3a |
| BG-24 | 3.6 v–B | 3.8 r–B | 4.3 k–B | 4.9 e–x | 4.7 g–z | 5.3 c–q | |
| BG-26 | 3.7 u–B | 4.2 o–B | 4.7 h–A | 5.3 c–q | 5.7 b–l | 7.2a | |
| BG-27 | 3.2AB | 3.6 v–B | 4.2 o–B | 4.4 j–B | 5.0 d–v | 5.3 c–p | |
| BG-31 | 3.8 r–B | 4.1 o–B | 4.6 i–A | 5.3 c–p | 5.5 c–p | 5.6 c–o | |
| BG-70 | 3.4 y–B | 3.5 x–B | 4.5 i–B | 5.2 c–s | 5.2 c–s | 6.1 a–h | |
| BG-80 | 3.1 B | 3.6 w–B | 4.1 p–B | 4.4 k–B | 4.8 f–y | 4.9 d–x | |
| BG-81 | 3.3 zAB | 3.8 s–B | 4.2 o–B | 4.4 j–B | 4.5 i–B | 5.1 c–t | |
| BG-100/GC | 3.7 t–B | 4.2 o–B | 4.6 i–A | 4.9 d–x | 5.5 c–p | 5.7 b–m | |
| DSI | 3.7 t–B | 4.4 k–B | 5.0 d–w | 5.7 b–n | 5.8 a–j | 5.9 a–i | |
| ESC | 3.4 y–B | 3.9 q–B | 4.5 i–B | 4.4 j–B | 5.9 a–i | 5.1 d–u | |
| KRI | 4.5 i–B | 4.9 d–x | 5.2 c–r | 5.9 a–i | 6.2 a–f | 6.6 abc | |
| KSC | 3.6 x–B | 4.3 l–B | 4.7 g–z | 5.7 b–m | 6.1 a–g | 6.1 a–g | |
| KSP | 4.1 o–B | 4.9 f–x | 5.7 b–k | 5.7 b–m | 6.3 a–e | 7.0 ab | |
| NqSC | NA | NA | NA | NA | NA | NA | |
| NRC | NA | NA | NA | NA | NA | NA | |
| NSRC | NA | NA | NA | NA | NA | NA | |
| NSRP | 3.8 r–B | 4.2 o–B | 4.8 f–y | 5.4 c–p | 5.7 b–l | 6.3 a–d | |
| FL | BG-19 | 15.9 t | 17.0 st | 19.7 o–t | 22.1 n–t | 27.6 i–t | 34.1 e–t |
| BG-24 | 26.0 k–t | 34.2 e–t | 45.2 d–p | 41.0 d–t | 42.0 d–t | 56.4 c–f | |
| BG-26 | 17.6 r–t | 21.1 o–t | 24.0 l–u | 27.2 j–t | 34.7 e–t | 42.7 d–s | |
| BG-27 | 25.9 k–t | 40.7 d–t | 49.7 c–l | 39.2 e–t | 59.3 cde | 102.5 a | |
| BG-31 | 19.0 p–t | 33.4 e–t | 37.6 e–t | 42.7 d–s | 49.3 c–m | 53.8 c–i | |
| BG-70 | 18.3 q–t | 24.1 l–t | 27.2 j–t | 29.1 g–t | 35.0 e–t | 43.5 d–r | |
| BG-80 | 21.6 o–t | 26.3 k–t | 29.9 g–t | 30.5 f–t | 33.2 e–t | 41.6 d–t | |
| BG-81 | 21.1 o–t | 24.2 l–t | 28.8 h–t | 28.4 h–t | 34.9 e–t | 40.5 d–t | |
| BG-100/GC | 18.8 q–t | 21.4 o–t | 23.3 m–t | 27.0 j–t | 28.7 h–t | 31.6 f–t | |
| DSI | 31.6 f–t | 36.1 e–t | 39.9 d–t | 43.8 d–r | 51.9 c–k | 74.5bc | |
| ESC | 33.3 e–t | 42.0 d–t | 51.4 c–k | 53.0 c–j | 44.6 d–q | 65.8cd | |
| KRI | 21.5 o–t | 24.1 l–t | 24.5 l–u | 26.8 j–t | 31.1 f–t | 33.3 e–t | |
| KSC | 27.9 i–t | 33.1 e–t | 33.8 e–t | 50.0 c–l | 48.4 c–n | 54.6 c–h | |
| KSP | 25.9 k–t | 45.3 d–p | 45.4 d–o | 49.7 c–l | 58.3cde | 55.2 c–g | |
| NqSC | NA | NA | NA | NA | NA | NA | |
| NRC | NA | NA | NA | NA | NA | NA | |
| NSRC | NA | NA | NA | NA | NA | NA | |
