Abstract
To learn about the gene structure, phylogenetic evolution, and function under biotic and abiotic stresses of BTB (Bric-a-Brac/Tramtrack/Broad Complex) genes in Paulownia fortunei, a whole-genome sequence evaluation was carried out, and a total of 62 PfBTB genes were identified. The phylogenetic analysis showed that PfBTB proteins are divided into eight groups, and these proteins are highly conserved. PfBTB genes were unevenly distributed on 17 chromosomes. The colinearity analysis found that fragment replication and tandem replication are the main modes of gene amplification in the PfBTB family. The analysis of cis-acting elements suggests that PfBTB genes may be involved in a variety of biological processes. The transcriptomic analysis results showed that PfBTB3/12/14/16/19/36/44 responded to Paulownia witches’ broom (PaWB), while PfBTB1/4/17/43 responded to drought stress, and the RT-qPCR results further support the reliability of transcriptome data. In addition, the association analysis between miRNA and transcriptome revealed a 91-pair targeting relationship between miRNAs and PfBTBs. In conclusion, the BTB genes in Paulownia are systematically identified in this research. This work provides useful knowledge to more fully appreciate the potential functions of these genes and their possible roles in the occurrence of PaWB and in response to stress.
1. Introduction
Currently, the BTB protein has been identified in many plants, including Arabidopsis thaliana [1,2,3], rice [4], tomato [5], corn [6], and peach [7]. In Arabidopsis thaliana, BTB proteins can be divided into different subfamilies based on their contained conserved domains other than the BTB domain, including BTB-MATH, BTB-Arm, BTB-only, BTB-TAZ, BTB-BACK, BTB-Ank, TPR, BTB-NPH3, and other [8,9,10,11,12,13]. The BTB (Bric-a-Brac/Tramtrack/Broad Complex) domain, also known as POZ (pox virus and zinc finger), is a conserved sequence of about 115 amino acids [14]. This domain was first discovered in the Bric-a-Brac, Tramtrack, and Broad Complex proteins of fruit flies (Drosophila melanogaster) [15].
In plants, proteins containing the BTB domain can participate in transcriptional inhibition, protein ubiquitination degradation, and signal transduction of plant hormones and play a critical role in stress and disease resistance [16,17]. In apples, the BTB family protein, MdBT2, promotes the ubiquitination and degradation of MdARF8 through the 26S proteasome pathway, negatively regulating the expression of MdGH3.1 and MdGH3.6, thereby inhibiting the formation of lateral roots [18]. Previous studies have shown that ETO1, EOL1, and EOL2 in the BTB family of Arabidopsis thaliana can regulate ethylene synthesis [19]. Systemic acquired resistance (SAR) in plants is mediated by the Arabidopsis thaliana BTB gene NPR1, which functions as a salicylic acid (SA) receptor [20,21]; when it is overexpressed in plants, resistance to biotic and abiotic stresses will be enhanced. As a homolog of NPR1, OsNPR1/NH1 contributes to rice’s resistance to Xanthomonas oryzae pv-caused bacterial blight [22]. In transgenic tomatoes, AtNPR1 overexpression exhibits broad-spectrum resistance to pathogens [20]. Research has shown that the SA receptors, NPR1 and NPR3/NPR4, in Arabidopsis thaliana have opposing functions in the transcriptional regulation of plant immunity [23]. Furthermore, the expressions of AtNPR1 in tomatoes and wheat enhances the resilience of the tomato plant to bacterial and fungal infections and the defense response against Fusarium head blight (FHB), respectively [20,24]. Similarly, GmBTB/POZ in soybean also plays a role in the plant response to Phytophthora soybean; its overexpression in this plant enhances its resistance to pathogen infection [25]. In addition, cold, drought, and salt stress control the expression of the cucumber BTB gene, which displays various patterns of expression in cucumber tissues [26]. These findings suggest that plant BTB genes regulate biotic and abiotic stress responses.
Paulownia is an important deciduous tree because of its fast growth and excellent wood, and it often is used in construction and making furniture and musical instruments and helps alleviate the shortage of wood. Its flowers, leaves, fruits, and bark can be used as medicine, as it has a high medicinal value. Paulownia has strong adaptability and resistance to improve the soil and ecological environment and manage saline and alkaline land [27]. Nevertheless, paulownias are susceptible to phytoplasma infection, leading to the occurrence of Paulownia witches’ broom (PaWB). Due to susceptibility to phytoplasma infection, the resulting symptoms include axillary bud clusters, plant dwarfing, and yellow flowers on leaves, causing slow growth or even death of large trees, which seriously affects the yield and economic benefits of paulownias [28]. This infection causes the plant to have overgrown axillary buds and dwarfism, which increase tree mortality, slow tree growth, and seriously affect the growth of Paulownia production. Therefore, it is important to identify the relevant genes against the occurrence of PaWB in Paulownia. At present, studies on the molecular mechanism of the pathogenesis of PaWB have made great progress [27,29,30,31]. The genomes of both Paulownia fortunei and PaWB phytoplasma have been decoded [27]. Despite the former, the members of the PfBTB gene family in this species have not yet been reported. Since the BTB family plays a crucial role in biological stress responses, it is very important to identify it. Based on the P. fortunei genome sequence, we can better understand the possible functions of BTB genes in PaWB. In this study, we adopt a bioinformatics approach to identify members of the BTB gene family on a genome-wide scale, investigating the physico-chemical properties, phylogenetic relationships, genetic structure, and cis-regulatory element and their expressions when stressed by biotic and abiotic factors and hormonal treatments. Altogether, our research provides the candidate BTB genes involved in PaWB response and stress-related pathways, providing a foundation for the genetic breeding of paulownias. At the same time, it provides new ideas and directions for the prevention and control of PaWB.
2. Results
2.1. Understanding the PfBTB Gene Family and Examining the Properties of Proteins
There were 62 genes found in the P. fortunei genome that were possible BTB gene family members. Then, we named these identified PfBTB genes according to the chromosomal location (Table 1). The PfBTB gene family has a molecular weight (MW) of 24.88 to 115.07 kDa (Kilodalton) and an isoelectric point (pI) of 4.64 to 9.52. Cell-PLoc 2.0 predicted the subcellular location of PfBTBs, and it was determined that the majority of PfBTBs were located in the nucleus (Table 1).

Table 1.
Information on the P. fortunei BTB gene family.
