The Population Divergence and Genetic Basis of Local Adaptation of Wild Soybean (Glycine soja) in China
Abstract
1. Introduction
2. Results
2.1. Phenotypic Variation in the Wild Soybean Population
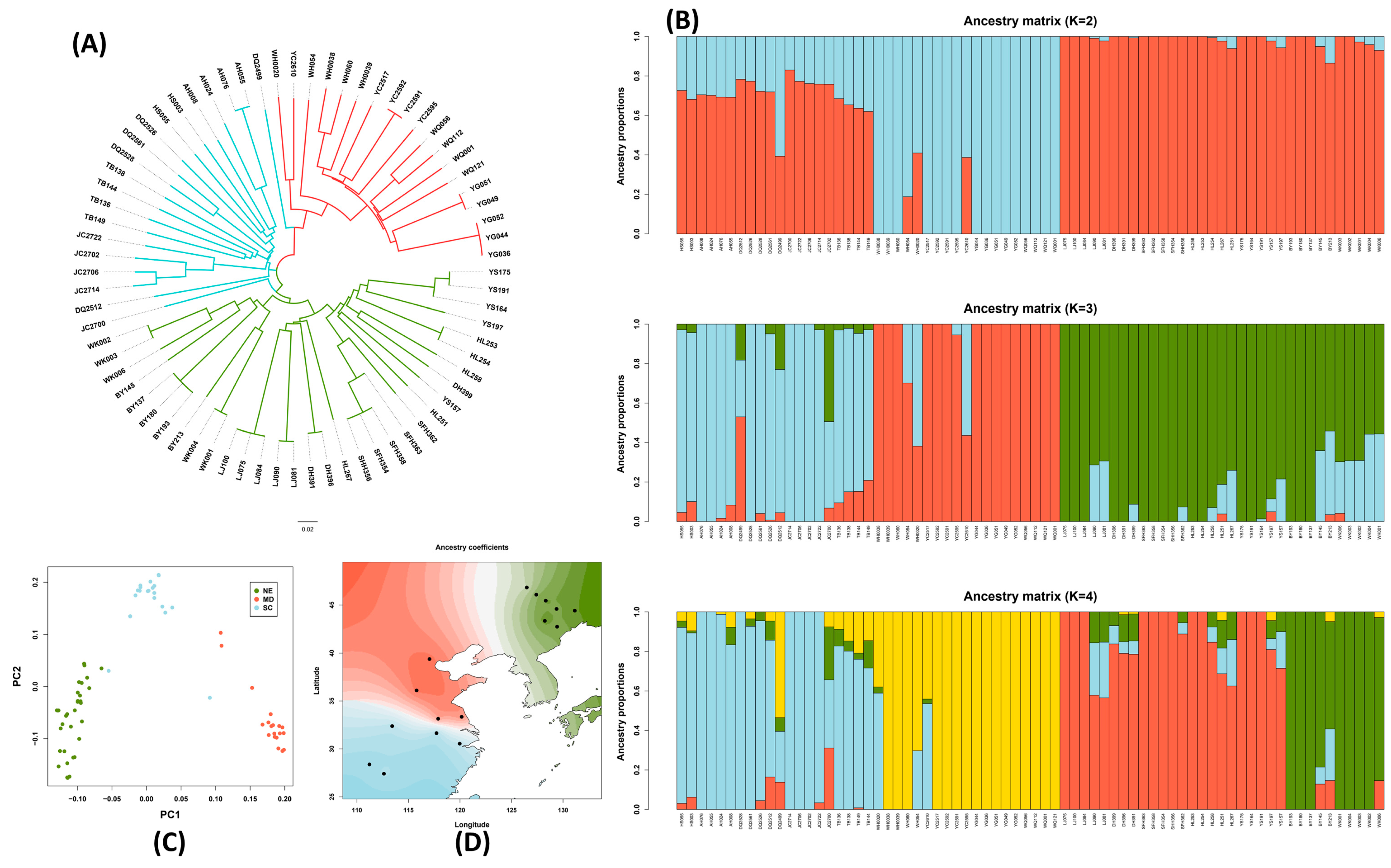
2.2. Genetic Divergence and Geographic Population Structure in Wild Soybean
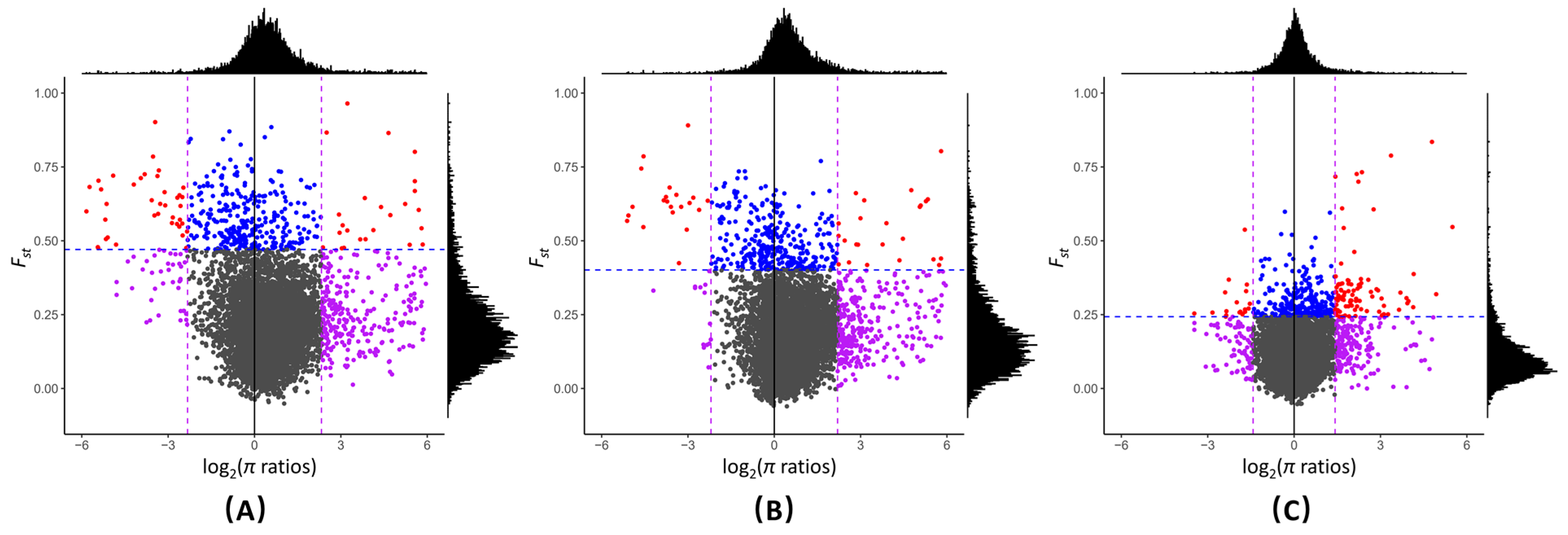
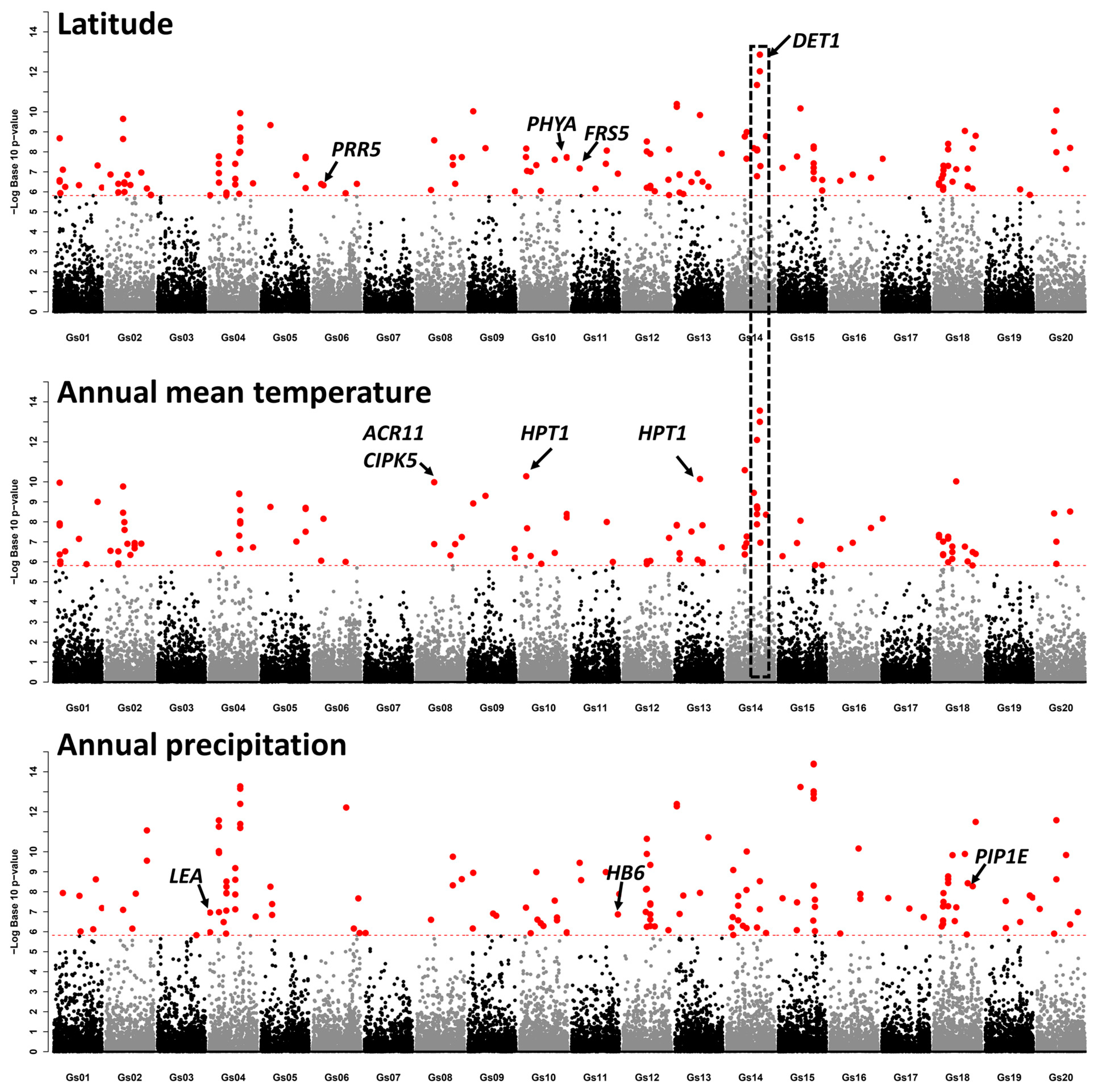
2.3. Selection Signatures between Genetic Groups and the Genetic Loci Associated with Local Adaptation in G. soja
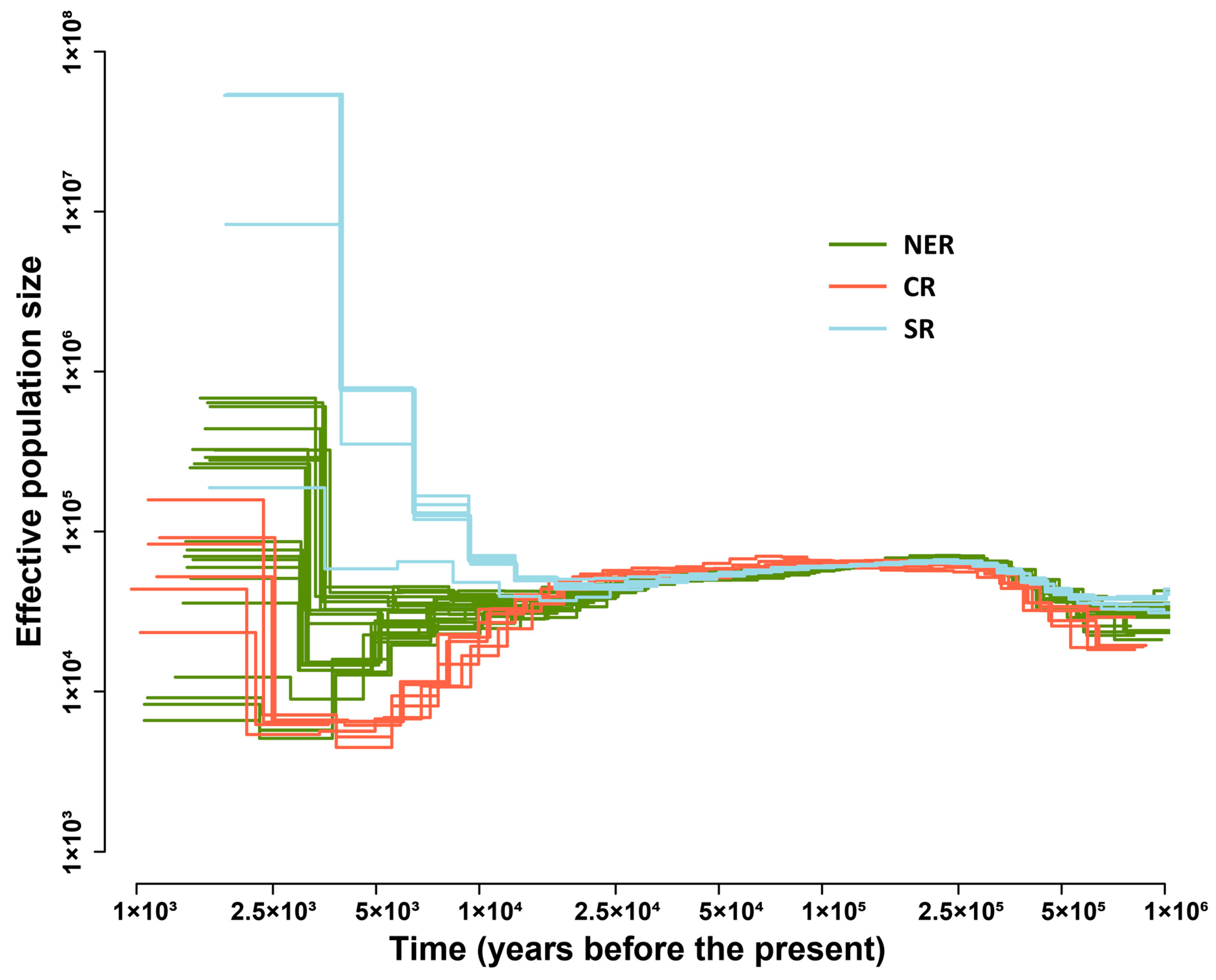
2.4. The Demographic History of the Three G. soja Genetic Groups
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. DNA Exaction, Genotyping by Sequencing (GBS), and Whole-Genome Sequencing (WGS)
4.3. Data Processing and Variant Calling
4.4. Phylogenetic Tree, PCA, and Population Structure
4.5. Mantel Test for Correlation between Genetic Distance and Geographic Distance
4.6. Identification of Selective Sweeps
4.7. Ecological Association Tests and Significantly Associated Loci (SALs)
4.8. Inference of Demographic History
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajjar, R.; Hodgkin, T. The use of wild relatives in crop improvement: A survey of developments over the last 20 years. Euphytica 2007, 156, 1–13. [Google Scholar] [CrossRef]
- Tanksley, S.D.; McCouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Tirnaz, S.; Zandberg, J.; Thomas, W.J.W.; Marsh, J.; Edwards, D.; Batley, J. Application of crop wild relatives in modern breeding: An overview of resources, experimental and computational methodologies. Front. Plant Sci. 2022, 13, 1008904. [Google Scholar] [CrossRef] [PubMed]