| NSRP | 35.4 e–t | 35.4 e–t | 41.2 d–t | 45.5 d–o | 56.5 c–f | 95.2ab | |
| FW | BG-19 | 15.2 n–t | 15.9 m–t | 21.2 h–t | 23.0 g–t | 28.5 e–o | 32.7 c–i |
| BG-24 | 15.1 n–t | 18.9 h–t | 27.7 e–r | 22.6 g–t | 22.7 g–t | 31.1 c–k | |
| BG-26 | 13.4 p–t | 18.2 i–t | 17.9 i–t | 20.8 h–t | 29.4 d–n | 36.9 c–g | |
| BG-27 | 13.0 q–t | 17.8 i–t | 26.3 e–s | 18.1 i–t | 25.5 f–t | 43.6bcd | |
| BG-31 | 11.1 t | 13.4 p–t | 17.3 j–t | 29.2 d–n | 40.8 b–e | 54.7ab | |
| BG-70 | 13.4 p–t | 16.0 l–t | 18.3 i–t | 18.0 i–t | 21.2 h–t | 24.2 g–t | |
| BG-80 | 14.0 o–t | 16.0 m–t | 18.2 i–t | 19.6 h–t | 23.9 g–t | 27.8 e–r | |
| BG-81 | 13.2 q–t | 15.5 n–t | 21.8 h–t | 22.1 g–t | 24.8 g–t | 31.5 c–j | |
| BG-100/GC | 13.8 o–t | 15.7 n–t | 17.1 j–t | 19.4 h–t | 21.6 h–t | 24.2 g–t | |
| DSI | 16.5 k–t | 24.5 g–t | 28.2 e–p | 33.9 c–h | 44.0bcd | 58.7a | |
| ESC | 10.8 tu | 13.8 o–t | 18.0 i–t | 22.2 g–t | 15.3 n–t | 24.4 g–t | |
| KRI | 17.3 j–t | 19.9 h–t | 20.9 h–t | 22.6 g–t | 25.1 f–t | 30.8 d–m | |
| KSC | 15.2 n–t | 21.7 h–t | 19.5 h–t | 23.9 g–t | 28.0 e–q | 40.0 b–f | |
| KSP | 11.4 st | 15.7 n–t | 27.5 e–r | 31.0 d–l | 14.4 n–t | 16.8 j–t | |
| NqSC | NA | NA | NA | NA | NA | NA | |
| NRC | NA | NA | NA | NA | NA | NA | |
| NSRC | NA | NA | NA | NA | NA | NA | |
| NSRP | 11.5 st | 13.0 rst | 14.0 o–t | 15.1 n–t | 17.1 j–t | 46.1abc | |
| Traits | Landraces | Days after Anthesis | |||
|---|---|---|---|---|---|
| 7 | 14 | 21 | 28 | ||
| PL | BG-24 | 78.67 a–e | 72.33 a–h | 69.33 b–j | 61.00 b–m |
| BG-27 | 42.83 f–n | 63.50 b–l | 75.83 a–g | 66.00 b–k | |
| BG-31 | 43.60 f–n | 47.67 d–n | 31.00lmn | 27.17mn | |
| BG-100/GC | 65.17 b–l | 63.00 b–l | 72.50 a–h | 62.55 b–l | |
| DSI | 81.17 a–d | 57.00 c–n | 88.00abc | 75.50 a–g | |
| ESC | 45.25 e–n | 74.00 a–g | 46.92 d–n | 49.67 d–n | |
| KRI | 61.83 b–m | 51.67 d–n | 44.67 e–n | 26.17n | |
| KSC | 69.83 a–j | 57.67 c–n | 67.00 b–j | 75.32 a–g | |
| KSP | 66.83 b–j | 104.00 a | 93.67 ab | 86.83 abc | |
| NqSC | 78.67 a–e | 66.33 b–j | 62.50 b–l | 55.00 c–n | |
| NRC | 92.50ab | 76.50 a–f | 73.50 a–h | 70.67 a–i | |
| NSRP | 35.67 j–n | 31.67 k–n | 41.22 g–n | 43.00f—n | |
| NSRC | 39.33 h–n | 72.67 a–h | 78.67 a–e | 36.50 i–n | |
| PW | BG-24 | 3.8tu | 4.7 q–u | 5.0 o–u | 7.2 d–k |
| BG-27 | 4.8 p–u | 3.6 u | 4.1 stu | 5.5 l–s | |
| BG-31 | 4.3 r–u | 5.0 o–u | 5.8 k–r | 7.0 e–l | |