2.2. Analysis of the Phylogenetic Tree of Members of the PfBTB Gene Family
To examine the evolutionary relations of the PfBTB gene family, we manufactured a phylogenetic tree (NJ, neighbor-joining) using the MEGA-X 10.2 tool based on the BTB proteins with P. fortunei (62 members) and Arabidopsis thaliana (80 members) (Figure 1). The phylogenetic tree’s findings indicate that 142 members of the BTB genes from the 2 species were gathered into 9 sub-groups, containing Ankyrin, MATH, Armadillo, BTB-only, BACK, TAZ, TPR, NPH3, and other. The number of BTB proteins between P. fortunei and Arabidopsis thaliana varied greatly in the same subfamily. The NPH3 subfamily has the largest number, including 21 Paulownia BTB proteins and 32 Arabidopsis thaliana BTB proteins (Figure 1).
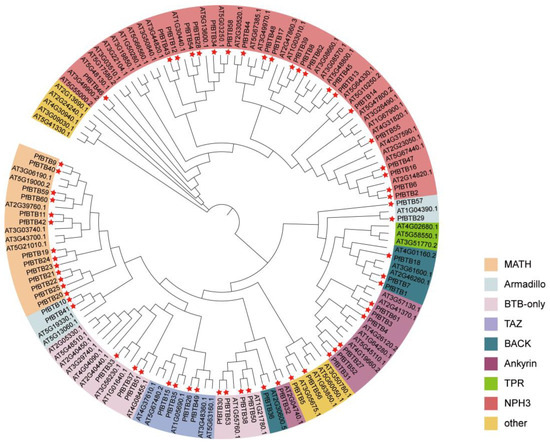
Figure 1.
The phylogenetic tree of the PfBTB gene family. A phylogenetic tree of the BTB proteins of the two species, P. fortunei and Arabidopsis thaliana. MEGA-X 10.2 software was used to build the phylogenetic tree using 1000 bootstraps. The red pentagon star is used to represent the BTB protein in P. fortunei. Two species contain all 142 BTB proteins that were clustered into 9 subgroups, named MATH, Armadillo, BTB-only, TAZ, BACK, Ankyrin, TPR, NPH3, and other.
2.3. Gene Structure and Conserved Motif Analysis
To further substantiate the evolutionary tree’s classification findings, we looked at the conserved motifs and gene architectures of P. fortunei’s PfBTB genes. The MEME suite (http://meme-suite.org/tools/meme (accessed on 13 September 2022)) was utilized to estimate 20 motifs, and we designated motifs 1 through 20 (Figure 2). Among them, Motif 2 was found among all clusters, signifying that it is highly conserved in all PfBTB proteins. According to the predicition analysis on the SMART website, it was found that the BTB domain is composed of Motif 1, 3 and 5. This result provides evidence of our accuracy in studying the BTB gene.
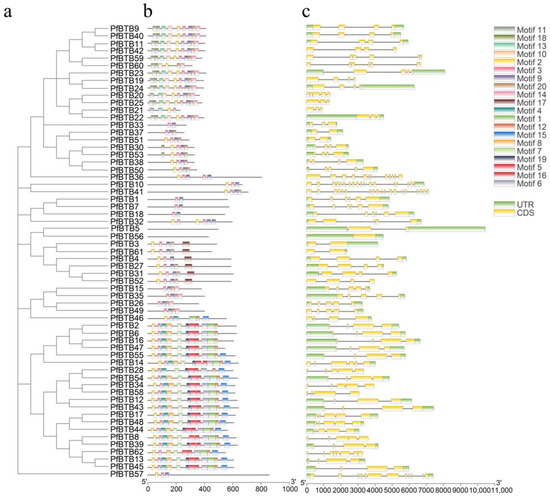
Figure 2.
The investigation of the conserved motifs of the P. fortunei BTB gene family’s genes. (a) Phylogenetic tree constructed using 62 PfBTB genes and neighbor-joining (NJ); (b) Examination of P. fortunei’s BTB genes’ conserved motifs. The MEME tool generated a total of 20 motifs, designated Motif 1–20. 200 aa is indicated by the scale bar; (c) Analysis of the UTR, intron, and exon regions in P. fortunei’s BTB genes. UTR is represented by the green rectangles, Exon by the yellow rectangles, and Intron by the grey lines. The scale bar shows a value of 2 kb.
To investigate the PfBTB gene structures of P. fortunei, from the P. fortunei database, we retrieved the exon–intron information for 62 PfBTB genes. Based on this information, the TBtools software (v. 2.019) was used to display these structures (Figure 2). The exon counts in PfBTB genes varied widely, ranging from 1 to 19. PfBTB41 has the most exons (19) out of the 62 BTB genes in Paulownias, while 26 BTB genes have four exons (41.93%).
Additionally, the exon and intron lengths were different. A total of 49 BTB genes were discovered to contain untranslated regions (UTR). Similar gene architectures among the BTB genes were clustered into one subclade, mirroring the results of the motif analysis. This finding demonstrates the possibility of the same groups sharing genomic architecture and conserved motifs across group members. These findings offer compelling proof that the results of the phylogenetic tree classification are accurate.
2.4. PfBTB Gene Locations on the Chromosome and Homologous Gene Analysis
TBtools (v. 2.019) was used to anticipate the PfBTB genome’s chromosomal distribution pattern (Figure 3a). Paulownias were used to derive the BTB gene’s location information. The results showed that no members of this gene family were mapped to chromosomes 1, 17, or 20, and that 57 PfBTB genes (91.93%) were unevenly distributed among the 20 Paulownias chromosomes. Therefore, we did not show them in our study. In addition, five other genes that were situated on scaffold contigs were likewise not displayed. Eight BTB genes were found on chromosome 8, with seven genes on chromosome 12 coming in second. Six BTB genes were located on chromosomes 5 and 9. There were four BTB genes on each of chromosomes 6 and 11. On chromosomes 2, 3, 4, 10, 13, 15, 16, and 18, two or three BTB genes were located. Chromosomes 7, 14, and 19 contained a single gene. Using the MCScanX program, we also located the homologous genes of the BTB gene family. The results show that the BTB gene family of paulownias, which has 37 identical genes, contained 24 homologous gene pairs. Between chromosomes 9 and 16, there were three homologous gene pairs (Figure 3b).
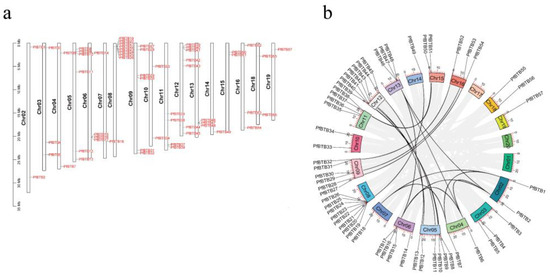
Figure 3.