- Sedivy, E.J.; Wu, F.Q.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Phytol. 2017, 214, 539–553. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Lin, X.; Chan, T.F.; Lam, H.M.; Baloch, F.S.; Ali, M.A.; Golokhvast, K.S.; Yang, S.H.; Chung, G. Genetic architecture of wild soybean (Glycine soja Sieb. and Zucc.) populations originating from different East Asian regions. Genet. Resour. Crop Evol. 2021, 68, 1577–1588. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.F.; Wang, Y.S.; Chen, J.J.; Li, Y.H.; Huang, H.W.; Qiu, L.J.; Wang, Y. Population structure of the wild soybean (Glycine soja) in China: Implications from microsatellite analyses. Ann. Bot. 2012, 110, 777–785. [Google Scholar] [CrossRef][Green Version]
- Dong, Y.S.; Zhuang, B.C.; Zhao, L.M.; Sun, H.; He, M.Y. The genetic diversity of annual wild soybeans grown in China. Theor. Appl. Genet. 2001, 103, 98–103. [Google Scholar] [CrossRef]
- Wang, Y.S.; Shahid, M.Q.; Ghouri, F.; Baloch, F.S.; Wang, Y.; Huang, H.W. Evaluation of the geographical pattern of genetic diversity of Glycine soja and Glycine max based on four single copy nuclear gene loci: For conservation of soybean germplasm. Biochem. Syst. Ecol. 2015, 62, 229–235. [Google Scholar] [CrossRef]
- Wang, J.; Hu, Z.B.; Liao, X.L.; Wang, Z.Y.; Li, W.; Zhang, P.P.; Cheng, H.; Wang, Q.; Bhat, J.A.; Wang, H.; et al. Whole-genome resequencing reveals signature of local adaptation and divergence in wild soybean. Evol. Appl. 2022, 15, 1820–1833. [Google Scholar] [CrossRef]
- Leamy, L.J.; Lee, C.R.; Song, Q.J.; Mujacic, I.; Luo, Y.; Chen, C.Y.; Li, C.B.; Kjemtrup, S.; Song, B.H. Environmental versus geographical effects on genomic variation in wild soybean (Glycine soja) across its native range in northeast Asia. Ecol. Evol. 2016, 6, 6332–6344. [Google Scholar] [CrossRef]
- He, S.L.; Wang, Y.S.; Li, D.Z.; Yi, T.S. Environmental and Historical Determinants of Patterns of Genetic Differentiation in Wild Soybean (Glycine soja Sieb. et Zucc). Sci. Rep. 2016, 6, 22795. [Google Scholar] [CrossRef]
