Effect of Plant Essential Oil Formulations on Bemisia tabaci MEAM1 (Gennadius) and Its Parasitoid Eretmocerus hayati (Zolnerowich and Rose)
Abstract
:1. Introduction
2. Results
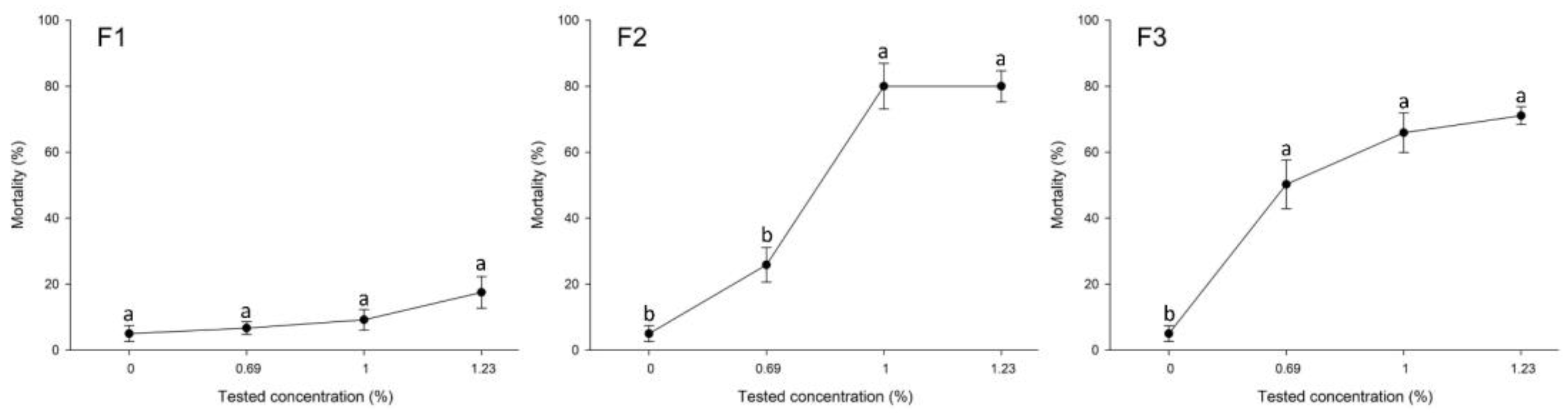
2.1. Toxicity Effects of the EO Formulations against the Eggs, Nymphal, and Adult Stages of B. tabaci
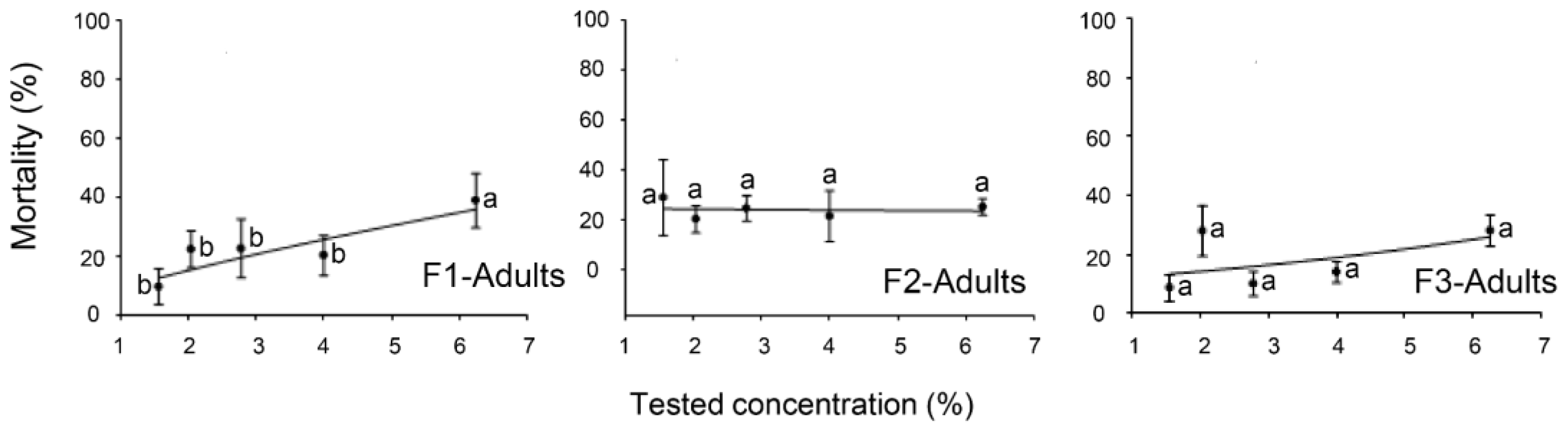
2.2. Toxicity of EOs Formulations against the Parasitoid E. hayati
3. Materials and Methods
3.1. Insects and Plants
3.2. Essential Oil Formulations
3.3. Toxicity of the EO Formulations against the Eggs, Nymphal, and Adult Stages of B. tabaci
3.4. Toxicity of EO Formulations against the Parasitoid E. hayati
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Perring, T.M. The Bemisia tabaci species complex. Crop Prot. 2001, 20, 725–737. [Google Scholar] [CrossRef]
- Ellsworth, P.C.; Martinez-Carrillo, J.L. IPM for Bemisia tabaci: A case study from North America. Crop Prot. 2001, 20, 853–869. [Google Scholar] [CrossRef]
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef]
- Abubakar, M.; Koul, B.; Chandrashekar, K.; Raut, A.; Yadav, D. Whitefly (Bemisia tabaci) Management (WFM) Strategies for Sustainable Agriculture: A Review. Agriculture 2022, 12, 1317. [Google Scholar] [CrossRef]
- Perring, T.M.; Stansly, P.A.; Liu, T.X.; Smith, H.A.; Andreason, S.A. Whiteflies: Biology, Ecology, and Management. In Sustainable Management of Arthropod Pests of Tomato; Academic Press: Cambridge, MA, USA, 2017; pp. 73–110. ISBN 9780128024416. [Google Scholar]
- Jones, D.R. Plant viruses transmitted by whiteflies. Eur. J. Plant Pathol. 2003, 109, 195–219. [Google Scholar] [CrossRef]
- Polston, J.E.; De Barro, P.; Boykin, L.M. Transmission specificities of plant viruses with the newly identified species of the Bemisia tabaci species complex. Pest Manag. Sci. 2014, 70, 1547–1552. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, S.E.; Ellsworth, P.C. The contribution of conservation biological control to integrated control of Bemisia tabaci in cotton. Biol. Control 2009, 51, 458–470. [Google Scholar] [CrossRef]