| BG-100/GC | 5.1 o–u | 5.3 m–s | 6.3 i–p | 8.7 abc | |
| DSI | 6.0 k–q | 8.0 a–h | 7.8 b–h | 8.7 abc | |
| ESC | 4.1stu | 4.0stu | 4.2 r–u | 5.0 o–u | |
| KRI | 7.1 e–k | 8.2 a–g | 8.5 a–e | 9.5 a | |
| KSC | 7.0 e–k | 8.0 a–h | 7.0 e–l | 7.0 e–k | |
| KSP | 6.0 k–q | 5.3 n–t | 7.1 e–k | 6.8 f–m | |
| NqSC | 3.8 tu | 6.7 g–n | 7.7 b–j | 9.0 abc | |
| NRC | 8.4 a–e | 8.3 a–f | 9.2 ab | 8.7 abc | |
| NSRP | 6.0 k–q | 6.5 h–o | 6.8 f–m | 7.0 e–l | |
| NSRC | 6.2 j–p | 7.6 c–j | 7.9 b–h | 7.0 e–l | |
| FL | BG-24 | 88.3 s–v | 128.7 o–t | 127.7 o–t | 138.0 n–s |
| BG-27 | 108.3 r–v | 127.8 o–t | 123.0 p–u | 220.5 f–l | |
| BG-31 | 119.7 p–u | 156.1 m–r | 207.0 g–m | 213.3 g–m | |
| BG-100/GC | 64.5 uv | 84.7 s–v | 108.3 r–v | 218.3 g–l | |
| DSI | 57.5 v | 177.0 k–p | 239.4 e–j | 256.7 e–h | |
| ESC | 96.7 s–v | 130.9 o–s | 227.9 e–k | 237.7 e–j | |
| KRI | 65.2 uv | 125.7 o–t | 192.0 i–n | 220.0 f–l | |
| KSC | 134.8 n–s | 286.0de | 351.0 bc | 374.6 bc | |
| KSP | 158.8 m–r | 161.8 l–r | 320.5 cd | 389.7 b | |
| NqSC | 88.3 s–v | 172.5 k–q | 200.2 h–m | 261.0 efg | |
| NRC | 69.7tuv | 113.3 q–v | 132.3 o–s | 226.7 f–k | |
| NSRP | 126.0 o–t | 182.3 j–o | 277.9 def | 597.0a | |
| NSRC | 139.3 n–s | 204.7 g–m | 245.3 e–i | 605.5a | |
| FW | BG-24 | 43.00 uvw | 83.00 m–p | 91.67 h–o | 120.00 def |
| BG-27 | 46.00 uvw | 73.67 n–s | 90.75 i–o | 83.00 m–p | |
| BG-31 | 40.00 vw | 60.67 q–v | 79.50 m–q | 96.33 g–m | |
| BG-100/GC | 51.67 t–w | 73.83 n–r | 88.17 k–o | 121.42 c–f | |
| DSI | 36.33 w | 95.00 g–n | 133.00 cd | 175.83 a | |
| ESC | 39.92 vw | 75.33 m–q | 79.04 m–q | 96.13 g–m | |
| KRI | 53.00 r–w | 90.83 i–o | 107.67 f–k | 142.50 bc | |
| KSC | 73.00 o–t | 89.00 k–o | 155.13 ab | 112.67 d–h | |
| KSP | 52.17 s–w | 72.33 o–t | 111.00 e–j | 165.17 a | |
| NqSC | 43.00 uvw | 89.50 j–o | 105.88 f–l | 130.50 cde | |
| NRC | 38.83 w | 73.83 n–r | 91.67 h–o | 127.33 c–f | |
| NSRP | 43.50 uvw | 62.73 p–u | 85.78 l–o | 111.50 d–i | |
| NSRC | 49.50 uvw | 88.17 k–o | 111.50 d–i | 114.50 d–g | |
| FM | BG-24 | 0.057 s | 0.270 k–s | 0.370 j–r | 0.690 f–i |
| BG-27 | 0.073 rs | 0.212 n–s | 0.354 j–s | 0.437 i–p | |
| BG-31 | 0.066 rs | 0.159 o–s | 0.347 j–s | 0.523 g–m | |
| BG-100/GC | 0.091 qrs | 0.252 l–s | 0.462 h–o | 1.257 cd | |
| DSI | 0.082 qrs | 0.553 g–l | 1.332 cd | 2.277 a | |