- Kim, M.S.; Lozano, R.; Kim, J.H.; Bae, D.N.; Kim, S.T.; Park, J.H.; Choi, M.S.; Kim, J.; Ok, H.C.; Park, S.K.; et al. The patterns of deleterious mutations during the domestication of soybean. Nat. Commun. 2021, 12, 97. [Google Scholar] [CrossRef]
- Bishop, J.G.; Ripoll, D.R.; Bashir, S.; Damasceno, C.M.B.; Seeds, J.D.; Rose, J.K.C. Selection on glycine β-1,3-endoglucanase genes differentially inhibited by a phytophthora glucanase inhibitor protein. Genetics 2005, 169, 1009–1019. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hu, Z.-A.; Jiang, G.-Q.; Deng, X.; Wang, H.-X. Molecular adaptation through diversity of retrotransposons and transcriptional factors in populations of wild soybean (Glycine soja). J. Plant Ecol. 2007, 31, 952–959. (In Chinese) [Google Scholar]
- Harrison, M.T.; Tardieu, F.; Dong, Z.S.; Messina, C.D.; Hammer, G.L. Characterizing drought stress and trait influence on maize yield under current and future conditions. Glob. Chang. Biol. 2014, 20, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Harrison, M.T.; Yan, H.L.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.Y.; Jaegermeyr, J.; et al. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef]
- Li, Y.H.; Qin, C.; Wang, L.; Jiao, C.Z.; Hong, H.L.; Tian, Y.; Li, Y.F.; Xing, G.N.; Wang, J.; Gu, Y.Z.; et al. Genome-wide signatures of the geographic expansion and breeding of soybean. Sci. China Life Sci. 2023, 66, 350–365. [Google Scholar] [CrossRef]
- Qi, X.P.; Li, M.W.; Xie, M.; Liu, X.; Ni, M.; Shao, G.H.; Song, C.; Yim, A.K.Y.; Tao, Y.; Wong, F.L.; et al. Identification of a novel salt tolerance gene in wild soybean by whole-genome sequencing. Nat. Commun. 2014, 5, 4340. [Google Scholar] [CrossRef]