- Lahey, Z.; Stansly, P. An Updated List of Parasitoid Hymenoptera Reared from the Bemisia tabaci Species Complex (Hemiptera: Aleyrodidae). Fla. Entomol. 2015, 98, 456–463. [Google Scholar] [CrossRef]
- Xu, H.Y.; Yang, N.W.; Chi, H.; Ren, G.D.; Wan, F.H. Comparison of demographic fitness and biocontrol effectiveness of two parasitoids, Encarsia sophia and Eretmocerus hayati (Hymenoptera: Aphelinidae), against Bemisia tabaci (Hemiptera: Aleyrodidae). Pest Manag. Sci. 2018, 74, 2116–2124. [Google Scholar] [CrossRef]
- Yang, N.W.; Wan, F.H. Host suitability of different instars of Bemisia tabaci biotype B for the parasitoid Eretmocerus hayati. Biol. Control 2011, 59, 313–317. [Google Scholar] [CrossRef]
- Goolsby, J.A.; Debarro, P.J.; Kirk, A.A.; Sutherst, R.W.; Canas, L.; Ciomperlik, M.A.; Ellsworth, P.C.; Gould, J.R.; Hartley, D.M.; Hoelmer, K.A.; et al. Post-release evaluation of biological control of Bemisia tabaci biotype “B” in the USA and the development of predictive tools to guide introductions for other countries. Biol. Control 2005, 32, 70–77. [Google Scholar] [CrossRef]
- De Barro, P.J.; Subramaniam, S.; Coombs, M.; Kay, I.; Heisswolf, S. Improved Management Strategies for Silverleaf Whitefly in Vegetables; Horticulture Australia Ltd.: North Sydney, Australia, 2005; Project Number VX02016. [Google Scholar]
- De Barro, P.J.; Coombs, M.T. Post-release evaluation of Eretmocerus hayati zolnerowich and rose in Australia. Bull. Entomol. Res. 2009, 99, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabou, S. Biological control of Bemisia tabaci Biotype “B” (Homoptera: Aleyrodidae) by introduction, release and establishment of Eretmocerus hayati (Hymenoptera: Aphelinidae). J. Pest Sci. 2004, 77, 91–94. [Google Scholar] [CrossRef]