The distribution of BTB genes in P. fortunei’s genome based on location and synteny analysis. (a) The location of PfBTBs on each of the 20 P. fortunei chromosomes. Chromosomes 1 and 20 do not contain any BTB genes, hence we did not display them in Figure 3.; (b) The BTB gene family’s distribution pattern synteny study. The synteny gene pairs of the BTB gene family are shown by the black lines.
2.5. Cis-Acting Element Prediction of PfBTB Genes
Cis-acting elements are necessary clues in predicting gene functions. By attaching to the cis-acting component of target genes in particular organic processes, transcription elements may affect the level of gene expression [32]. In addition, to further study the function of the PfBTBs, we used the Plant CARE database to predict the cis-acting elements of the promoter regions. As a consequence, 62 cis-acting elements were recognized, of which we chose 12 interesting ones for more research. These are related to stress, hormones, and plant growth and development. According to Figure 4a, numerous distribution patterns of cis-acting elements were discovered in the promoter region of PfBTB genes, demonstrating the importance of the BTB gene family of Paulownia in a range of biological processes. In the meanwhile, we discovered that all PfBTB genes contain the cis-acting elements associated with the regulation of hormones, including responsiveness factors to salicylic acid (SA), gibberellin (GA), auxin (IAA), and methyl jasmonate (MeJA). The ABA-responsive element, or ABRE, is one of the most crucial cis-acting elements in the promoter sequence that controls the expression of the ABA-inducible gene in response to ABA therapy [33]. In total, 37 PfBTB genes were identified in our analysis as ABA responsiveness factors, indicating that the BTB gene family may be involved in ABA signal transduction (Figure 4b). Additionally, 30 PfBTB genes were found to contain cold-related cis-acting regions, suggesting that they might have particular resistance under low-temperature treatment.
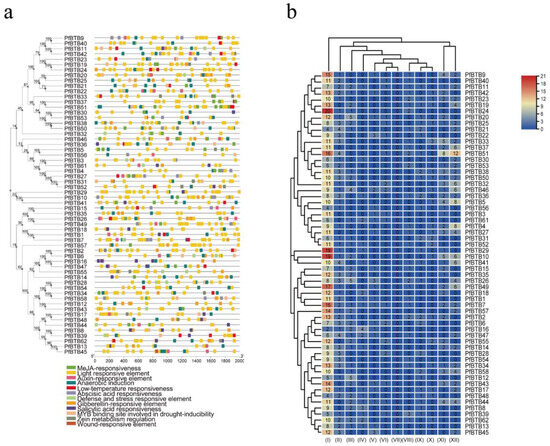
Figure 4.
Analysis of the potential promoter of PfBTB genes’ cis-acting elements. (a) There are 12 cis-acting elements in the putative PfBTB gene promoter. The color scale at the top right indicates the number of cis-acting components. The color represents the wide range of cis-acting influences on BTB members. There are 12 cis-acting components, including the following: (I) light responsive element; (II) anaerobic induction; (III) gibberellin-responsive element; (IV) salicylic acid responsiveness; (V) auxin-responsive element; (VI) defense and stress responsive element; (VII) wound-responsive element; (VIII) low-temperature responsiveness; (IX) MYB binding site involved in drought-inducibility; (X) zein metabolism regulation; (XI) abscisic acid responsiveness; (XII) MeJA-responsiveness. (b) The distribution pattern of 12 cis-acting regions of the BTB gene family’s putative promoter in Paulownia.
2.6. PfBTB Genes Response to Phytoplasma and Expression Patterns in Different Tissues/Organs
PaWB infection is one of the main causes of P. fortunei mortality [27]. Studies have revealed that the BTB gene has a significant defensive role against biological stress. To identify key disease resistance genes, we examined the amounts of BTB gene transcription in PaWB-infected P. fortunei (PFI) and healthy P. fortunei (PF). From the transcriptome determined in the laboratory, we found that 7 of the 62 PfBTB genes are significantly different in PF/PFI, where PfBTB12, PfBTB14, and PfBTB44 were significantly upregulated and PfBTB3, PfBTB16, PfBTB19, and PfBTB36 were significantly downregulated.
Seven PfBTB genes were chosen for an RT-qPCR experiment to look at the expression levels in two separate P. fortunei samples in order to confirm the validity of the transcriptome sequencing analysis. As shown in Figure 5b, PfBTB12, PfBTB14, and PfBTB44 were significantly upregulated and PfBTB3, PfBTB16, PfBTB19, and PfBTB36 were significantly downregulated. Therefore, we hypothesize that these genes might have a significant impact on PaWB.
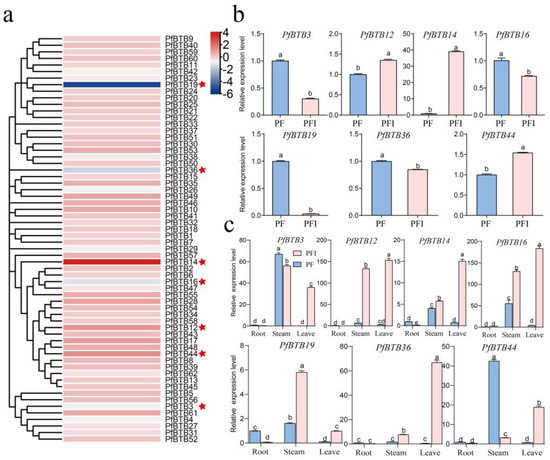
Figure 5.
Expression levels of BTB gene family members of Paulownia foutunei in healthy and phytoplasma-infected seedlings. (a) Heat maps are plotted using log2RPKM and 0 to 1 scale methods to visualize differences in expression levels. Red star indicates seven genes with significant differences in PF/PFI. (b) Seven differential genes were picked for an RT-qPCR to validate transcriptome data correctness. (c) PfBTB genes’ relative expression in the stems, roots, and leaves in PF and PFI. Values are presented as the averages ± SE of three biological replicates. Each gene’s distinct letters indicate significant differences (p < 0.05). PF: healthy seedlings of P. fortunei. PFI: P. fortunei seedlings infected by PaWB phytoplasma.
To study the roles of seven BTB genes in P. fortunei growth and development, we examined the expression levels of the different developmental tissues/organs. As Figure 5c illustrates, we found that the transcripts of these genes are expressed in all tissues, with PfBTB12 showing high levels of expression in the stem and leaves of PFI, while other genes are highly expressed only in the leaves. According to the findings, the seven genes may be essential for the growth and development of P. fortunei.