- Shen, X.J.; Wang, Y.Y.; Zhang, Y.X.; Guo, W.; Jiao, Y.Q.; Zhou, X.A. Overexpression of the Wild Soybean R2R3-MYB Transcription Factor Enhances Resistance to Salt Stress and in Transgenic. Int. J. Mol. Sci. 2018, 19, 3958. [Google Scholar] [CrossRef]
- Wang, H.J.; Yin, X.L.; Du, D.; Liang, Z.Y.; Han, Z.Z.; Nian, H.; Ma, Q.B. GsMYB7 encoding a R2R3-type MYB transcription factor enhances the tolerance to aluminum stress in soybean (Glycine max L.). BMC Genom. 2022, 23, 529. [Google Scholar] [CrossRef]
- Ning, W.F.; Zhai, H.; Yu, J.Q.; Liang, S.; Yang, X.; Xing, X.Y.; Huo, J.L.; Pang, T.; Yang, Y.L.; Bai, X. Overexpression of Glycine soja WRKY20 enhances drought tolerance and improves plant yields under drought stress in transgenic soybean. Mol. Breed. 2017, 37, 19. [Google Scholar] [CrossRef]
- Xun, H.W.; Qian, X.Y.; Wang, M.; Yu, J.X.; Zhang, X.; Pang, J.S.; Wang, S.C.; Jiang, L.L.; Dong, Y.S.; Liu, B. Overexpression of a Cinnamyl Alcohol Dehydrogenase-Coding Gene, GsCAD1, from Wild Soybean Enhances Resistance to Soybean Mosaic Virus. Int. J. Mol. Sci. 2022, 23, 15206. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.D.; Li, S.C.; Wang, L.S.; Su, T.; Zhang, C.B.; Bi, Y.D.; Lai, Y.C.; Kong, L.P.; Wang, F.; Pei, X.X.; et al. The genetic basis of high-latitude adaptation in wild soybean. Curr. Biol. 2023, 33, 252–262.e4. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.D.; Cheng, Q.; Fang, C.; Kong, L.P.; Yang, H.; Hou, Z.H.; Li, Y.L.; Nan, H.Y.; Zhang, Y.H.; Chen, Q.S.; et al. Parallel selection of distinct Tof5 alleles drove the adaptation of cultivated and wild soybean to high latitudes. Mol. Plant 2022, 15, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.H.; Fang, C.; Liu, B.H.; Yang, H.; Kong, F.J. Origin, variation, and selection of natural alleles controlling flowering and adaptation in wild and cultivated soybean. Mol. Breed. 2023, 43, 36. [Google Scholar] [CrossRef]