- Ma, D.; Gorman, K.; Devine, G.; Luo, W.; Denholm, I. The biotype and insecticide-resistance status of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae), invading cropping systems in Xinjiang Uygur Autonomous Region, northwestern China. Crop Prot. 2007, 26, 612–617. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Hagler, J.R. Conservation of natural enemies in cotton: Role of insect growth regulators in management of Bemisia tabaci. Biol. Control 2004, 30, 52–72. [Google Scholar] [CrossRef]
- Erdogan, C.; Moores, G.D.; Oktay Gurkan, M.; Gorman, K.J.; Denholm, I. Insecticide resistance and biotype status of populations of the tobacco whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) from Turkey. Crop Prot. 2008, 27, 600–605. [Google Scholar] [CrossRef]
- Byrne, F.J.; Oetting, R.D.; Bethke, J.A.; Green, C.; Chamberlin, J. Understanding the dynamics of neonicotinoid activity in the management of Bemisia tabaci whiteflies on poinsettias. Crop Prot. 2010, 29, 260–266. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Kontsedalov, S.; Ishaaya, I. Dynamics of resistance to the neonicotinoids acetamiprid and thiamethoxam in Bemisia tabaci (Homoptera: Aleyrodidae). J. Econ. Entomol. 2004, 97, 2051–2056. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, B.; Qiu, X.; Chen, M.; Ma, Z.; Yu, X. Distribution and risk assessment of 82 pesticides in Jiulong River and estuary in South China. Chemosphere 2016, 144, 1177–1192. [Google Scholar] [CrossRef]
- Hopkinson, J. New Insecticides for the Control of Silverleaf Whitefly (SLW) in Cotton, and Considerations for Resistance Management|the Beatsheet. Available online: https://thebeatsheet.com.au/new-insecticides-for-the-control-of-silverleaf-whitefly-slw-in-cotton/ (accessed on 26 July 2023).
- Hopkinson, J. Impact of Insecticides on Silverleaf Whitefly Parasitoid|the Beatsheet. Available online: https://thebeatsheet.com.au/impact-of-insecticides-on-silverleaf-whitefly-parasitoid/ (accessed on 26 July 2023).