2.7. Expression Patterns with Different Stress Treatments
To further confirm whether the PfBTB gene expression is affected by abiotic stress and hormone treatment, the expression profile of PfBTB under such conditions was analyzed using an RT-qPCR. We comprehensively analyzed the expression levels of PfBTBs during drought stress using transcriptome sequencing data. After drought treatment, PfBTB12 and PfBTB21 were upregulated, and PfBTB7/14/17/19/48 was 7- to 11-fold-lower than the control, indicating that PfBTB7/12/14/17/19/21/48 is a drought-sensitive gene (Figure 6a). We subsequently randomly selected four genes to verify the transcriptome data, and they all fit the transcriptome trend (Figure 6b). In addition, our further analysis of the evolutionary tree of the PfBTB gene family and the homologs of NPR1 compared the six PfBTB3/4/27/31/52/61 genes plus the seven genes that were significantly different in PF/PFI using an RT-qPCR. The outcomes demonstrated that these gene expressions were upregulated after treatment with exogenous SA (Figure 6c). The transcriptome sequencing analysis indicated that the PfBTB expression was affected by drought stress, and differences in PfBTB expression levels suggest that they may additionally have one-of-a-kind features in response to drought stress.
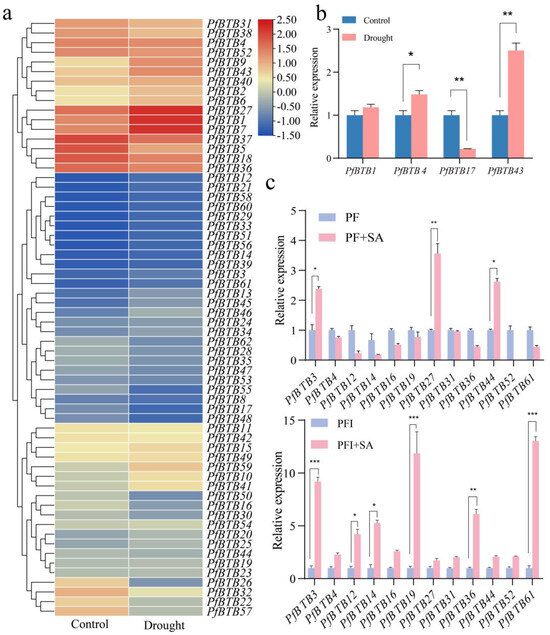
Figure 6.
Expression analysis of PfBTBs under abiotic stress and hormone treatment. (a) Heatmap of PfBTB genes expressions in response to drought. The color scale represents the RPKM values normalized by log2(RPKM + 1). Red color represents high expression, while blue represents low expression. (b) RT-qPCR of PfBTBs genes expression in response to drought. (c) RT-qPCR of PfBTBs genes expression in response to SA treatment. The bars display the mean and standard error (n = 3). (* p < 0.05, ** p < 0.01 and *** p < 0.001).
2.8. Association Analysis of PfBTB and miRNA
Using the online tool, psRNATarget, we predicted targeting relationships between the pfmiRNA sequence and the 62 members of the PfBTB family (Table S2) and demonstrated targeting pairs with anticipated values less than four (Figure 7a). This allowed us to confirm the regulatory role of pfmiRNA in PfBTB expression. The findings revealed 95 targeting relationships with expected values less than 4, including 42 members of the PfBTB family and 74 pfmiRNAs. The findings also revealed that the same pfmiRNA could target more than one PfBTB and that the same PfBTB could be controlled by two or more pfmiRNAs. The pf-miR166 family, which has 12 pairs of targeting relationships and 12 members, is the largest family of pfmiRNAs in this range. Ten of the twelve members of the family can simultaneously target the same PfBTB16. Only eight pairs of these targeting relationships are controlled by transcriptional repression, while the remaining relationships all block PfBTB translation into proteins via the breaking mode. The data from the PF and PFI seedlings shoots were included in the analysis of the associated pfmiRNAs (Figure 7b). The outcomes demonstrated that the expression trends of various pfmiRNAs regulating PfBTB16 in the seedlings, pf-miR166c/pf-miR166e/pf-miR166u/pf-miR166f, were inconsistent with each other, whereas pf-miR166a/pf-miR166d/pf-miR166h/pf-miR44 had a downward trend. This suggests that the pfmiR166 family has synergistic and functional interactions and plays a significant role in controlling PfBTB expression.
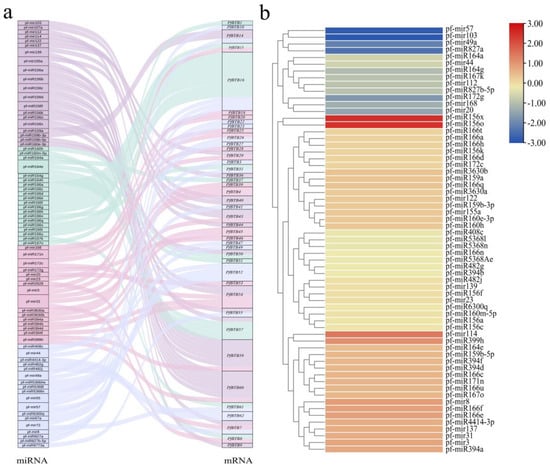
Figure 7.
Prediction of the targeting relationship between PfBTBs and miRNAs and analysis of the expression of miRNAs under PaWB infection. (a) Sankey diagrams demonstrate the targeted binding relationship between PfBTBs and miRNAs. (b) Heatmap of miRNAs expression in response to PaWB infection. The depth of color represents the changes of the value of log2 (PFI/PF), and red represents upregulation while blue represents downregulation. PF: healthy seedlings of P. fortunei. PFI: P. fortunei seedlings infected by PaWB phytoplasma.
3. Discussion
The number of BTB family members in plants varies greatly among different species: 69 members in grape [34], 49 members in sugar beet [35], 38 members in tomato [5], and 158 members in rice [4]. Using the recent determination of the P. fortunei genome, we identified 62 BTB members for the first time in Paulownia (Table 1). These differences in the number of BTB genes correlate with the size of the genomes of different plants, and it is likely that BTB genes have changed during the long-term evolution of plants.