- Yang, G.; Li, W.; Fan, C.; Liu, M.; Liu, J.X.; Liang, W.W.; Wang, L.; Di, S.F.; Fang, C.; Li, H.Y.; et al. Genome-wide association study uncovers major genetic loci associated with flowering time in response to active accumulated temperature in wild soybean population. BMC Genom. 2022, 23, 749. [Google Scholar] [CrossRef]
- Kim, D.Y.; Heo, J.H.; Pack, I.S.; Park, J.H.; Um, M.S.; Kim, H.J.; Park, K.W.; Nam, K.H.; Oh, S.D.; Kim, J.K.; et al. Natural hybridization between transgenic and wild soybean genotypes. Plant Biotechnol. Rep. 2021, 15, 299–308. [Google Scholar] [CrossRef]
- Wang, X.T.; Chen, L.Y.; Ma, J.X. Genomic introgression through interspecific hybridization counteracts genetic bottleneck during soybean domestication. Genome Biol. 2019, 20, 22. [Google Scholar] [CrossRef]
- Maeda, H.; Song, W.; Sage, T.L.; DellaPenna, D. Tocopherols play a crucial role in low-temperature adaptation and phloem loading in. Plant Cell 2006, 18, 2710–2732. [Google Scholar] [CrossRef]
- Goulas, E.; Schubert, M.; Kieselbach, T.; Kleczkowski, L.A.; Gardeström, P.; Schröder, W.; Hurry, V. The chloroplast lumen and stromal proteomes of Arabidopsis thaliana show differential sensitivity to short- and long-term exposure to low temperature. Plant J. 2006, 47, 720–734. [Google Scholar] [CrossRef]
- Li, R.F.; Zhang, J.W.; Wei, J.H.; Wang, H.Z.; Wang, Y.Z.; Ma, R.C. Functions and mechanisms of the CBL-CIPK signaling system in plant response to abiotic stress. Prog. Nat. Sci.-Mater. 2009, 19, 667–676. [Google Scholar] [CrossRef]