- Feng, Y.; Zhang, A. A Floral Fragrance, Methyl Benzoate, is An Efficient Green Pesticide. Sci. Rep. 2017, 7, 42168. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Jhan, P.K.; Shim, J.K.; Lee, K.Y. Methyl benzoate exhibits insecticidal and repellent activities against Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). PLoS ONE 2018, 13, e0208552. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Insecticidal efficacy of three benzoate derivatives against Aphis gossypii and its predator Chrysoperla carnea. Ecotoxicol. Environ. Saf. 2019, 184, 109653. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Zhang, A. Commercially Available Natural Benzyl Esters and Their Synthetic Analogs Exhibit Different Toxicities against Insect Pests. Sci. Rep. 2018, 8, 7902. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Shim, J.K.; Hwang, H.S.; Bunch, H.; Lee, K.Y. Acaricidal effects of methyl benzoate against Tetranychus urticae Koch (Acari: Tetranychidae) on common crop plants. Pest Manag. Sci. 2020, 76, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Hassan, E.; Mostafiz, M.M.; Iramu, E.T.; George, D.; Lee, K.-Y. Evaluation of the Effect of Fungatol and Gamma-T-ol on the Emergence and Adult Parasitoid Survival of Mummies of Cotton Aphids Parasitized by Aphidius colemani. Insects 2021, 13, 38. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Shim, J.K.; Lee, K.Y. Lethal and sublethal effects of methyl benzoate on the predatory bug Nesidiocoris tenuis. Insects 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Güncan, A.; Lee, K.Y. Evaluation of Lethal and Sublethal Effects of Methyl Benzoate on the Generalist Predator Orius laevigatus (Fieber). J. Econ. Entomol. 2022, 115, 1911–1920. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Paes, J.L.; Faroni, L.R.D.A.; Dhingra, O.D.; Cecon, P.R.; Silva, T.A. Insecticidal fumigant action of mustard essential oil against Sitophilus zeamais in maize grains. Crop Prot. 2012, 34, 56–58. [Google Scholar] [CrossRef]
- Konecka, E.; Kaznowski, A.; Marcinkiewicz, W.; Tomkowiak, D.; Maciąg, M.; Stachowiak, M. Insecticidal activity of Brassica alba mustard oil against lepidopteran pests Cydia pomonella (Lepidoptera: Tortricidae), Dendrolimus pini (Lepidoptera: Lasiocampidae), and Spodoptera exigua (Lepidoptera: Noctuidae). J. Plant Prot. Res. 2018, 58, 206–209. [Google Scholar] [CrossRef]
- Liu, T.X.; Stansly, P.A. Insecticidal activity of surfactants and oils against silverleaf whitefly (Bemisia argentifolii) nymphs (Homoptera: Aleyrodidae) on collards and tomato. Pest Manag. Sci. 2000, 56, 861–866. [Google Scholar] [CrossRef]
- Cis, J.; Nowak, G.; Kisiel, W. Antifeedant properties and chemotaxonomic implications of sesquiterpene lactones and syringin from Rhaponticum pulchrum. Biochem. Syst. Ecol. 2006, 34, 862–867. [Google Scholar] [CrossRef]
- Chowdhury, N.Y.; Islam, W.; Khalequzzaman, M. Insecticidal activity of compounds from the leaves of Vitex negundo (Verbenaceae) against Tribolium castaneum (Coleoptera: Tenebrionidae). Int. J. Trop. Insect Sci. 2011, 31, 174–181. [Google Scholar] [CrossRef]
- Saguez, J.; Attoumbré, J.; Giordanengo, P.; Baltora-Rosset, S. Biological activities of lignans and neolignans on the aphid Myzus persicae (Sulzer). Arthropod. Plant. Interact. 2013, 7, 225–233. [Google Scholar] [CrossRef]
- McKenzie, C.L.; Weathersbee, A.A.; Puterka, G.J. Toxicity of sucrose octanoate to egg, nymphal, and adult Bemisia tabaci (Hemiptera: Aleyrodidae) using a novel plant-based bioassay. J. Econ. Entomol. 2005, 98, 1242–1247. [Google Scholar] [CrossRef]