In order to classify the functional genes of the BTB family in P. fortunei, this research used a cluster analysis of the 62 members of P. fortunei together with the 80 members of Arabidopsis thaliana. Based on the BTB domain together with other conserved domains, 62 PfBTB members were classified into eight subfamilies, compared with the Arabidopsis thaliana BTB subfamily classification, P. fortunei lacks the TPR subfamily (Figure 1). We also found that the high degree of conservation of BTB family members allows genes localized in the same subfamily to have the same or similar functions. For example, the Ankyrin subfamily can respond to drought, salt stress, and ABA treatment, and PfBTB3/4/31/32/27/52/61 in P. fortunei belongs to the same subfamily, so we infer that these genes may be involved in similar plant stress tolerance processes. In addition, members of the MATH subfamily have been reported to be involved in plant resistance to biotic stress processes, such as CaBPM4 (CaBTB51) of the MATH subfamily of pepper, which is highly regulated under the Phytophthora capsici infection [36]. These studies suggest that within the different subfamilies of BTB, each subfamily member is involved in plant-specific biological processes.
In terms of gene structure, research has shown that gene structure (intron–exon structure) is a typical marker of gene family evolution. In this article, we found that the exon count of PfBTB varies greatly, ranging from 1 to 19. Among the 62 PfBTB genes, PfBTB41 in the Armadillo subfamily has the most exons (19), while PfBTB56 in the other subfamily only has one exon (Figure 2). We observed very different patterns of intron–exon structure distributions in tomato [5], grape [34], sugar beet [35], and rice [4] from those of the BTB members we studied in Paulownia. Thus, the differences and diversity in these structures emphasize that the evolutionary patterns between different species are complex and diverse. This gives rise to specific genomic features and regulatory mechanisms for genes.
As is well known, cis-acting elements are sequences that exist on gene promoters and play a crucial role in the transcriptional regulation of genes [37]. The analysis of the cis- acting elements of the PfBTB genes promoter revealed the presence of light-responsive elements, stress-responsive elements, and hormone-responsive elements (Figure 4). Among them, light-responsive elements are ubiquitous and most abundant, which is consistent with the analysis results of different species of grape [34], sugar beet [35], and tomato [5], indicating that the transcriptional regulation of PfBTB may be generally affected by light signals. Meanwhile, hormone-responsive elements, such as SA, GA, IAA, MeJA, and ABA, were also contained on the PfBTB gene promoter. We also found that 20 PfBTBs contained SA-responsive elements in the promoter region, among which members of the NPH3 subfamily were the most numerous, suggesting that NPH3 members may be involved in the transduction process of SA signals. In addition, some cold-related, defense and stress-responsive, and wound-responsive elements were also found in the promoter region of PfBTBs. These results suggest that PfBTB genes may be involved in a variety of biological processes during the growth and development of paulownias.
In addition, previous reports have shown that the BTB protein can promote plant resistance to various abiotic and biological stresses [38]. This protein affects the synthesis of related hormones in plants by participating in the Cul3 ubiquitin pathway, thereby regulating the growth, development, and stress resistance response of plants [38]. Numerous plant BTB/POZ protein family members control hormone-mediated processes and phytologically related signal network reactions, such as JA [39], SA [23,40], ABA [41,42], and GA3 [43,44], thereby regulating a series of physiological and biochemical processes including the germination of plants and metabolism, growth, and development.
Based on the RNA-seq and RT-qPCR analyses of PF and PFI, we found that the expression of some PfBTB genes in P. fortunei was significantly influenced by PaWB (Figure 5). The expression level of PfBTB12/14/44 increased during the formation of PaWB by P. fortunei, while the expression level of PfBTB3/16/19/36 decreased. These findings indicate that the BTB family was responsive to PaWB phytoplasma infection. We know that plants synthesize a large amount of SA when infected by pathogens, and the accumulation of SA can activate the accumulation of the BTB member NPR1 in the nucleus, thereby regulating the expression of downstream defense-related genes [45,46]. Thus, we performed an RT-qPCR on the 12 selected genes, and the results showed that they were induced to be expressed in SA-treated infected seedlings. Among them, PfBTB3/12/14/19/36 genes were significantly expressed, indicating that these genes might be crucial for the process of defense.
Moreover, BTB genes are crucial for plant defense against abiotic stress. In P. fortunei under drought stress, many PfBTBs were stress response groups; in particular, PfBTB12/14/19 genes were sensitive to both biotic and abiotic pressure (Figure 6a,b). MicroRNAs (miRNAs), a class of small non-coding RNAs, play significant roles in biotic and abiotic stressors by controlling their target genes [47]. According to studies on the targeting relationship between miRNAs and BTB genes, miRNA families are linked to development and stress target BTB genes [34]. By comparing the miRNA and transcriptome before and after PaWB, 74 pf-miRNAs targeting 42 PfBTBs were found. We found that PfBTB14, their target gene, was increased in diseased seedlings, while pf-miRNA159a and pf-miRNA159b-3p were downregulated in diseased seedlings [48]. Infected seedlings had increased pf-miRNA114 expression, and its PfBTB44 target gene had increased expression as well. Therefore, we speculate that pf-miRNA114-mediated PfBTB44 may play an important role in PaWB.
4. Materials and Methods
4.1. Plant Materials and Treatment
Tissue seedlings of PaWB-infected P. fortunei (PFI) and healthy P. fortunei (PF) were cultivated for 30 days as this study’s materials. The culture of the materials was conducted according to the method by FAN et al. [49]. For hormone treatment, the PFI seedlings were treated by adding 0.2 mM SA to the medium without SA as a control. For drought treatment seedlings (PF), the soil water content was 25%, the soil water content was 75% as control, and the second round of leaves were collected for 30 days during the drought stress. The samples were collected and stored in −80 °C.
4.2. Identification of BTB Gene Family Members in P. fortunei’s Genome
To discover the workable members of the BTB gene, we first downloaded the P. fortunei genome from NCBI (https://www.ncbi.nlm.nih.gov/ (accessed on 27 June 2022)). Then, using the HMM search software (v. 3.1), we searched for the putative BTB genes in the P. fortunei protein database using the seed file for the BTB domain (PF00651). To check the accuracy of the BTB conserved domain, all candidate proteins were then uploaded to the SMART website (http://smart.embl-heidelberg.de/ (accessed on 29 June 2022)). Additionally, the PfBTB proteins’ pI and MW were determined using ExPASy ProtParam (accessed on 13 July 2022), an online analysis tool. Furthermore, we investigated the subcellular locations of PfBTBs using the WoLF PSORT website (https://www.genscript.com/wolf-psort.html (accessed on 23 July 2022)).