- Tang, R.J.; Wang, C.; Li, K.L.; Luan, S. The CBL-CIPK Calcium Signaling Network: Unified Paradigm from 20 Years of Discoveries. Trends Plant Sci. 2020, 25, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, Y.M.; Xiong, L.Z. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol. 2007, 144, 1416–1428. [Google Scholar] [CrossRef] [PubMed]
- Abdula, S.E.; Lee, H.J.; Ryu, H.; Kang, K.K.; Nou, I.; Sorrells, M.E.; Cho, Y.G. Overexpression of BrCIPK1 Gene Enhances Abiotic Stress Tolerance by Increasing Proline Biosynthesis in Rice. Plant Mol. Biol. Rep. 2016, 34, 501–511. [Google Scholar] [CrossRef]
- Hu, D.G.; Ma, Q.J.; Sun, C.H.; Sun, M.H.; You, C.X.; Hao, Y.J. Overexpression of MdSOS2L1, a CIPK protein kinase, increases the antioxidant metabolites to enhance salt tolerance in apple and tomato. Physiol. Plant. 2016, 156, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.X.; Ding, Y.L.; Zhao, T.J.; Gai, J.Y. Genetic diversity and peculiarity of annual wild soybean (G. soja Sieb. et Zucc.) from various eco-regions in China. Theor. Appl. Genet. 2009, 119, 371–381. [Google Scholar] [CrossRef]
- Li, X.H.; Wang, K.J.; Jia, J.Z. Genetic diversity and differentiation of Chinese wild soybean germplasm (G. soja Sieb. & Zucc.) in geographical scale revealed by SSR markers. Plant Breed. 2009, 128, 658–664. [Google Scholar]
- Osman, M.B.; Tierney, J.E.; Zhu, J.; Tardif, R.; Hakim, G.J.; King, J.; Poulsen, C.J. Globally resolved surface temperatures since the Last Glacial Maximum. Nature 2021, 599, 239–244. [Google Scholar] [CrossRef]
- Carter, T.E.J.; Hymowitz, T.; Nelson, R.L. Biogeography, Local Adaptation, Vavilov, and Genetic Diversity in Soybean. In Biological Resources and Migration; Werner, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 47–59. [Google Scholar] [CrossRef]
- Hyten, D.L.; Song, Q.J.; Zhu, Y.L.; Choi, I.Y.; Nelson, R.L.; Costa, J.M.; Specht, J.E.; Shoemaker, R.C.; Cregan, P.B. Impacts of genetic bottlenecks on soybean genome diversity. Proc. Natl. Acad. Sci. USA 2006, 103, 16666–16671. [Google Scholar] [CrossRef]