| ESC | 0.053 s | 0.231 m–s | 0.296 k–s | 0.471 h–n | |
| KRI | 0.080 qrs | 0.382 j–q | 0.788fg | 1.258 cd | |
| KSC | 0.152 p–s | 0.630 f–j | 1.145cde | 1.434 bc | |
| KSP | 0.108 qrs | 0.190 n–s | 0.897 ef | 1.655 b | |
| NqSC | 0.057 s | 0.320 k–s | 0.762 fgh | 1.310 cd | |
| NRC | 0.068 rs | 0.322 k–s | 0.563 g–k | 1.345 cd | |
| NSRP | 0.080 qrs | 0.530 g–m | 0.642 f–j | 1.110 de | |
| NSRC | 0.105 qrs | 0.532 g–m | 1.133 cde | 1.180 cde | |
| (a) | |||||||||
| Traits | 42 DAS | 49 DAS | 56 DAS | 63 DAS | |||||
| SL × SW | 0.43 *** | 0.57 *** | 0.21 * | 0.40 *** | |||||
| SL × SFM | 0.62 *** | 0.83 *** | 0.41 *** | 0.63 *** | |||||
| SL × SDM | 0.73 *** | 0.82 *** | 0.48 *** | 0.40 *** | |||||
| SL × SMC | 0.63 *** | 0.82 *** | 0.32 *** | 0.21 * | |||||
| SW × SFM | 0.22 ** | 0.51 *** | ns | 0.60 *** | |||||
| SW × SDM | 0.45 *** | 0.52 *** | ns | 0.42 *** | |||||
| SW × SMC | 0.74 *** | 0.63 *** | 0.21 * | 0.19 * | |||||
| SFM × SDM | 0.79 *** | 0.93 *** | 0.43 *** | 0.68 *** | |||||
| SFM × SMC | 0.52 *** | 0.72 *** | 0.41 *** | 0.32 ** | |||||
| SDM × SMC | 0.58 *** | 0.77 *** | −0.33 ** | −0.42 *** | |||||
| (b) | |||||||||
| Traits | 0 DAA | 1 DAA | 2 DAA | 3 DAA | 4 DAA | 5 DAA | |||
| PL × PW | Ns | ns | −0.18 * | −0.22 ** | −0.20 * | −0.35 *** | |||
| PL × FL | Ns | ns | ns | ns | ns | ns | |||
| PL × FW | Ns | ns | ns | ns | −0.19 * | −0.17 * | |||
| PW × FL | Ns | ns | ns | ns | 0.26 ** | ns | |||
| PW × FW | 0.27 *** | ns | ns | 0.17 * | ns | Ns | |||
| FL × FW | Ns | 0.23 ** | 0.48 *** | 0.60 *** | ns | 0.60 *** | |||
| (c) | |||||||||
| Variables | SL | SW | SFM | SDM | SMC | PL | PW | FL | FW |
| SW | 0.878 | ||||||||
| SFM | 0.845 | 0.695 | |||||||
| SDM | 0.875 | 0.813 | 0.907 | ||||||
| SMC | 0.949 | 0.828 | 0.725 | 0.807 | |||||
| PL | 0.070 | 0.093 | 0.133 | 0.091 | 0.171 | ||||
| PW | −0.239 | −0.098 | −0.045 | −0.062 | −0.416 | −0.582 | |||
| FL | 0.351 | 0.392 | 0.215 | 0.333 | 0.377 | 0.092 | 0.124 | ||
| FW | 0.207 | 0.271 | 0.108 | 0.208 | 0.221 | 0.072 | 0.225 | 0.984 | |
| FM | 0.003 | 0.035 | 0.269 | 0.266 | −0.105 | 0.151 | 0.460 | 0.605 | 0.662 |
| Variables | PC1 | PC2 | PC3 |
|---|---|---|---|
| SL | 0.943 | −0.245 | −0.133 |
| SW | 0.888 | −0.135 | −0.136 |
| SFM | 0.854 | −0.137 | −0.215 |
| SDM | 0.922 | −0.091 | −0.201 |