4.3. PfBTB Proteins Underwent Phylogenetic Analysis
The BTB protein sequences of Arabidopsis thaliana were downloaded from TAIR (https://www.Arabidopsis.org/browse/genefamily/pub.jsp (accessed on 27 July 2022)). All BTB protein sequences of P. fortunei and Arabidopsis were compared using ClustalW. MEGA-X 10.2 (Method, NJ; Bootstrap, 1000) was then used to build the phylogenetic tree. Finally, the evolutionary tree of the BTB proteins was modified using the iTOL online website, (https://itol.embl.de/ (accessed on 30 August 2022)).
4.4. Analysis of Gene Structures, Motifs, and Cis-Acting Elements
Gene structure data was taken from the P. fortunei whole genome database in order to identify and represent the introns, exons, and UTR structural organization of the PfBTB gene family. The unique conserved motifs of PfBTB genes were discovered using the MEME suite (http://meme-suite.org/tools/meme (accessed on 13 September 2022)). For the analysis, 20 motifs were used in total, with a maximum of 200 amino acids for motif width. TBtools (v. 2.019) was used to visualize the results of the gene structure and conserved motif analyses [50].
The 2000 bp upstream location of PfBTBs was once described as a putative promoter sequence. We obtained such sequences of PfBTBs using TBtools (v. 2.019). Cis-acting factors of the putative promoter location of these genes were estimated using PlantCare (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 27 September 2022)) [51]. According to the characteristic annotations of the cis-acting elements, the intriguing portions have been saved for later research, and the cis-acting element using equal functional annotations were built-in into the equal group.
4.5. Chromosomal Localization and Synteny Analysis
PfBTB homologous gene pairs were discovered using an all-vs-all blast method and blast software. The synteny areas were then determined by MCScanX program utilizing the all-vs-all blast results. To display the distribution of synteny gene pairs, we plotted circus images. TBtools (v. 2.019) carried out the analysis of the chromosomal locations [50].
4.6. Prediction of the miRNA and PfBTB Targeting Relationship
Using the psRNATarget online software (https://www.zhaolab.org/psRNATarget/ (accessed on 15 March 2023)), the regulatory link between PfBTB and pfmiRNA was predicted with a mismatch value of one, an expectation value less than or equal to four, and other analytic parameters set to the system default [52]. Tbtools (v. 2.019) was used to evaluate and create graphs from the transcriptome data of all predicted pfmiRNAs in sick seedlings.
4.7. Investigation of PfBTB Expression Patterns
The RNA-seq data under different treatments of P. fortunei were obtained from our previous study [27]. We downloaded these data from NCBI (accession number: SRR11787938, SRR11787927, SRR11787916, SRR11787905, SRR11787894, SRR11787883, PRJNA221355) [53]. Then, heat maps were plotted using TBtools (v. 2.019).
The PF and PFI samples’ total RNA was extracted using a total RNA extraction kit. (Tiangen Biotech Co., Beijing, China). Then, the RNA was reverse-transcribed into cDNA using the PrimeScriptTM RT reagent kit (GenStar, Beijing, China). A quantitative real-time PCR (RT-qPCR) analysis was undertaken using a 2×RealStar Fast SYBR qPCR Mix (Low ROX) (GenStar, Beijing, China). With the aid of the Primer 5.0 software, we created fifteen pairs of precise primers, which were then validated using NCBI (Table S1). The relative expression of genes was determined using the 2−∆∆CT technique with actin as an internal reference.
4.8. Statistical Analysis
All results were collected from three parallel experiments. By using the analysis of variance (ANOVA) and Duncan’s multiple range test with significant differences (p < 0.05), data were compared with the control group or between treatments. For the Student’s t test, * p < 0.05; ** p < 0.01, and *** p < 0.001. Graphs were plotted using GraphPad Prism 8.0.
5. Conclusions
We found 62 PfBTB members of the P. fortunei genome in this investigation. Based on the phylogenetic tree analysis, nine groups of this species’ BTB gene family were discovered. Analyses of conserved motifs and gene structures offered compelling support for the classification outcomes obtained from the phylogenetic trees. The cis-acting element analysis revealed that the BTB family genes contain various sensitive elements that allow P. fortunei to respond to biotic and abiotic factors. The transcriptional sequencing data analysis revealed different expression levels of the PfBTB gene. Candidate genes implicated in biotic and abiotic stressors were subsequently identified using an RT-qPCR. Our analysis provides a solid foundation for future studies on the molecular mechanisms of PfBTB genes in response to biotic and abiotic stresses.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants12244144/s1, Table S1: Primer sequence for RT-qPCR. Table S2: Prediction of targeting relationship between pfmiRNA and PfBTB.
Author Contributions
G.F. conceived and designed the experiments; P.Z. and Y.F. performed the experiments and wrote the paper; P.X. contributed reagents and analyzed the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Academic Scientist Fund for Zhongyuan Scholars of Henan Province (grant 2018 [99]), the 73rd batch of China Postdoctoral Science Foundation (2023M730989) and 2022 Postdoctoral research grant from Henan Province (HN2022129).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We appreciate the reviewers’ and editors’ diligent reading of and constructive criticism on this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Figueroa, P.; Gusmaroli, G.; Serino, G.; Habashi, J.; Ma, L.; Shen, Y.; Feng, S.; Bostick, M.; Callis, J.; Hellmann, H.; et al. Arabidopsis has two redundant Cullin3 proteins that are essential for embryo development and that interact with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligase complexes in vivo. Plant Cell 2005, 17, 1180–1195. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.; Bernhardt, A.; Dieterle, M.; Hano, P.; Mutlu, A.; Estelle, M.; Genschik, P.; Hellmann, H. Arabidopsis AtCUL3a and AtCUL3b form complexes with members of the BTB/POZ-MATH protein family. Plant Physiol. 2005, 137, 83–93. [Google Scholar] [CrossRef]