- Li, Y.H.; Li, W.; Zhang, C.; Yang, L.A.; Chang, R.Z.; Gaut, B.S.; Qiu, L.J. Genetic diversity in domesticated soybean (Glycine max) and its wild progenitor (Glycine soja) for simple sequence repeat and single-nucleotide polymorphism loci. New Phytol. 2010, 188, 242–253. [Google Scholar] [CrossRef]
- Lam, H.M.; Xu, X.; Liu, X.; Chen, W.B.; Yang, G.H.; Wong, F.L.; Li, M.W.; He, W.M.; Qin, N.; Wang, B.; et al. Resequencing of 31 wild and cultivated soybean genomes identifies patterns of genetic diversity and selection. Nat. Genet. 2010, 42, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Caye, K.; Jay, F.; Michel, O.; François, O. Fast Inference of Individual Admixture Coefficients Using Geographic Data. Ann. Appl. Stat. 2018, 12, 586–608. [Google Scholar] [CrossRef]
- Frichot, E.; François, O. LEA: An R package for landscape and ecological association studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Jing, C.Y.; Zhang, F.M.; Wang, X.H.; Wang, M.X.; Zhou, L.; Cai, Z.; Han, J.D.; Geng, M.F.; Yu, W.H.; Jiao, Z.H.; et al. Multiple domestications of Asian rice. Nat. Plants 2023, 9, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Proc, G.P.D. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Haubold, B.; Mitchell-Olds, T. Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis, and related genera (Brassicaceae). Mol. Biol. Evol. 2000, 17, 1483–1498. [Google Scholar] [CrossRef] [PubMed]
| Trait | Phenotype | NER a | CR b | SR c |
|---|---|---|---|---|
| Flower color | Purple | 32 | 16 | 16 |
| Pubescence color | Brown | 32 | 16 | 16 |
| Seed coat color | Black | 32 | 16 | 16 |
| Leaf shape | Lanceolate | 6 | 2 | 2 |
| Ovoid | 15 | 2 | 3 | |
| Ellipse | 11 | 12 | 11 | |