- McKenzie, C.L.; Weathersbee, A.A.; Hunter, W.B.; Puterka, G.J. Sucrose Octanoate Toxicity to Brown Citrus Aphid (Homoptera: Aphididae) and the parasitoid Lysiphlebus testaceipes (Hymenoptera: Aphidiidae). J. Econ. Entomol. 2009, 97, 1233–1238. [Google Scholar] [CrossRef]
- Sterk, G.; Hassan, S.A.; Baillod, M.; Bakker, F.; Bigler, F.; Blümel, S.; Bogenschütz, H.; Boller, E.; Bromand, B.; Brun, J.; et al. Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS-Working Group ‘Pesticides and Beneficial Organisms’. BioControl 1999, 44, 99–117. [Google Scholar] [CrossRef]
- Fiume, M.M.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Decyl Glucoside and Other Alkyl Glucosides as Used in Cosmetics. Int. J. Toxicol. 2013, 32, 22S–48S. [Google Scholar] [CrossRef] [PubMed]
- Grygier, A. Mustard Seeds as a Bioactive Component of Food. Food Rev. Int. 2023, 39, 4088–4101. [Google Scholar] [CrossRef]
- Peng, C.; Zhao, S.Q.; Zhang, J.; Huang, G.Y.; Chen, L.Y.; Zhao, F.Y. Chemical composition, antimicrobial property and microencapsulation of Mustard (Sinapis alba) seed essential oil by complex coacervation. Food Chem. 2014, 165, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Frauenkron, M.; Melder, J.-P.; Ruider, G.; Rossbacher, R.; Höke, H. Ethanolamines and Propanolamines. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Avery, P.B.; Faull, J.; Simmonds, M.S.J. Effect of different photoperiods on the growth, infectivity and colonization of Trinidadian strains of Paecilomyces fumosoroseus on the greenhouse whitefly, Trialeurodes vaporariorum, using a glass slide bioassay. J. Insect Sci. 2004, 4, 38. [Google Scholar] [CrossRef]
- Yang, N.W.; Li, A.L.; Wan, F.H.; Liu, W.X.; Johnson, D. Effects of plant essential oils on immature and adult sweetpotato whitefly, Bemisia tabaci biotype B. Crop Prot. 2010, 29, 1200–1207. [Google Scholar] [CrossRef]
- Al-mazra’awi, M.S.; Ateyyat, M. Insecticidal and repellent activities of medicinal plant extracts against the sweet potato whitefly, Bemisia tabaci (Hom.: Aleyrodidae) and its parasitoid Eretmocerus mundus (Hym.: Aphelinidae). J. Pest Sci. 2009, 82, 149–154. [Google Scholar] [CrossRef]
- Kim, S.I.; Chae, S.H.; Youn, H.S.; Yeon, S.H.; Ahn, Y.J. Contact and fumigant toxicity of plant essential oils and efficacy of spray formulations containing the oils against B- and Q-biotypes of Bemisia tabaci. Pest Manag. Sci. 2011, 67, 1093–1099. [Google Scholar] [CrossRef]
- Simmons, A.M.; Shaaban, A.R. Populations of predators and parasitoids of Bemisia tabaci (Hemiptera: Aleyrodidae) after the application of eight biorational insecticides in vegetable crops. Pest Manag. Sci. 2011, 67, 1023–1028. [Google Scholar] [CrossRef]
- Kumar, P.; Whitten, M.; Thoeming, G.; Borgemeister, C.; Poehling, H.M. Effects of bio-pesticides on Eretmocerus warrae (Hym., Aphelinidae), a parasitoid of Bemisia tabaci (Hom., Aleyrodidae). J. Appl. Entomol. 2008, 132, 605–613. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar]
- Zacharia, J.T. Identity, Physical and Chemical Properties of Pesticides. In Pesticides in the Modern World—Trends in Pesticides Analysis; IntechOpen: Rijeka, Croatia, 2011; ISBN 978-953-307-437-5. [Google Scholar]
- Rodriguez-Saona, C.; Wanumen, A.C.; Salamanca, J.; Holdcraft, R.; Kyryczenko-Roth, V. Toxicity of insecticides on various life stages of two tortricid pests of cranberries and on a non-target predator. Insects 2016, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Boulamtat, R.; Mesfioui, A.; El-Fakhouri, K.; Oubayoucef, A.; Sabraoui, A.; Aasfar, A.; El-Bouhssini, M. Chemical composition, and insecticidal activities of four plant essential oils from Morocco against larvae of Helicoverpa armigera (Hub.) under field and laboratory conditions. Crop Prot. 2021, 144, 105607. [Google Scholar] [CrossRef]