- Weber, H.; Hellmann, H. Arabidopsis thaliana BTB/ POZ-MATH proteins interact with members of the ERF/AP2 transcription factor family. FEBS J. 2009, 276, 6624–6635. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, D.J.; Hanada, K.; Shiu, S.H.; Vierstra, R.D. Large-scale, lineage-specific expansion of a bric-a-brac/tramtrack/broad complex ubiquitin-ligase gene family in rice. Plant Cell 2007, 19, 2329–2348. [Google Scholar] [CrossRef]
- Li, J.; Su, X.; Wang, Y.; Yang, W.; Pan, Y.; Su, C.; Zhang, X. Genome-wide identification and expression analysis of the BTB domain-containing protein gene family in tomato. Genes Genom. 2018, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Juranič, M.; Srilunchang, K.O.; Krohn, N.G.; Leljak-Levanic, D.; Sprunck, S.; Dresselhaus, T. Germline-specific MATH-BTB substrate adaptor MAB1 regulates spindle length and nuclei identity in maize. Plant Cell 2012, 24, 4974–4991. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Lian, X.; Cheng, J.; Zeng, W.; Zheng, X.; Wang, W.; Ye, X.; Li, J.; Li, Z.; Zhang, L.; et al. Genome-wide identification and transcriptome profiling reveal that E3 ubiquitin ligase genes relevant to ethylene, auxin and abscisic acid are differentially expressed in the fruits of melting flesh and stony hard peach varieties. BMC Genom. 2019, 20, 892. [Google Scholar] [CrossRef]
- Adams, J.; Kelso, R.; Cooley, L. The kelch repeat superfamily of proteins: Propellers of cell function. Trends Cell Biol. 2000, 10, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Puccetti, E.; Sennewald, B.; Fosca-Ferrara, F.; Boehrer, S.; Bianchini, A.; Hoelzer, D.; Ottmann, O.G.; Nervi, C.; Ruthardt, M. Down-stream regions of the POZ-domain influence the interaction of the t(11;17)-associated PLZF/RARalpha fusion protein with the histone-deacetylase recruiting co-repressor complex. Hematol. J. 2001, 2, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Rivero, F.; Dislich, H.; Glockner, G.; Noegel, A.A. The Dictyostelium discoideum family of Rho-related proteins. Nucleic Acids Res. 2001, 29, 1068–1079. [Google Scholar] [CrossRef]
- Stogios, P.J.; Prive, G.G. The BACK domain in BTB-kelch proteins. Trends Biochem. Sci. 2004, 29, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Salas-Vidal, E.; Meijer, A.H.; Cheng, X.; Spaink, H.P. Genomic annotation and expression analysis of the zebrafish Rho small GTPase family during development and bacterial infection. Genomics 2005, 86, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Downs, G.S.; Jauhal, J.J.S.; Nandra, S.K.; Prive, G.G. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005, 6, R82. [Google Scholar] [CrossRef] [PubMed]
- DiBello, P.R.; Withers, D.A.; Bayer, C.A.; Fristrom, J.W.; Guild, G.M. The Drosophila Broad-Complex encodes a family of related proteins containing zinc fingers. Genetics 1991, 129, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Zollman, S.; Godt, D.; Privé, G.G.; Couderc, J.L.; Laski, F.A. The BTB domain, found primarily in zinc finger proteins, defines an evolutionarily conserved family that includes several developmentally regulated genes in Drosophila. Proc. Natl. Acad. Sci. USA 1994, 91, 10717–10721. [Google Scholar] [CrossRef]
- Bomont, P.; Cavalier, L.; Blondeau, F.; Ben Hamida, C.; Belal, S.; Tazir, M.; Demir, E.; Topaloglu, H.; Korinthenberg, R.; Tüysüz, B.; et al. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat. Genet. 2000, 26, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Stone, J.R.; Williams, A.J. All in the family: The BTB/POZ, KRAB, and SCAN domains. Mol. Cell. Biol. 2001, 21, 3609–3615. [Google Scholar] [CrossRef]
- Ji, X.L.; Li, H.L.; Qiao, Z.W.; Zhang, J.C.; Sun, W.J.; You, C.X.; Hao, Y.J.; Wang, X.F. The BTB protein MdBT2 recruits auxin signaling components to regulate adventitious root formation in apple. Plant Physiol. 2022, 189, 1005–1020. [Google Scholar] [CrossRef]
- Christians, M.J.; Gingerich, D.J.; Hansen, M.; Binder, B.M.; Kieber, J.J.; Vierstra, R.D. The BTB ubiquitin ligases ETO1, EOL1 and EOL2 act collectively to regulate ethylene biosynthesis in Arabidopsis by controlling type-2 ACC synthase levels. Plant J. 2009, 57, 332–345. [Google Scholar] [CrossRef]
- Lin, W.C.; Lu, C.F.; Wu, J.W.; Cheng, M.L.; Lin, Y.M.; Yang, N.S.; Black, L.; Green, S.K.; Wang, J.F.; Cheng, C.P. Transgenic tomato plants expressing the Arabidopsis NPR1 gene display enhanced resistance to a spectrum of fungal and bacterial diseases. Transgenic Res. 2004, 13, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.X.; Cao, L.; Li, J.; Duan, C.J.; Luo, X.M.; Le, N.; Wei, H.; Liang, S.; Chu, C.; Pan, Q. Involvement of OsNPR1/NH1 in rice basal resistance to blast fungus Magnaporthe oryzae. Eur. J. Plant Pathol. 2011, 131, 221–235. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhong, S.; Li, Q.; Zhu, Z.; Lou, Y.; Wang, L.; Wang, J.; Wang, M.; Li, Q.; Yang, D.; et al. Functional analysis of rice NPR1-like genes reveals that OsNPR1/NH1 is the rice orthologue conferring disease resistance with enhanced herbivore susceptibility. Plant Biotechnol. J. 2007, 5, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467.e1415. [Google Scholar] [CrossRef] [PubMed]
- Makandar, R.; Essig, J.S.; Schapaugh, M.A.; Trick, H.N.; Shah, J. Genetically engineered resistance to Fusarium head blight in wheat by expression of Arabidopsis NPR1. Mol. Plant-Microbe Interact. MPMI 2006, 19, 123–129. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Q.; Wang, H.; Gao, H.; Fang, X.; Chen, X.; Zhao, M.; Wei, W.; Song, B.; Liu, S.; et al. GmBTB/POZ promotes the ubiquitination and degradation of LHP1 to regulate the response of soybean to Phytophthora sojae. Commun. Biol. 2021, 4, 372. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, G.; Zhang, L.; Xu, J.; Liu, S. Comprehensive genomic analysis and expression profiling of the BTB and TAZ (BT) genes in cucumber (Cucumis sativus L.). Czech J. Genet. Plant Breed. 2019, 56, 15–23. [Google Scholar] [CrossRef]