| Pubescence type | Erect | 0 | 0 | 5 |
| Oblique | 15 | 14 | 8 | |
| Flat | 17 | 2 | 3 | |
| Seed shape | Round | 1 | 0 | 0 |
| Oblate | 6 | 0 | 1 | |
| Ellipse | 1 | 0 | 0 | |
| Flat ellipse | 24 | 16 | 13 | |
| Long ellipse | 0 | 0 | 2 | |
| Hilum color | Yellow | 1 | 1 | 2 |
| Light black | 1 | 3 | 4 | |
| Black | 30 | 12 | 10 | |
| Seed coat bloom | No | 0 | 0 | 3 |
| Yes | 32 | 16 | 13 | |
| Seed luster | No | 28 | 11 | 9 |
| Weak | 4 | 5 | 7 | |
| Pod shape | Straight | 10 | 3 | 2 |
| Bent | 22 | 13 | 14 | |
| Pod color | Brown | 5 | 6 | 7 |
| Dark brown | 15 | 7 | 8 | |
| Black | 12 | 3 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, P.; Ding, X.; Wang, Y.; Qi, G.; Yu, J.; Zeng, Y.; Cai, D.; Yang, X.; Yang, J.; et al. The Population Divergence and Genetic Basis of Local Adaptation of Wild Soybean (Glycine soja) in China. Plants 2023, 12, 4128. https://doi.org/10.3390/plants12244128
Liu X, Li P, Ding X, Wang Y, Qi G, Yu J, Zeng Y, Cai D, Yang X, Yang J, et al. The Population Divergence and Genetic Basis of Local Adaptation of Wild Soybean (Glycine soja) in China. Plants. 2023; 12(24):4128. https://doi.org/10.3390/plants12244128
Chicago/Turabian StyleLiu, Xiaodong, Peiyuan Li, Xiaoyang Ding, Ying Wang, Guangxun Qi, Jiaxin Yu, Yong Zeng, Dezhi Cai, Xuhang Yang, Jiahui Yang, and et al. 2023. "The Population Divergence and Genetic Basis of Local Adaptation of Wild Soybean (Glycine soja) in China" Plants 12, no. 24: 4128. https://doi.org/10.3390/plants12244128
APA StyleLiu, X., Li, P., Ding, X., Wang, Y., Qi, G., Yu, J., Zeng, Y., Cai, D., Yang, X., Yang, J., Xu, C., Liu, B., Dong, Y., & Zhao, N. (2023). The Population Divergence and Genetic Basis of Local Adaptation of Wild Soybean (Glycine soja) in China. Plants, 12(24), 4128. https://doi.org/10.3390/plants12244128