- Ma, D.L.; Gordh, G.; Zalucki, M.P. Biological effects of azadirachtin on Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) fed on cotton and artificial diet. Aust. J. Entomol. 2000, 39, 301–304. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Flinn, P.W. Integrated Pest Management; CRC Press: Boca Raton, FL, USA, 2018; Volume 4, ISBN 9789400777965. [Google Scholar]
- Hajek, A.E. Natural Enemies: An Introduction to Biological Control; Cambridge University Press: Cambridge, UK, 2004; ISBN 9780521652957. [Google Scholar]
- Souto, A.L.; Sylvestre, M.; Tölke, E.D.; Tavares, J.F.; Barbosa-Filho, J.M.; Cebrián-Torrejón, G. Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: Prospects, applications and challenges. Molecules 2021, 26, 4835. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D. Botanical pesticides for eco-friendly pest management: Drawbacks and limitations. In Pesticides in Crop Production: Physiological and Biochemical Action; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 181–193. ISBN 9781119432241. [Google Scholar]


| Formulations | SLW Stage | n | LC50 | LC90 | p | ||
|---|---|---|---|---|---|---|---|
| (%) (±SE) | 95% CI | (%) (±SE) | 95% CI | ||||
| F1 | Egg | 600 | 0.73 (±0.026) | 0.684–0.789 | 1.59 (±0.117) | 1.405–1.885 | <0.001 |
| F2 | Egg | 599 | 1.02 (±0.062) | 0.913–1.169 | 3.43 (±0.601) | 2.580–5.299 | <0.001 |
| F3 | Egg | 600 | 1.05 (±0.052) | 0.958–1.168 | 2.69 (±0.348) | 2.172–3.685 | <0.001 |
| F1 | YN | 600 | 0.69 (±0.042) | 0.608–0.778 | 3.15 (±0.60) | 2.317–5.061 | <0.001 |
| F2 | YN | 600 | 0.65 (±0.023) | 0.608–0.698 | 1.36 (±0.086) | 1.213–1.565 | <0.001 |
| F3 | YN | 600 | 0.88 (±0.028) | 0.830–0.942 | 1.68 (±0.114) | 1.500–1.971 | <0.001 |
| F1 | ON | 600 | 1.03 (±0.048) | 0.944–1.139 | 2.53 (±0.305) | 2.076–3.390 | <0.001 |
| F2 | ON | 600 | 0.91 (±0.031) | 0.854–0.976 | 1.81 (±0.136) | 1.589–2.152 | <0.001 |
| F3 | ON | 589 | 0.90 (±0.041) | 0.829–0.994 | 2.40 (±0.285) | 1.968–3.187 | <0.001 |
| Formulation 1 | Formulation 2 | Formulation 3 | % |
|---|---|---|---|
| Lauryl glucoside | Laureth carboxylate | Lauryl glucoside | 20 |
| MW 100 emulsifier | MW 100 emulsifier | MW 100 emulsifier | 40 |
| Mustard oil | Mustard oil | Mustard oil | 20 |
| Cellosolve acetate | Monoethanolamine | Monoethanolamine | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, E.; Obaidoon, Y.; Mostafiz, M.M.; Senior, L. Effect of Plant Essential Oil Formulations on Bemisia tabaci MEAM1 (Gennadius) and Its Parasitoid Eretmocerus hayati (Zolnerowich and Rose). Plants 2023, 12, 4137. https://doi.org/10.3390/plants12244137
Hassan E, Obaidoon Y, Mostafiz MM, Senior L. Effect of Plant Essential Oil Formulations on Bemisia tabaci MEAM1 (Gennadius) and Its Parasitoid Eretmocerus hayati (Zolnerowich and Rose). Plants. 2023; 12(24):4137. https://doi.org/10.3390/plants12244137
Chicago/Turabian StyleHassan, Errol, Yasir Obaidoon, Md Munir Mostafiz, and Lara Senior. 2023. "Effect of Plant Essential Oil Formulations on Bemisia tabaci MEAM1 (Gennadius) and Its Parasitoid Eretmocerus hayati (Zolnerowich and Rose)" Plants 12, no. 24: 4137. https://doi.org/10.3390/plants12244137
APA StyleHassan, E., Obaidoon, Y., Mostafiz, M. M., & Senior, L. (2023). Effect of Plant Essential Oil Formulations on Bemisia tabaci MEAM1 (Gennadius) and Its Parasitoid Eretmocerus hayati (Zolnerowich and Rose). Plants, 12(24), 4137. https://doi.org/10.3390/plants12244137