- Cao, Y.B.; Sun, G.L.; Zhai, X.Q.; Xu, P.L.; Ma, L.M.; Deng, M.J.; Zhao, Z.L.; Yang, H.B.; Dong, Y.P.; Shang, Z.H.; et al. Genomic insights into the fast growth of paulownias and the formation of Paulownia witches’ broom. Mol. Plant 2021, 14, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.N.; Wu, Y.F.; Shi, Y.Z.; Wu, K.K.; Li, Y.R. First Report of Paulownia Witches’-Broom Phytoplasma in China. Plant Dis. 2008, 92, 1134. [Google Scholar] [CrossRef]
- Yang, H.; Zhai, X.; Zhao, Z.; Fan, G. Comprehensive analyses of the SPL transcription factor family in Paulownia fortunei and their responses to biotic and abiotic stresses. Int. J. Biol. Macromol. 2023, 226, 1261–1272. [Google Scholar] [CrossRef]
- Zhao, X.; Li, B.; Zhai, X.; Liu, H.; Deng, M.; Fan, G. Genome-Wide Analysis of Specific PfR2R3-MYB Genes Related to Paulownia Witches’ Broom. Genes 2022, 14, 7. [Google Scholar] [CrossRef]
- Deng, M.; Dong, Y.; Xu, S.; Huang, S.; Zhai, X.; Fan, G. Genome-Wide Identification and Expression of the Paulownia fortunei MADS-Box Gene Family in Response to Phytoplasma Infection. Genes 2023, 14, 696. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Taganna, J. Genome-wide analysis of the U-box E3 ubiquitin ligase enzyme gene family in tomato. Sci. Rep. 2020, 10, 9581. [Google Scholar] [CrossRef] [PubMed]
- Grill, E.; Himmelbach, A. ABA signal transduction. Curr. Opin. Plant Biol. 1998, 1, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Bhuria, M.; Verma, D.; Garewal, N.; Singh, K. Genome-Wide Identification of BTB Domain-Containing Gene Family in Grapevine (Vitis vinifera L.). Agriculture 2023, 13, 252. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Zhao, C.L.; Wang, X.; Ding, G.Z.; Li, Y.L.; Chen, L. Genome-Wide Identification and Expression Analysis of the BTB Domain-Containing Protein Gene Family in Sugar Beet. Agronomy 2022, 12, 253. [Google Scholar] [CrossRef]
- YuMei, H.; KeKe, L.; HuaiXia, Z.; GuoXin, C.; Muhammad, A.; Saeed Ul, H.; AiMin, W.; ZhenHui, G. Contribution of CaBPM4, a BTB Domain–Containing Gene, to the Response of Pepper to Phytophthora capsici Infection and Abiotic Stresses. Agronomy 2019, 9, 417. [Google Scholar] [CrossRef]
- Santner, A.; Estelle, M. Recent advances and emerging trends in plant hormone signalling. Nature 2009, 459, 1071–1078. [Google Scholar] [CrossRef]
- Johnson, C.; Boden, E.; Arias, J. Salicylic acid and NPR1 induce the recruitment of trans-activating TGA factors to a defense gene promoter in Arabidopsis. Plant Cell 2003, 15, 1846–1858. [Google Scholar] [CrossRef]
- Chico, J.M.; Lechner, E.; Fernandez-Barbero, G.; Canibano, E.; García-Casado, G.; Franco-Zorrilla, J.M.; Hammann, P.; Zamarreño, A.M.; García-Mina, J.M.; Rubio, V.; et al. CUL3(BPM) E3 ubiquitin ligases regulate MYC2, MYC3, and MYC4 stability and JA responses. Proc. Natl. Acad. Sci. USA 2020, 117, 6205–6215. [Google Scholar] [CrossRef]
- Silva, K.J.P.; Brunings, A.; Peres, N.A.; Mou, Z.L.; Folta, K.M. The Arabidopsis NPR1 gene confers broad-spectrum disease resistance in strawberry. Transgenic Res. 2015, 24, 693–704. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.H.; Seo, D.H.; Chung, S.; Kim, S.W.; Lee, J.S.; Kim, W.T.; Lee, J.H. ABA-HYPERSENSITIVE BTB/POZ PROTEIN 1 functions as a negative regulator in ABA-mediated inhibition of germination in Arabidopsis. Plant Mol. Biol. 2016, 90, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Wang, Y.; Tu, G.; Chen, P.; Luan, S.; Lan, W. Type A2 BTB Members Decrease the ABA Response during Seed Germination by Affecting the Stability of SnRK2.3 in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 3153. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhao, Q.; Yang, Y.; Zhang, R.; Wang, X.; Zhang, T.; You, C.; Huo, H.; Hao, Y. Interaction of BTB-TAZ protein MdBT2 and DELLA protein MdRGL3a regulates nitrate-mediated plant growth. Plant Physiol. 2021, 186, 750–766. [Google Scholar] [CrossRef]
- Woodger, F.J.; Jacobsen, J.V.; Gubler, F. GMPOZ, a BTB/POZ domain nuclear protein, is a regulator of hormone responsive gene expression in barley aleurone. Plant Cell Physiol. 2004, 45, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhai, H.; He, S.; Zhu, H.; Gao, S.; Xing, S.; Wei, Z.; Zhao, N.; Liu, Q. The Sweetpotato BTB-TAZ Protein Gene, IbBT4, Enhances Drought Tolerance in Transgenic Arabidopsis. Front. Plant Sci. 2020, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, D.J.; Gagne, J.M.; Salter, D.W.; Hellmann, H.; Estelle, M.; Ma, L.G.; Vierstra, R.D. Cullins 3a and 3b assemble with members of the broad complex/tramtrack/bric-a-brac (BTB) protein family to form essential ubiquitin-protein ligases (E3s) in Arabidopsis. J. Biol. Chem. 2005, 280, 18810–18821. [Google Scholar] [CrossRef]
- Song, G.; Zhang, R.; Zhang, S.; Li, Y.; Gao, J.; Han, X.; Chen, M.; Wang, J.; Li, W.; Li, G. Response of microRNAs to cold treatment in the young spikes of common wheat. BMC Genom. 2017, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhai, X.; Cao, Y.; Zhao, H.; Wang, Z.; Liu, H.; Fan, G. Transcriptome and Small RNA Sequencing Analysis Revealed Roles of PaWB-Related miRNAs and Genes in Paulownia fortunei. Forests 2018, 9, 397. [Google Scholar] [CrossRef]
- Fan, G.; Cao, X.; Zhao, Z.; Deng, M. Transcriptome analysis of the genes related to the morphological changes of Paulownia tomentosa plantlets infected with phytoplasma. Acta Physiol. Plant. 2015, 37, 202. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Wang, Z.; Zhai, X.; Cao, Y. ceRNA Cross-Talk in Paulownia Witches’ Broom Disease. Int. J. Mol. Sci. 2018, 19, 2463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Niu, S.; Fan, G.; Deng, M.; Wang, Y. Genome-Wide Analysis of Gene and microRNA Expression in Diploid and Autotetraploid Paulownia fortunei (Seem) Hemsl. under Drought Stress by Transcriptome, microRNA, and Degradome Sequencing. Forests 2018, 9, 88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