| SMC | 0.906 | −0.311 | 0.046 |
| PL | 0.193 | −0.164 | 0.850 |
| PW | −0.163 | 0.650 | −0.672 |
| FL | 0.564 | 0.707 | 0.274 |
| FW | 0.438 | 0.805 | 0.272 |
| FM | 0.268 | 0.818 | 0.096 |
| Eigenvalue | 4.723 | 2.468 | 1.457 |
| Variability (%) | 47.233 | 24.681 | 14.574 |
| Cumulative (%) | 47.233 | 71.915 | 86.488 |
| Variables | δ2g | δ2e | δ2p | GM | PCV | GCV | ECV% | h2 | GA |
|---|---|---|---|---|---|---|---|---|---|
| SL | 7296.5 | 4156.0 | 11452.5 | 312.1 | 60.6 | 48.4 | 36.5 | 36.3 | 0.4 |
| SW | 0.1 | 0.09 | 0.14 | 0.7 | 4.5 | 2.8 | 3.5 | 60.5 | 0.3 |
| SFM | 47.4 | 35.0 | 82.3 | 16.4 | 22.4 | 17.0 | 14.6 | 42.5 | 0.4 |
| SDM | 0.4 | 0.3 | 0.6 | 1.8 | 5.8 | 4.4 | 3.7 | 41.9 | 0.4 |
| SMC | 342.5 | 6.9 | 349.5 | 83.8 | 20.4 | 20.2 | 2.9 | 2.0 | 0.5 |
| PLi | 5.2 | 1.8 | 7.0 | 4.1 | 13.1 | 11.3 | 6.6 | 25.6 | 0.4 |
| PWi | 3.1 × 10−2 | 5.6 × 10−3 | 3.7 × 10−2 | 0.4 | 3.0 | 2.7 | 1.1 | 15.0 | 0.4 |
| FLi | 3.4 | 1.9 | 5.2 | 3.3 | 12.6 | 10.1 | 7.5 | 35.5 | 0.4 |
| FWi | 1.2 | 0.6 | 1.8 | 2.0 | 9.6 | 7.9 | 5.5 | 32.5 | 0.4 |
| PLh | 177.2 | 218.1 | 395.3 | 61.9 | 25.3 | 16.9 | 18.8 | 55.2 | 0.3 |
| PWh | 1.6 × 10−2 | 4.2 × 10−2 | 2.0 × 10−2 | 0.7 | 1.7 | 1.5 | 0.8 | 21.2 | 0.4 |
| FLh | 7771.7 | 639.4 | 8411.1 | 193.9 | 65.9 | 63.3 | 18.2 | 7.6 | 0.5 |
| FWh | 676.1 | 84.0 | 760.1 | 87.8 | 29.4 | 27.7 | 9.8 | 11.1 | 0.5 |
| FMh | 0.2 | 1.7 × 10−2 | 0.2 | 0.6 | 5.5 | 5.2 | 1.7 | 10.0 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buthelezi, L.G.; Mavengahama, S.; Sibiya, J.; Ntuli, N.R. Variation in Shoot, Peduncle and Fruit Growth of Lagenaria siceraria Landraces. Plants 2023, 12, 532. https://doi.org/10.3390/plants12030532
Buthelezi LG, Mavengahama S, Sibiya J, Ntuli NR. Variation in Shoot, Peduncle and Fruit Growth of Lagenaria siceraria Landraces. Plants. 2023; 12(3):532. https://doi.org/10.3390/plants12030532
Chicago/Turabian StyleButhelezi, Lungelo Given, Sydney Mavengahama, Julia Sibiya, and Nontuthuko Rosemary Ntuli. 2023. "Variation in Shoot, Peduncle and Fruit Growth of Lagenaria siceraria Landraces" Plants 12, no. 3: 532. https://doi.org/10.3390/plants12030532
APA StyleButhelezi, L. G., Mavengahama, S., Sibiya, J., & Ntuli, N. R. (2023). Variation in Shoot, Peduncle and Fruit Growth of Lagenaria siceraria Landraces. Plants, 12(3), 532. https://doi.org/10.3390/plants12030532






